Significance
Cells are mechanosensitive: a cell’s adaptive responses to mechanical inputs will determine its morphology, gene expression, and posttranslational protein modification. Focal adhesions (FAs), the integrin-containing complexes positioned between the extracellular matrix and the cell, are key in transmitting the mechanosignal to initiate these downstream adaptive responses. In this study we made the unique observation that FA and cellular morphology changes in response to sustained uniaxial stretch are orientation specific. Our results also suggest orientation-specific responses at the molecular level in individual FAs propagate up to regulate cell morphology, which may in turn mediate adaptive responses that promote tissue growth.
Keywords: mechanosensing, integrin, tissue mechanics
Abstract
Cells are mechanosensitive to extracellular matrix (ECM) deformation, which can be caused by muscle contraction or changes in hydrostatic pressure. Focal adhesions (FAs) mediate the linkage between the cell and the ECM and initiate mechanically stimulated signaling events. We developed a stretching apparatus in which cells grown on fibronectin-coated elastic substrates can be stretched and imaged live to study how FAs dynamically respond to ECM deformation. Human bone osteosarcoma epithelial cell line U2OS was transfected with GFP-paxillin as an FA marker and subjected to sustained uniaxial stretching. Two responses at different timescales were observed: rapid FA growth within seconds after stretching, and delayed FA disassembly and loss of cell polarity that occurred over tens of minutes. Rapid FA growth occurred in all cells; however, delayed responses to stretch occurred in an orientation-specific manner, specifically in cells with their long axes perpendicular to the stretching direction, but not in cells with their long axes parallel to stretch. Pharmacological treatments demonstrated that FA kinase (FAK) promotes but Src inhibits rapid FA growth, whereas FAK, Src, and calpain 2 all contribute to delayed FA disassembly and loss of polarity in cells perpendicular to stretching. Immunostaining for phospho-FAK after stretching revealed that FAK activation was maximal at 5 s after stretching, specifically in FAs oriented perpendicular to stretch. We hypothesize that orientation-specific activation of strain/stress-sensitive proteins in FAs upstream to FAK and Src promote orientation-specific responses in FA growth and disassembly that mediate polarity rearrangement in response to sustained stretch.
Tissues of the pulmonary, circulatory, musculoskeletal, digestive, and renal systems are subject to mechanical perturbations such as cyclic or continuous stretch. Cells within these tissues are mechanosensitive, responding to these mechanical inputs by changing ion channel configurations (1–3), cytoskeleton organization (4–6), mRNA splicing (7), gene expression (8–10), and posttranslational protein modification (11–13). Although many forms of mechanical stimuli occur physiologically, the most well studied are the responses of cells to cyclic stretch to specifically simulate the contraction/relaxation cycles that occur within the cardiovascular and pulmonary systems (14–17). However, sustained stretch also commonly occurs in tissues—e.g., when injury-induced swelling causes local hydrostatic pressure increase (18), in long-term blood pressure increase (16, 19), during prolonged muscle contraction (20, 21), or when the bladder retains large volume of urine (22, 23). In such situations, tissue stretching may last for minutes or hours without regular relaxation intervals, and likely generates cellular responses that lead to tissue adaptation. However, the cellular responses to sustained stretching are not well studied.
Cells exhibit stereotypical morphological responses to stretch. Cyclic uniaxial stretch induces cells to reorient their long axes perpendicular to the direction of stretch. This process is accompanied by a similar reorientation of actomyosin stress fibers and integrin-mediated focal adhesions (FAs) and is thought to minimize tissue resistance to stretch (5, 6, 24, 25). FAs serve as mechanical conduits, transmitting forces generated by stress fibers to the ECM to drive cell movement or ECM remodeling, and also transmitting forces generated in tissues into the cell. The mechanosensitivity of FA assembly and downstream signaling is well documented, and thus they are prime candidates for mediating cellular responses to stretch (26). Indeed, the cytoskeletal reorientation that occurs in response to cyclic stretch requires several proteins that localize to FA, including integrins (27), zyxin (28), paxillin (25), Src, and p130 Crk-associated substrate (p130Cas) (29), and involves sliding reorganization of FAs (6). However, the effects of sustained stretch on FA organization and dynamics have not been explored.
Here, we sought to characterize the dynamic response of FAs and cell morphologies to sustained uniaxial stretch. We found FAs to be directionally sensitive to sustained stretching. FA disassembly and loss of cell polarity occurred in cells with their long axes perpendicular to stretch, but not in cells with their long axes parallel to stretch. We show that FA kinase (FAK), Src family kinases (SFKs), and calpain 2 all contribute to FA disassembly and loss of cell polarity, and that FAK is activated specifically in FAs oriented perpendicular to stretch. We hypothesize that putative stress/strain sensing proteins in FAs upstream to FAK and Src align with the long axes of FAs, whereas a majority of FAs aligns with the cellular long axis, resulting in orientation-specific responses. Our findings show that sensitivity of strain/stress orientation at the molecular level propagates up to regulate cell polarity in response to sustained mechanical stimulus.
Results
Sustained Uniaxial Stretching Promotes Rapid FA Growth and Slow, Orientation-Specific FA Disassembly and Loss of Cell Polarity.
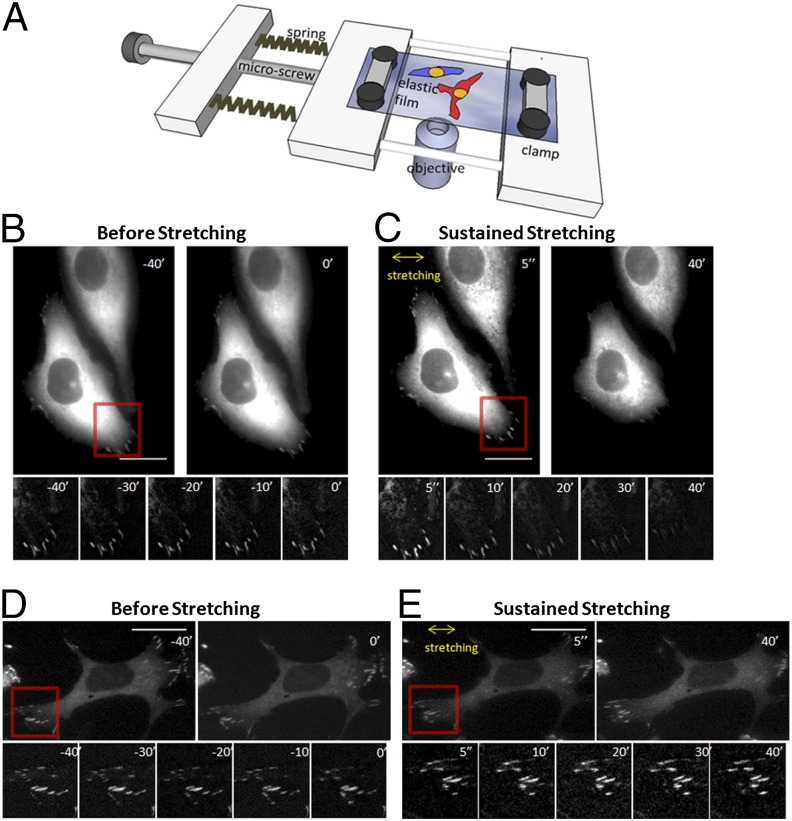
To investigate FA dynamics in response to sustained uniaxial stretching, we developed a stretching apparatus in which a piece of fibronectin-coated elastic film is fixed by two parallel clamps (Fig. 1A). A high-precision adjustment microscrew allows fine adjustment of the distance between the clamps. By manipulating the microscrew, the clamped elastic film is uniaxially stretched along the axis of the microscrew. The elastic film used in this study was made of silicon, 50 kPa in stiffness, 100 µm thick, and transparent, allowing us to perform live-cell imaging of FA dynamics before and after the introduction of uniaxial stretching. We used human bone osteosarcoma epithelial cells (line U2OS) stably expressing GFP-paxillin as an FA marker to study the effects of stretching on FA dynamics. Paxillin was used as an FA marker because it is recruited to FAs at the earliest stage of FA formation and is constantly present through FA maturation and disassembly (30–32). Cells were seeded onto the fibronectin-coated elastic film and allowed to adhere for 4 h. Sustained uniaxial stretching was then applied to the cells, and the FA dynamics before and after stretching was recorded for subsequent analyses by wide-field epifluorescence microscopy at one frame per minute for 40 min before and after stretching. We analyzed cells with no significant protrusion or retraction when unstretched, so that FA turnover was in equilibrium.
Fig. 1.
Orientation-specific response of FAs to 5% sustained uniaxial stretching. (A) Schematic diagram of the stretching apparatus used in this study. (B–E) U2OS cells with GFP-paxillin–labeled FAs were subjected to 5% sustained uniaxial stretching in the direction indicated by the double-headed yellow arrows in C and E. The time relative to the stretching initiation is indicated. The regions highlighted by the red square are shown in magnified details below in smaller panels. (B and C) Example of a cell with its long axis oriented perpendicular to stretch (long axis 60° or higher relative to the stretching). (D and E) Example of a cell with its long axis oriented parallel to stretch (long axis 30° or less relative to the stretching). (Scale bar: 10 µm.) See also Movies S1 and S2.
Qualitative examination of images from the time-lapse movies (Fig. 1 B–E; Movies S1 and S2) showed that immediately after 5% stretch, there was a marked increase of GFP-paxillin in FAs. In a subset of cells, this early response to stretch was followed, about tens of minutes later, by FA disassembly and cell edge retraction. Further inspection of many time-lapse movies suggested that the cell orientation relative to the stretching direction may be an important factor in the fate of FAs. When the cell’s long axis was oriented nearly perpendicular to the stretching direction, FAs underwent disassembly and the cell edge retracted (Fig. 1 B and C), whereas no apparent FA disassembly and edge retraction was observed in cells whose long axes were nearly parallel to the stretching direction (Fig. 1 D and E).
To verify the notion of orientation-dependent effects induced by stretch on cell adhesion and FAs, four parameters were devised to quantitatively characterize the responses of cells to sustained uniaxial stretch: (i) rapid induction in FA growth (Fig. 2C); (ii) changes in FA orientation (Fig. 2D); (iii) changes in steady-state FA disassembly/assembly (Fig. 2E); and (iv) changes in cell polarity (Fig. 2F). These responses were then compared for cells and/or FAs that we categorized in terms of their orientations with respect to the direction of stretching. A cell or FA was considered perpendicular when its long axis and the stretching direction formed an angle larger than 60°, and parallel when this angle was less than 30°. The long axis of cells or FAs was defined by best fit of an ellipse to the cell or FA, and cells with no distinct long axis (aspect ratio <1.2) or with angles between 30° and 60° were disregarded.
Fig. 2.
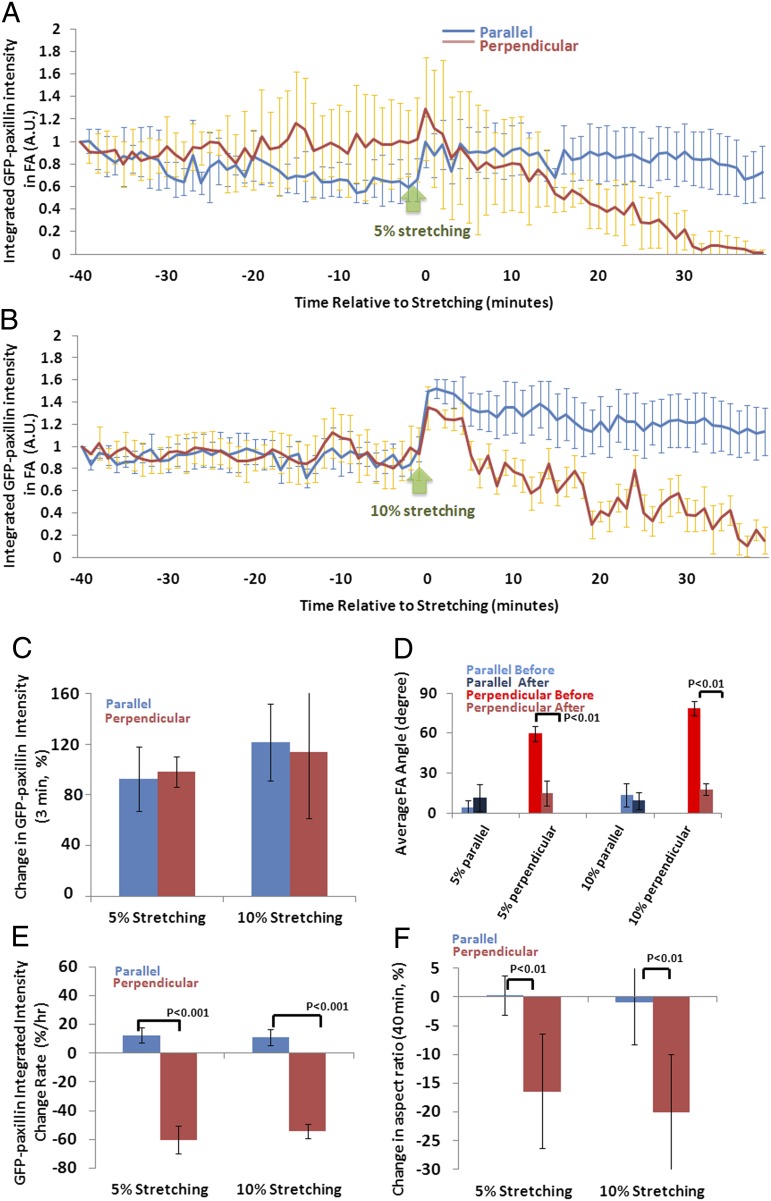
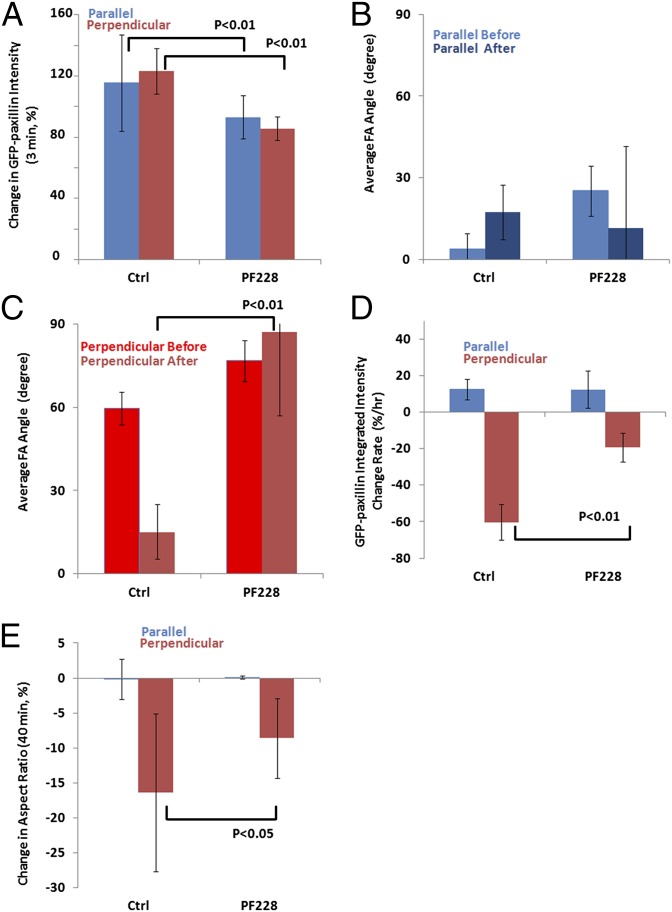
A 5% sustained uniaxial stretching results in immediate paxillin recruitment and delayed orientation-specific FA disassembly. U2OS cells expressing GFP-paxillin–labeled FAs were imaged at 1-min intervals for 40 min before and after being subjected to 5% or 10% sustained uniaxial stretching. A cell or FA was considered perpendicular when the long axis of a best-fit ellipse and the stretching direction formed an angle larger than 60°, and parallel when this angle was less than 30°. GFP-paxillin–labeled FAs were segmented in images. Time course of change in GFP-paxillin intensity over time; time point when 5% (A, perpendicular cells: n = 12, parallel cells: n = 9) or 10% (B, perpendicular cells: n = 8, parallel cells: n = 7) uniaxial stretching was applied is indicated by the green arrow. (C) Immediate paxillin recruitment induced by stretch, quantified as the percent change in the average GFP-paxillin intensity in all FAs within a cell in the last three frames before stretching relative to that of the first three in-focus frames after stretching. (D) Average FA angles (parallel to stretching: 0°, perpendicular: 90°) before and after stretching. (E) The rate of FA disassembly/assembly (percent change per hour) over the 40 min before and after stretching, determined by linear fitting of the integrated GFP-paxillin intensity vs. time plot in A. (F) Percent change of cell polarity, determined by comparing the aspect ratios between before and after stretching. Positive values indicate increased polarity, and negative values indicate loss of polarity. Error bars indicate SE.
We first evaluated the effects of stretching on rapid induction of FA growth and its orientation dependency by measuring the recruitment of GFP-paxillin to FAs within 3 min after stretching in cells oriented either perpendicular or parallel to stretch. We plotted the integrated intensity of GFP-paxillin within all segmented FAs in a cell over time (Fig. 2 A and B), and determined the ratio of the average intensity in the last three frames before stretching relative to that of first three in-focus frames after stretching. Comparison of rapid paxillin recruitment in cells oriented parallel and perpendicular to stretch showed that GFP-paxillin intensity increased by ∼20% (16.5 ± 3.5% in perpendicular cells and 21.3 ± 9.9% in parallel cells) after stretching, regardless of the cell orientation (Fig. 2C). Thus, uniaxial stretching induces rapid FA growth independent of cell orientation relative to stretch.
We next evaluated the average FA orientation in cells parallel and perpendicular to stretch, and determined how this orientation changed after stretching. FA orientation was determined by averaging the angle of the long axis of all individual segmented FAs relative to stretch before and after stretching, and was compared in cells parallel and perpendicular to stretch. This analysis revealed that before stretching, FAs had a similar orientation as the cellular orientation, with FAs oriented on average at 65° relative to the direction of stretching in cells perpendicular to stretch, and FAs oriented on average at 5° relative to the direction of stretching in cells parallel to stretch. Forty minutes after stretching, the average FA angle in cells perpendicular to stretch became more parallel, decreasing from 65° to 15° (Fig. 2D), whereas the average FA angle did not significantly change (<5°, P > 0.01) in cells parallel to stretch. These results show that 5% sustained stretching induces FA realignment in an orientation-dependent manner, and suggest an orientation-specific change in FA assembly or disassembly that results in a net realignment of FAs to more parallel to the direction of stretching.
We next sought to determine the effects of 5% sustained uniaxial stretching on FA disassembly/assembly dynamics in cells oriented either perpendicular or parallel to stretch. The rate of FA disassembly/assembly over 40 min before and after stretching was determined by linear fitting of the integrated GFP-paxillin intensity vs. time plot (Fig. 2 A and B). This analysis showed that cells perpendicular to the stretching direction exhibited significant FA disassembly over the course of 40 min after stretching, whereas a very slight increase in FA assembly was detected in cells parallel to the stretching direction over a similar time period (Fig. 2E). Examination of images indicated that FA disassembly occurred selectively in regions where most FAs appeared oriented perpendicular to the stretching direction (Fig. 1 B and C). FAs in such regions slid and disassembled simultaneously with cell edge retraction (Fig. 1 B and C). Thus, sustained uniaxial stretching specifically promotes FA disassembly in cells oriented perpendicular to the stretching direction.
Next we assessed the effects of 5% sustained uniaxial stretching on cell polarity in cells oriented either perpendicular or parallel to stretch. We measured the aspect ratio of a cell as the ratio between the long axis and short axis of its best-fitted ellipse. The change of cell polarity was determined by comparing the percent change in the aspect ratios between before and after stretching. This analysis showed that cells perpendicular to stretch reduced their aspect ratio by 15–20% and therefore became less polarized (Figs. 1 B and C and 2E), whereas no change could be detected in cells oriented parallel to stretch (Figs. 1 D and E and 2F). Together, our results show that individual FAs are capable of orientation-specific responses to sustained uniaxial stretching and specific disassembly of perpendicular FAs promotes stretching-induced loss of cell polarity.
To determine if cellular responses depend on the strain magnitude, we analyzed the effects of 10% strain on FAs and cell morphology. Evaluation of rapid induction of FA growth (Fig. 2 B and C), FA orientation changes (Fig. 2D), changes in steady-state FA disassembly/assembly (Fig. 2 B and E) as well as changes in cell polarity (Fig. 2F) revealed no significant differences in cellular responses to 10% strain compared with the effects of 5% strain. Because the responses to 5% and 10% sustained stretching were similar, 5% sustained stretching was performed in the rest of this study.
Calpain 2 Activity Is Required for Orientation-Specific FA Disassembly and Loss of Cell Polarity in Response to Stretching.
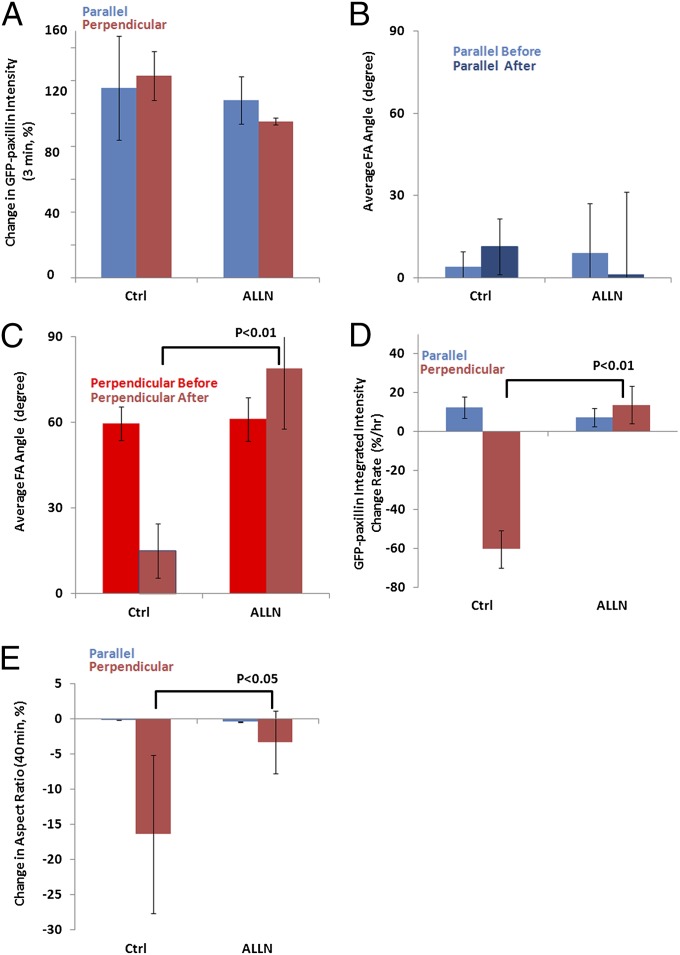
FA disassembly is thought to result from calpain 2-mediated proteolysis of FA components, including talin (33), paxillin (34), and FAK (35). Thus, we sought to determine whether calpain 2 is responsible for the orientation-specific FA disassembly observed in this study. To examine the involvement of calpain 2 in FA and cell responses to stretching, cells were treated with the calpain 2-inhibiting peptide N-[N-(N-Acetyl-L-leucyl)-L-leucyl]-L-norleucine (ALLN) (Ki = 220 nM, 50 µM) (36) for 2 h followed by analysis of orientation-dependent effects on FA growth, orientation, turnover, and cell polarity in response to sustained uniaxial stretching.
Analysis of GFP-paxillin intensity immediately before and after stretching in ALLN-treated cells showed that, compared with untreated controls, calpain 2 inhibition caused no effect on the rapid stretching-induced recruitment of GFP-paxillin to FAs in cells parallel to stretch, and induced a minor, although significant, reduction in rapid FA growth in cells perpendicular to the stretch (Fig. 3A). Thus, calpain 2-mediated proteolysis likely plays a minimal role in stretching-induced rapid FA growth.
Fig. 3.
Calpain 2 activity is required for orientation-specific FA disassembly in response to sustained stretching. U2OS cells expressing GFP-paxillin were treated with calpain 2-inhibiting peptide ALLN (50 µM) for 2 h, then imaged at 1-min intervals for 40 min before and after stretching (perpendicular cells: n = 8, parallel cells: n = 7). A cell or FA was considered perpendicular when the long axis of a best-fit ellipse and the stretching direction formed an angle larger than 60°, and parallel when this angle was less than 30°. GFP-paxillin–labeled FAs were segmented in images. (A) Immediate paxillin recruitment induced by stretch, quantified as the percent change in the average GFP-paxillin intensity in all FAs within a cell in the last three frames before stretching relative to that of the first three in-focus frames after stretching. (B and C) Average FA angles (B, parallel to stretching: 0°; C, perpendicular: 90°) before and after stretching. (D) The rate of FA disassembly/assembly (percent change per hour) over the 40 min before and after stretching, determined by linear fitting of the integrated GFP-paxillin intensity vs. time plot. (E) Percent change of cell polarity, determined by comparing the aspect ratios between before and after stretching. Positive values represent increased polarity, and negative values loss of polarity. Error bars indicate SE.
Next, the effects of calpain 2 inhibition were analyzed on stretching-induced FA reorientation, turnover, and loss of cell polarity. This analysis showed that ALLN treatment had no significant effect on FA orientation in cells parallel to the stretching direction (Fig. 3B), but blocked the FA reorientation induced by stretching perpendicular to the long axis of the cell (Fig. 3C). Consistent with this finding, calpain 2 inhibition also reduced the FA disassembly rate in cells perpendicular to stretch compared with control (Fig. 3D). As a result of reduced FA disassembly and edge retraction, the aspect ratio in calpain 2-inhibited cells either perpendicular or parallel to stretch did not change significantly (Fig. 3E), as opposed to control cells, where 20% reduction in aspect ratio was detected in cells perpendicular to stretching. Thus, calpain 2 activity is critical to the orientation-specific responses to sustained uniaxial stretching, promoting FA disassembly, and loss of cell polarity in cells oriented perpendicular to stretch.
SFKs Inhibit Rapid FA Growth but Promote Orientation-Specific FA Disassembly and Loss of Cell Polarity in Response to Stretching.
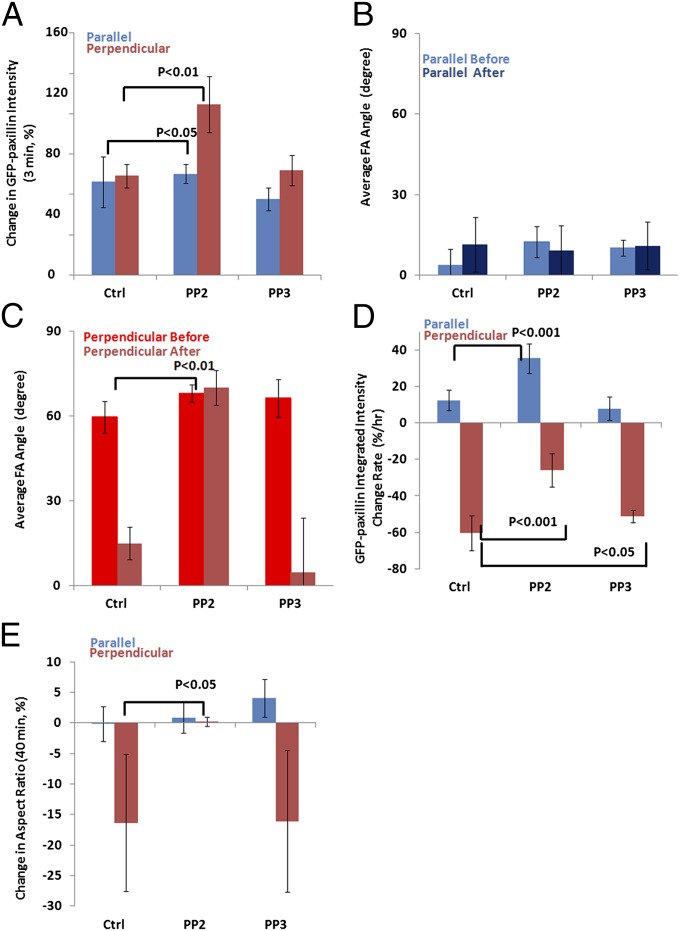
SFK signaling is thought to regulate FA turnover (30) and is activated in response to cyclic stretching (37, 38). We speculated that SFKs might also be involved in the cellular response to sustained stretching. To determine the role of SFKs in the response of cells to sustained stretching, we treated cells expressing GFP-paxillin for 2 h with 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo(3,4-d)pyrimidine (PP2; 10 µM), an SFK inhibitor (39), or its inactive analog 4-amino-7-phenylpyrazolo[3,4-d]pyrimidine (PP3; 10 µM) as a control, and then imaged FA and cell response to sustained stretching followed by analysis of orientation-dependent effects on rapid FA growth, orientation, turnover, and cell polarity.
Analysis of immediate GFP-paxillin recruitment showed that compared with PP3-treated or untreated controls, SFK inhibition enhanced the rapid stretching-induced FA growth (Fig. 4A) in cells perpendicular to stretch, where GFP-paxillin intensity increased twofold after stretching, compared with an insignificant 1.2-fold increase in cells parallel to stretch (Fig. 4A). These results reveal an orientation-specific effect of uniaxial stretching on rapid FA growth, and suggest that SFKs act to inhibit stretching-induced FA growth, and may preferentially be activated in cells perpendicular to the stretching direction.
Fig. 4.
Src activity inhibits immediate paxillin recruitment but is required for orientation-specific FA disassembly in response to sustained stretching. U2OS cells expressing GFP-paxillin were treated with SFK inhibitor PP2 (10 µM, perpendicular cells: n = 10, parallel cells: n = 8) or its inactive analog PP3 (10 µM, perpendicular cells: n = 7, parallel cells: n = 5), then imaged at 1-min intervals for 40 min before and after stretching. A cell or FA was considered perpendicular when the long axis of a best-fit ellipse and the stretching direction formed an angle larger than 60°, and parallel when this angle was less than 30°. GFP-paxillin–labeled FAs were segmented in images. (A) Immediate paxillin recruitment induced by stretching, quantified as the percent change in the average GFP-paxillin intensity in all FAs within a cell in the last three frames before stretching relative to that of the first three in-focus frames after stretching. (B and C) Average FA angles (B, parallel to stretching: 0°; C, perpendicular: 90°) before and after stretching. (D) The rate of FA disassembly/assembly (percent change per hour) over the 40 min before and after stretching, determined by linear fitting of the integrated GFP-paxillin intensity vs. time plot. (E) Percent change of cell polarity, determined by comparing the aspect ratios between before and after stretching. Positive values represent increased polarity, and negative values loss of polarity. Error bars indicate SE.
To test the role of SFK activity in effects of sustained stretching on FAs, we measured changes in average FA orientation, net FA assembly/disassembly rate, and aspect ratio in cells treated with PP2 or PP3. Our findings showed that PP2 had no effect on FA orientation in cells parallel to stretch (Fig. 4B), but blocked the FA reorientation induced by stretching perpendicular to the long axis of the cell (Fig. 4C). Consistent with this, SFK inhibition also reduced the FA disassembly rate induced by perpendicular stretching, and actually promoted FA assembly in cells parallel to stretch (Fig. 4D). As expected, the aspect ratio of PP2-pretreated cells both perpendicular or parallel to stretch did not change significantly in response to stretching (Fig. 4E). The results from PP3 treatment showed a similar tendency to those of untreated controls. In summary, SFKs play an important role in the orientation-specific responses of cells to sustained stretching, inhibiting rapid FA growth and promoting FA disassembly that leads to subsequent polarity loss in cells perpendicular to stretch.
FAK Activity Is Required for Rapid FA Growth, Orientation-Specific FA Disassembly, and Loss of Cell Polarity in Response to Stretching.
FAK is thought to inhibit FA growth and promote FA turnover at least in part by triggering calpain 2-dependent proteolysis of FA components, and is also known to be activated by mechanical stimulus of FAs (30, 33–36, 40). To test if FAK is involved in the responses to sustained stretching, morphological and FA responses to stretching were assessed in cells treated with the specific FAK inhibitor PF228 (10 µM) for 2 h (41). Compared with untreated controls, PF228 treatment slightly decreased the rapid FA growth induced by stretching (P < 0.05; Fig. 5A), regardless of cell orientation. FAK inhibition had no effect on FA orientation (Fig. 5B) or assembly/disassembly rate in cells parallel to the direction of stretching (Fig. 5D), but blocked the stretching-induced FA reorientation (Fig. 5C), FA disassembly, and loss of polarity in cells perpendicular to stretch (Fig. 5 D and E). In summary, FAK contributes to stretching-induced rapid FA growth independent of orientation, and is required in the orientation-specific FA disassembly and loss of cell polarity in cells perpendicular to stretch.
Fig. 5.
FAK is required for immediate paxillin recruitment and slow orientation-specific FA disassembly in response to sustained stretching. U2OS cells expressing GFP-paxillin were treated with FAK inhibitor PF228 (10 µM, perpendicular cells: n = 9, parallel cells: n = 7), then imaged at 1-min interval for 40 min before and after stretching. A cell or FA was considered perpendicular when the long axis of a best-fit ellipse and the stretching direction formed an angle larger than 60°, and parallel when this angle was less than 30°. GFP-paxillin–labeled FAs were segmented in images. (A) Immediate paxillin recruitment induced by stretch, quantified as the percent change in the average GFP-paxillin intensity in all FAs within a cell in the last three frames before stretching relative to that of the first three in-focus frames after stretching. (B and C) Average FA angles (B, parallel to stretching: 0°; C, perpendicular: 90°) before and after stretching. (D) The rate of FA disassembly/assembly (percent change per hour) over the 40 min before and after stretching, determined by linear fitting of the integrated GFP-paxillin intensity vs. time plot. (E) Percent change of cell polarity, determined by comparing the aspect ratios between before and after stretching. Positive values represent increased polarity, and negative values loss of polarity. Error bars indicate SE.
Stretching Induces Two Phases of FAK Activation in FAs: Fast, Orientation-Specific Response, Followed by a Slow, Orientation-Independent Response.
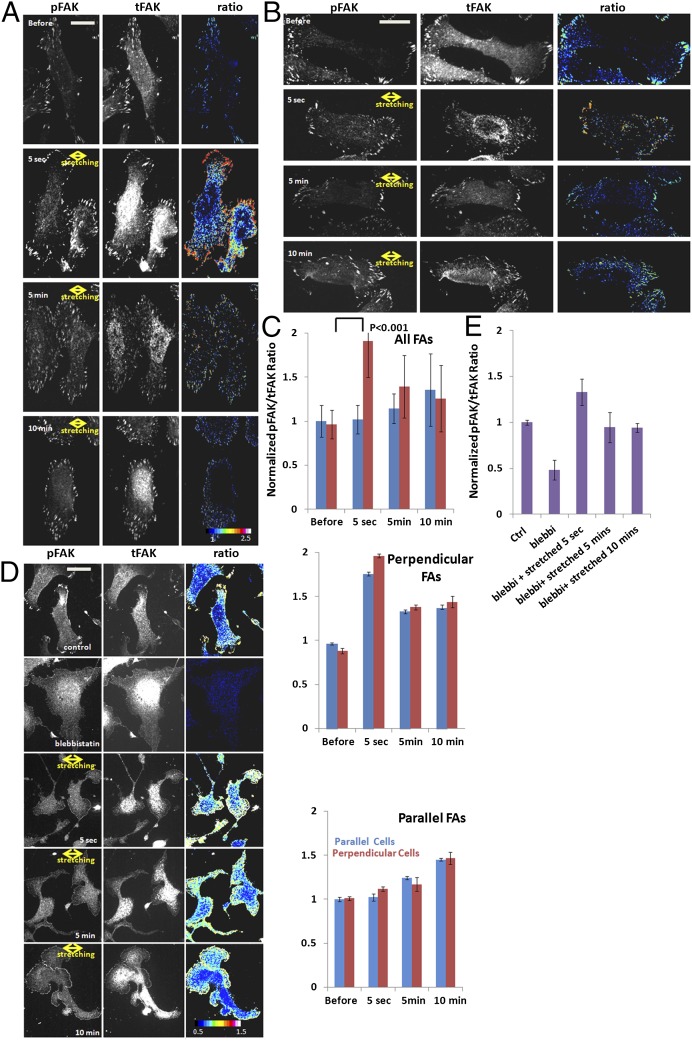
Our results indicate that FAK activity is required for FA disassembly in cells perpendicular to stretch (Fig. 5), suggesting that FAK may be activated in response to stretching in an orientation-specific manner. To test this hypothesis, we evaluated FAK activation in FAs in response to stretching by immunostaining cells with antibodies that specifically recognize activated FAK that is phosphorylated on tyrosine 397 (Fig. 6). U2OS cells were fixed and stained for total FAK (tFAK) and phosphorylated FAKTyr397 (pFAK) at different time points relative to the time of stretching (unstretched and 5 s, 5 min, or 10 min after stretching). The fluorescence intensities of pFAK and tFAK were quantified and normalized followed by the determination the pFAK/tFAK ratio within all segmented FAs, as wells as color encoding of images with the ratio values on a pixel-by-pixel basis. We first compared the average ratio at the whole-cell level for cells that were oriented perpendicular or parallel to stretch. This analysis revealed that the average pFAK/tFAK ratio in FAs in cells perpendicular to stretch increased about twofold at 5 s after stretching (Fig. 6 A and C), and dissipated slowly, still remaining elevated by ∼40% over prestretching values at 10 min after stretching (Fig. 6 A and C). In contrast, the pFAK/tFAK ratio in FAs in cells parallel to stretch did not significantly increase after stretching (Fig. 6 B and C). Thus, the kinetics of FAK activation in FAs in response to stretching differed in an orientation-specific manner at the whole-cell level.
Fig. 6.
FAK is rapidly activated in FAs oriented perpendicular to the direction of stretching, independent of myosin II activity. Unstretched cells (before) and cells that were oriented with their long axes perpendicular (A) or parallel (B) to stretch (for 5 s, 5 min, or 10 min) were fixed and immunostained with antibodies specific to pFAK and tFAK. A cell or FA was considered perpendicular when the long axis of a best-fit ellipse and the stretching direction formed an angle larger than 60°, and parallel when this angle was less than 30°. (A and B) Immunostained cells (Left and Center); the ratio between pFAK and tFAK was determined on a pixel-by-pixel basis and color-coded according to the heat-scale bar (Bottom Right). (C) Quantification of the average pFAK/tFAK ratio in segmented FAs in whole cells (C, Top, perpendicular cells: n = 24; parallel cells: n = 21) or FAs oriented perpendicular (C, Center) or parallel (C, Bottom) to stretch. FAs were sorted by their orientation relative to stretch in C Center and C Bottom, regardless of the orientation of the cells to which the FAs belonged. (D and E) U2OS cells were treated with 10 µM blebbistatin (blebbi) to inhibit myosin II ATPase for 2 h before stretching, followed by fixing and staining for tFAK and pFAK at the indicated time. Control shows untreated, unstretched cells. Because blebbistatin induces lamellipodial protrusion and loss of cell polarity, no distinct cell or FA axis could be identified, and the pFAK/tFAK ratio was only quantified at the whole-cell level without grouping cells and FAs into parallel and perpendicular categories. (D) The ratio between pFAK and tFAK was determined on a pixel-by-pixel basis and color-coded according to the heat-scale bar (Bottom Right). (E) Quantification of the average pFAK/tFAK ratio in segmented FAs in whole cells (n = 24). (Scale bars: 10 µm.) Error bars indicate SE.
Examination of immunostaining showed that like paxillin, the level of FAK increased substantially in FA at 5 s after stretching, indicating that stretching induces rapid FAK recruitment to FAs. Furthermore, pFAK/tFAK ratiometric images confirmed that the increase in FAK phosphorylation in stretched cells was localized to FAs, and suggested that the orientation-specific response to stretching may occur at the level of individual FAs (Fig. 6). To test this, we sorted FAs by their orientation relative to stretch, regardless of the orientation of the cells to which they belonged, and compared the pFAK/tFAK ratio of the two groups (Fig. 6C). This analysis showed that the pFAK/tFAK ratio in FAs perpendicular to the stretch increased rapidly by approximately twofold at 5 s after stretching, dissipated to ∼40% higher than prestretching value at 5 min, and remained elevated to this level at 10 min after stretching (Fig. 6C). In contrast, in FAs parallel to stretch, the pFAK/tFAK ratio did not increase rapidly at 5 s after stretching, but increased above prestretching values by ∼20% at 5 min and 40% at 10 min after stretching, respectively (Fig. 6C). No increase in pFAK/tFAK ratio was observed in stretched cells that had been pretreated with the FAK inhibitor, regardless of orientation (Fig. S1). Because the majority of FAs aligned with the long axis of the whole cell (Fig. 2C), orientation-specific changes in pFAK/tFAK ratios at the cell level resembled values at the individual FA level. Together these results show that FAK recruitment to FAs and phosphorylation rapidly peaks after stretching in FAs perpendicular to stretch, whereas FAK phosphorylation increases gradually over 10 min after stretching in FAs both parallel and perpendicular to stretch. This finding suggests that sustained uniaxial stretching induces two phases of FAK activation in FAs: a fast, orientation-specific response, followed by slow, orientation-independent response.
Stretching-Induced FAK Activation in FAs Is Myosin II Independent.
FAK activity in FA is known to be at least in part dependent on actomyosin contractility (42). To test the hypothesis that FAK activation in response to stretching requires myosin II-mediated contractility, we evaluated the effect of stretching on FAK activation in FAs in cells pretreated with the myosin II inhibitor blebbistatin (10 µM for 2 h; Fig. 6 D and E). Staining for tFAK and pFAK and analysis showed that in the absence of stretching, as expected, myosin II inhibition caused cells to lose polarity, extend wild lamellipodia, reduce their FA size, and decrease the average pFAK/tFAK ratio by 50% (Fig. 6 D and E). Blebbistatin-treated cells were then subjected to 5% stretch followed by fixing and staining for tFAK and pFAK at 5 s, 5 min, or 10 min after stretching. Analysis showed that within 5 s after stretching, the pFAK/tFAK ratio increased to threefold higher compared with unstretched blebbistatin-treated cells, and dissipated slowly, remaining elevated for 10 min by ∼50% over prestretching values (Fig. 6E). Examination of ratiometric images showed that this activation concentrated along the edges of lamellipodia, likely in nascent FAs, independent of the orientation of the cell edge relative to stretch (Fig. 6D). However, because cells lost polarity, and FA size was reduced by blebbistatin, we were unable to evaluate the orientation specificity of the response. These results indicate that stretching is sufficient to rapidly activate FAK and keep it elevated for an extended time, independent of myosin II contractility.
Stretching Induces Slow, Orientation-Independent Src Activation in FAs.
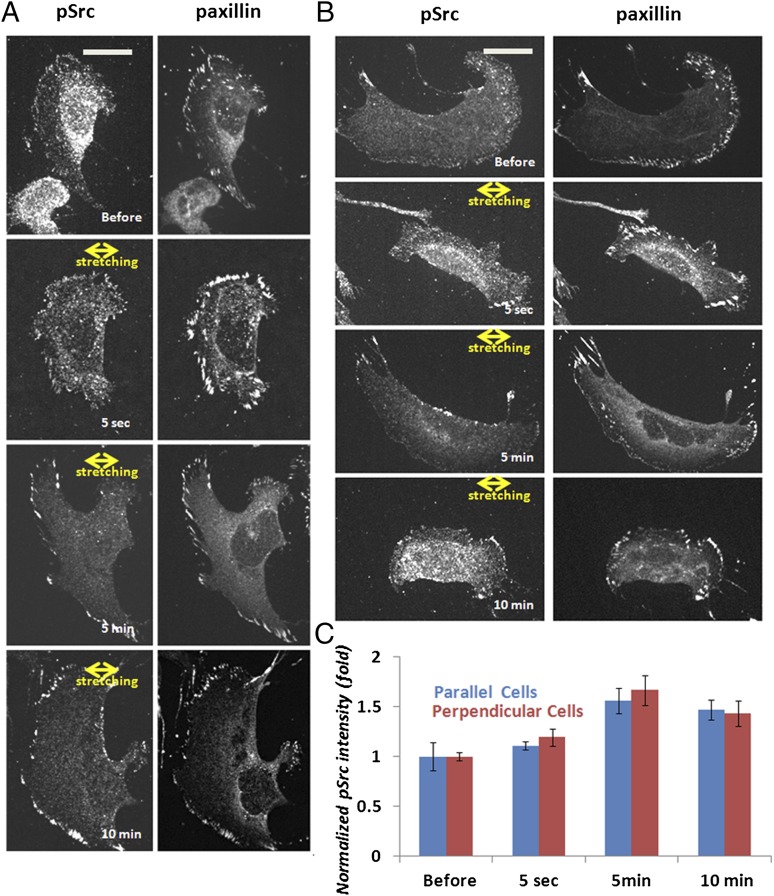
Because we found that SFK activity perturbation affects cellular responses to sustained stretching, we sought to determine whether stretching affects the timing, location, and orientation specificity of Src activation in cells. We fixed cells at 5 s, 5 min, or 10 min after stretching and immunostained with antibodies specific to the activated form of Src that is phosphorylated on tyrosine 418 (pSrc), along with antibodies to paxillin to mark the location of FA (Fig. 7 A and B). Unfortunately, antibodies to total Src (tSrc) did not work for immunofluorescence in our cells. Quantification of the fluorescence intensity of pSrc in segmented FAs showed that, unlike pFAK, which increased 5 s after stretching, there was no difference in pSrc staining in FA between unstretched controls and in cells 5 s after stretching (Fig. 7C). However, pSrc level in FAs increased at 5 min after stretching, and remained elevated above prestretch values at 10 min after stretching. Comparison of pSrc staining in cells of different orientations relative to stretch showed that there was no orientation dependence of pSrc level in FAs (Fig. 7C). These results indicate that stretching induces slow, orientation-independent Src activation in FAs.
Fig. 7.
Stretching induces slow, orientation-independent Src activation in FAs. Unstretched cells (before) and cells that were oriented with their long axes perpendicular (A) or parallel (B) to 5% sustained uniaxial stretch (for 5 s, 5 min, or 10 min) were fixed and immunostained with antibodies specific to pSrc and paxillin. A cell was considered perpendicular when the long axis of a best-fit ellipse and the stretching direction formed an angle larger than 60°, and parallel when this angle was less than 30°. (A and B) Immunostained cells. (C) Quantification of the average pSrc intensity in segmented FAs in whole cells (perpendicular cells: n = 21, parallel cells: n = 21).
Discussion
We analyzed the organization and dynamics of integrin-based FAs after sustained uniaxial stretching to understand how cells adapt to prolonged mechanical stimuli. We found sustained stretching affects FA dynamics on two time scales: a rapid induction of FA growth/reinforcement, followed by slow FA disassembly. Surprisingly, we found these two responses differ in their orientation dependence relative to stretch. Stretching-induced rapid FA growth is orientation independent, but delayed disassembly only occurs in FAs perpendicular to stretch. The disassembly of perpendicular FAs caused the edges facing along the cellular long axis to retract, leading to polarity loss specifically in cells perpendicular to stretch. By perturbing molecules known to play a role in FA dynamics, we found that SFK inhibits, and FAK is required, for rapid FA growth, whereas calpain 2, SFK, and FAK activities are required for delayed FA disassembly and subsequent loss of polarity in cells perpendicular to stretch. By analyzing FAK and Src phosphorylation in individual FAs, we found that FAK is activated rapidly in FAs oriented perpendicular to stretch, whereas both FAK and Src activities increase gradually in FAs independent of orientation. This finding suggests that baseline Src activity and a threshold of FAK activity within individual FAs may be required to mediate delayed Src-, FAK-, and calpain-dependent FA disassembly. Stretching in the absence of myosin contractility indicated that mechanical stimulus, be it supplied by internal cellular contractility or an external perturbation, is sufficient to activate FAK. Together, our results suggest that mechanosensitive molecules within FAs are activated by a single, sustained applied stress (or strain) of a specific orientation to mediate rapid FAK activation and induce FA disassembly, loss of cell polarity, and cell reorientation.
The fact that orientation relative to stretch at the whole-cell level can determine the fate of FAs implies that cellular organization is intrinsically aligned across different spatial scales: the long axes of the mechanosensitive molecules inside FAs, of individual FAs, and of whole cells. Indeed, we observed that the majority of FAs are aligned with the long axis of the cell (Fig. 2). At the molecular level, it is plausible that putative strain/stress sensing FA proteins may also align with the long axis of the FAs and the cell. These mechanosensitive molecules must be capable of sensing directionally applied stress or strain and converting this mechanical signal to biochemical signals.
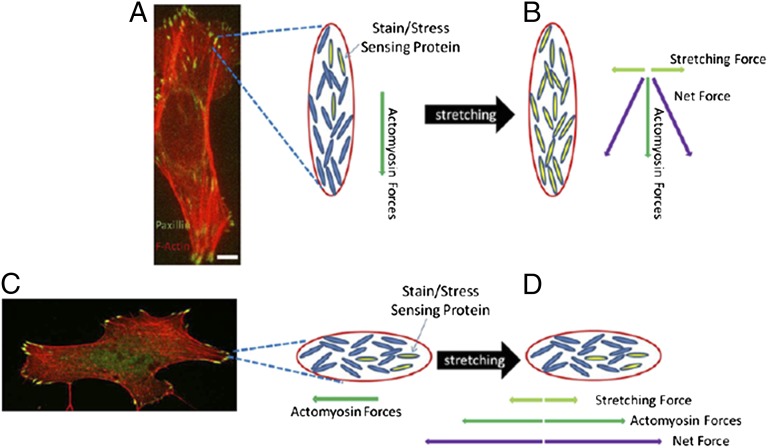
We speculate the following scenario (Fig. 8): Before uniaxial stretching, the only force putative strain/stress sensors in FAs experience is the actomyosin pulling force transmitted through the stress fiber attached to the FA (Fig. 8 A and C). Molecules that are aligned with the stress fiber will experience the highest magnitude of pulling force, whereas those slightly misaligned molecules experience only a fraction of such magnitude, which equals the projected value in the direction of the long axis. As a result, only the well-aligned fraction of the strain/stress sensing molecules experience enough force to overcome their activation criteria. Thus, only a fraction of their downstream targets (FAK, SFKs, calpain 2) are activated (Fig. 8 A and C). Upon being stretched perpendicular to the cell’s long axis, the slightly misaligned, inactive strain/stress sensing molecules in FAs experience an additional strain in the orthogonal direction (Fig. 8B). The net force on these misaligned molecules may then reach the level required for activation. Activation of additional sensor molecules pushes the FA over the threshold required to fully initiate downstream events, including FAK, SFK, and calpain 2 activation, leading to FA disassembly. In contrast, in FAs parallel to stretch, the stretching force would not supply the orthogonal vector component required to overcome the activation criteria of those misaligned sensors (Fig. 8D). Because FAs are generally coaligned with the cellular long axis, the orientation-specific FA disassembly was more prominent in cells perpendicular to stretch compared with cells parallel to stretch, where most FAs were also parallel to stretch and most strain/stress sensing molecules would thus remain inactive. In the absence of actomyosin contractility, the sensors would be inactive and of isotropic orientation in nascent FA, and the fraction of these sensors that happened to be aligned with stretch would be activated. As for the identity of the putative strain/stress sensor, a likely candidate is integrin, which promotes FAK activation (43) and is thought to be activated by directional force transmitted from actin retrograde flow driven by polarized filament assembly at the leading edge (44, 45). Alternatively, talin, which has been shown to be stretched along the axis of stress fibers (46) and binds to FAK (47), also could be involved.
Fig. 8.
Model for a putative mechanism of stretch orientation-specific effects on FA stress/strain sensing proteins. Our model depicts that the net force vector, which combines the actomyosin pulling forces (A) and the stretching force in an FA oriented perpendicular to externally applied stretching (B), is sufficient to overcome the activation threshold of slightly misaligned stress/strain sensing proteins, leading to higher FAK activation downstream to promote Src, FAK, and calpain 2-dependent FA disassembly, whereas the net force vector in an FA oriented parallel to externally applied stretching (C and D) lacks the orthogonal component to activate its slightly misaligned stress/strain sensing proteins and overcome the criteria to trigger delayed FA disassembly. Such discrepancy scales up to the cell level because the majority of FAs coalign with the long axis of the cell, resulting in orientation-specific FA disassembly and loss of polarity of cells oriented perpendicular to stretch.
Our study shows that the rapid response of FA growth to stretching and the delayed response of FA disassembly may be differentially regulated by the timing and magnitudes of SFK and FAK signaling in FAs. For the rapid response, we found that whereas FAK promotes rapid FA growth independent of orientation, SFKs suppress rapid FA growth in cells perpendicular to stretch. This observation revealed an orientation-specific regulation of the rapid response of FAs to stretch. Based on our results, we suggest that SFKs may phosphorylate and activate an orientation-dependent and FAK-independent inhibitor of rapid FA growth in FAs perpendicular to stretch. It is known that the caveolin-mediated endocytosis of membrane-associated proteins, such as FA proteins (48, 49), is promoted by Src but not FAK (50–52). Thus, it is possible that inhibition of SFKs could promote rapid FA growth through the inactivation of the caveolin pathway. Indeed, mechanoregulation of the caveolin pathway has been documented (53, 54). In contrast, although our immunofluorescence results indicate that FAK activity increases rapidly in FAs perpendicular to stretch, pretreatment with FAK inhibitor blocked both rapid FA growth (independent of FA orientation) and delayed FA disassembly (in perpendicular FAs), which suggests that baseline FAK activity is required for rapid FA growth in all FAs, but reaching a high threshold of FAK activity is required for the delayed FA disassembly. Based on our model proposed above, we suggest that FAK only reaches this threshold level in FAs whose orientation-sensitive strain/stress sensors are misaligned with the FA axis, but can be activated specifically by stretching that is perpendicular to this axis. To summarize, the rapid FA growth is regulated by two distinct signaling pathways: one is activated only in FAs perpendicular to stretch and can be suppressed by a baseline SFK activity, and the other requires baseline FAK activity. Delayed FA disassembly requires both SFK activity and a high threshold of FAK activity, which can only be achieved in an orientation-dependent manner.
Materials and Methods
Cell Culture, Transfection, and Reagents.
U2OS cells were obtained from American Type Culture Collection and maintained in DMEM supplemented with 10% (vol/vol) FBS (Invitrogen) at 37 °C and 5% (vol/vol) CO2. For stable expression of paxillin-GFP, U2Os cells were transfected with 1 μg of vector using Amaxa Nucleofector Solution V. Transfected cells were then selected for 2 wk in media supplemented with 0.8 mg/mL G-418 (Invitrogen), and cells expressing low level of paxillin-GFP collected by FACS. Cells stably expressing paxillin-GFP stable were analyzed by fluorescent microscopy and Western blot. We verified paxillin localization at focal adhesions and equal expression of paxillin-GFP and endogenous paxillin. For live-cell imaging of FA dynamics, 4 h before experiments, 105 cells were seeded on an area of 15 × 30 mm elastic film that had been coated with 5 µg/mL fibronectin (EMD Millipore) in PBS for 2 h at 37 °C. Cells were imaged live in DMEM without phenol red and supplemented with 10% FBS, 25 mM Hepes, and 10 U/mL Oxyrase. The following pharmacological inhibitors were used: 10 µM myosin II inhibitor blebbistatin (EMD Millipore), 10 µM SFK inhibitor PP2 (EMD Millipore), 10 µM FAK inhibitor PF-228 (Pfizer), and 50 µM ALLN (Sigma). The following antibodies were used: rabbit anti-pY418Src (BD), mouse anti-paxillin (Invitrogen), rabbit anti–pY397-FAK (Invitrogen), mouse anti-FAK (Invitrogen), donkey anti-rabbit IgG Alexa 555 (Jackson ImmunoResearch Laboratories, Inc.), and goat anti-mouse IgG Alexa 488 (Jackson ImmunoResearch Laboratories, Inc).
Stretching Cells.
The stretching apparatus we built consisted of a rectangular frame fit to the motorized microscope stage (Applied Scientific Instruments), a high-precision adjustment microscrew (Newport), a pair of screw-on clamps, and a pair of springs (Fig. 1A). The microscrew allowed fine adjustment of the distance between the clamps that hold the cell-seeded elastic film. The pair of springs provides the balancing force while stretching the elastic film. By manipulating the microscrew, the clamped elastic film was uniaxially stretched along the moving axis of the microscrew. The silicon-based elastic film was 50 kPa in stiffness and 100 µm thick in its unstretched state (Specialty Manufacturing, Inc.). Cells were seeded onto the fibronectin-coated elastic film and allowed to adhere for 4 h. The elastic film was mounted on an adaptor in such a way that 3 mL of medium would cover the area where cells grew. The adaptor with the elastic film was transferred to the stretcher, and the film was clamped directly to the stretcher and the adapter removed before imaging. The stretcher was then mounted to the motorized microscope stage for live imaging, and then sustained uniaxial stretching was applied to the film by manually turning the microscrew.
Immunofluorescence.
Cells grown on the elastic film were prefixed and permeabilized for 1 min at 37 °C in 0.01% Triton X-100, 0.25% paraformaldehyde (Electron Microscopy Science) in cytoskeleton buffer (CB; 10 mM MES, 3 mM MgCl2, 138 mM KCl, and 2 mM EGTA) and postfixed in 3% paraformaldehyde in CB for 20 min at 37 °C. After fixation, cells were further permeabilized with 0.25% Triton X-100 in CB. Free aldehydes were reacted with 0.1 M glycine for 5 min, and cells were washed three times for 10 min in TBS and blocked in blocking solution (2% BSA IgG free and protease free; Jackson ImmunoResearch Laboratories, Inc.) for at least 1 h. Fixed cells were then incubated with primary antibodies diluted in blocking solution (1:300 for rabbit anti-pY397-FAK, 1:200 for mouse anti-FAK, 1:150 for rabbit anti–pY418-Src, 1:300 for mouse anti-paxillin) overnight in a humid chamber at 4 °C. After primary antibody incubation, cells were washed four times for 10 min in Tris-buffered saline with 0.5% Tween-20 (Invitrogen) and incubated with fluorophore-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc.), diluted 1:250 in blocking solution for 1 h, and washed again. The elastic film containing the stained cells was then covered with 15% glycerol in PBS and imaged within 12 h.
Microscopy.
Live cell imaging for focal adhesion dynamics was performed using wide-field epifluorescence microscopy on an inverted microscope system (TE2000E2; Nikon) using a 40× 0.95 N.A. Plan Apo differential interference contrast objective lens (Nikon). Illumination was provided by a 100-W mercury arc lamp. Illumination wavelength and exposure time were controlled using a multibandpass dichromatic mirror (Semrock) and bandpass excitation and emission filters (Chroma) in electronic filterwheel/shutter devices (Sutter Instruments). Stage position and temperature (37 °C) were controlled with a linear-encoded robotic stage (MS-2000; Applied Scientific Instruments) and an airstream incubator (Nevtek), respectively. Images were captured at 1-min intervals for 40 min before stretching and 40 min after stretching with a cooled CCD camera (CoolSNAP HQ2; Photometrics). Microscope system automation was controlled with Metamorph software (Molecular Devices).
Immunostained fixed cells were imaged by spinning-disk confocal microscopy using the microscope system described in Shin et al. (55) using a 60× 1.2 N.A. water-immersion objective lens on a Nikon TE2000 equipped with the Perfect Focus System, an electronic shutter (Sutter Instruments) for transmitted illumination, a linear-encoded robotic stage with a piezo-driven z-axis top plate (ASI Technologies), and a Yokogawa CSU-X spinning disk confocal scan head equipped with a multibandpass dichromatic mirror (Semrock) and bandpass filters (Chroma) in an electronic filterwheel for selection of green and red emission. The 561- and 488-nm laser illumination was provided by a custom-built laser combiner module (Spectral Applied Research) containing solid-state lasers (488 nm: Coherent; 561 nm: MPB Communications) that were shuttered with electronic shutters and attenuated and/or directed to a fiber-coupled output port with an acoustooptical tunable filter (Neos Technologies) and directed to the confocal scan head via a single-mode optical fiber (Oz Optics). Images were acquired using a CoolSNAP HQ2 cooled CCD (Photometrics). The microscope system automation was controlled with Metamorph software (Molecular Devices).
Image Analysis.
The orientation of a cell was determined using the following procedure: Images of cells were first converted to binarized masks representative of the shapes of the cells using appropriate threshold selected manually. Then the Fit Ellipse function in ImageJ (National Institutes of Health) was used to determine the minimal ellipse that encompasses the binarized cell image; and the angles and the lengths of the two axes (long axis and short axis) of the ellipse were then recorded for later analysis. We measured the aspect ratio of a cell as the ratio between the lengths of the long axis and short axis in the best-fitted ellipse. The change of cell polarity was determined by the percent change of the aspect ratio before and after stretch, with positive values indicating increased polarization and negative values indicating loss of polarization.
To segment FAs, images of GFP-paxillin at each time point were median filtered with a three-pixel square kernel, background flattened with a 13-pixel square kernel, and binarized to create a mask based on a manually selected threshold level that allowed all FAs containing GFP-paxillin. The mask was then applied to the original fluorescent images of GFP-paxillin–labeled FA at the corresponding time point for segmentation. The intensity of GFP-paxillin in segmented FAs was then measured and recorded for each time point. To determine the immediate changes in GFP-paxillin recruitment to FA, we determined the ratio of the average intensity in the last three frames before stretching relative to that of first three frames after stretching. FA orientation was determined using the morphometry measurement function for fitting an ellipse in Metamorph software (Molecular Devices) and averaging the angle of the long axis of all individual segmented FAs relative to the stretch direction. The rate of FA disassembly/assembly over 40 min before and after stretching was determined by linear fitting of the integrated GFP-paxillin intensity vs. time plot (Fig. 2A).
A cell or FA was considered perpendicular when its long axis and the stretching direction formed an angle larger than 60°, and parallel when this angle was less than 30°. The long axis of cells or FAs was defined by best fit of an ellipse to the cell or FA, and cells with no distinct long axis (aspect ratio <1.2) or with angles between 30° and 60° were disregarded.
Analysis of the ratio between pFAK and tFAK in FAs was performed as follows. FAs were segmented in images of total FAK as described above for GFP-paxillin. The fluorescence intensity of pFAK and tFAK staining in every segmented FA was background subtracted before dividing the pFAK intensity by the total FAK intensity. The values of pFAK/tFAK ratio were normalized to the ratio in control cells parallel to the stretching direction. Statistical significance of differences was determined using Student t test.
Supplementary Material
Acknowledgments
We thank Sergey Plotinikov, Robert Fischer, and Ingo Thievessen for helpful discussions; Bill Shin for maintaining microscopes in the C.M.W. laboratory; and Ruperto Villadiego for helping build the cell stretcher. This work was supported by the National Research Council Fellowship (to Y.C.) and the intramural programs of the National Heart, Lung, and Blood Institute (A.M.P. and C.M.W.) and the National Institute of Neurological Disorders and Stroke (Y.C. and A.P.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. T.L. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221637110/-/DCSupplemental.
References
- 1.Liu W, et al. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol. 2003;285(5):F998–F1012. doi: 10.1152/ajprenal.00067.2003. [DOI] [PubMed] [Google Scholar]
- 2.Althaus M, Bogdan R, Clauss WG, Fronius M. Mechano-sensitivity of epithelial sodium channels (ENaCs): Laminar shear stress increases ion channel open probability. FASEB J. 2007;21(10):2389–2399. doi: 10.1096/fj.06-7694com. [DOI] [PubMed] [Google Scholar]
- 3.Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol. 1984;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang JH, Yang G, Li Z, Shen W. Fibroblast responses to cyclic mechanical stretching depend on cell orientation to the stretching direction. J Biomech. 2004;37(4):573–576. doi: 10.1016/j.jbiomech.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Hsu H-J, Lee CF, Locke A, Vanderzyl SQ, Kaunas R. Stretch-induced stress fiber remodeling and the activations of JNK and ERK depend on mechanical strain rate, but not FAK. PLoS ONE. 2010;5(8):e12470. doi: 10.1371/journal.pone.0012470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldyn AM, Rioja BA, Spatz JP, Ballestrem C, Kemkemer R. Force-induced cell polarisation is linked to RhoA-driven microtubule-independent focal-adhesion sliding. J Cell Sci. 2009;122(Pt 20):3644–3651. doi: 10.1242/jcs.054866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Y, Beqaj S, Kemp P, Ariel I, Schuger L. Stretch-induced alternative splicing of serum response factor promotes bronchial myogenesis and is defective in lung hypoplasia. J Clin Invest. 2000;106(11):1321–1330. doi: 10.1172/JCI8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vittal V, Rose A, Gregory KE, Kelley MJ, Acott TS. Changes in gene expression by trabecular meshwork cells in response to mechanical stretching. Invest Ophthalmol Vis Sci. 2005;46(8):2857–2868. doi: 10.1167/iovs.05-0075. [DOI] [PubMed] [Google Scholar]
- 9.Li YS, et al. The Ras-JNK pathway is involved in shear-induced gene expression. Mol Cell Biol. 1996;16(11):5947–5954. doi: 10.1128/mcb.16.11.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCormick SM, et al. DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc Natl Acad Sci USA. 2001;98(16):8955–8960. doi: 10.1073/pnas.171259298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadoshima J, Izumo S. Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: Potential involvement of an autocrine/paracrine mechanism. EMBO J. 1993;12(4):1681–1692. doi: 10.1002/j.1460-2075.1993.tb05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torsoni AS, Marin TM, Velloso LA, Franchini KG. RhoA/ROCK signaling is critical to FAK activation by cyclic stretch in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2005;289(4):H1488–H1496. doi: 10.1152/ajpheart.00692.2004. [DOI] [PubMed] [Google Scholar]
- 13.Ishida T, Peterson TE, Kovach NL, Berk BC. MAP kinase activation by flow in endothelial cells. Role of beta 1 integrins and tyrosine kinases. Circ Res. 1996;79(2):310–316. doi: 10.1161/01.res.79.2.310. [DOI] [PubMed] [Google Scholar]
- 14.Porter KE, Turner NA. Cardiac fibroblasts: At the heart of myocardial remodeling. Pharmacol Ther. 2009;123(2):255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Birukov KG. Cyclic stretch, reactive oxygen species, and vascular remodeling. Antioxid Redox Signal. 2009;11(7):1651–1667. doi: 10.1089/ars.2008.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ando J, Yamamoto K. Effects of shear stress and stretch on endothelial function. Antioxid Redox Signal. 2011;15(5):1389–1403. doi: 10.1089/ars.2010.3361. [DOI] [PubMed] [Google Scholar]
- 17.Curtis M, Russell B. Micromechanical regulation in cardiac myocytes and fibroblasts: Implications for tissue remodeling. Pflugers Arch Eur J Physiol. 2011;462(1):105–117. doi: 10.1007/s00424-011-0931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostrow LW, Sachs F. Mechanosensation and endothelin in astrocytes—hypothetical roles in CNS pathophysiology. Brain Res Brain Res Rev. 2005;48(3):488–508. doi: 10.1016/j.brainresrev.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Lemarié CA, Tharaux P-L, Lehoux S. Extracellular matrix alterations in hypertensive vascular remodeling. J Mol Cell Cardiol. 2010;48(3):433–439. doi: 10.1016/j.yjmcc.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Place N, Yamada T, Bruton JD, Westerblad H. Muscle fatigue: From observations in humans to underlying mechanisms studied in intact single muscle fibres. Eur J Appl Physiol. 2010;110(1):1–15. doi: 10.1007/s00421-010-1480-0. [DOI] [PubMed] [Google Scholar]
- 21.Harridge SDR. Plasticity of human skeletal muscle: Gene expression to in vivo function. Exp Physiol. 2007;92(5):783–797. doi: 10.1113/expphysiol.2006.036525. [DOI] [PubMed] [Google Scholar]
- 22.Lewis SA, Hanrahan JW, Sidney Fleischer BF. Physiological approaches for studying mammalian urinary bladder epithelium. Methods Enzymol. 1990;192:632–650. doi: 10.1016/0076-6879(90)92100-r. [DOI] [PubMed] [Google Scholar]
- 23.Araki I, et al. Roles of mechanosensitive ion channels in bladder sensory transduction and overactive bladder. Int J Urol. 2008;15(8):681–687. doi: 10.1111/j.1442-2042.2008.02052.x. [DOI] [PubMed] [Google Scholar]
- 24.Katanosaka Y, et al. Analysis of cyclic-stretching responses using cell-adhesion-patterned cells. J Biotechnol. 2008;133(1):82–89. doi: 10.1016/j.jbiotec.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Huang W, Sakamoto N, Miyazawa R, Sato M. Role of paxillin in the early phase of orientation of the vascular endothelial cells exposed to cyclic stretching. Biochem Biophys Res Commun. 2012;418(4):708–713. doi: 10.1016/j.bbrc.2012.01.083. [DOI] [PubMed] [Google Scholar]
- 26.Bershadsky AD, et al. Assembly and mechanosensory function of focal adhesions: experiments and models. Eur J Cell Biol. 2006;85(3-4):165–173. doi: 10.1016/j.ejcb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Kaneko D, et al. Temporal effects of cyclic stretching on distribution and gene expression of integrin and cytoskeleton by ligament fibroblasts in vitro. Connect Tissue Res. 2009;50(4):263–269. doi: 10.1080/03008200902846270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshigi M, Hoffman LM, Jensen CC, Yost HJ, Beckerle MC. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J Cell Biol. 2005;171(2):209–215. doi: 10.1083/jcb.200505018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niediek V, et al. Cyclic stretch induces reorientation of cells in a Src family kinase- and p130Cas-dependent manner. Eur J Cell Biol. 2012;91(2):118–128. doi: 10.1016/j.ejcb.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Webb DJ, et al. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6(2):154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 31.Zaidel-Bar R, Cohen M, Addadi L, Geiger B. Hierarchical assembly of cell-matrix adhesion complexes. Biochem Soc Trans. 2004;32(Pt3):416–420. doi: 10.1042/BST0320416. [DOI] [PubMed] [Google Scholar]
- 32.Zimerman B, Volberg T, Geiger B. Early molecular events in the assembly of the focal adhesion-stress fiber complex during fibroblast spreading. Cell Motil Cytoskeleton. 2004;58(3):143–159. doi: 10.1002/cm.20005. [DOI] [PubMed] [Google Scholar]
- 33.Franco SJ, et al. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol. 2004;6(10):977–983. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- 34.Cortesio CL, Boateng LR, Piazza TM, Bennin DA, Huttenlocher A. Calpain-mediated proteolysis of paxillin negatively regulates focal adhesion dynamics and cell migration. J Biol Chem. 2011;286(12):9998–10006. doi: 10.1074/jbc.M110.187294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan KT, Bennin DA, Huttenlocher A. Regulation of adhesion dynamics by calpain-mediated proteolysis of focal adhesion kinase (FAK) J Biol Chem. 2010;285(15):11418–11426. doi: 10.1074/jbc.M109.090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westhoff MA, Serrels B, Fincham VJ, Frame MC, Carragher NO. SRC-mediated phosphorylation of focal adhesion kinase couples actin and adhesion dynamics to survival signaling. Mol Cell Biol. 2004;24(18):8113–8133. doi: 10.1128/MCB.24.18.8113-8133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naruse K, Sai X, Yokoyama N, Sokabe M. Uni-axial cyclic stretch induces c-src activation and translocation in human endothelial cells via SA channel activation. FEBS Lett. 1998;441(1):111–115. doi: 10.1016/s0014-5793(98)01528-2. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki M, et al. Up-regulation of integrin beta 3 expression by cyclic stretch in human umbilical endothelial cells. Biochem Biophys Res Commun. 1997;239(2):372–376. doi: 10.1006/bbrc.1997.7364. [DOI] [PubMed] [Google Scholar]
- 39.Hanke JH, et al. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271(2):695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 40.Carragher NO, Frame MC. Focal adhesion and actin dynamics: A place where kinases and proteases meet to promote invasion. Trends Cell Biol. 2004;14(5):241–249. doi: 10.1016/j.tcb.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 41.Slack-Davis JK, et al. Cellular characterization of a novel focal adhesion kinase inhibitor. J Biol Chem. 2007;282(20):14845–14852. doi: 10.1074/jbc.M606695200. [DOI] [PubMed] [Google Scholar]
- 42.Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J Cell Biol. 2010;188(6):877–890. doi: 10.1083/jcb.200906012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sieg DJ, et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2(5):249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 44.Zhu J, et al. Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol Cell. 2008;32(6):849–861. doi: 10.1016/j.molcel.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Browe DM, Baumgarten CM. Stretch of beta 1 integrin activates an outwardly rectifying chloride current via FAK and Src in rabbit ventricular myocytes. J Gen Physiol. 2003;122(6):689–702. doi: 10.1085/jgp.200308899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Margadant F, et al. Mechanotransduction in vivo by repeated talin stretch-relaxation events depends upon vinculin. PLoS Biol. 2011;9(12):e1001223. doi: 10.1371/journal.pbio.1001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen H-C, et al. Interaction of focal adhesion kinase with cytoskeletal protein talin. J Biol Chem. 1995;270(28):16995–16999. doi: 10.1074/jbc.270.28.16995. [DOI] [PubMed] [Google Scholar]
- 48.del Pozo MA, et al. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005;7(9):901–908. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sottile J, Chandler J. Fibronectin matrix turnover occurs through a caveolin-1–dependent process. Mol Biol Cell. 2005;16(2):757–768. doi: 10.1091/mbc.E04-08-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grande-García A, et al. Caveolin-1 regulates cell polarization and directional migration through Src kinase and Rho GTPases. J Cell Biol. 2007;177(4):683–694. doi: 10.1083/jcb.200701006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nabi IR, Le PU. Caveolae/raft-dependent endocytosis. J Cell Biol. 2003;161(4):673–677. doi: 10.1083/jcb.200302028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shajahan AN, Tiruppathi C, Smrcka AV, Malik AB, Minshall RD. Gbetagamma activation of Src induces caveolae-mediated endocytosis in endothelial cells. J Biol Chem. 2004;279(46):48055–48062. doi: 10.1074/jbc.M405837200. [DOI] [PubMed] [Google Scholar]
- 53.Gervásio OL, Phillips WD, Cole L, Allen DG. Caveolae respond to cell stretch and contribute to stretch-induced signaling. J Cell Sci. 2011;124(Pt 21):3581–3590. doi: 10.1242/jcs.084376. [DOI] [PubMed] [Google Scholar]
- 54.Kawamura S, Miyamoto S, Brown JH. Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: Cytoskeletal regulation of ERK translocation. J Biol Chem. 2003;278(33):31111–31117. doi: 10.1074/jbc.M300725200. [DOI] [PubMed] [Google Scholar]
- 55. Shin WD, et al. (2010) A versatile, multi-color total internal reflection fluorescence and spinning disk confocal microscope system for high-resolution live cell imaging. Live Cell Imaging: A Laboratory Manual, ed Goldman D (Cold Spring Harbor Lab Press, Cold Spring Harbor, NY), 2nd Ed, Chap 8.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.