Background: Ectonucleoside triphosphate diphosphohydrolases (ENTPDs) hydrolyze extracellular ATP and diminish ATP signaling.
Results: ENTPD inhibition increases the fibrotic phenotype of cardiac fibroblasts (CFs) by enhancing pro-fibrotic response to ATP and attenuating anti-fibrotic response to adenosine.
Conclusion: ENTPDs integrate ATP and adenosine signaling to regulate the CF phenotype.
Significance: ENTPD is an important regulatory component controlling ATP- and adenosine-mediated CF homeostasis.
Keywords: Adenosine Receptor, ATPases, Fibroblast, Myofibroblast, Purinergic Receptor, ENTPD, P2Y
Abstract
The establishment of set points for cellular activities is essential in regulating homeostasis. Here, we demonstrate key determinants of the fibrogenic set point of cardiac fibroblasts (CFs) by focusing on the pro-fibrotic activity of ATP, which is released by CFs. We tested the hypothesis that the hydrolysis of extracellular ATP by ectonucleoside triphosphate diphosphohydrolases (ENTPDs) regulates pro-fibrotic nucleotide signaling. We detected two ENTPD isoforms, ENTPD-1 and -2, in adult rat ventricular CFs. Partial knockdown of ENTPD-1 and -2 with siRNA increased basal extracellular ATP concentration and enhanced the pro-fibrotic effect of ATP stimulation. Sodium polyoxotungstate-1, an ENTPD inhibitor, not only enhanced the pro-fibrotic effects of exogenously added ATP but also increased basal expression of α-smooth muscle actin, plasminogen activator inhibitor-1 and transforming growth factor (TGF)-β, collagen synthesis, and gel contraction. Furthermore, we found that adenosine, a product of ATP hydrolysis by ENTPD, acts via A2B receptors to counterbalance the pro-fibrotic response to ATP. Removal of extracellular adenosine or inhibition of A2B receptors enhanced pro-fibrotic ATP signaling. Together, these results demonstrate the contribution of basally released ATP in establishing the set point for fibrotic activity in adult rat CFs and identify a key role for the modulation of this activity by hydrolysis of released ATP by ENTPDs. These findings also imply that cellular homeostasis and fibrotic response involve the integration of signaling that is pro-fibrotic by ATP and anti-fibrotic by adenosine and that is regulated by ENTPDs.
Introduction
The regulation of the remodeling of extracellular matrix (ECM)2 is essential for tissue homeostasis during normal development and also in tissue healing after injury (1, 2). In the heart, cardiac fibroblasts (CFs) maintain myocardial ECM turnover and are critical contributors to the remodeling that occurs after injury, such as myocardial infarction (3–5). Organization of myocardial structure by the ECM is also essential for normal cardiac contraction and electrical conduction (6, 7). Activation of fibroblasts by certain cytokines and hormones causes their transformation into pro-fibrogenic myofibroblasts and can result in tissue fibrosis through excessive deposition of collagens (primarily types I and III) and other ECM proteins (5, 8, 9).
Myofibroblasts express α-smooth muscle actin (α-SMA), a contractile protein that aids in wound contraction (10), and numerous pro-fibrotic cytokines, including plasminogen activator inhibitor (PAI)-1 and transforming growth factor (TGF)-β. PAI-1 inhibits the activation of plasmin and matrix metalloproteinases and is linked to the development of tissue fibrosis (11, 12). TGF-β receptor/Smad signaling in fibroblasts is strongly pro-fibrotic and induces the synthesis of ECM and expression of α-SMA (13, 14).
Understanding tissue fibrosis requires identification of the stimuli that regulate the homeostatic phenotype of fibroblasts and that trigger fibrosis. Extracellular ATP has been shown to promote monocyte and inflammatory cell recruitment to sites of injury in the lung (15) and liver (16). Consistent with such effects, we have found that extracellular ATP and UTP and their signaling through P2Y2 receptors are pro-fibrotic in adult rat and mouse ventricular fibroblasts grown in primary culture (17). The activation of P2Y2 receptors by these nucleotides increases collagen synthesis and the expression of α-SMA, PAI-1, and TGF-β. Furthermore, ATP, released from CFs via connexin-43 and -45 hemi-channels, activates P2Y2 receptors in an autocrine/paracrine manner (18). Data in the latter study also revealed that the release of and signaling by ATP contribute to the basal fibrotic state of CFs as follows: hydrolysis of extracellular ATP, by addition of the nucleotidase apyrase, decreased basal collagen synthesis, and the expression of α-SMA stress fibers. Such data indicate that the release of cellular ATP and subsequent activation of P2Y2 receptors contribute to fibroblast phenotypes and suggest that nucleotide signaling is an important pro-fibrotic mechanism in CFs and potentially in fibroblasts in other tissues.
Extracellular ATP catabolism is important in regulating signal transduction by nucleotides. Ectonucleoside triphosphate diphosphohydrolases (ENTPDs) are a family of nucleotidases that hydrolyze tri- and di-phosphate nucleotides. ENTPD activity in vascular endothelial cells is essential for the regulation of inflammation and thrombosis (19, 20) and for vascular tone (21). Furthermore, ENTPD activity in hepatic portal fibroblasts may limit fibroblast and endothelial cell proliferation, thus implicating nucleotide catabolism in the regulation of liver fibrosis (22).
The strong anti-fibrotic effects of apyrase on activities of CFs led us to ask if CFs endogenously express nucleotidases that produce similar effects and thereby provide a “brake” on the pro-fibrotic actions of released ATP. Of the four extracellular ENTPD isoforms (ENTPD-1, -2, -3, and -8) (23), ENTPD-1 has been the most studied in the heart, but almost exclusively in the context of cardioprotection following injury (24). No previous information is available regarding expression of ENTPDs by CFs nor have the potential effects of ATP hydrolysis by ENTPDs been studied with respect to the transformation of CFs to pro-fibrogenic myofibroblasts.
We hypothesized that ENTPD activity is a mechanism that inhibits the pro-fibrotic autocrine/paracrine pathway stimulated by ATP-P2Y receptor signaling, such that both ATP release and its hydrolysis determine fibrotic response. In this study, we investigated ENTPD expression in adult rat CFs, the effect of ENTPD inhibition on CF phenotype, and the role of these enzymes in modulating pro-fibrotic nucleotide signaling. We also assessed whether adenosine, a product generated by the hydrolysis of ATP, activates adenosine receptors and also regulates CF phenotypes. The results reveal a key integrative role for ENTPD in ATP-mediated pro-fibrotic and adenosine-mediated anti-fibrotic responses.
EXPERIMENTAL PROCEDURES
Isolation and Culture of Adult Rat Cardiac Fibroblasts
Approval for the ethical care and use of animals for this study was granted by the University of California at San Diego Institutional Animal Care and Use Committee and was in compliance with the guiding principles of the American Physiological Society. CFs were isolated from adult (8–10 weeks), male Sprague-Dawley (SD) rats, as described previously (8). Briefly, SD rats were anesthetized via intraperitoneal injection of 100 mg/kg ketamine, 10 mg/kg xylazine. The heart was removed, cannulated on a modified Langendorff apparatus, and perfused with collagenase II (Worthington). CFs were separated from cardiac myocytes by gravity separation and grown to confluency in 10-cm culture dishes at 37 °C, 10% CO2 in DMEM containing 10% FBS, 1% penicillin, 1% streptomycin. CFs were then split to appropriate-sized culture dishes, allowed to adhere overnight, and serum-starved in DMEM, 0% FBS for 24 h prior to treatment.
Extracellular ATP Quantification
Growth media (100 μl) were carefully removed by placing a pipette as close to the fluid surface as possible to avoid perturbation of the cells. ATP concentration was measured using a luciferase-based ATP assay kit (ENLITEN ATP Assay; Promega, Madison, WI) and TD-20/20 luminometer (Turner Biosystems, Sunnyvale, CA) according to the manufacturers' instructions. Assays were conducted at room temperature. Standard curves using DMEM supplemented with known ATP concentrations were generated and used to quantify ATP concentration in the experimental samples.
Malachite Green Assay
ATP hydrolysis was measured by quantifying inorganic phosphate accumulation using the SensoLyte malachite green assay kit (AnaSpec, Fremont, CA) following the manufacturer's instructions. CFs were cultured in phosphate- and serum-free DMEM. One hour after addition of 30 μm exogenous ATP, media were gently removed and filtered (to remove free cells). Media samples (80 μl) or standards consisting of DMEM containing phosphate standards were mixed with 20 μl of assay reagent in a 96-well plate. Following incubation at room temperature on a rotary shaker for 20 min, the absorbance was measured at 620 nm using a DTX 800 multimode detector (Beckman Coulter). The concentration of inorganic phosphate in CF-conditioned media was calculated by comparison with standard curves.
siRNA Transfection
Targeted siRNA sequences for rat ENTPD-1 (s134179), ENTPD-2 (s134120) and negative control siRNA were purchased from Ambion (Grand Island, NY). Cells were transfected with 5 nm siRNA using RNAiMAX (Invitrogen) for 8 h according to the manufacturer's instructions. The media containing transfection reagent were then replaced with fresh serum-free DMEM, and CFs were incubated for 24 h.
Quantitative Real Time PCR (qPCR)
Total RNA was isolated by TRIzol extraction (Invitrogen), and cDNA was generated using the Superscript III cDNA synthesis system (Invitrogen) according to the manufacturer's instructions. The qScript One-Step qRT-PCR kit (Quanta Biosciences, Gaithersburg, MD) and a DNA Engine Opticon 2 (Bio-Rad) qPCR machine were used for gene expression analyses. Primers for PCR amplification (Table 1) were designed based on the nucleotide sequences of the respective gene target using Primer3Plus software. When possible, each forward and reverse primer set was designed between multiple exons. Amplification efficiency of each primer pair was tested prior to analysis, and relative gene expression levels were determined using the ΔΔCT method with 18 S as the reference gene (25).
TABLE 1.
Primer sequences used for real time qPCR
| Gene | Forward, 5′–3′ | Reverse, 5′–3′ |
|---|---|---|
| α-SMA | CATCAGGAACCTCGAGAAGC | TCGGATACTTCAGGGTCAGG |
| PAI-1 | GGAGAAGCGAAACAGGAGTG | TCCAGAAGGGGATATGTTGC |
| TGF-β | CCTGGAAAGGGCTCAACA | GTTGGTTGTAGAGGGCAAGG |
| ENTPD-1 | AGGAGCCTGAAGAGCTACCC | GTCTGATTTAGGGGCACGAA |
| ENTPD-2 | CTTCGGGATGTACCCAGAGA | CAGCAGGTAGTTGGCAGTCA |
| 18 S | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
Immunoblot Analysis
Whole cell lysates were prepared in 150 mm Na2CO3 buffer (pH 11) and homogenized by sonication. Protein concentration was measured using a Bradford protein assay (Bio-Rad), and equal amounts of protein were separated by SDS-PAGE using 10% polyacrylamide precast gels (Invitrogen) and transferred to a poly(vinylidenedifluoride) membrane with the iBlot system (Invitrogen). Membranes were blocked in PBS Tween (1%) containing 5% nonfat dry milk and incubated with primary antibody overnight at 4 °C. Bound antibodies were visualized using horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) and ECL reagent (Amersham Biosciences). Bands were compared with molecular weight standards to confirm migration of proteins at the appropriate size. Quantification of the densitometry of protein expression was performed using ImageJ software (National Institutes of Health). Antibodies for α-SMA were purchased from Invitrogen, PAI-1 from BD Biosciences, GAPDH from Abcam, and p-ERK and total ERK from Cell Signaling (Danvers, MA).
Collagenase-sensitive [3H]Proline Incorporation
Collagenase-sensitive [3H]proline incorporation assays were used to quantify collagen accumulation and were performed as described previously (8, 18). CFs cultured on 12-well plates were serum-starved for 24 h followed by the addition of 1 μCi/ml [3H]proline (PerkinElmer Life Sciences; 1 Ci = 37 GBq) along with compounds of interest and incubated at 37 °C for 24 h. Cells were lysed with 0.5 n NaOH, and following neutralization with HCl, protein was precipitated overnight with 10% trichloroacetic acid (TCA). Samples were pelleted and washed in 5% TCA, dissolved in 0.2 n NaOH, and neutralized with HCl. Collagenase II (2 mg/ml; Worthington) in Tris/CaCl2/N-ethylmaleimide buffer was added to each sample, and samples were incubated at 37 °C for 1 h. Protein was then precipitated and centrifuged. The supernatant was collected and the radioactivity quantified using a liquid scintillation counter.
Collagen Gel Contraction Assay
Collagen gels were prepared using rat tail collagen 1 (BD Biosciences) neutralized with NaOH and supplemented with 1× DMEM (Sigma). Gels contained 2–3 mg/ml rat tail collagen, depending on experimental protocols, in 0.5-ml volumes. CFs were seeded at a density of 1.8 × 105 cells per gel, and the gels were allowed to polymerize at 37 °C for 1 h. Following polymerization, 0.5 ml of serum-free DMEM was added, and the gels were detached and suspended in liquid culture. Treatments were done for 24 h, and images were taken for surface area quantification.
Reagents
ATP, adenosine, suramin, POM-1, H89, (Rp)-cAMPS, PSB 603, and SCH 442416 were purchased from Tocris Bioscience (Minneapolis, MN). Apyrase and adenosine deaminase were purchased from Sigma.
Statistical Analysis
Calculations and statistics were performed using GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA). Numerical values are presented as mean ± S.E. Analysis of numerical data from experiments with multiple comparisons were done using analysis of variance with Tukey's test. p < 0.05 was considered significant.
RESULTS
ATP Signaling Regulates Basal α-SMA Expression and Contractile Tone in Cardiac Fibroblasts
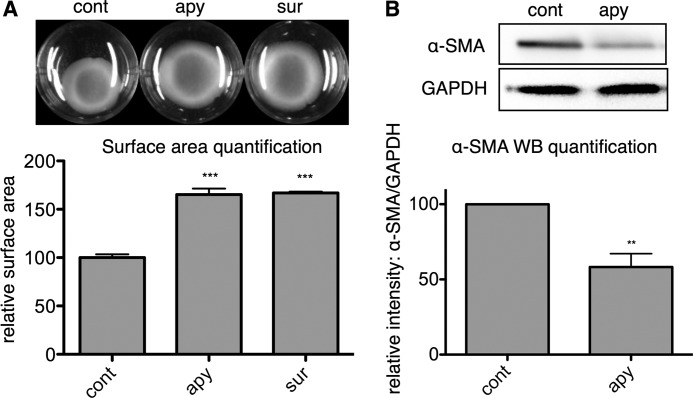
The expression of α-SMA is a hallmark of myofibroblast transformation (10) and confers a contractile phenotype to CFs (26). In previous studies, we showed that ATP released from CFs regulates collagen synthesis and that addition of exogenous ATP increases formation of α-SMA-expressing fibers in CFs via P2Y2 receptor signaling (17, 18). Those results suggested that extracellular ATP helps determine CF α-SMA expression and contraction. To assess the extent of basally released ATP in regulating CF contractile tone, we seeded CFs into gels containing 2.5 mg/ml collagen, which CFs spontaneously contract. Hydrolysis of extracellular ATP, produced by addition of 1 unit/ml apyrase, decreased this contraction and increased gel surface area (by 65%, p < 0.001) while also decreasing basal α-SMA expression (by 42%, p < 0.01) (Fig. 1, A and B). Suramin (50 μm), a P2 nucleotide receptor inhibitor, also increased collagen gel surface area (by 67%, p < 0.001). Thus, removal of ambient extracellular ATP by apyrase or blocking P2 receptors reduced basal α-SMA expression and CF contraction, indicating in addition to our previous findings related to P2Y2 signaling in CFs (18) that tonic ATP-P2Y2 signaling promotes myofibroblast transformation of CFs. These results led us to ask if nucleotidase activity expressed by CFs may regulate CF homeostasis by hydrolyzing basally released ATP and attenuating this pro-fibrotic phenotype.
FIGURE 1.
Basal ATP signaling stimulates α-SMA expression and CF contraction. A, CFs seeded in 2.5 mg/ml rat tail collagen spontaneously contracted the collagen gels by 24 h. Subsequent 24 h of treatment with apyrase (apy) (1 unit/ml) and P2 inhibition with suramin (sur) (50 μm) reversed spontaneous collagen gel contraction and increased collagen gel surface area compared with the 48-h untreated controls (cont) by 65 and 67%, respectively. B, apyrase (1 unit/ml for 24 h) decreased α-SMA protein expression by 42%. **, p < 0.01; ***, p < 0.001 versus untreated controls; quantitative data are presented as mean ± S.E. of three independent experiments.
ENTPD Expression in Rat CFs, siRNA-mediated Knockdown of Endogenous NTPases Increases Extracellular ATP Concentration and Enhances the Pro-fibrotic Effect of ATP Stimulation
CFs, along with numerous cell types in the myocardium, release ATP in response to physical or chemical stimuli (18, 27–29), but the fate of this released ATP is not well defined. We hypothesized that CFs may endogenously express enzymes with nucleotide hydrolytic activity that might result in effects akin to those we observed by adding apyrase and thereby, provide a brake on pro-fibrotic ATP signaling. Kauffenstein et al. (21) described that the mouse vasculature expresses two NTPase isoforms, ENTPD-1 and -2. Using real time qPCR analysis of isolated rat ventricular CFs, we detected ENTPD-1 and -2 at approximately equal expression levels (Fig. 2A). ENTPD-3 was not detected (data not shown).
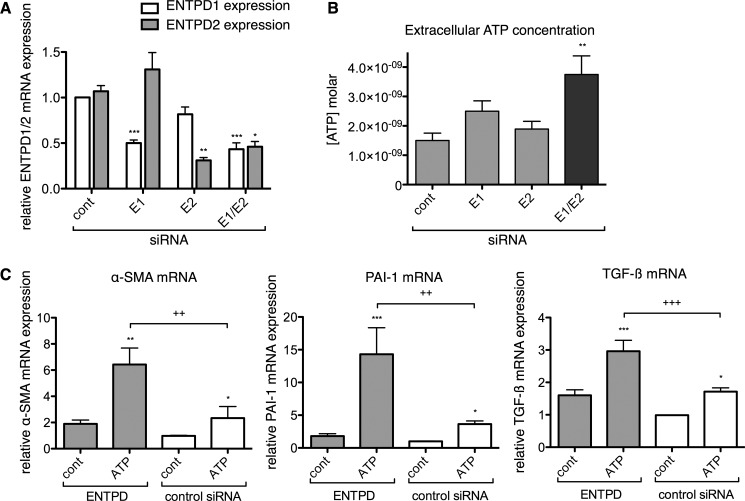
FIGURE 2.
Simultaneous siRNA knockdown of ENTPD1 (E1) and ENTPD2 (E2) increases extracellular ATP concentration and enhances the pro-fibrotic effect of ATP. A, ENTPD-1 and -2 were detected in similar abundance in rat ventricular CFs. Co-transfection with siRNA for ENTPD-1 and -2 (E1/E2) decreased expression by 57 and 54%, respectively. B, ENTPD-1/-2 knockdown increased basal extracellular ATP concentration by 2.5-fold. C, knockdown of ENTPD-1/-2 significantly enhanced the pro-fibrotic effect of 10 μm ATP on CFs. CFs transfected with siRNA targeting both ENTPD-1/-2 up-regulated α-SMA, PAI-1, and TGF-β expression by 2.7-, 4.0-, and 1.7-fold, respectively, in response to a 4-h incubation with ATP as compared with control (cont) siRNA-transfected CFs incubated with ATP. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus identically transfected, untreated samples; +, p < 0.05; ++, p < 0.01; +++, p < 0.001 between groups indicated. Gene expression data are presented as mean ± S.E. of three independent experiments; ATP assay data are presented as mean ± S.E. of six independent experiments.
We used siRNA to selectively decrease expression of each ENTPD isoform. Although single knockdown of ENTPD-1 or -2 did not substantially change extracellular ATP concentration, knockdown of both ENTPD-1 and -2 decreased their expression by 57 and 54%, respectively, and significantly increased basal extracellular ATP concentration (by 2.5-fold, p < 0.01) (Fig. 2B). The requirement to knock down both ENTPD-1 and -2 implies that both enzymes contribute to ATP hydrolytic activity in CFs.
Assessment of changes in pro-fibrotic marker expression (by qPCR) was used to investigate the functional impact of knockdown of ENTPD-1 and -2 and thus of decreased ATP hydrolytic activity in CFs. Although we found increased expression of α-SMA, PAI-1, and TGF-β following ENTPD-1/-2 knockdown, these increases were not statistically significant (p > 0.05). However, addition of 10 μm ATP to ENTPD-deficient CFs had a significantly greater stimulatory effect on α-SMA, PAI-1, and TGF-β expression (2.7-, 4.0-, and 1.7-fold, respectively) than did ATP treatment of control siRNA-transfected CFs (Fig. 2C). Thus, a decrease in ENTPD expression in CFs enhanced the pro-fibrotic response to ATP.
POM-1, an ENTPD Inhibitor, Enhances Basal Pro-fibrotic and ERK Activity in CFs
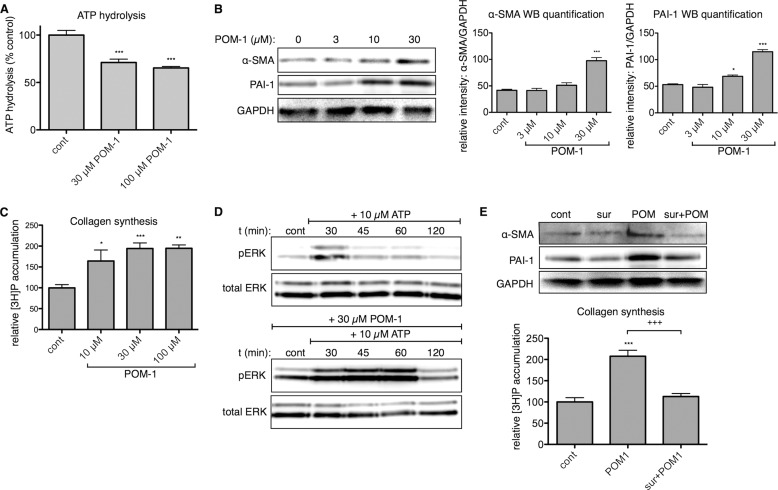
Because of the partial knockdown that we achieved with siRNA and the need to target both ENTPD-1 and -2 to decrease ATP hydrolysis, we tested a second approach to inhibit ENTPDs as follows: treatment of CFs with a polyoxometalate-derived compound, which is an extracellular ENTPD inhibitor (30, 31). Sodium polyoxotungstate (POM)-1 prominently inhibits ENTPD activity in endothelial and smooth muscle cells and in mouse models of cardiac and renal ischemia (24, 32). Because POM-1 interferes with luciferase-based methods for ATP quantification, we used a malachite green assay to measure accumulation of inorganic phosphate resulting from ATP hydrolysis (31, 33–35). Addition of 30 μm POM-1 inhibited ATP hydrolysis by ∼30% (p < 0.001) (Fig. 3A); 100 μm POM-1 did not produce a significantly greater inhibitory effect. As a result of ENTPD inhibition, we found that treatment of CFs with POM-1 produced a concentration-dependent increase in α-SMA and PAI-1 protein expression and collagen synthesis (Fig. 3, B and C), thus indicating that POM-1 enhances the basal transformation of CFs to myofibroblasts.
FIGURE 3.
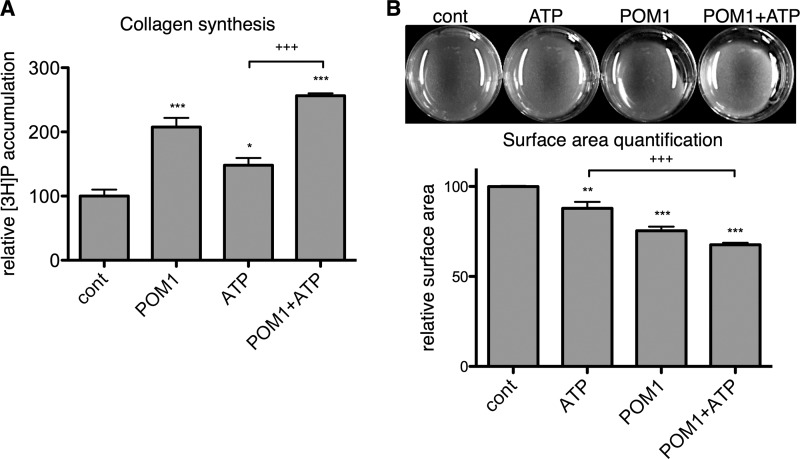
Inhibition of endogenous NTPase activity by POM-1 is pro-fibrotic. A, ATP hydrolysis was measured using a malachite green assay. Addition of 30 μm POM-1 inhibited ENTPD-mediated hydrolysis of exogenously added ATP (30 μm) after 1 h. POM-1 treatment for 24 h increased CF α-SMA and PAI-1 protein expression (B) and collagen synthesis (C) in a concentration-dependent manner. D, POM-1 pretreatment (1 h) increased basal ERK phosphorylation and prolonged 10 μm ATP-stimulated ERK phosphorylation. E, stimulatory effects of 30 μm POM-1 on collagen synthesis and expression of α-SMA and PAI-1 protein were blocked by 50 μm suramin (sur). *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus untreated controls; +++, p < 0.001 between groups indicated. Quantitative data are presented as mean ± S.E. of at least three independent experiments. cont, control; WB, Western blot.
Previous data indicate that the P2Y2 receptor is the most highly expressed P2Y subtype in rat ventricular fibroblasts and that P2Y2 mediates the majority of pro-fibrotic responses of rat CFs in response to ATP stimulation (17, 18). P2Y2 receptor stimulation can lead to rapid and transient activation of MAPK/ERK (36, 37); rat CFs deficient in P2Y2 receptors lack this response to exogenously added ATP (18). Consistent with the ability of ENTPD inhibition to enhance ATP-mediated signaling, a 1-h pretreatment of CFs with 30 μm POM-1 increased basal phospho-ERK levels and prominently prolonged ATP-stimulated activation of ERK compared with the response of CFs treated with ATP alone (Fig. 3D). The P2 receptor antagonist suramin blocked the stimulatory effect of 30 μm POM-1 on collagen synthesis and on the protein expression of α-SMA and PAI-1 (Fig. 3E), indicating that the pro-fibrotic effects of POM-1 require P2, most likely P2Y2 receptor activation (17, 18).
POM-1 Enhances the Pro-fibrotic Effects of ATP
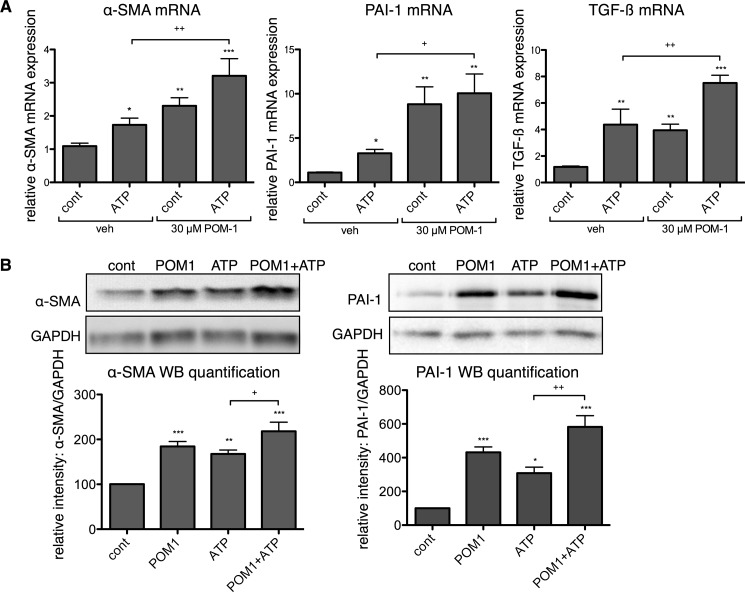
In addition to stimulating basal pro-fibrotic signaling, POM-1 enhanced the pro-fibrotic effects of exogenously added ATP. Treatment with 30 μm POM-1 significantly increased ATP-promoted mRNA expression of α-SMA, PAI-1, and TGF-β by 1.9-fold (p < 0.01), 3.0-fold (p < 0.05), and 1.7-fold (p < 0.01), respectively (Fig. 4A). Similarly, protein expressions of α-SMA and PAI-1 were increased 30% (p < 0.05) and 89% (p < 0.01), respectively, and collagen synthesis was increased 73% (p < 0.001) in CFs treated with POM-1 plus ATP compared with responses of cells incubated only with ATP (Figs. 4B and 5A). CF-mediated collagen gel contraction was also enhanced, and POM-1 increased ATP-stimulated gel contraction by 23% (p < 0.001) (Fig. 5B).
FIGURE 4.
POM-1 enhances the effects of ATP on pro-fibrotic marker expression of CFs. A, incubation of CFs with POM-1 together with ATP for 4 h increased expression of α-SMA, PAI-1, and TGF-β mRNA by 1.9-, 3.0-, and 1.7-fold, respectively, compared with CFs incubated only with ATP. B, incubation of CFs with POM-1 for 24 h increased α-SMA and PAI-1 protein expression by 30 and 89%, respectively, as compared with CFs incubated only with ATP. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus untreated controls (cont); +, p < 0.05; ++, p < 0.01 between groups indicated. Data are presented as mean ± S.E. of at least three independent experiments. veh, vehicle.
FIGURE 5.
POM-1 enhances the stimulatory effect of ATP on collagen synthesis and gel contraction. A, incubation of CFs with 30 μm POM-1 increased the effect of 10 μm ATP on collagen synthesis 73% after 24 h as compared with CFs incubated only with ATP. B, POM-1 increased the 12% reduction in collagen gel surface area in response to a 24-h incubation with 10 μm ATP to 32%. *, p < 0.05; **, p < 0.01, ***, p < 0.001 versus untreated controls (cont); +++, p < 0.001 between groups indicated. Quantitative data are presented as mean ± S.E. of three independent experiments.
Together, these data indicate that the pro-fibrotic effects mediated by ATP are enhanced by inhibiting extracellular ENTPD activity. POM-1 increased the basal pro-fibrotic state of CFs and enhanced the stimulatory effect of ATP on collagen synthesis and the expression of pro-fibrotic markers, α-SMA, PAI-1, and TGF-β. Thus, ENTPD activity is a brake that regulates a basal pro-fibrotic pathway driven by constitutive nucleotide release and signaling.
Adenosine Reverses the Pro-fibrotic Effects of POM-1
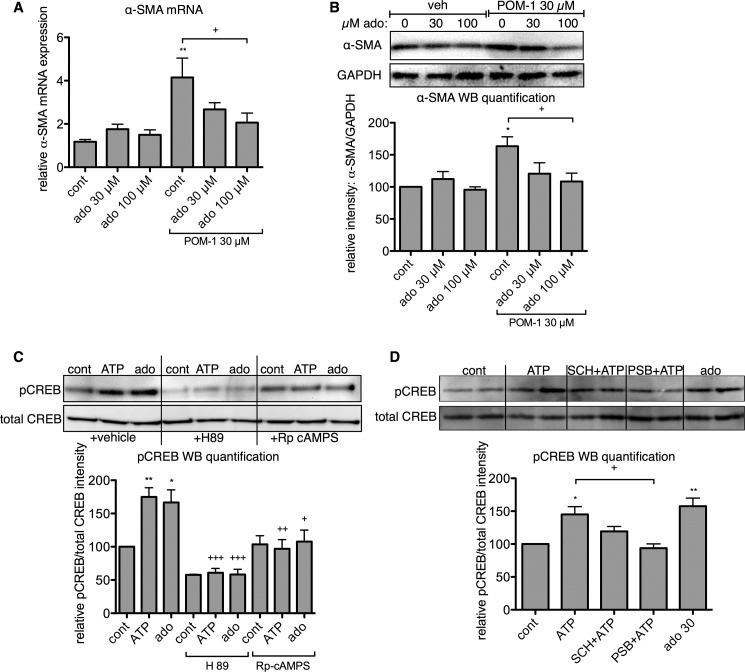
Adenosine signaling in rat CFs is anti-fibrotic, mediated by increased intracellular cAMP via activation of Gs-coupled A2 adenosine receptors (38–40). ENTPD activity, in combination with the activity of 5′-nucleotidases (e.g. CD73) and alkaline phosphatase, produces extracellular adenosine (24, 41, 42). Thus, we speculated that the generation of adenosine by ATP hydrolysis might represent a counterbalancing anti-fibrotic mechanism in CFs that functions via A2 receptor activation. Indeed, we found that addition of 100 μm adenosine significantly reduces the stimulatory effect of POM-1 on α-SMA mRNA and protein expression (p < 0.05) (Fig. 6, A and B).
FIGURE 6.
Adenosine signaling counteracts the pro-fibrotic effects of ATP. Adenosine (ado) decreased POM-1-stimulated α-SMA mRNA (A) and protein (B) expression in a concentration-dependent manner. C, incubation with ATP (10 μm) and adenosine (ado, 30 μm) for 4 h increased CREB phosphorylation, an effect that was blunted with the PKA inhibitors, H89 (10 μm) and (Rp)-cAMPS (50 μm). D, inhibition of A2B adenosine receptors with PSB 603 (300 nm) blocked ATP-stimulated CREB phosphorylation; A2A receptor inhibition (300 nm SCH 442416) had no significant effect. *, p < 0.05; **, p < 0.01 versus untreated controls (cont); +, p < 0.05; ++, p < 0.01; +++, p < 0.001 between groups indicated or as compared with control CFs not treated with inhibitors (C). Data are presented as mean ± S.E. of at least three independent experiments. WB, Western blot; veh, vehicle.
The anti-fibrotic effects of adenosine in CFs occur via an increase in intracellular cAMP in response to Gs-coupled A2 receptor activation (38, 40). We therefore assessed PKA-dependent CREB phosphorylation resulting from ATP and adenosine treatment. Stimulation of CFs for 4 h with either 10 μm ATP or 30 μm adenosine significantly increased phospho-CREB levels in a PKA (and thus, cAMP)-dependent manner (Fig. 6C). Consistent with this idea, inhibition of PKA with 10 μm H89 or 50 μm (Rp)-cAMPS blocked CREB phosphorylation stimulated by ATP and adenosine. Furthermore, antagonism of A2B receptors with 300 nm PSB 603 blocked the stimulatory effect of ATP on CREB phosphorylation (p < 0.05; Fig. 6D), whereas the A2A receptor inhibitor SCH 442416 had no significant effect (p > 0.05). These results show that ATP-stimulated CREB phosphorylation is dependent on A2B receptors and activation of the cAMP-PKA pathway. Thus, ATP can activate Gs-coupled A2B receptors, an effect resulting from the hydrolysis of extracellular ATP into adenosine.
Extracellular Adenosine Deamination and Adenosine A2B Receptor Inhibition Enhances Pro-fibrotic ATP Signaling
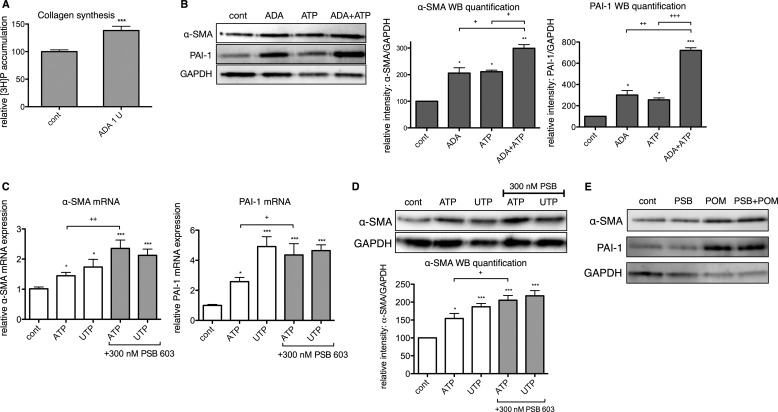
If the hydrolysis of pro-fibrotic ATP via the generation of adenosine produces a counterbalancing anti-fibrotic response in CFs, interventions that decrease extracellular adenosine should enhance the pro-fibrotic phenotype. To test this idea, we assessed α-SMA and PAI-1 protein expression in response to ATP in the presence of adenosine deaminase (ADA). ADA (1 unit/ml) increased collagen synthesis by 38% (p < 0.001) and the expression of α-SMA and PAI-1 protein by 2- and 3-fold, respectively (p < 0.05) (Fig. 7, A and B). Addition of ADA also enhanced response to ATP, incubation of CFs with ADA and ATP increased α-SMA and PAI-1 protein expression 42% (p < 0.05) and 182% (p < 0.001) more than ATP alone (Fig. 7B).
FIGURE 7.
Extracellular adenosine deamination and A2B receptor inhibition enhances pro-fibrotic ATP signaling. Incubation with adenosine deaminase (ADA) increased collagen synthesis by 38% (after 24 h) (A) and the expression of α-SMA and PAI-1 protein 2- and 3-fold, respectively (after 4 h) (B). Incubation of CFs with ADA enhanced the stimulation produced by 10 μm ATP in the expression of α-SMA (42%) and PAI-1 (182%). C, incubation with 300 nm PSB 603 (PSB) enhanced the stimulation by ATP (10 μm, 4 h treatment) of α-SMA and PAI-1 gene expression by 62 and 69%, respectively. D, incubation for 24 h with PSB produced a 33% increase in ATP-promoted enhancement in α-SMA protein levels. PSB did not enhance the UTP-promoted increases in α-SMA or PAI-1 expression. E, effects of 30 μm POM-1 were not enhanced by pretreatment with PSB. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus untreated controls (cont); +, p < 0.05; ++, p < 0.01; +++, p < 0.001 between groups indicated. Data are presented as mean ± S.E. of three independent experiments. WB, Western blot.
As an alternative approach to block adenosine action, we incubated CFs with ATP or UTP in the presence of PSB 603. PSB 603 (300 nm) enhanced responses to ATP (10 μm), ATP-promoted increases in α-SMA and PAI-1 mRNA expression were 62% (p < 0.01) and 69% (p < 0.05) greater, respectively, in the presence of PSB 603 (Fig. 7C), and α-SMA protein levels were 33% (p < 0.05) higher than if incubated only with ATP (Fig. 7D). By contrast, response to 10 μm UTP was not enhanced by the addition of PSB 603. This result is as expected because although ATP and UTP activate P2Y2 receptors with equal affinity (43, 44), UTP hydrolysis does not yield adenosine, and thus A2B receptor inhibition should have no effect. Furthermore, PSB 603 did not potentiate the effects of POM-1 on α-SMA or PAI-1 expression (p > 0.05) (Fig. 7E), indicating a robust pro-fibrotic response to ENTPD inhibition and the absence of adenosine signaling. Thus, pro-fibrotic ATP signaling is amplified in CFs by interventions that block the counterbalancing anti-fibrotic pathway mediated by adenosine.
DISCUSSION
Based on previous data that identified a role for constitutively released ATP in regulating fibrotic activity of CFs (18), we hypothesized that ATP hydrolysis by endogenous nucleotidases might be an important mechanism that controls the activation of pro-fibrogenic pathways by basal nucleotide signaling in CFs. We demonstrate results consistent with this hypothesis as follows: endogenous ENTPD activity attenuates pro-fibrotic nucleotide signaling in CFs, thus establishing a stimulatory mechanism (i.e. ATP release) and an inhibitory mechanism (ATP hydrolysis by ENTPD) that work in concert to establish the set point for extracellular ATP levels and P2Y2-mediated pro-fibrotic tone. Inhibition of ENTPD activity (such as with POM-1) increases pro-fibrotic autocrine/paracrine effects of basal nucleotide signaling, thus identifying the importance of these nucleotidases in attenuating this pathway.
Previous reports have described the use of POM-1 as an ENTPD inhibitor (24, 31, 32), and one study showed that POM-1 inhibits cardioprotection via adenosine generation (24). However, previous studies have not addressed whether ENTPD inhibition perturbs fibrogenic responses. We found that treatment of CFs with POM-1 inhibits hydrolysis of extracellular ATP by CFs, increases basal expression of the pro-fibrotic markers α-SMA, PAI-1, and TGF-β, and stimulates collagen synthesis. We did not observe statistically significant effects on basal CF gene expression with siRNA-mediated ENTPD knockdown, likely because we only achieved partial knockdown of ENTPD expression. Unfortunately, we found that ENTPD siRNA concentrations higher than 5 nm were not well tolerated by CFs. Even so, CFs transfected with siRNA targeting ENTPD-1 and -2 were significantly more pro-fibrotic in response to exogenously added ATP than were control siRNA-transfected CFs.
The effects observed with POM-1 treatment were blunted by P2 receptor inhibition, consistent with the involvement of extracellular nucleotide-P2Y receptor signaling in the pro-fibrotic response to POM-1 (17, 18). Incubation with POM-1 also enhanced and prolonged the transient effects of ATP on ERK activation, indicating that POM-1 enhances ATP signaling. Consistent with this idea, treatment with POM-1 significantly increased the ATP-promoted increase in expression of α-SMA, PAI-1, and TGF-β, cellular collagen synthesis, and CF-mediated gel contraction.
In addition to attenuating pro-fibrotic ATP signaling, nucleotidase activity, via the generation of adenosine, initiates a counterbalancing, anti-fibrotic response mediated by A2B receptor activation. Numerous studies have found that ENTPD activity facilitates the production of adenosine and that ENTPD inhibition decreases extracellular adenosine concentration (24, 31, 32, 45). We found that ATP stimulates PKA-dependent CREB phosphorylation, a response that is sensitive to inhibition of A2B receptors, indicating that these effects are a result of adenosine generation and signaling. Furthermore, treating CFs with agents that blunt adenosine levels (ADA) or response (an A2B receptor antagonist) enhanced the pro-fibrotic activity of CFs, increasing the pro-fibrotic effect of ATP on α-SMA and PAI-1 expression. Thus, the anti-fibrotic component of ATP signaling is initiated by ecto-nucleotidase-mediated hydrolysis. Consistent with this mechanism, we found that A2B inhibition did not further potentiate the pro-fibrotic effects of POM-1, indicating a strong induction of ATP signaling by ENTPD inhibition and the absence of adenosine signaling.
Together, our findings indicate that a decrease in ENTPD expression or activity in CFs will enhance pro-fibrogenic activity and potentially promote the development of cardiac fibrosis, which can result from two effects: prolonged ATP signaling and decreased adenosine generation. Of note, such effects are not limited to the heart. Similar mechanisms may regulate hepatic stellate cell and portal fibroblast proliferation and activity in the liver (22, 46). Our data demonstrate that autocrine/paracrine signaling that results from nucleotide release and hydrolysis appear to be essential for determining the set point of the CF phenotype and perhaps in other tissues as well.
In the cardiovascular system, further studies are required to investigate whether disease settings result in altered extracellular ATP catabolism and the impact of this effect on CF activity. Mice that overexpress ENTPD1/CD39 are protected from ischemia-reperfusion injury as a consequence of adenosine production and signaling in cardiac myocytes (24, 33). Furthermore, differential ENTPD1/CD39 activities have been observed in patients with coronary artery disease and ischemic heart disease (47, 48). Our data suggest that ENTPD up-regulation may protect against cardiac fibrosis in vivo and that conversely down-regulation or decreased activity of ENTPD may exacerbate the fibrotic phenotype.
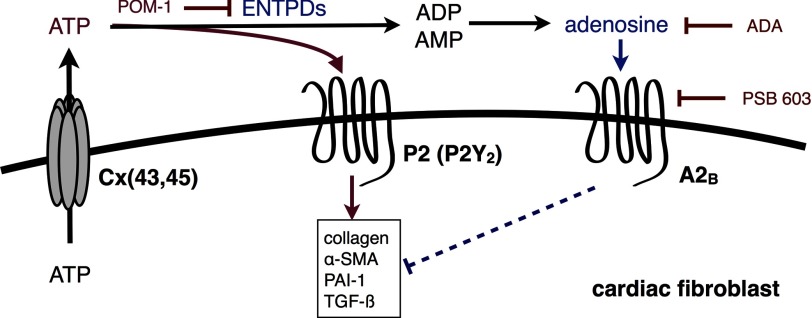
Together with previous findings (18, 22, 46, 49), the data presented here demonstrate the important role of basally released ATP and nucleotide hydrolysis in mediating cellular homeostasis and physiology (e.g. proliferation, contraction, migration, and collagen synthesis) (Fig. 8). ENTPDs play an essential role in the attenuation of ATP signaling but also initiate ADP (and UDP) and adenosine-driven responses (20, 49). We find that the generation of adenosine, an anti-fibrotic mediator in CFs (38, 40), also helps determine the set point of CFs. The release of cellular nucleotides and the activity of ENTPDs and possibly other ecto-nucleotidases thus mediate counteracting ATP-P2Y and adenosine-P1 pathways that regulate cellular homeostasis.
FIGURE 8.
Model of the counterbalancing pro-fibrotic ATP-P2Y (P2Y2) and anti-fibrotic adenosine-P1 signaling pathways initiated by cellular ATP release and regulated by ENTPD and nucleotidase activity.
This work was supported, in whole or in part, by National Institutes of Health Grants 1R01HL091071-01 and GM066232 (to P. A. I.) and 1F31AG039992–01 (to D. L.).
- ECM
- extracellular matrix
- ADA
- adenosine deaminase
- α-SMA
- α-smooth muscle actin
- CF
- cardiac fibroblast
- CREB
- cAMP response element-binding protein
- ENTPD
- ectonucleoside triphosphate diphosphohydrolase
- PAI-1
- plasminogen activator inhibitor-1
- POM-1
- polyoxotungstate-1
- RT-qPCR
- real time quantitative PCR
- TGF-β
- transforming growth factor-β.
REFERENCES
- 1. Cossu G., Bianco P. (2003) Mesoangioblasts–vascular progenitors for extravascular mesodermal tissues. Curr. Opin. Genet. Dev. 13, 537–542 [DOI] [PubMed] [Google Scholar]
- 2. van den Borne S. W., Diez J., Blankesteijn W. M., Verjans J., Hofstra L., Narula J. (2010) Myocardial remodeling after infarction: the role of myofibroblasts. Nat. Rev. Cardiol. 7, 30–37 [DOI] [PubMed] [Google Scholar]
- 3. Camelliti P., Borg T. K., Kohl P. (2005) Structural and functional characterisation of cardiac fibroblasts. Cardiovasc. Res. 65, 40–51 [DOI] [PubMed] [Google Scholar]
- 4. Zeisberg E. M., Kalluri R. (2010) Origins of cardiac fibroblasts. Circ. Res. 107, 1304–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldsmith E. C., Bradshaw A. D., Spinale F. G. (2013) Cellular mechanisms of tissue fibrosis. 2. Contributory pathways leading to myocardial fibrosis: moving beyond collagen expression. Am. J. Physiol. Cell Physiol. 304, C393–C402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kakkar R., Lee R. T. (2010) Intramyocardial fibroblast myocyte communication. Circ. Res. 106, 47–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maleckar M. M., Greenstein J. L., Giles W. R., Trayanova N. A. (2009) Electrotonic coupling between human atrial myocytes and fibroblasts alters myocyte excitability and repolarization. Biophys. J. 97, 2179–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Swaney J. S., Roth D. M., Olson E. R., Naugle J. E., Meszaros J. G., Insel P. A. (2005) Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc. Natl. Acad. Sci. U.S.A. 102, 437–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spinale F. G. (2007) Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol. Rev. 87, 1285–1342 [DOI] [PubMed] [Google Scholar]
- 10. Souders C. A., Bowers S. L., Baudino T. A. (2009) Cardiac fibroblast: the renaissance cell. Circ. Res. 105, 1164–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghosh A. K., Vaughan D. E. (2012) PAI-1 in tissue fibrosis. J. Cell. Physiol. 227, 493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takeshita K., Hayashi M., Iino S., Kondo T., Inden Y., Iwase M., Kojima T., Hirai M., Ito M., Loskutoff D. J., Saito H., Murohara T., Yamamoto K. (2004) Increased expression of plasminogen activator inhibitor-1 in cardiomyocytes contributes to cardiac fibrosis after myocardial infarction. Am. J. Pathol. 164, 449–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dobaczewski M., Bujak M., Li N., Gonzalez-Quesada C., Mendoza L. H., Wang X. F., Frangogiannis N. G. (2010) Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ. Res. 107, 418–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leask A., Abraham D. J. (2004) TGF-β signaling and the fibrotic response. FASEB J. 18, 816–827 [DOI] [PubMed] [Google Scholar]
- 15. Elliott M. R., Chekeni F. B., Trampont P. C., Lazarowski E. R., Kadl A., Walk S. F., Park D., Woodson R. I., Ostankovich M., Sharma P., Lysiak J. J., Harden T. K., Leitinger N., Ravichandran K. S. (2009) Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461, 282–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ayata C. K., Ganal S. C., Hockenjos B., Willim K., Vieira R. P., Grimm M., Robaye B., Boeynaems J. M., Di Virgilio F., Pellegatti P., Diefenbach A., Idzko M., Hasselblatt P. (2012) Purinergic P2Y(2) receptors promote neutrophil infiltration and hepatocyte death in mice with acute liver injury. Gastroenterology 143, 1620–1629 [DOI] [PubMed] [Google Scholar]
- 17. Braun O. O., Lu D., Aroonsakool N., Insel P. A. (2010) Uridine triphosphate (UTP) induces profibrotic responses in cardiac fibroblasts by activation of P2Y2 receptors. J. Mol. Cell. Cardiol. 49, 362–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu D., Soleymani S., Madakshire R., Insel P. A. (2012) ATP released from cardiac fibroblasts via connexin hemichannels activates profibrotic P2Y2 receptors. FASEB J. 26, 2580–2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marcus A. J., Broekman M. J., Drosopoulos J. H., Islam N., Alyonycheva T. N., Safier L. B., Hajjar K. A., Posnett D. N., Schoenborn M. A., Schooley K. A., Gayle R. B., Maliszewski C. R. (1997) The endothelial cell ecto-ADPase responsible for inhibition of platelet function is CD39. J. Clin. Invest. 99, 1351–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marcus A. J., Broekman M. J., Drosopoulos J. H., Islam N., Pinsky D. J., Sesti C., Levi R. (2003) Metabolic control of excessive extracellular nucleotide accumulation by CD39/ecto-nucleotidase-1: implications for ischemic vascular diseases. J. Pharmacol. Exp. Ther. 305, 9–16 [DOI] [PubMed] [Google Scholar]
- 21. Kauffenstein G., Drouin A., Thorin-Trescases N., Bachelard H., Robaye B., D'Orléans-Juste P., Marceau F., Thorin E., Sévigny J. (2010) NTPDase1 (CD39) controls nucleotide-dependent vasoconstriction in mouse. Cardiovasc. Res. 85, 204–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jhandier M. N., Kruglov E. A., Lavoie E. G., Sévigny J., Dranoff J. A. (2005) Portal fibroblasts regulate the proliferation of bile duct epithelia via expression of NTPDase2. J. Biol. Chem. 280, 22986–22992 [DOI] [PubMed] [Google Scholar]
- 23. Robson S. C., Sévigny J., Zimmermann H. (2006) The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal. 2, 409–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Köhler D., Eckle T., Faigle M., Grenz A., Mittelbronn M., Laucher S., Hart M. L., Robson S. C., Müller C. E., Eltzschig H. K. (2007) CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation 116, 1784–1794 [DOI] [PubMed] [Google Scholar]
- 25. Pfaffl M. W. (2001) A new mathematical model for relative quantification in real time RT-PCR. Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hinz B., Celetta G., Tomasek J. J., Gabbiani G., Chaponnier C. (2001) α-Smooth muscle actin expression up-regulates fibroblast contractile activity. Mol. Biol. Cell 12, 2730–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Montalbetti N., Leal Denis M. F., Pignataro O. P., Kobatake E., Lazarowski E. R., Schwarzbaum P. J. (2011) Homeostasis of extracellular ATP in human erythrocytes. J. Biol. Chem. 286, 38397–38407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nishida M., Sato Y., Uemura A., Narita Y., Tozaki-Saitoh H., Nakaya M., Ide T., Suzuki K., Inoue K., Nagao T., Kurose H. (2008) P2Y6 receptor-Gα12/13 signalling in cardiomyocytes triggers pressure overload-induced cardiac fibrosis. EMBO J. 27, 3104–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamamoto K., Furuya K., Nakamura M., Kobatake E., Sokabe M., Ando J. (2011) Visualization of flow-induced ATP release and triggering of Ca2+ waves at caveolae in vascular endothelial cells. J. Cell Sci. 124, 3477–3483 [DOI] [PubMed] [Google Scholar]
- 30. Müller C. E., Iqbal J., Baqi Y., Zimmermann H., Röllich A., Stephan H. (2006) Polyoxometalates–a new class of potent ecto-nucleoside triphosphate diphosphohydrolase (NTPDase) inhibitors. Bioorg. Med. Chem. Lett. 16, 5943–5947 [DOI] [PubMed] [Google Scholar]
- 31. Wall M. J., Wigmore G., Lopatár J., Frenguelli B. G., Dale N. (2008) The novel NTPDase inhibitor sodium polyoxotungstate (POM-1) inhibits ATP breakdown but also blocks central synaptic transmission, an action independent of NTPDase inhibition. Neuropharmacology 55, 1251–1258 [DOI] [PubMed] [Google Scholar]
- 32. Grenz A., Zhang H., Hermes M., Eckle T., Klingel K., Huang D. Y., Müller C. E., Robson S. C., Osswald H., Eltzschig H. K. (2007) Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J. 21, 2863–2873 [DOI] [PubMed] [Google Scholar]
- 33. Cai M., Huttinger Z. M., He H., Zhang W., Li F., Goodman L. A., Wheeler D. G., Druhan L. J., Zweier J. L., Dwyer K. M., He G., d'Apice A. J., Robson S. C., Cowan P. J., Gumina R. J. (2011) Transgenic overexpression of ectonucleotide triphosphate diphosphohydrolase-1 protects against murine myocardial ischemic injury. J. Mol. Cell. Cardiol. 51, 927–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clayton A., Al-Taei S., Webber J., Mason M. D., Tabi Z. (2011) Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J. Immunol. 187, 676–683 [DOI] [PubMed] [Google Scholar]
- 35. Corriden R., Chen Y., Inoue Y., Beldi G., Robson S. C., Insel P. A., Junger W. G. (2008) Ecto-nucleoside triphosphate diphosphohydrolase 1 (E-NTPDase1/CD39) regulates neutrophil chemotaxis by hydrolyzing released ATP to adenosine. J. Biol. Chem. 283, 28480–28486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Neary J. T., Kang Y., Willoughby K. A., Ellis E. F. (2003) Activation of extracellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. J. Neurosci. 23, 2348–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang L., Cranson D., Trinkaus-Randall V. (2004) Cellular injury induces activation of MAPK via P2Y receptors. J. Cell. Biochem. 91, 938–950 [DOI] [PubMed] [Google Scholar]
- 38. Chen Y., Epperson S., Makhsudova L., Ito B., Suarez J., Dillmann W., Villarreal F. (2004) Functional effects of enhancing or silencing adenosine A2b receptors in cardiac fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 287, H2478–H2486 [DOI] [PubMed] [Google Scholar]
- 39. Dubey R. K., Gillespie D. G., Zacharia L. C., Mi Z., Jackson E. K. (2001) A(2b) receptors mediate the antimitogenic effects of adenosine in cardiac fibroblasts. Hypertension 37, 716–721 [DOI] [PubMed] [Google Scholar]
- 40. Epperson S. A., Brunton L. L., Ramirez-Sanchez I., Villarreal F. (2009) Adenosine receptors and second messenger signaling pathways in rat cardiac fibroblasts. Am. J. Physiol. Cell Physiol. 296, C1171–C1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaczmarek E., Koziak K., Sévigny J., Siegel J. B., Anrather J., Beaudoin A. R., Bach F. H., Robson S. C. (1996) Identification and characterization of CD39/vascular ATP diphosphohydrolase. J. Biol. Chem. 271, 33116–33122 [DOI] [PubMed] [Google Scholar]
- 42. Zimmermann H., Zebisch M., Sträter N. (2012) Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 8, 437–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Abbracchio M. P., Burnstock G., Boeynaems J. M., Barnard E. A., Boyer J. L., Kennedy C., Knight G. E., Fumagalli M., Gachet C., Jacobson K. A., Weisman G. A. (2006) International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 58, 281–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. von Kügelgen I. (2006) Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol. Ther. 110, 415–432 [DOI] [PubMed] [Google Scholar]
- 45. Fausther M., Lecka J., Soliman E., Kauffenstein G., Pelletier J., Sheung N., Dranoff J. A., Sévigny J. (2012) Coexpression of ecto-5′-nucleotidase/CD73 with specific NTPDases differentially regulates adenosine formation in the rat liver. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G447–G459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dranoff J. A., Ogawa M., Kruglov E. A., Gaça M. D., Sévigny J., Robson S. C., Wells R. G. (2004) Expression of P2Y nucleotide receptors and ectonucleotidases in quiescent and activated rat hepatic stellate cells. Am. J. Physiol. Gastrointest Liver Physiol 287, G417–G424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bagatini M. D., Martins C. C., Gasparetto D., Spanevello R. M., Becker L. V., Rosa C. S., Battisti V., Bellé L., Gonçalves J. F., Schetinger M. R., Dos Santos R. B., Oliveira L. Z., Morsch V. M. (2011) Enzymes that hydrolyze adenine nucleotides in patients with ischemic heart disease. Clin. Chim. Acta 412, 159–164 [DOI] [PubMed] [Google Scholar]
- 48. El-Omar M. M., Islam N., Broekman M. J., Drosopoulos J. H., Roa D. C., Lorin J. D., Sedlis S. P., Olson K. E., Pulte E. D., Marcus A. J. (2005) The ratio of ADP- to ATP-ectonucleotidase activity is reduced in patients with coronary artery disease. Thromb. Res. 116, 199–206 [DOI] [PubMed] [Google Scholar]
- 49. Corriden R., Insel P. A. (2010) Basal release of ATP: an autocrine-paracrine mechanism for cell regulation. Sci. Signal. 3, re1. [DOI] [PMC free article] [PubMed] [Google Scholar]