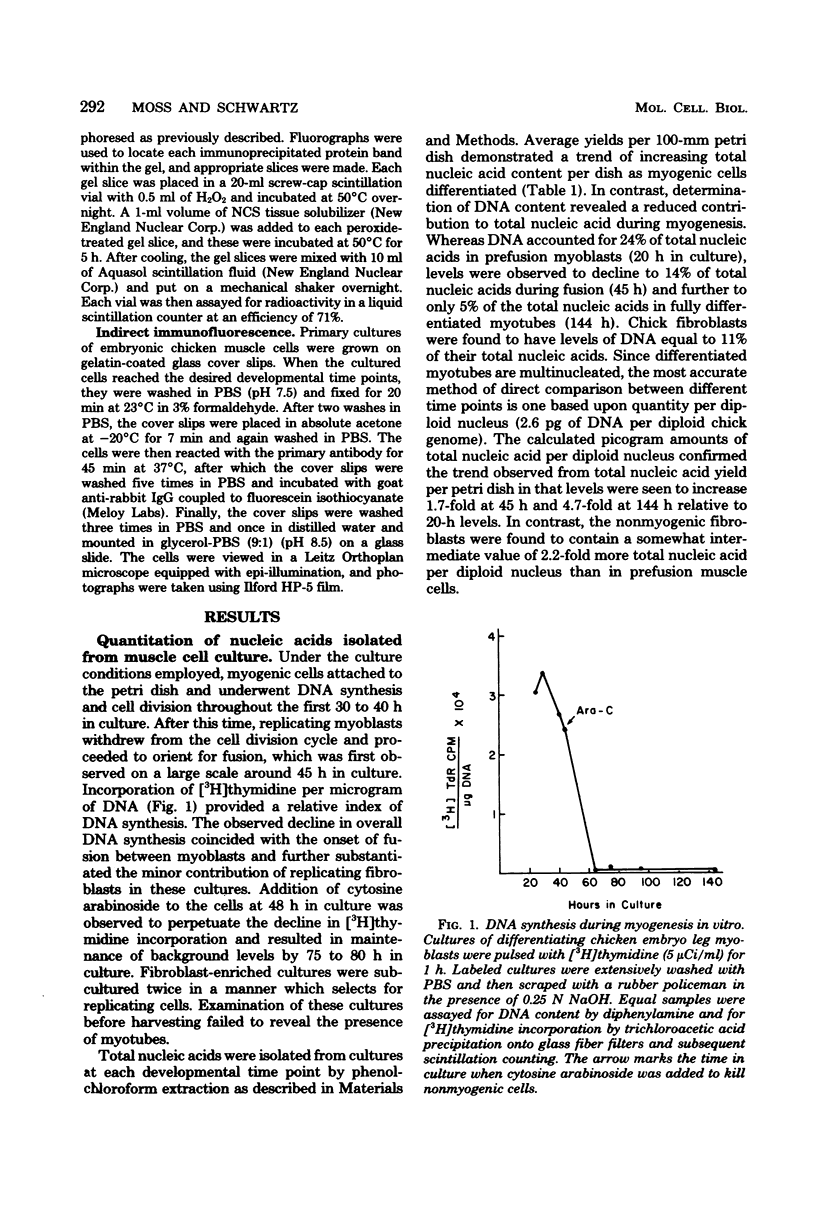
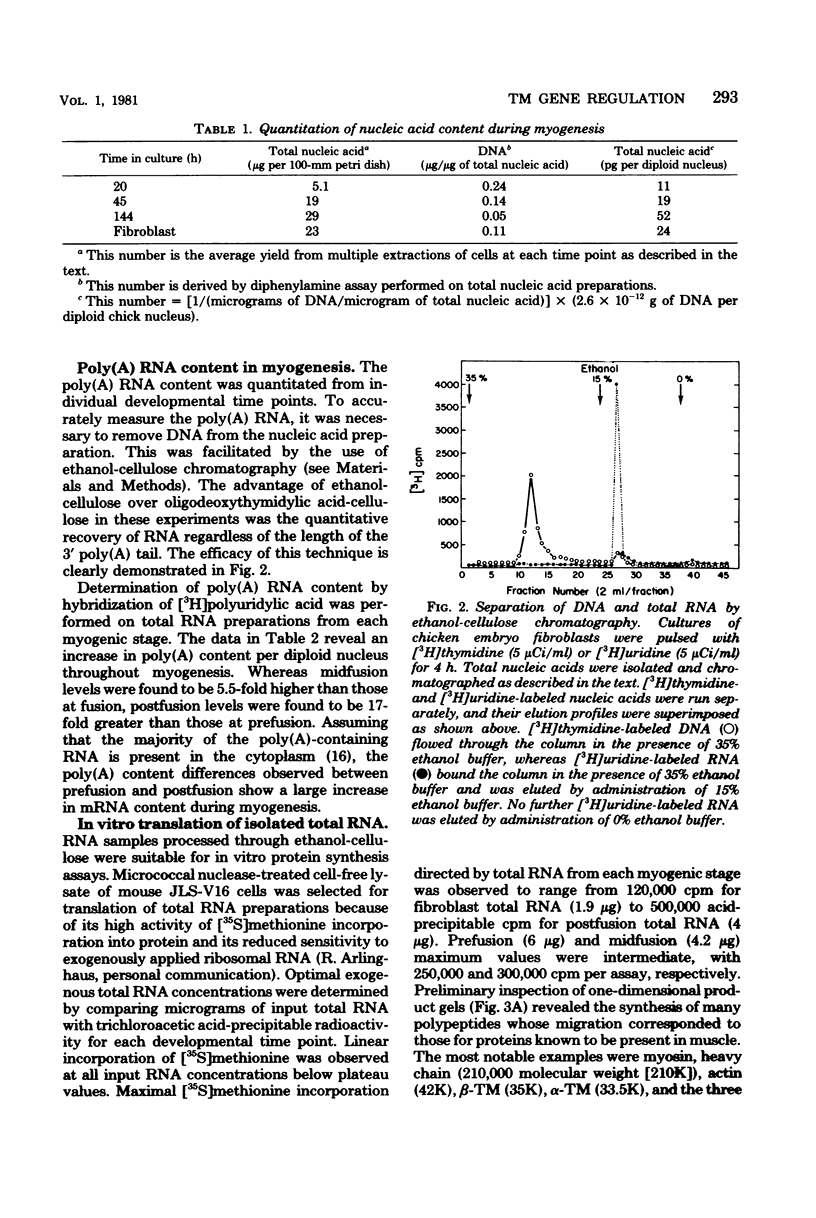
Abstract
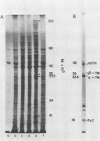
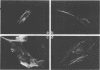
In skeletal muscle, tropomyosin has a critical role in transduction of calcium-induced contraction. Presently, little is known about the regulation of tropomyosin gene expression during myogenesis. In the present study, qualitative and quantitative changes in the nucleic acid populations of differentiating chicken embryo muscle cells in culture have been examined. Total nucleic acid content per nucleus increased about fivefold in fully developed myotubes as compared to mononucleated myoblasts. The contribution of deoxyribonucleic acid to the total nucleic acid population decreased from 24% in myoblasts to 5% of total nucleic acid in myotubes. Concomitant with the decrement in deoxyribonucleic acid contribution to total nucleic acid was an increase in polyadenylated ribonucleic acid (RNA) content per cell which reached levels in myotubes that were 17-fold higher than those of myoblasts. Specific changes in the RNA population during myogenesis were further investigated by quantitation of the synthetic capacity (messenger RNA levels) per cell for alpha- and beta-tropomyosin. Cell-free translation and immunoprecipitation demonstrated an approximately 40-fold increase in messenger RNA levels per nucleus for alpha- and beta-tropomyosin after fusion in the terminally differentiated myotubes. Indirect immunofluorescence with affinity-purified tropomyosin antibodies demonstrated the presence of tropomyosin-containing filaments in cells throughout myogenesis. Thus, the tropomyosin genes are constitutively expressed during muscle differentiation through the production of tropomyosin messenger RNA and translation into tropomyosin protein.
Full text
PDF


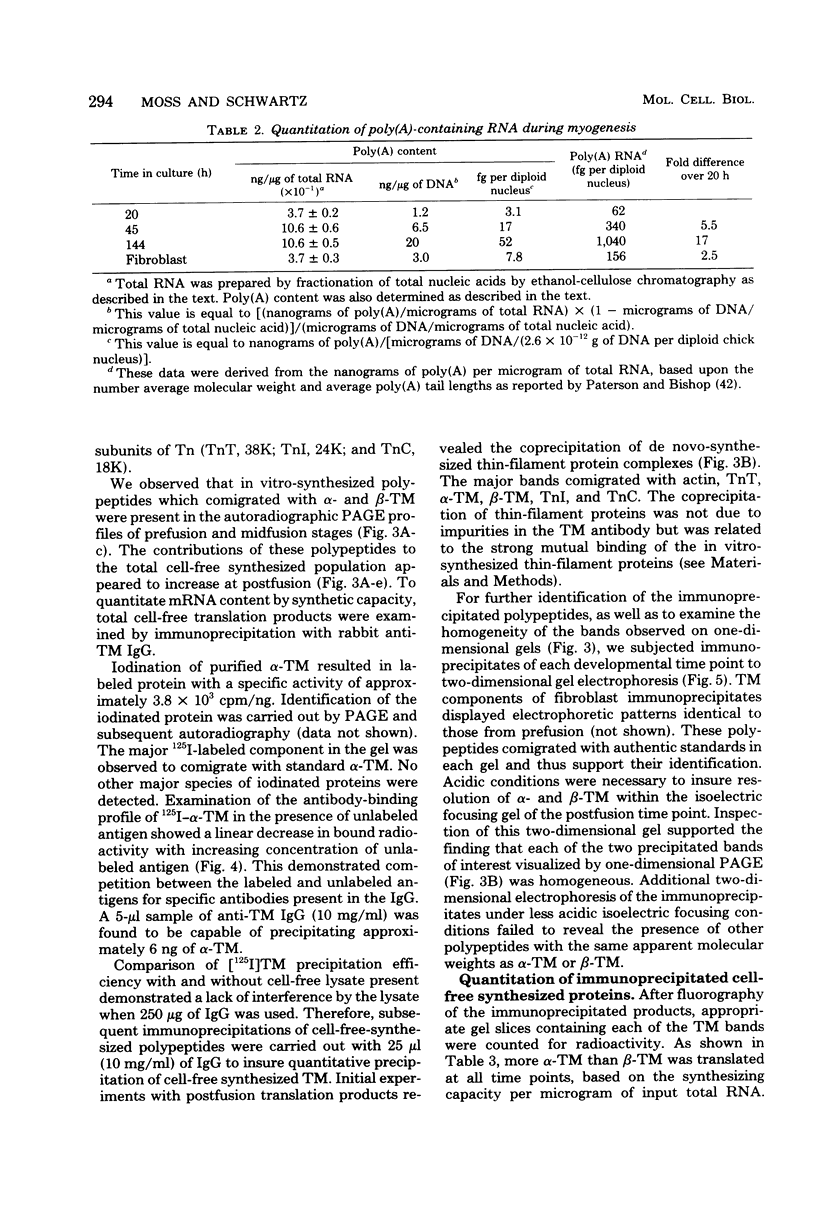
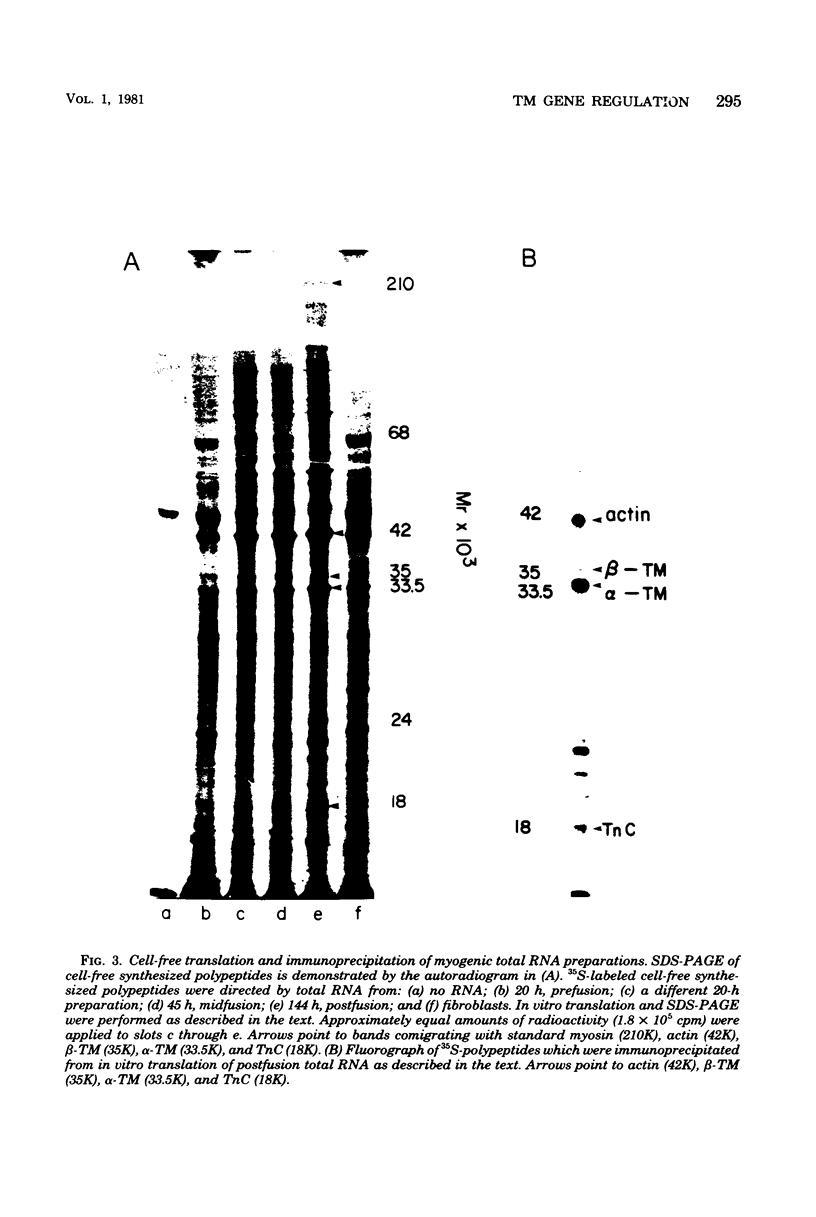
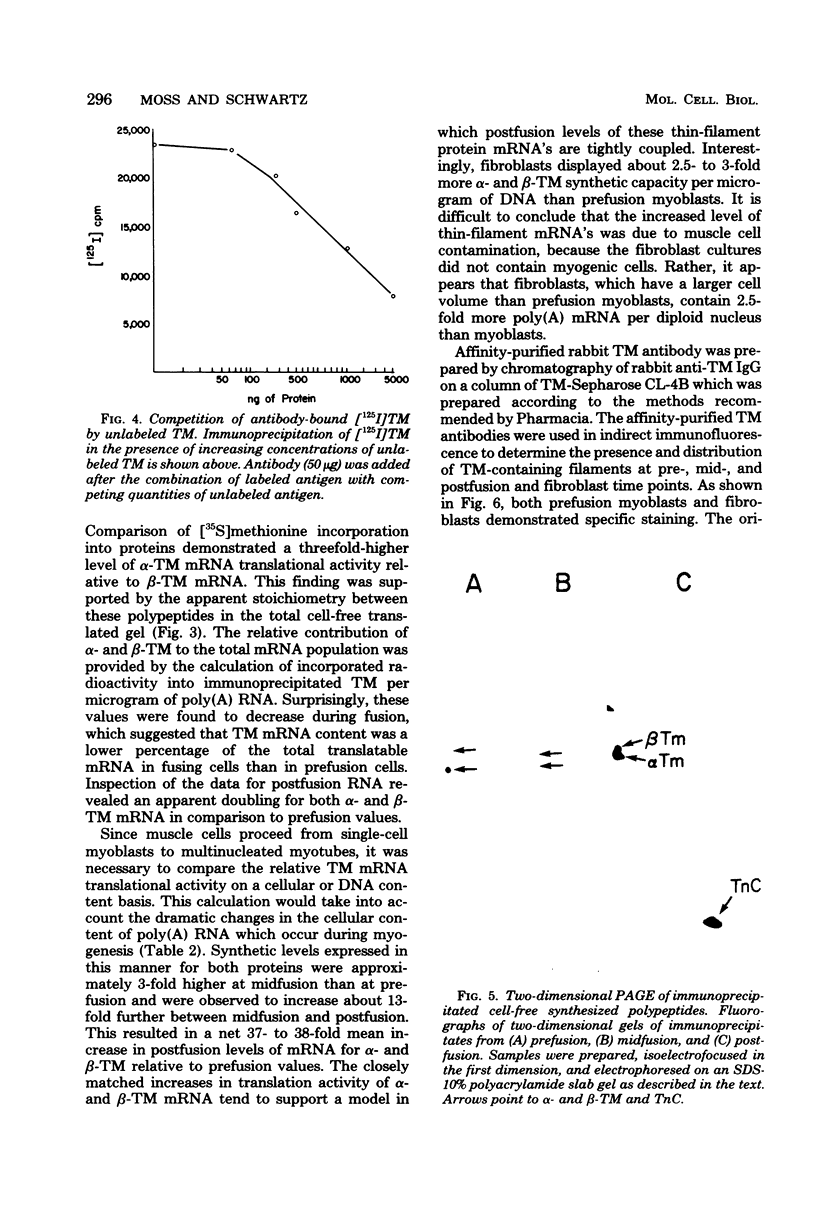
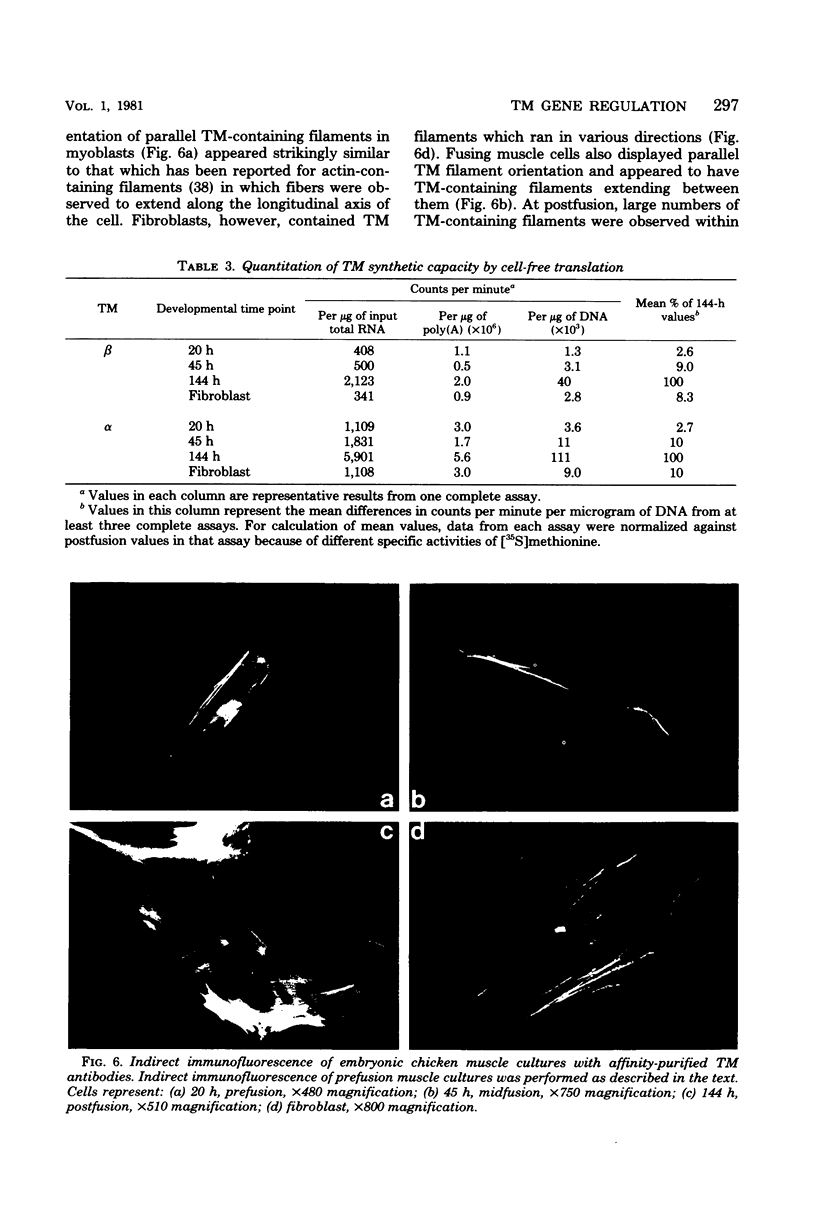




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. E., Stromer M. H., Goll D. E., Robson R. M. Sythesis of tropomyosin in cultures of differentiating muscle cells. J Cell Biol. 1978 Jan;76(1):98–104. doi: 10.1083/jcb.76.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bowman L. H., Emerson C. P., Jr Post-transcriptional regulation of ribosome accumulation during myoblast differentiation. Cell. 1977 Apr;10(4):587–596. doi: 10.1016/0092-8674(77)90091-5. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Tropomyosin from bovine brain contains two polypeptide chains of slightly different molecular weights. FEBS Lett. 1978 Jan 1;85(1):145–148. doi: 10.1016/0014-5793(78)81267-8. [DOI] [PubMed] [Google Scholar]
- Buckingham M. E., Caput D., Cohen A., Whalen R. G., Gros F. The synthesis and stability of cytoplasmic messenger RNA during myoblast differentiation in culture. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1466–1470. doi: 10.1073/pnas.71.4.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmon Y., Neuman S., Yaffe D. Synthesis of tropomyosin in myogenic cultures and in RNA-directed cell-free systems: qualitative changes in the polypeptides. Cell. 1978 Jun;14(2):393–401. doi: 10.1016/0092-8674(78)90124-1. [DOI] [PubMed] [Google Scholar]
- Chamley-Campbell J., Campbell G. R., Gröschel-Stewart U., Burnstock G. FITC-labelled antibody staining of tropomyosin-containing fibrils in smooth, cardiac and skeletal muscle cells, prefusion myoblasts, fibroblasts, endothelial cells and 3T3 cells in culture. Cell Tissue Res. 1977 Sep 26;183(2):153–166. doi: 10.1007/BF00226616. [DOI] [PubMed] [Google Scholar]
- Clarke M., Spudich J. A. Nonmuscle contractile proteins: the role of actin and myosin in cell motility and shape determination. Annu Rev Biochem. 1977;46:797–822. doi: 10.1146/annurev.bi.46.070177.004053. [DOI] [PubMed] [Google Scholar]
- Clissold P., Cole R. J. Regulation of ribosomal RNA synthesis during mammalian myogenesis in culture. Exp Cell Res. 1973 Jul;80(1):159–169. doi: 10.1016/0014-4827(73)90287-5. [DOI] [PubMed] [Google Scholar]
- Colbert D. A., Coleman J. R. Transcriptional expression of non-repetitive DNA during normal and BUdR-mediated inhibition of myogenesis in culture. Exp Cell Res. 1977 Oct 1;109(1):31–42. doi: 10.1016/0014-4827(77)90041-6. [DOI] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. The subunits and biological activity of polymorphic forms of tropomyosin. Biochem J. 1973 Aug;133(4):765–777. doi: 10.1042/bj1330765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr The origin of mRNA and the structure of the mammalian chromosome. Harvey Lect. 1973;(69):1–47. [PubMed] [Google Scholar]
- Davidson E. H., Britten R. J. Organization, transcription, and regulation in the animal genome. Q Rev Biol. 1973 Dec;48(4):565–613. doi: 10.1086/407817. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate accumulation of contractile protein mRNAs during myoblast differentiation. Dev Biol. 1979 Mar;69(1):202–216. doi: 10.1016/0012-1606(79)90286-0. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate regulation of contractile protein synthesis during myoblast differentiation. Cell. 1978 Apr;13(4):599–611. doi: 10.1016/0092-8674(78)90211-8. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D. Synapse formation between dissociated nerve and muscle cells in low density cell cultures. Dev Biol. 1972 Jun;28(2):407–429. doi: 10.1016/0012-1606(72)90023-1. [DOI] [PubMed] [Google Scholar]
- Franklin R. M. Purification and properties of the replicative intermediate of the RNA bacteriophage R17. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1504–1511. doi: 10.1073/pnas.55.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert J. M., Anderson W. F. Cell-free hemoglobin synthesis. II. Characteristics of the transfer ribonucleic acid-dependent assay system. J Biol Chem. 1970 May 10;245(9):2342–2349. [PubMed] [Google Scholar]
- Harris H. Switching on the muscle genes. Nature. 1980 Aug 21;286(5775):758–759. doi: 10.1038/286758a0. [DOI] [PubMed] [Google Scholar]
- Herrmann H., Heywood S. M., Marchok A. C. Reconstruction of muscle development as a sequence of macromolecular synthesis. Curr Top Dev Biol. 1970;5:181–234. [PubMed] [Google Scholar]
- Heywood S. M., Kennedy D. S. Purification of myosin translational control RNA and its interaction with myosin messenger RNA. Biochemistry. 1976 Jul 27;15(15):3314–3319. doi: 10.1021/bi00660a023. [DOI] [PubMed] [Google Scholar]
- Hitchcock S. E. The appearance of a functional contractile apparatus in developing muscle. Dev Biol. 1970 Nov;23(3):399–423. doi: 10.1016/0012-1606(70)90106-5. [DOI] [PubMed] [Google Scholar]
- Hodges R. S., Smillie L. B. Chemical evidence for chain heterogeneity in rabbit muscle tropomyosin. Biochem Biophys Res Commun. 1970 Nov 25;41(4):987–994. doi: 10.1016/0006-291x(70)90182-8. [DOI] [PubMed] [Google Scholar]
- Hunter T., Garrels J. I. Characterization of the mRNAs for alpha-, beta- and gamma-actin. Cell. 1977 Nov;12(3):767–781. doi: 10.1016/0092-8674(77)90276-8. [DOI] [PubMed] [Google Scholar]
- John H. A., Patrinou-Georgoulas M., Jones K. W. Detection of myosin heavy chain mRNA during myogenesis in tissue culture by in vitro and in situ hybridization. Cell. 1977 Oct;12(2):501–508. doi: 10.1016/0092-8674(77)90126-x. [DOI] [PubMed] [Google Scholar]
- Kennedy D. S., Siegel E., Heywood S. M. Purification of myosin mRNP translational control RNA and its inhibition of myosin and globin messenger translation. FEBS Lett. 1978 Jun 15;90(2):209–214. doi: 10.1016/0014-5793(78)80370-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Tropomyosin antibody: the specific localization of tropomyosin in nonmuscle cells. J Cell Biol. 1975 Jun;65(3):549–561. doi: 10.1083/jcb.65.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss M., Asch B., Schwartz R. Differentiation of actin-containing filaments during chick skeletal myogenesis. Exp Cell Res. 1979 Jun;121(1):167–178. doi: 10.1016/0014-4827(79)90457-9. [DOI] [PubMed] [Google Scholar]
- Moss M., Norris J. S., Peck E. J., Jr, Schwartz R. J. Alterations in iodinated cell surface proteins during myogenesis. Exp Cell Res. 1978 May;113(2):445–450. doi: 10.1016/0014-4827(78)90388-9. [DOI] [PubMed] [Google Scholar]
- Murphy E. C., Jr, Arlinghaus R. B. Cell-free synthesis of Rauscher murine leukemia virus "gag" and "gag-pol" precursor polyproteins from virion 35 S RNA in a mRNA-dependent translation system derived from mouse tissue culture cells. Virology. 1978 May 15;86(2):329–343. doi: 10.1016/0042-6822(78)90074-0. [DOI] [PubMed] [Google Scholar]
- Măn N. T., Cole R. J. Quantitative changes in chromosomal activity during chicken myogenesis in vitro. a DNA-RNA hybridisation study. Exp Cell Res. 1974 Feb;83(2):328–334. doi: 10.1016/0014-4827(74)90346-2. [DOI] [PubMed] [Google Scholar]
- Nguyen-thi-Mân, Cole R. J. RNA synthesis during mammalian myogenesis in culture. Exp Cell Res. 1972 Aug;73(2):429–435. doi: 10.1016/0014-4827(72)90068-7. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Paterson B. M., Bishop J. O. Changes in the mRNA population of chick myoblasts during myogenesis in vitro. Cell. 1977 Nov;12(3):751–765. doi: 10.1016/0092-8674(77)90275-6. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Yaffe D. Determination of actin messenger RNA in cultures of differentiating embryonic chick skeletal muscle. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4467–4471. doi: 10.1073/pnas.71.11.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter J. D., Gergely J. Troponin, tropomyosin, and actin interactions in the Ca2+ regulation of muscle contraction. Biochemistry. 1974 Jun 18;13(13):2697–2703. doi: 10.1021/bi00710a007. [DOI] [PubMed] [Google Scholar]
- Strohman R. C., Moss P. S., Micou-Eastwood J., Spector D., Przybyla A., Paterson B. Messenger RNA for myosin polypeptides: isolation from single myogenic cell cultures. Cell. 1977 Feb;10(2):265–273. doi: 10.1016/0092-8674(77)90220-3. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]