Abstract
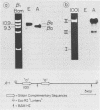
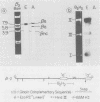
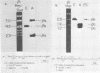
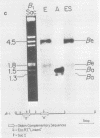
We used recombinant chicken deoxyribonucleic acid clones containing embryonic and adult beta-globin genes and "runoff" endogenous nuclear transcription to investigate the expression of embryonic and adult beta-globin genes during hematopoiesis in the developing chicken embryo. Purified, cloned deoxyribonucleic acids were digested with various restriction enzymes, separated on agarose gels, blotted to nitrocellulose, and annealed with purified nuclear [32P]ribonucleic acid synthesized in vitro from embryonic or adult red cell nuclei. Transcription of the respective globin genes was assayed by hybridization of nuclear [32P]ribonucleic acid to specific restriction fragments containing adult or embryonic coding sequences. Our results indicate that little, if any, transcription from the adult or embryonic beta-globin genes is detectable in the heterologous red cell nuclei, even under conditions in which ribonucleic acid processing probably does not occur.
Full text
PDF

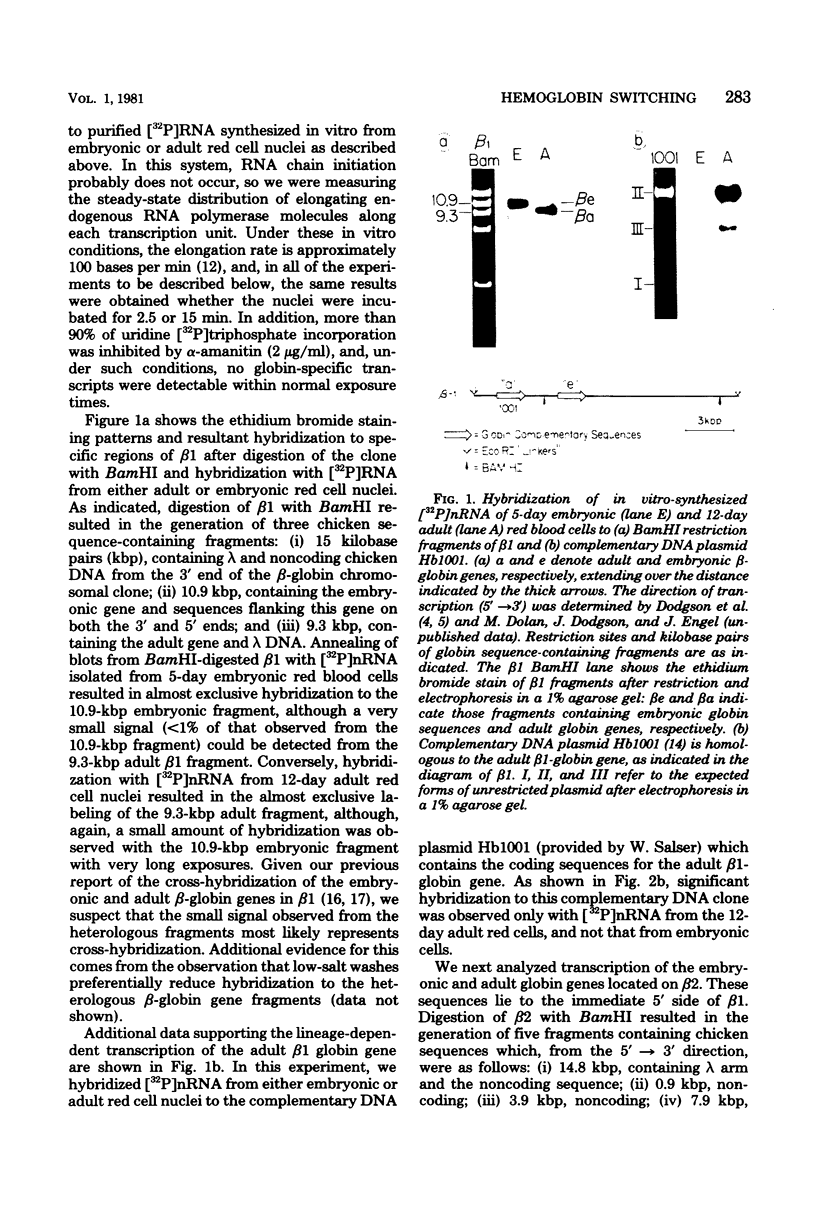


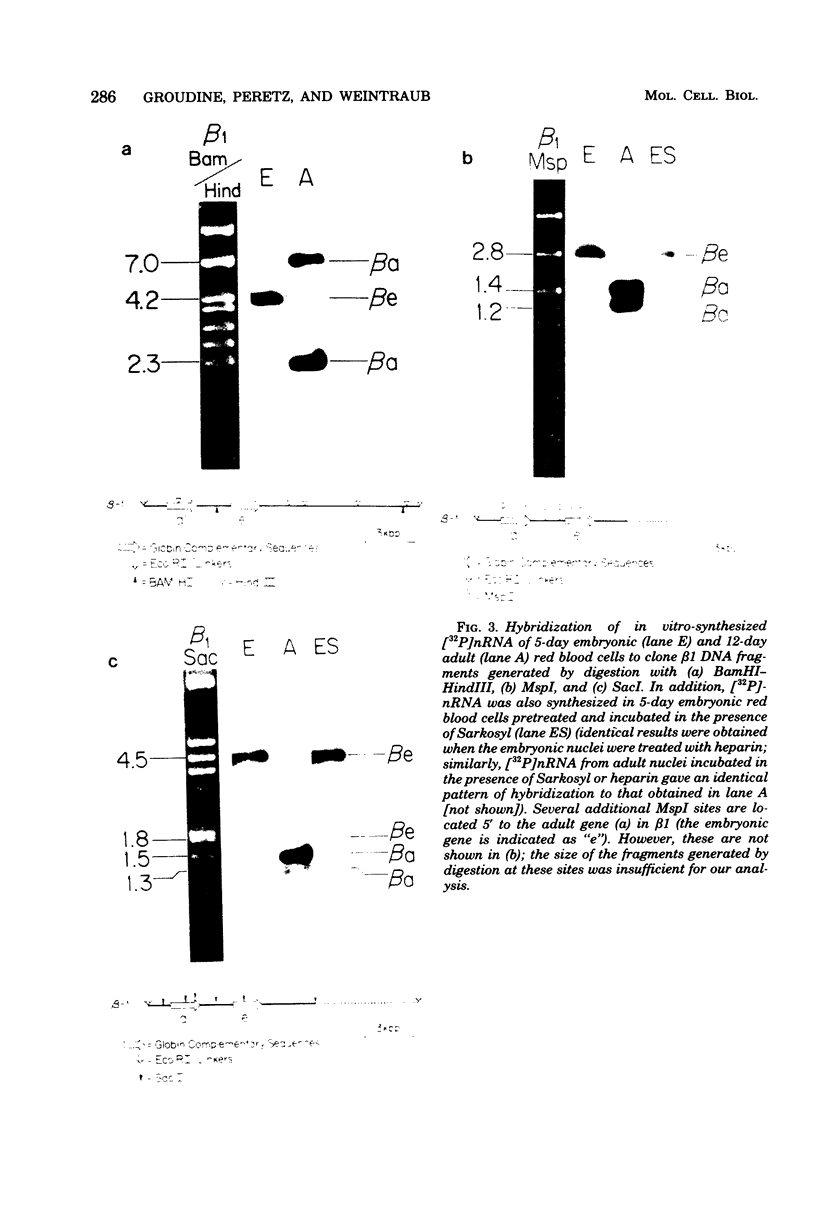


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. L., Ingram V. M. Structural studies on chick embryonic hemoglobins. J Biol Chem. 1974 Jun 25;249(12):3960–3972. [PubMed] [Google Scholar]
- Chapman B. S., Tobin A. J. Distribution of developmentally regulated hemoglobins in embryonic erythroid populations. Dev Biol. 1979 Apr;69(2):375–387. doi: 10.1016/0012-1606(79)90298-7. [DOI] [PubMed] [Google Scholar]
- Cox R. F. Quantitation of elongating form A and B RNA polymerases in chick oviduct nuclei and effects of estradiol. Cell. 1976 Mar;7(3):455–465. doi: 10.1016/0092-8674(76)90176-8. [DOI] [PubMed] [Google Scholar]
- Dodgson J. B., Strommer J., Engel J. D. Isolation of the chicken beta-globin gene and a linked embryonic beta-like globin gene from a chicken DNA recombinant library. Cell. 1979 Aug;17(4):879–887. doi: 10.1016/0092-8674(79)90328-3. [DOI] [PubMed] [Google Scholar]
- Ferencz A., Seifart K. H. Comparative effect of heparin on RNA synthesis of isolated rat-liver nucleoli and purified RNA polymerase A. Eur J Biochem. 1975 May 6;53(2):605–613. doi: 10.1111/j.1432-1033.1975.tb04104.x. [DOI] [PubMed] [Google Scholar]
- Gariglio P., Buss J., Green M. H. Sarkosyl activation of RNA polymerase activity in mitotic mouse cells. FEBS Lett. 1974 Aug 30;44(3):330–333. doi: 10.1016/0014-5793(74)81170-1. [DOI] [PubMed] [Google Scholar]
- Gariglio P. Effect of Sarkosyl on chromatin and viral RNA synthesis. The isolation of SV40 transcription complex. Differentiation. 1976 Jun 4;5(2-3):179–183. doi: 10.1111/j.1432-0436.1976.tb00912.x. [DOI] [PubMed] [Google Scholar]
- Ginder G. D., Wood W. I., Felsenfeld G. Isolation and characterization of recombinant clones containing the chicken adult beta-globin gene. J Biol Chem. 1979 Sep 10;254(17):8099–8102. [PubMed] [Google Scholar]
- Groudine M., Holtzer H., Scherrer K., Therwath A. Lineage-dependent transcription of globin genes. Cell. 1974 Nov;3(3):243–247. doi: 10.1016/0092-8674(74)90138-x. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Palmiter R. D., Christensen A. K., Schimke R. T. Organization of polysomes from pre-existing ribosomes in chick oviduct by a secondary administration of either estradiol or progesterone. J Biol Chem. 1970 Feb 25;245(4):833–845. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stalder J., Groudine M., Dodgson J. B., Engel J. D., Weintraub H. Hb switching in chickens. Cell. 1980 Apr;19(4):973–980. doi: 10.1016/0092-8674(80)90088-4. [DOI] [PubMed] [Google Scholar]
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil P. A., Luse D. S., Segall J., Roeder R. G. Selective and accurate initiation of transcription at the Ad2 major late promotor in a soluble system dependent on purified RNA polymerase II and DNA. Cell. 1979 Oct;18(2):469–484. doi: 10.1016/0092-8674(79)90065-5. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Yang V. W., Binger M. H., Flint S. J. Transcription of adenoviral genetic information in isolated nuclei. Characterization of viral RNA sequences synthesized in vitro. J Biol Chem. 1980 Mar 10;255(5):2097–2108. [PubMed] [Google Scholar]