Abstract
Compulsive behavior is a debilitating clinical feature of many forms of neuropsychiatric disease, including Tourette syndrome, obsessive-compulsive spectrum disorders, eating disorders, and autism. Although several studies link striatal dysfunction to compulsivity, the pathophysiology remains poorly understood. Here, we show that both constitutive and induced genetic deletion of the gene encoding the melanocortin 4 receptor (MC4R), as well as pharmacologic inhibition of MC4R signaling, normalize compulsive grooming and striatal electrophysiologic impairments in synapse-associated protein 90/postsynaptic density protein 95-associated protein 3 (SAPAP3)-null mice, a model of human obsessive-compulsive disorder. Unexpectedly, genetic deletion of SAPAP3 restores normal weight and metabolic features of MC4R-null mice, a model of human obesity. Our findings offer insights into the pathophysiology and treatment of both compulsive behavior and eating disorders.
Keywords: anxiety disorders, conditional knockout mice, metabolism, synaptic transmission
Investigation of the synapse-associated protein 90/postsynaptic density protein 95-associated protein 3 (SAPAP3) and the melanocortin 4 receptor (MC4R) have provided important insights into the pathophysiology of compulsivity and obesity, respectively. Genetic variations in SAPAP3 have been identified in some patients with OCD-spectrum disorders, such as trichotillomania (1–3), and SAPAP3 is enriched in the striatum where it influences excitatory synaptic function (4). Importantly, SAPAP3-null mice display compulsive grooming that can be ameliorated by fluoxetine, a mainstay treatment for patients with obsessive–compulsive disorder (OCD) (4). Thus, SAPAP3-null mice are a valuable model of compulsive behavior in neuropsychiatric disease.
MC4R signaling, on the other hand, regulates feeding behavior and energy expenditure (5). Mutations in the MC4R are the most common monogenic cause of hyperphagia and morbid obesity in people, and MC4R-null mice thus provide a useful model of human obesity (6). MC4Rs have long been known to be highly active in the hypothalamus, and it has recently been shown that MC4Rs also operate within D1 medium spiny neurons of the ventral striatum to mediate procedural memory and affective responses to stress (7, 8). In addition, previous results have suggested that stimulation of MC4R-signaling induces compulsive grooming in rats (9). These findings, combined with the well-established and critical role of striatal circuitry in mediating compulsive behavior (10–12), prompted us to hypothesize that loss of MC4R signaling might ameliorate compulsive grooming in SAPAP3-null mice.
Results
Constitutive Genetic Elimination of MC4R Normalizes Grooming in SAPAP3-Null Mice.
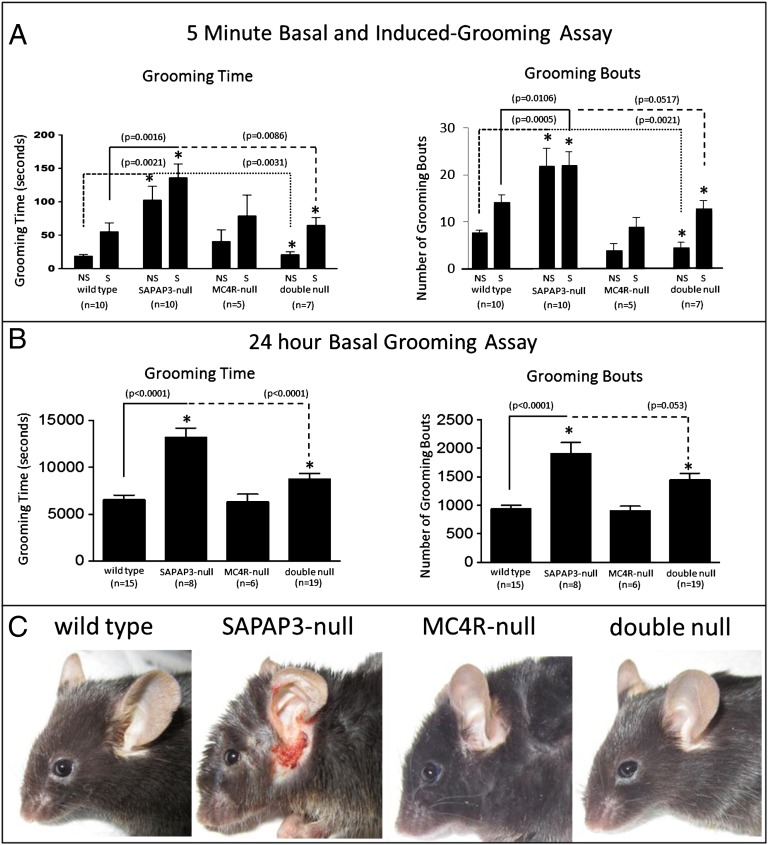
After demonstrating that MC4R agonists induce compulsive grooming in wild-type mice (Fig. S1) as was previously shown in rats (9), we addressed our hypothesis by crossing SAPAP3-null mice with MC4R-null mice to generate four experimental groups for analysis of compulsive grooming behavior: wild-type (Mc4r+/+, Sapap3+/+), SAPAP3-null (Mc4r+/+, Sapap3−/−), MC4R-null (Mc4r−/−, Sapap3+/+), and double null (Mc4r−/−, Sapap3−/−). As expected (4), SAPAP3-null mice showed increased grooming time in the standard 5-min spray test of induced grooming, due to increased bouts of grooming, both at baseline and after stimulation by water spray (Fig. 1A). However, mice lacking both SAPAP3 and MC4R displayed wild-type levels of grooming time and bouts at both baseline and after spray, whereas MC4R-null mice showed no difference from wild type (Fig. 1A).
Fig. 1.
Genetic elimination of both MC4R and SAPAP3 normalizes excessive grooming observed in singly deficient SAPAP3-null mice. (A) SAPAP3-null mice showed increased grooming time and number of grooming bouts at baseline (NS = “no spray”) and after grooming stimulus (S = “spray”) in the 5-min grooming assay, compared with wild-type and MC4R-null mice. (B) SAPAP3-null mice showed increased grooming time and number of grooming bouts relative to wild-type and MC4R-null mice in the 24-h home cage basal grooming assay. Time spent grooming and number of grooming bouts was normalized in double null mice in all aspects of 5-min and 24-h assays (data presented as mean ± SEM). (C) Double deletion of MC4R and SAPAP3 prevent acquisition of self-induced facial lesions, which are characteristic of SAPAP3-null mice.
We then tested grooming more rigorously using the Laboras system, which allows 24-h automated collection of nonstimulated, basal grooming behavior using a vibration sensitive plate to monitor fine motor activity. Consistent with spray test results, SAPAP3-null mice exhibited significantly increased total grooming time and bouts over 24 h, whereas double null mice again showed normalized grooming time and bouts (Fig. 1B). Grooming in MC4R-null mice was the same as in wild-type mice. Loss of MC4R in SAPAP3 null mice rescued compulsive grooming by normalizing the number of grooming bouts rather than by shortening the duration of individual bouts, which were the same across all four groups (average time of bouts: 7.01 ± 0.32 s for wild type, 7.23 ± 0.62 s for SAPAP3-null, 6.83 ± 0.51 s for MC4R-null, 6.23 ± 0.42 s for double null). We additionally observed that double deletion of MC4R and SAPAP3 prevented acquisition of self-induced facial lesions, which are otherwise characteristic of SAPAP3-null mice (Fig. 1C).
Pharmacologic Inhibition of MC4R Signaling Normalizes Compulsive Grooming in SAPAP3-Null Mice.
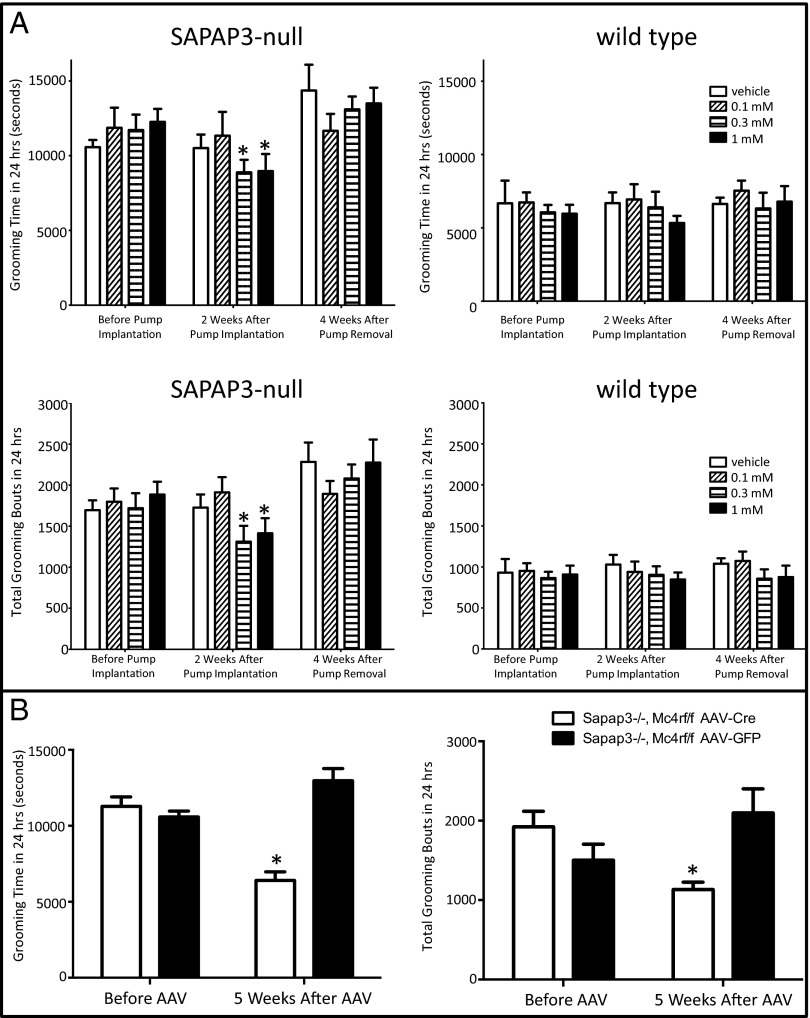
We next tested whether normalization of grooming behavior in double null mice was due to interactions between MC4R signaling and SAPAP3 function in mature neuronal circuitry, rather than simply a developmental effect. Specifically, we assessed the efficacy of pharmacologic inhibition or genetic elimination of MC4R signaling in adult SAPAP3-null mice. First, grooming was evaluated in the 24-h Laboras grooming assay following 2 wk of intracerebroventricular (ICV) delivery of the MC4R antagonist HS014 via Alzet osmotic minipumps into adult SAPAP3 null mice. HS014 is a cyclic melanocyte stimulating hormone analog that serves as an MC4R ligand (Ki of 3.2 nM) with 17-fold MC4/MC3 receptor selectivity (13). Without directly measuring brain tissue drug levels, it is impossible to determine the brain concentration of HS014 after 2 wk of continuous ICV delivery. Previously in the literature, however, others have determined that an ICV infusion rate of 0.17 nmol of HS014 per hour for 2 wk is sufficient to induce hyperphagia and obesity in rodents (14). Accordingly, we conservatively infused at lower doses. The Alzet osmotic minipumps we used dispel their contents at a rate of 0.11 μL/h. Thus, at a concentration of 1 mM HSO14 in the pump, drug delivery occurred at a rate of 0.11 nmol/h. Accordingly, 0.3 mM concentration in the pump effected delivery of 0.033 nmol/h, and 0.1 mM concentration in the pump effected delivery at a rate of 0.011 nmol/h. Although the lowest dose of 0.011 nmol/h of HS014 had no effect on grooming behavior, higher dosages of 0.033 and 0.11 nmol/h significantly reduced grooming time and bouts in SAPAP3-null mice (Fig. 2A). Pumps and cannulae were then removed and mice were allowed to recover for 4 wk. Subsequent reevaluation in the 24-h Laboras grooming assay at this later time point showed that grooming time and bouts returned to the high levels typically seen in SAPAP3-null mice. Analogous experiments were conducted on wild-type littermate mice, and no grooming differences were seen at any time point. Thus, acute pharmacologic inhibition of MC4Rs significantly ameliorated compulsive grooming in SAPAP3-null mice, but did not affect grooming in wild-type mice.
Fig. 2.
Pharmacologic inhibition and genetic elimination of MC4R in SAPAP3-null mice normalizes excessive grooming. (A) Intracerebroventricular delivery of the MC4R antagonist HS014 to SAPAP3-null mice significantly reduced grooming time and number of grooming bouts in the 24-h Laboras grooming assay (*P < 0.01, Student’s t test) at 0.3 and 1.0 mM concentrations, and this effect was reversed following cessation of HS014 delivery. Concentrations refer to loading concentration of the drug in the osmotic minipump. Based on the delivery rate of the pump, the following rates of HS014 infusion into the left lateral ventricle of the brain were implemented over a 2-wk time period: 1mM HS014 in the pump = 0.11 nmol/h, 0.3 mM HS014 in the pump = 0.033 nmol/h, and 0.1 mM HS014 in the pump = 0.011 nmol/h. (B) Cre-mediated deletion of Mc4r in Sapap3−/−:Mc4rlox/lox mice normalizes grooming time and number of grooming bouts (*P = 0.0002, Student’s t test) in the 24-h Laboras grooming assay.
Induced Genetic Elimination of Mc4r Normalizes Grooming in SAPAP3-Null Mice.
We next generated Sapap3−/−:Mc4rlox/lox experimental mice by crossing SAPAP3-null mice with mice in which the single exon of the mc4r gene was flanked by lox P sites (15). Sixteen- to 17-wk-old Sapap3−/−:Mc4rlox/lox mice were screened in the Laboras 24-h grooming assay to ensure that these new mice displayed compulsive grooming (Fig. 2B). Then, mice were randomly and equally divided into two groups, and key components of corticostriatal circuitry were targeted stereotaxically and bilaterally by administering adeno-associated virus (AAV) vector encoding either Cre recombinase and green fluorescent protein (GFP) (AAV-Cre-GFP), or GFP alone (AAV-GFP; “control group”) into the orbitofrontal cortex and nucleus accumbens shell, a component of the ventral striatum. Both regions were targeted for elimination of MC4R to test our hypothesis of a role for MC4R signaling in compulsive behavior secondary to loss of SAPAP3 in the striatum. Five weeks later, mice were reevaluated in the 24-h Laboras grooming assay. Mice that received AAV-Cre-GFP showed reduced grooming time and bouts to wild-type levels (Fig. 2B). Subsequently, brains were examined both histologically for GFP expression to verify efficient viral delivery (Fig. S2) and by in situ hybridization for MC4R transcript expression to verify viral-mediated elimination of intact Mc4r (Fig. S3) in both orbitofrontal cortex and nucleus accumbens shell. These results confirm the specificity of our previously demonstrated effect of pharmacological inhibition of MC4Rs on compulsive grooming, and provide further evidence that inhibition of MC4R signaling helps normalize grooming in adult SAPAP3-null mice. In future studies it will be informative to delineate pre- vs. postsynaptic roles of MC4R signaling by selectively eliminating MC4R in the nucleus accumbens, while leaving expression intact in the orbitofrontal cortex.
Elimination of MC4R Restores Normal Ventral Striatal Synaptic Transmission in SAPAP3-Null Mice.
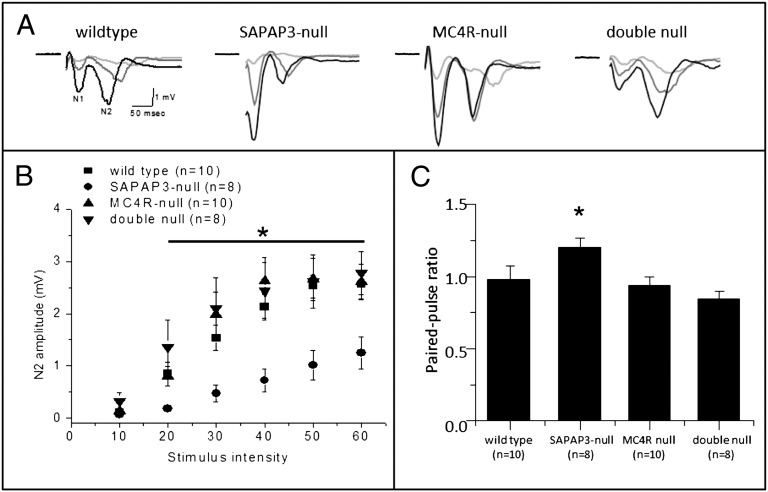
SAPAP3-null mice have reduced AMPA receptor (AMPAR)-mediated synaptic transmission and an increased number of silent synapses in the dorsal striatum (16, 17). Furthermore, activation of MC4R signaling within ventral striatal neurons reduces synaptic strength by increasing endocytosis of AMPARs (8). We thus hypothesized that normalization of grooming behavior in double null mice might correlate with restoration of normal ventral striatal neuron AMPAR activity. Accordingly, we recorded input-output curves of extracellular field potential responses in acute ventral striatal slices from SAPAP3-null, MC4R-null, double null, and wild-type littermate mice. Consistent with previous results in the dorsal striatum, field potential amplitudes recorded in ventral striatal slices from SAPAP3-null mice exhibited a decreased, synaptically mediated N2 field potential response relative to wild type. By contrast, peak N2 amplitudes were the same in slices obtained from MC4R-null and wild-type mice. However, N2 field potential amplitudes in double null mice were restored to wild-type levels (Fig. 3 A and B). No differences were observed in the N1 component of field potential recordings (Fig. S4), which reflects the direct excitability of axons and cells in the slice (18). These results suggest that similar to the dorsal striatum, decreased AMPAR-mediated synaptic transmission occurs in SAPAP3-null mice and that this synaptic modification, like compulsive grooming, is rescued by MC4R deletion. We also found a significant increase in paired-pulse ratio (PPR) in SAPAP3-null mice, which was absent in both MC4R-null and double null mice (Fig. 3C). Although these measures using field potential recordings from striatal slices reflect a mixture of presynaptic and postsynaptic events (18), these results provide further evidence that deletion of MC4R signaling rescues synaptic changes caused by loss of SAPAP3 in the striatum.
Fig. 3.
MC4R deletion rescues ventral striatal synaptic transmission deficits in SAPAP3-null mice. (A) Representative traces of field potential recordings at 10-, 30-, and 50-µA stimulus intensities in wild-type, SAPAP3-null, MC4R-null, and double null ventral striatum (N1 first negative peak representing axonal stimulation, N2 second negative peak representing AMPAR antagonist sensitive potential). (B) Current-voltage relationship plot of ventral striatal N2 field potentials show reduced SAPAP3-null responses are rescued in double null acute slices. (C) Paired-pulse ratios at 50-ms interstimulus intervals are increased in SAPAP3-null mice and rescued in double null mice.
Elimination of SAPAP3 Restores Normal Weight and Metabolism in MC4R-Null Mice.
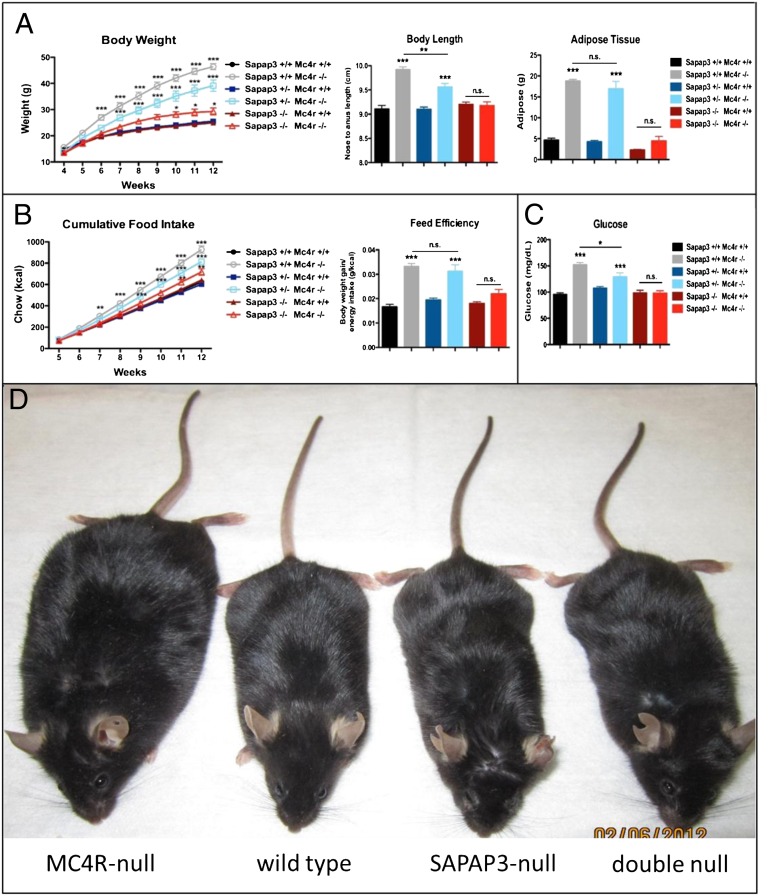
Because loss of function MC4R mutations cause obesity in humans (19) and rodents (20), we evaluated body composition to ensure that changes in grooming were not secondary to changes in body habitus. Quite surprisingly, we observed that although MC4R-null mice were obese as expected, double null mice displayed body weight similar to wild-type and SAPAP3-null mice (Fig. 4A). Consistent with the rescue in body weight, deletion of SAPAP3 from MC4R-null mice also restored normal body length, adiposity, food intake and fasting blood glucose (Fig. 4 A–C). Taken together, our results confirm that the reduction in grooming in double null mice relative to SAPAP3-null mice does not result from nonspecific metabolic or locomotor aberrations, as double-null mice exhibit the same lean phenotype (Fig. 4) and locomotor activity (Fig. S5) as SAPAP3-null mice. Importantly, our results also demonstrate an unanticipated interaction of SAPAP3–MC4R signaling in feeding behavior and metabolism.
Fig. 4.
SAPAP3 is required for the metabolic deficits characteristic of MC4R-null mice. (A) Loss of SAPAP3 causes a gene dosage-dependent rescue of body weight (significant time × genotype interaction, F40,679 = 12.27, P < 0.001), and body length (F1,67 = 26.91, P < 0.001), whereas complete loss of SAPAP3 rescues adiposity (F1,72 = 83.46, P < 0.001). (B) Loss of SAPAP3 reduces food intake (significant time × genotype interaction, F35,565 = 9.14, P < 0.001) and feed efficiency (F1,76 = 22.88, P < 0.001) in MC4R-null mice. (C) Loss of SAPAP3 causes a gene dosage-dependent rescue of fasting glucose (F1,71 = 19.56, P < 0.001; data are presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001). (D) Representative pictures of 20-wk-old MC4R-null, wild-type, SAPAP3-null, and double null mice illustrate that elimination of SAPAP3 restores normal weight in the MC4R-null background.
Discussion
We have identified a biologically relevant interaction of MC4R–SAPAP3 signaling in regulating compulsive behavior and body weight. In the case of compulsive behavior as measured by excessive grooming, this effect correlates well with modifications of AMPAR-mediated synaptic transmission in ventral striatal neurons. Our findings also suggest that regulation of excitatory synapse strength is a critical function of MC4R signaling in the ventral striatum.
On first glance, metabolism and compulsive behavior may appear unlikely partners for a neuronally mediated mechanistic link. However, several clinically relevant intersections of compulsivity and food intake do occur. For example, a common obsession in OCD is fear of contamination (21), typically associated with compulsive cleansing rituals, such as washing of hands or objects. Nutrient-sensing pathways might thus regulate compulsivity to maximize assessment of food quality and safety. MC4R signaling is known to increase during periods of caloric abundance (22), and augmentation of compulsive concerns of cleanliness and food safety would be beneficially adaptive during periods of plentiful food availability. Conversely, MC4R signaling is decreased during periods of starvation (22), which could diminish these compulsive concerns when it is more important to survive by consuming scarcely available nutrition. Symptoms centering on anxiety and food intake are also comorbid in eating disorders. For example, patients with anorexia nervosa have much higher rates of anxiety and compulsivity than the general population (23), and OCD-like symptoms generally precede the onset of an eating disorder (24).
Our findings also identify an unanticipated role for synaptic plasticity in MC4R-signaling control of body weight. In the context of reduced strength of excitatory synapses observed in SAPAP3-null mice, loss of MC4R signaling failed to elicit either hyperphagia or obesity, indicating that modulation of glutamatergic signaling may be a key factor in MC4R-mediated feeding behavior. This observation extends the earlier finding that MC4R signaling acts presynaptically within the nucleus tractus solitarius to modulate glutamatergic synaptic transmission (5), and is consistent with the recent demonstration that MC4R signaling within the striatum reduces strength of excitatory synapses (8). In addition, others have previously demonstrated that loss of SAPAP3 elicits postsynaptic endocytosis of AMPARs via increased metabotropic glutamate receptor 5 (mGluR5) activity (25), which ultimately reduces AMPAR-mediated synaptic transmission in the striatum. Here, we show that inhibition or genetic ablation of the MC4R-signaling system also normalizes levels of AMPAR-mediated synaptic activity. Taken together, these results suggest that distinct mGluR5 and MC4R signaling pathways converge to affect the same synaptic property within striatal neurons. Further dissection of the relative contribution and molecular events of these pathways to this common outcome could thus contribute to identification of potential targets for therapeutic intervention in compulsive behavior.
In summary, we have found an interaction of striatal MC4R and SAPAP3 signaling that may help foster development of new pharmacologic treatments for patients with pathologically compulsive behavior or eating disorders. It will be important to further analyze the relationship between MC4R-SAPAP3 interactions and specific subtypes of human illness. Loss of MC4R function models human monogenic obesity, which is particularly sensitive to hyperphagia of calorically dense foods (26). By contrast, human mutations in SAPAP3 have been associated with trichotillomania, a rare disorder that sometimes involves ingestion of a nonnutrient object (hair) (2). These polar opposites in human behavior suggest that the interaction of MC4R-SAPAP3 signaling may be critical for the evaluation and selection of nutrients for consumption. Although the SAPAP3 model of compulsive grooming has good face validity and predictive validity for OCD-like behaviors (27), caution should be applied when interpreting the results. Compulsive or stereotypic grooming has also been linked to numerous stress-inducing events, including stimulant exposure, feeding, sexual behavior, social interactions, and exploration of a novel environment (28). Furthermore, repetitive movements or behaviors are observed in multiple conditions, including intellectual disability, autism, dementia, Tourette syndrome, and temporal lobe epilepsy (29), indicating that multiple distinct forms of neural dysfunction may result in a common behavioral manifestation. Future work will need to delineate the specificity of the interaction of SAPAP3-MC4R signaling in diverse disease states.
Materials and Methods
Animal procedures were performed in accordance with University of Texas Southwestern Medical Center Institutional Animal Care and Use Committee guidelines. Mice were handled in accordance with the Guide for the Care and Use of Laboratory Animals as adopted by the US National Institutes of Health. Specific protocols were approved by the Institutional Animal Care and Use Committee. SAPAP3-null mice were provided by Guoping Feng (Massachusetts Institute of Technology, Cambridge, MA), and MC4R-null mice were provided by Joel Elmquist of University of Texas Southwestern Medical Center. For a detailed description of methods, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Noelle Williams, Joel Elmquist, Héctor De Jesús-Cortés, James Potash, and John Wemmie for critical review of the manuscript. We thank Aaron Burket for technical assistance. We thank Guoping Feng for generously providing breeding pairs of SAPAP3-null mice. This work was supported by funds from The Hartwell Foundation (to A.A.P.); National Institutes of Health (NIH) Grants DK081185-01, DK081182-01, and MH084058-01A1 (to M.L.); the Dylan Tauber Researcher Award from the Brain and Behavior Foundation (to M.L.); the STARS Summer Research Program (Kathryn and Ashley H. Priddy Award for Young Scientists) (to R.H.); NIH grants (to R.C.M.); NIH Grants R01DK075632, P30DK046200, and P30DK057521 (to B.B.L.); and Grant F3DK078478 (to L.V.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308195110/-/DCSupplemental.
References
- 1.Boardman L, et al. Investigating SAPAP3 variants in the etiology of obsessive-compulsive disorder and trichotillomania in the South African white population. Compr Psychiatry. 2011;52(2):181–187. doi: 10.1016/j.comppsych.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Züchner S, et al. Multiple rare SAPAP3 missense variants in trichotillomania and OCD. Mol Psychiatry. 2009;14(1):6–9. doi: 10.1038/mp.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bienvenu OJ, et al. Sapap3 and pathological grooming in humans: Results from the OCD collaborative genetics study. Am J Med Genet. 2009;150B(5):710–720. doi: 10.1002/ajmg.b.30897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welch JM, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448(7156):894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balthasar N, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123(3):493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 6.Farooqi IS. Monogenic human obesity. Front Horm Res. 2008;36:1–11. doi: 10.1159/000115333. [DOI] [PubMed] [Google Scholar]
- 7.Cui H, et al. Melanocortin 4 receptor signaling in dopamine 1 receptor neurons is required for procedural memory learning. Physiol Behav. 2012;106(2):201–210. doi: 10.1016/j.physbeh.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487(7406):183–189. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvaro JD, Taylor JR, Duman RS. Molecular and behavioral interactions between central melanocortins and cocaine. J Pharmacol Exp Ther. 2003;304(1):391–399. doi: 10.1124/jpet.102.040311. [DOI] [PubMed] [Google Scholar]
- 10.Bourne SK, Eckhardt CA, Sheth SA, Eskandar EN. Mechanisms of deep brain stimulation for obsessive compulsive disorder: Effects upon cells and circuits. Front Int Neurosci. 2012;6:29. doi: 10.3389/fnint.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu K, Hanna GL, Rosenberg DR, Arnold PD. The role of glutamate signaling in the pathogenesis and treatment of obsessive-compulsive disorder. Pharmacol Biochem Behav. 2012;100(4):726–735. doi: 10.1016/j.pbb.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor JL, Rajbhandari AK, Berridge KC, Aldridge JW. Dopamine receptor modulation of repetitive grooming actions in the rat: potential relevance for Tourette syndrome. Brain Res. 2010;1322:92–101. doi: 10.1016/j.brainres.2010.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schiöth HB, Mutulis F, Muceniece R, Prusis P, Wikberg JES. Discovery of novel melanocortin4 receptor selective MSH analogues. Br J Pharmacol. 1998;124(1):75–82. doi: 10.1038/sj.bjp.0701804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kask A, et al. Long-term administration of MC4 receptor antagonist HS014 causes hyperphagia and obesity in rats. Neuroreport. 1999;10(4):707–711. doi: 10.1097/00001756-199903170-00009. [DOI] [PubMed] [Google Scholar]
- 15.Sohn JW, et al. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell. 2013;152(3):612–619. doi: 10.1016/j.cell.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan Y, Feng G, Calakos N. Sapap3 deletion causes mGluR5-dependent silencing of AMPAR synapses. J Neurosci. 2011;31(46):16685–16691. doi: 10.1523/JNEUROSCI.2533-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Backs J, et al. Selective repression of MEF2 activity by PKA-dependent proteolysis of HDAC4. J Cell Biol. 2011;195(3):403–415. doi: 10.1083/jcb.201105063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malenka RC, Kocsis JD. Presynaptic actions of carbachol and adenosine on corticostriatal synaptic transmission studied in vitro. J Neurosci. 1988;8(10):3750–3756. doi: 10.1523/JNEUROSCI.08-10-03750.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farooqi IS, et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348(12):1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- 20.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27(7):736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 21.Bloch MH, Landeros-Weisenberger A, Rosario MC, Pittenger C, Leckman JF. Meta-analysis of the symptom structure of obsessive-compulsive disorder. Am J Psychiatry. 2008;165(12):1532–1542. doi: 10.1176/appi.ajp.2008.08020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellacott KL, Cone RD. The role of the central melanocortin system in the regulation of food intake and energy homeostasis: lessons from mouse models. Philos Trans R Soc Lond B Biol Sci. 2006;361(1471):1265–1274. doi: 10.1098/rstb.2006.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaye WH, Bulik CM, Thornton L, Barbarich N, Masters K. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am J Psychiatry. 2004;161(12):2215–2221. doi: 10.1176/appi.ajp.161.12.2215. [DOI] [PubMed] [Google Scholar]
- 24.Bulik CM, Sullivan PF, Fear JL, Joyce PR. Eating disorders and antecedent anxiety disorders: A controlled study. Acta Psychiatr Scand. 1997;96(2):101–107. doi: 10.1111/j.1600-0447.1997.tb09913.x. [DOI] [PubMed] [Google Scholar]
- 25.Wan S, et al. Presynaptic melanocortin-4 receptors on vagal afferent fibers modulate the excitability of rat nucleus tractus solitarius neurons. J Neurosci. 2008;28(19):4957–4966. doi: 10.1523/JNEUROSCI.5398-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler AA, et al. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci. 2001;4(6):605–611. doi: 10.1038/88423. [DOI] [PubMed] [Google Scholar]
- 27.Yang XW, Lu XH. Molecular and cellular basis of obsessive-compulsive disorder-like behaviors: emerging view from mouse models. Curr Opin Neurol. 2011;24(2):114–118. doi: 10.1097/WCO.0b013e32834451fb. [DOI] [PubMed] [Google Scholar]
- 28.Audet MC, Goulet S, Doré FY. Repeated subchronic exposure to phencyclidine elicits excessive atypical grooming in rats. Behav Brain Res. 2006;167(1):103–110. doi: 10.1016/j.bbr.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 29.Kelley AE. Measurement of rodent stereotyped behavior. Curr Protoc Neurosci. 2001;8:8.8. doi: 10.1002/0471142301.ns0808s04. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.