Abstract
Epicardial mapping and ablation is increasingly being performed for the treatment of complex arrhythmias. Right ventricular (RV) puncture remains the most common complication, with damage to surrounding non-cardiac structures also a concern. We describe the standard techniques used in our lab essential for safe epicardial access, as well as a novel technique incorporating electroanatomic mapping (EAM) guidance.
In a series of 8 patients referred for ventricular tachycardia ablation, an RV endocardial voltage map was created using EAM systems. EAM images were fused with pre-procedure CT scans when available. A 17G Tuohy needle was integrated with the EAM system by attaching the needle to sterile electrode clamps. EAM location points were used in conjunction with standard access techniques until epicardial access was obtained. Epicardial access was successfully obtained in 8/8 (100%) patients. Successful access without RV puncture was achieved in 7/8 (88%) cases. This proof of concept study demonstrates that EAM systems can be used as an adjunct to standard access techniques to visualize and facilitate pericardial access.
Keywords: ventricular tachycardia, catheter ablation, epicardial ablation, electroanatomic mapping
INTRODUCTION
Experience with epicardial access for mapping and ablation has increased significantly since its first description for the ablation of epicardial ventricular tachycardia (VT) substrates in patients with Chagas disease by Sosa and colleagues in 1996. 1
In many centers, endocardial ablation is attempted initially and epicardial mapping and ablation is performed as a second procedure for recurrent VT. However, recent evidence suggests this may not always be the optimal approach. In experienced centers including our own, all nonischemic cardiomyopathy (NICM) and arrhythmogenic right ventricular cardiomyopathy (ARVC) VT cases routinely undergo combined endocardial and epicardial mapping during the index procedure, as the use of this combined approach to ablation of VT is believed to have the potential to increases the initial procedure success.
COMPLICATIONS OF EPICARDIAL ACCESS
The overall complication rates for such an approach are relatively low in experienced centers2, 3 with reported rates of 4–7%. However, as the technique is used more frequently in lower volume, less experienced centers the complication rates may be higher. Pericardial bleeding due to right ventricular (RV) puncture remains the most common complication and can manifest as a dry puncture (RV entry without tamponade) or with hemopericardium. Pericardial bleeding can be even more problematic when significant RV pathology, such as aneurysmal dilation and free wall thinning is present, such as in ARVC.
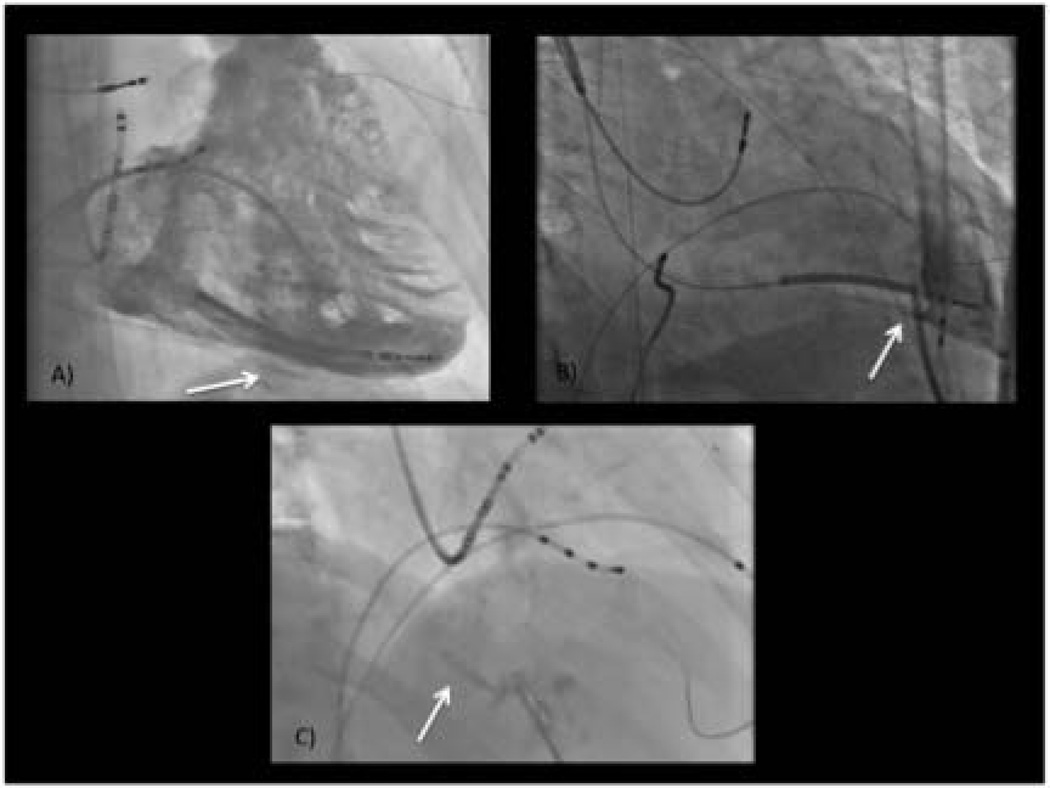
In addition, the risk of ventricular psuedoaneurysm, ventricular hematoma and coronary venous or coronary artery laceration exist. Further, damage to surrounding thoracic and abdominal structures have been reported, even in experienced centers, including abdominal vessel bleeding and hepatic hematoma 4 (Figure 1). These extra-cardiac structures are not adequately visualized on fluoroscopy. Techniques to minimize these risks and allow for real-time visualization of the relationship between the access needle and the surrounding structures may be helpful.
Figure 1.
Potential complications associated with attempted epicardial access (arrows) including: A) persistent intra-myocardial staining suggesting intra-myocardial hematoma; B) RV perforation and dilator placement in RV; C) hepatic trauma (hepatic venous angiogram shown).
There are numerous procedural strategies that can be used to improve safety and are currently available clinically. Here we describe the standard techniques used in our lab. While these standard techniques provide a certain level of safety, they have not changed significantly since the first descriptions by Sosa and colleagues.
To this end, new equipment and technologies for pericardial access are being sought and alternative means of access have been described. However, when and if these techniques and technologies will be incorporated into clinical practice is not yet clear and therefore attempts should be made to utilize all currently available tools. We describe a new technique in development in our lab that we believe has the potential to add an additional layer of safety.
DESCRIPTION OF STANDARD APPROACH
After appropriate sterile prep of the subxiphoid region and use of local anesthesia, a 3 ½ or 6 inch 17G Tuohy needle (Havel’s, Inc., Cincinnati, OH) is used to puncture at a site between the xiphoid process and the left rib with the needle initially tip directed at the left shoulder. The initial projection is shallow just under the rib and then a steeper angle is taken to allow for a posterior access to the ventricle. The angle of access is adjusted based on factors such as the position of the liver and the extent to which the hemi-diaphragm extends into the thorax. These adjustments often lead to the needle angle being aligned more in parallel with the sternum.
USE OF FLUOROSCOPY
Biplane fluoroscopy with RAO and LAO views is essential for safe epicardial access. Placement of basic diagnostic catheters including a HIS catheter, right ventricular (RV) catheter as well as a coronary sinus catheter helps delineate the septal plane and AV groove. As the pericardium is approached and the fibrous pericardium is indented, contrast is injected to demonstrate tenting of the pericardium. (Video 1). Additional contrast is then injected once the pericardium is punctured demonstrating layering in the pericardial space.
IMPORTANCE OF UNDERSTANDING CARDIAC/PERICARDIAL ANATOMY
A detailed understanding of cardiac anatomy, specifically the pericardial recesses, is essential to ensure correct interpretation of pericardial contrast imaging and assess wire/sheath/catheter placement. Once the pericardium is punctured, a J-tipped guide wire is advanced into the pericardial space. (Video 2) Observation of the wire advancing in the LAO view should show the wire advancing along the left heart border and preferably across the transverse sinus.
USE OF PERICARDIOGRAM
A pericardiogram verifies appropriate pericardial access but also can help determine if significant pericardial adhesions are present that may limit epicardial mapping and ablation. Once we have verified epicardial access with J-wire position, we advance a 4F soft dilator over the wire and the wire is removed for pericardial imaging with injection of approximately 5–10cc of contrast. Once imaging is completed an exchange wire is advanced and the 4F dilator is exchanged for an 8F SLO sheath (St. Jude Medical, Minnetonka, MN).
NOVEL STRATEGY TO IMPROVE SAFETY
Electroanatomic mapping systems (EAM) (Ensite Velocity [NavX], St. Jude Medical, Minneapolis, MN or CARTO III, Biosense Webster, Diamond Bar, CA) are increasingly used during cardiac electrophysiologic procedures. These systems have not been validated for use defining anatomic points in the soft-tissue outside of the vasculature or epicardial space.
We recently sought to assess the incorporation of these systems to help guide epicardial access. In order to do so, an RV endocardial voltage map was created with the EAM system in a series of patients. Attention was made to delineate the RV apex as this often represents the most anterior portion of the RV. When available, the endocardial voltage map was fused with the previously obtained CT scan using the superior and inferior vena cava and xiphoid process as reference points.
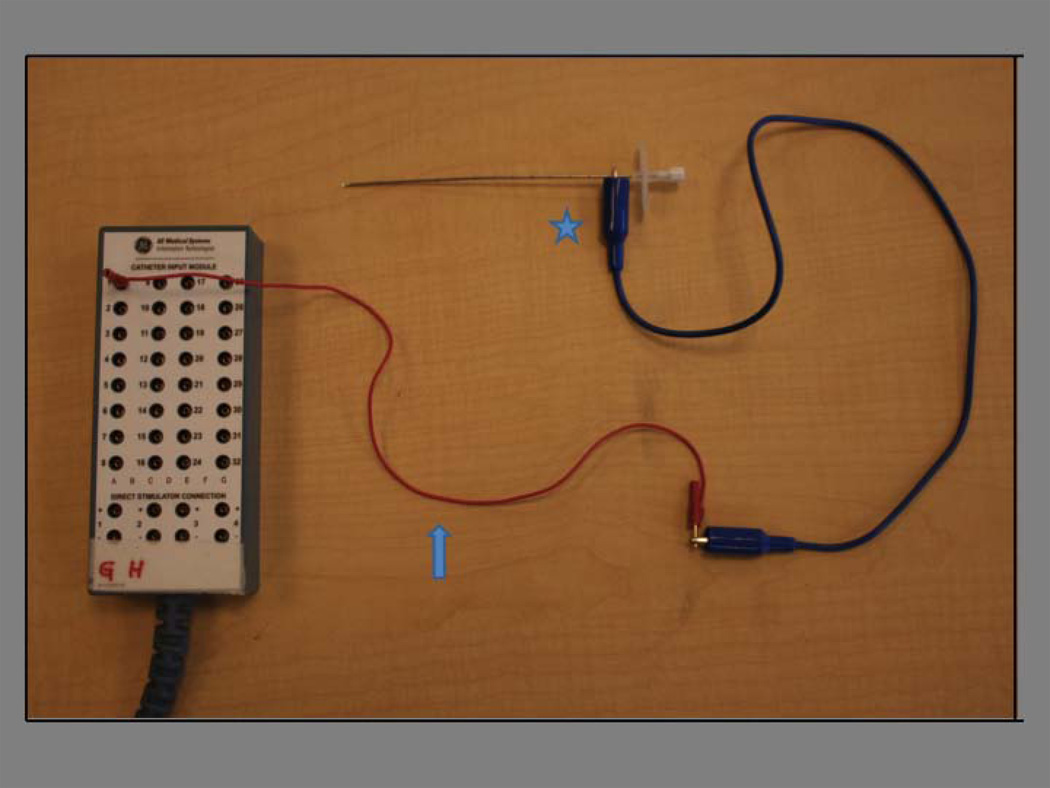
For epicardial access, a Tuohy needle attached to sterile electrode clamps (alligator clips) was used, allowing for integration of the needle with the EAM system. The alligator clips were attached to the recording system pin box via electrode cables. (Figure 2) Due to EAM system limitations, the tip of the needle functioned as a unipolar electrode with the system reference patch as the indifferent electrode for the Ensite (NavX) system, while the tip functioned as a bipolar electrode with the Carto mapping system.
Figure 2.
Schematic of set-up for integration of epicardial access needle into EAM system. A “jumper cable” connects the pin box to the “alligator clip” (blue arrow). The “alligator clip” connects to the Tuohy needle for epicardial access (blue star).
Prior to attempting access, the tip of the Tuohy needle was placed in direct contact with the xiphoid process within the soft tissue for a reference point and to verify that the system located the needle tip at the site of the xiphoid process seen on CT fusion in available cases. Mapping points were taken during advancement of the needle until epicardial access was obtained and verified fluoroscopically. (Figure 3)
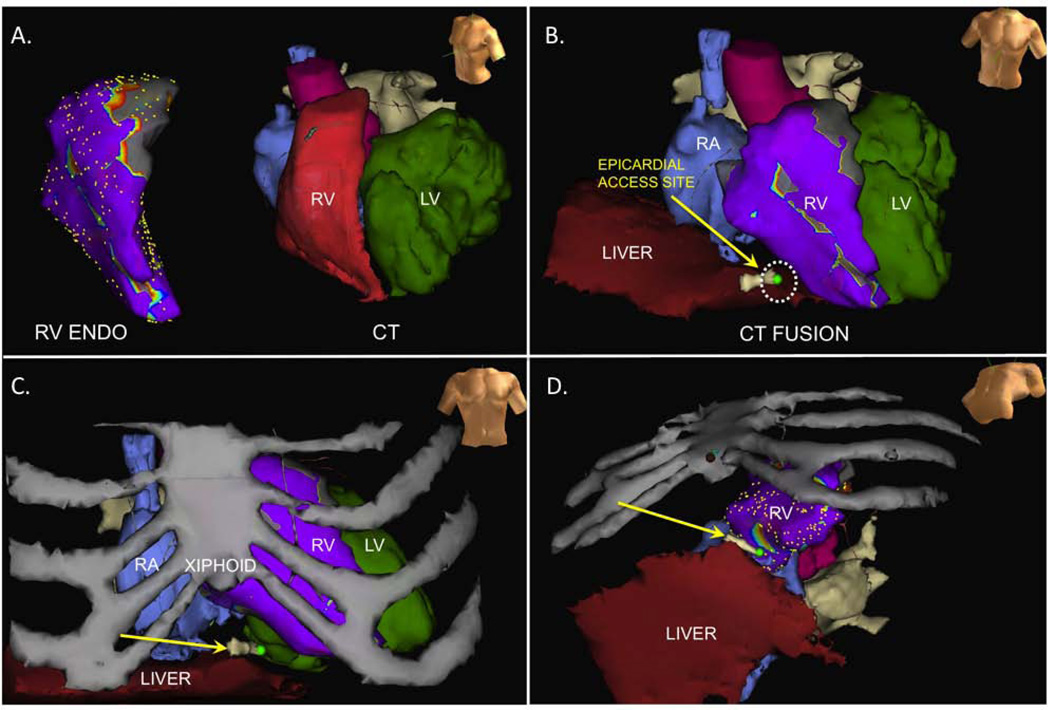
Figure 3.
A) RV endocardial voltage map and CT scan prior to fusion; B) LAO cranial view of epicardial access site superimposed on CT fusion including cardiac and hepatic structures; C) AP view of epicardial access site superimposed on CT fusion/RV voltage map with addition of ribs; D) LAO caudal view of epicardial access site.
The ability of the system to locate the tip of a non-insulated needle or wire is a known potential limitation of this system modification. Therefore, the following steps were taken in our initial studies: First, the needle tip was positioned on the xiphoid process and correlation with this anatomic position was seen with images seen on CT fusion as noted above. Second, we verified that the anatomic point being collected when a J-wire was advanced through the needle within the pericardial space correlated with the most distal aspect of the wire. This was done by connecting the sterile electrode clamps to the epicardial wire after access was obtained. The tip of the wire was visualized only after it exited the sheath in the pericardium. The wire was tracked as it progressed along the lateral border of the heart as verified fluoroscopically. The anatomic point taken by the EAM system correlated with the position of the wire tip fluoroscopically, not with a position along the shaft of the wire.
Patient Series
A total of 8 patients (75% male) with a mean age 55±14 years referred for endocardial/epicardial mapping and VT ablation were studied. (Table 1) Seven patients (87.5%) had documented sustained monomorphic VT leading to recurrent implantable cardioverter-defibrillator therapy. Of these 7 patients, 4 (57%) had NICM and 3 (43%) had ARVC. One additional patient had a high burden of symptomatic premature ventricular contractions thought to be contributing to an underlying NIDCM resistant to previous endocardial ablation.
TABLE I.
Baseline characteristics
| Patient | Age | Sex | Cardiomyopathy etiology |
Ejection fraction % (LV/RV) |
ICD? | Antiarrhythmic medication |
Previous ablation? (#) |
Mapping system |
|---|---|---|---|---|---|---|---|---|
| 1 | 54 | F | ARVC | 30/15 | yes | Amiodarone Mexiletine |
No | NavX |
| 2 | 39 | M | ARVC | 55/45 | yes | Amidoarone | Yes (3) | NavX |
| 3 | 65 | M | NICM | 45/50 | no | none | Yes (1) | NavX |
| 4 | 44 | M | NICM | 30/55 | yes | Amiodarone Mexiletine |
Yes (1) | NavX |
| 5 | 80 | M | NICM | 25/30 | no | Amiodarone | Yes (1) | NavX |
| 6 | 67 | M | NICM | 20/30 | yes | Amiodarone | Yes (1) | NavX |
| 7 | 42 | M | NICM | 25/30 | yes | Amiodarone | Yes (1) | Carto |
| 8 | 49 | F | ARVC | 45/25 | yes | Mexiletine | Yes (1) | Carto |
Epicardial access was successfully obtained in 8/8 (100%) patients. Successful access of the pericardial space, with point of access seen external to the RV endocardial shell on EAM, was achieved in 7/8 (87.5%) of the patients at the first attempt. In one case, patient had expected difficult epicardial access based on two previous epicardial access procedures for cardiac tamponade and pericarditis related to a perforated right ventricular lead. In this case, the needle tip was seen adjacent to the RV endocardium during tenting of the pericardium as expected. The position was documented on contrast injection during fluoroscopy. However, on puncture of the pericardium the needle-tip as located by EAM was shown as crossing the RV endocardial border. (Figure 4) To verify the position, a J-tipped wire was advanced through the needle. The position of the wire was not clear fluoroscopically as it could not be freely advanced along the left heart border as is our protocol. Given the patients’ known pericardial adhesions it was not clear from fluoroscopic assessment alone if this limited motion was due to the adhesions or due to RV puncture.
Figure 4.
Demonstration of RV perforation with attempted epicardial needle access in a patient with difficult pericardial access due to adhesions. CT fusion with RV EAM with voltage points removed in the LAO view is shown. The access point crosses heart border (green circle with red halo). The red halo demonstrates component initially crossing RV wall. Path of wire as advanced into the RV outflow is shown (purple tract). green=right atrium, yellow=aorta, red=left atrium. aqua=liver.
On further assessment of EAM images with the electrode clamps attached to the wire (figure 4), the wire was clearly seen advancing into the RV endocardial shell. The needle was withdrawn and redirected and the wire was seen advancing through the needle into the epicardial space on both fluoroscopy and EAM. No bleeding was seen consistent with a ‘dry’ tap, but, as a precaution the case was terminated and rescheduled.
The remaining 7 patients had successful epicardial mapping and ablation as appropriate. Eighty-six percent (6/7) of these patients underwent ablation utilizing predominantly a substrate-based approach due to hemodynamically untolerated VT or noninducible VT at the time of study. One (14%) patient with ARVC was in incessant tachycardia during the procedure. VT was terminated transiently with epicardial ablation, but terminated permanently after endocardial ablation at the corresponding RV free wall site. Acute success was obtained in 7/7 (100%) patients who underwent VT ablation. The mean duration of follow-up was 79±71 days. Follow-up was available at the time of submission for 88% of the patients, with an 86% success rate at short-term follow-up.
Conclusion
Data for the overall safety and efficacy of epicardial mapping and ablation for arrhythmias such as VT ablation continues to accumulate. Safe epicardial access options are essential as epicardial mapping and ablation of VT increases. In addition, it will become even more essential as new procedures are developed that require epicardial access.
In this report we describe our techniques for minimizing the risk of epicardial access including new data on the use of EAM systems as a supplement to standard techniques.
Our data provides a proof of concept that a currently available tool, EAM, can be used in conjunction with CT fusion and fluoroscopy to visualize and facilitate pericardial access. Such a technique has the potential to decrease the risk of RV perforation and damage to surrounding structures that are not visualized fluoroscopically. While development of new technology for epicardial access is needed, further adaptation of currently available technology may provide more tools to improve the safety of epicardial access. This may be of particular utility in difficult access cases when standard fluoroscopic verification of epicardial access is limited by pericardial adhesions.
Supplementary Material
Demonstration of epicardial access technique with tenting of the pericardium prior to puncture.
Demonstration of the J-tipped wire advancing along the left heart border after successful epicardial access.
Acknowledgments
Dr. Shivkumar receives grant support from the National Heart Lung and Blood Institute (R01HL084261).
Footnotes
No other disclosures.
REFERENCES
- 1.Sosa E, Scanavacca M, d'Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996;7:531–536. doi: 10.1111/j.1540-8167.1996.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 2.Sacher F, Roberts-Thomson K, Maury P, Tedrow U, Nault I, Steven D, Hocini M, Koplan B, Leroux L, Derval N, Seiler J, Wright MJ, Epstein L, Haissaguerre M, Jais P, Stevenson WG. Epicardial ventricular tachycardia ablation a multicenter safety study. J Am Coll Cardiol. 55:2366–2372. doi: 10.1016/j.jacc.2009.10.084. [DOI] [PubMed] [Google Scholar]
- 3.Della Bella P, Brugada J, Zeppenfeld K, Merino J, Neuzil P, Maury P, Maccabelli G, Vergara P, Baratto F, Berruezo A, Wijnmaalen AP. Epicardial ablation for ventricular tachycardia: A European multicenter study. Circ Arrhythm Electrophysiol. 4:653–659. doi: 10.1161/CIRCEP.111.962217. [DOI] [PubMed] [Google Scholar]
- 4.Koruth JS, Aryana A, Dukkipati SR, Pak HN, Kim YH, Sosa EA, Scanavacca M, Mahapatra S, Ailawadi G, Reddy VY, d'Avila A. Unusual complications of percutaneous epicardial access and epicardial mapping and ablation of cardiac arrhythmias. Circ Arrhythm Electrophysiol. 4:882–888. doi: 10.1161/CIRCEP.111.965731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demonstration of epicardial access technique with tenting of the pericardium prior to puncture.
Demonstration of the J-tipped wire advancing along the left heart border after successful epicardial access.