Abstract
Laccases, as early as 1959, were proposed to catalyze the oxidative polymerization of monolignols. Genetic evidence in support of this hypothesis has been elusive due to functional redundancy of laccase genes. An Arabidopsis double mutant demonstrated the involvement of laccases in lignin biosynthesis. We previously identified a subset of laccase genes to be targets of a microRNA (miRNA) ptr-miR397a in Populus trichocarpa. To elucidate the roles of ptr-miR397a and its targets, we characterized the laccase gene family and identified 49 laccase gene models, of which 29 were predicted to be targets of ptr-miR397a. We overexpressed Ptr-MIR397a in transgenic P. trichocarpa. In each of all nine transgenic lines tested, 17 PtrLACs were down-regulated as analyzed by RNA-seq. Transgenic lines with severe reduction in the expression of these laccase genes resulted in an ∼40% decrease in the total laccase activity. Overexpression of Ptr-MIR397a in these transgenic lines also reduced lignin content, whereas levels of all monolignol biosynthetic gene transcripts remained unchanged. A hierarchical genetic regulatory network (GRN) built by a bottom-up graphic Gaussian model algorithm provides additional support for a role of ptr-miR397a as a negative regulator of laccases for lignin biosynthesis. Full transcriptome–based differential gene expression in the overexpressed transgenics and protein domain analyses implicate previously unidentified transcription factors and their targets in an extended hierarchical GRN including ptr-miR397a and laccases that coregulate lignin biosynthesis in wood formation. Ptr-miR397a, laccases, and other regulatory components of this network may provide additional strategies for genetic manipulation of lignin content.
Lignin, an abundant biological polymer affecting the ecology of the terrestrial biosphere, is vital for the integrity of plant cell walls, the strength of stems, and resistance against pests and pathogens (1). Lignin is also a major barrier in the pulping and biomass-to-ethanol processes (2–4). For extracting cellulose (pulping) or for enzymatic degradation of cellulose for bioethanol, harsh chemical or physical treatments are used to reduce interactions with lignin or other cell wall components (2–4). Reducing lignin content or altering lignin structure to reduce its recalcitrance are major goals for more efficient processing.
Lignin is polymerized primarily from three monolignol precursors, p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol (1, 5). Over five decades, efforts have been made to understand the biosynthesis of the primary monolignols and to modify the quantity or composition of lignin. The polymerization of monolignols into a lignin polymer has long been thought to occur through oxidative polymerization catalyzed by either laccases or peroxidases (6). The mechanisms and specificity of the roles of the oxidative enzymes in lignin polymerization have been controversial (7).
Laccases (EC. 1.10.3.2) are multicopper oxidoreductases. Plant laccase was the first enzyme shown to be able to polymerize lignin monomers in vitro (6), and the expression of some laccase genes is closely correlated with lignin deposition in xylem (8–10). Very few genetic experiments have been published supporting the involvement of laccases in lignin polymerization. Ranocha et al. (11) isolated five laccase cDNAs (lac1, lac2, lac3, lac90, and lac110) from the stem-differentiating xylem of Populus trichocarpa. Suppression of the lac3 gene resulted in no significant alteration in lignin content and composition, although an alteration of the xylem fiber cell walls was observed in antisense suppressed lac3, lac90, or lac110 (12). Only recently has genetic data been obtained implicating laccases in lignin polymerization in Arabidopsis thaliana. Simultaneous disruption of LAC4 and the other laccase, LAC17, resulted in a reduction of lignin content in Arabidopsis stems, whereas single-gene mutations in LAC4 or LAC17 caused only a modest reduction in lignin content (13). Zhou et al. (14) identified MYB58 and MYB63 to be transcriptional activators of lignin biosynthesis and MYB58 directly activates LAC4. In Arabidopsis, there are 17 laccase genes, and 8 are expressed in stems (13). These results suggest functional redundancy of laccases in lignin polymerization, requiring multiple mutations to observe significant effects.
Trees are particularly suitable for studying lignin biosynthesis because of the abundance of lignin in wood and the role of lignin in the structure and physiology of the plant. We used P. trichocarpa as a model woody plant to investigate the laccase gene family and its regulators related to lignin biosynthesis. Previously, we identified a subset of 28 laccase genes to be targets of miR397 (15). MIR397 is a family of small and noncoding microRNAs (miRNAs) conserved in dicots, monocots, and gymnosperms (16). We found three MIR397 gene models in the P. trichocarpa genome: Ptr-MIR397a, b, and c (17). Only ptr-miR397a was sufficiently abundant to be detected by high throughput sequencing and real-time quantitative reverse transcription PCR (qRT-PCR) (17, 18). All of the tissues analyzed had detectable levels of mature ptr-miR397a. The levels in phloem, mature leaves, and stem differentiating xylem (SDX) were higher than those in young leaves, young stems, and roots (17). MiR397-directed cleavage of laccase transcripts has been observed in Arabidopsis (19) and P. trichocarpa (15). MiR397 could be a negative regulator of lignin content.
We characterized the P. trichocarpa laccase gene family and created transgenics overexpressing in Ptr-MIR397a. Twenty-three SDX-expressed laccases are targets of ptr-miR397a in P. trichocarpa. Overexpression of Ptr-MIR397a in P. trichocarpa resulted in a reduction in lignin content, whereas levels of monolignol biosynthetic gene transcripts remained unchanged, verifying the involvement of laccases in activation of monolignols for polymerization. We conclude that ptr-miR397a is a master regulator of polymerization in lignin biosynthesis. Using gene expression data and bioinformatic analysis of transcription factors (TFs), we constructed a hierarchical genetic regulatory network including ptr-miR397a, laccases, and associated TFs and their targets.
Results
Genomewide Characterization of Laccase Genes in P. trichocarpa.
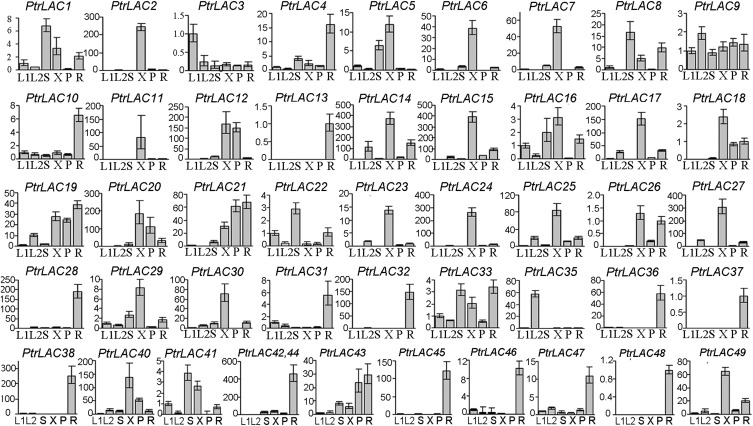
Plant laccases, which are members of a large family of multicopper oxidases, consist of three blue copper protein domains with signature sequences (20). We used 17 Arabidopsis laccase protein sequences to blast the P. trichocarpa genome (v2.2) and found 49 laccase gene models (PtrLACs) with Cu-oxidase domains (Table S1) that fall into six clades (Fig. S1). Suppression of Arabidopsis AtLAC4 and AtLAC17 resulted in reduction of lignin content (13). Seven PtrLACs are closely related to AtLAC4 in clade 1, and 11 are clustered with AtLAC17 in clade 2 (Fig. S1). Analysis of laccase mRNA abundance using qRT-PCR revealed transcripts of many laccase genes in each tissue examined and some show specificity for SDX (Fig. 1). Of a total of 49 gene models, transcripts for 30 PtrLACs were detected in SDX, of which 17 are abundant. These 17 belong to clades 1, 2, and 5, where the two lignin-related Arabidopsis laccases (AtLAC4 and AtLAC17) are also clustered. The abundance of multiple laccases within SDX suggests functional redundancy of laccases for lignin biosynthesis in wood formation.
Fig. 1.
Expression of PtrLAC genes in tissues of P. trichocarpa. Fold changes of transcript levels in young leaves (L1), mature leaves (L2), young stems (S), SDX (X), phloem (P), and young roots (R) are shown. Transcript levels in young leaves were arbitrarily set to 1, except PtrLAC13, PtrLAC18, PtrLAC26, and PtrLAC37, whose levels in young roots were set to 1.
Twenty-Nine PtrLACs Are Targets of ptr-miR397a.
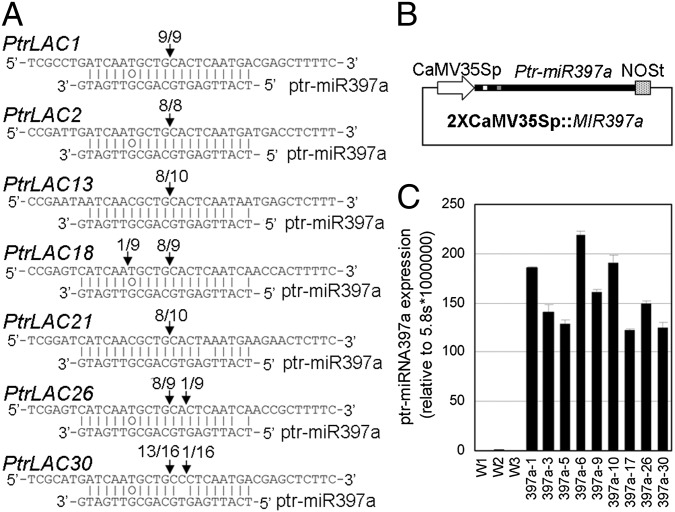
To determine whether any of these 49 laccases are regulated by ptr-miR397a, we screened for ptr-miR397a target sequences in the laccase transcripts using psRNATarget (21). Twenty-nine PtrLACs were predicted targets (Fig. S2A). The targets of ptr-miR397a are in a sequence encoding a conserved Cu-oxidase domain (Fig. S2A). We randomly selected 7 PtrLACs (1, 2, 13, 18, 21, 26, and 30) from the 29 predicted targets and characterized them by the modified 5′-rapid amplification of cDNA ends (RACE) (22). All seven were authentic targets of ptr-miR397a (Fig. 2A). This experiment verified the computational prediction and supported a regulatory role of ptr-miR397a in suppressing these laccases. The 29 PtrLACs targeted by ptr-miR397a belong to clades 1, 2, 4, and 5 (Fig. S1). No predicted targets were found in clades 3 and 6. The seven P. trichocarpa homologs of AtLAC4 in clade 1, and the 11 P. trichocarpa homologs of AtLAC17 in clade 2 are all predicted targets of ptr-miR397a. Among the 17 PtrLACs abundantly expressed in SDX, 13 are targets of ptr-miR397a (Fig. S1), suggesting a role of ptr-miR397a in secondary cell wall biosynthesis.
Fig. 2.
Ptr-miR397a targets 29 PtrLACs for cleavage and overexpression in P. trichocarpa. (A) Experimentally validated targets of ptr-miR397a. The cleavage sites were determined by the modified 5′ RNA ligase-mediated RACE. PtrLAC sequence of each complementary site from 5′ to 3′ and ptr-miR397a sequence from 3′ to 5′ are shown. Watson–Crick pairing (vertical dashes) and G:T wobble pairing (circles) are indicated. Vertical arrows indicate the 5′ termini of miRNA-guided cleavage products, as identified by 5′ RACE, with the frequency of clones shown. (B) The 2XCaMV35Sp::MIR397a plasmid used for overexpression of Ptr-MIR397a in P. trichocarpa. (C) Ptr-MIR397a expression in the three WT plants (W1–W3) and nine transgenic lines.
Cloning and Overexpression of the Full-Length Ptr-MIR397a.
To further elucidate the roles of ptr-miR397a and its target PtrLACs, we cloned the full-length Ptr-MIR397a cDNA using 5′ and 3′ RACE PCR, followed by full cDNA amplification. The primary Ptr-MIR397a transcript is 1,387 bases. The mature ptr-miR397a and ptr-miR397a* are close to the 5′-end of Ptr-MIR397a (Fig. S2 B and C). We prepared a pBI121-based construct to overexpress Ptr-MIR397a under the control of a double Cauliflower Mosaic Virus (CaMV) 35S promoter (2×CaMV35Sp::MIR397a; Fig. 2B). The construct was introduced into P. trichocarpa using Agrobacterium (23). Thirty-two transgenic lines were obtained. Significant overexpression of Ptr-MIR397a was found in all nine randomly selected lines (Fig. 2C).
Overexpression of Ptr-MIR397a Reduced Lignin Content.
All plants from the nine overexpression transgenic lines were maintained in a greenhouse and harvested at the age of 6 mo. No growth differences were observed compared with WT plants, and SDX cells did not show any anatomical difference compared with WT (Fig. S3). Based on the highest level of ptr-miR397a, four lines were selected for analysis of lignin content. Lignin content of the four lines, 397a-1, 397a-6, 397a-9, and 397a-10, showed reduction in Klason lignin content ranging from 12% to 22% (P < 0.005; Table 1). Significant increases (P < 0.05) in xylan content and decreases of mannan and uronic acid content were also found (Table 1). Other hemicelluloses, including rhamnan, arabinan, and galactan, as well as cellulose, showed no significant changes (Table 1).
Table 1.
Composition of WT and transgenic plants
| WT1 | WT2 | 397-1 | 397-6 | 397-9 | 397-10 | Prob > |t| | |
| Lignin* | |||||||
| Klason | 20.5 ± 0.0 | 20.0 ± 1.2 | 17.8 ± 0.4 | 16.2 ± 0.0 | 15.8 ± 0.1 | 16.2 ± 0.7 | 0.005 |
| Acid-soluble | 3.3 ± 0.1 | 3.9 ± 0.2 | 4.7 ± 0.4 | 5.0 ± 0.0 | 4.4 ± 0.2 | 4.6 ± 0.4 | 0.015 |
| Total lignin | 23.7 ± 0.1 | 23.9 ± 1.4 | 22.5 ± 0.0 | 21.2 ± 0.1 | 20.1 ± 0.1 | 20.8 ± 1.0 | 0.022 |
| Polysaccharide* | |||||||
| Rhamnan | 0.4 ± 0.0 | 0.5 ± 0.2 | 0.6 ± 0.1 | 0.5 ± 0.0 | 0.4 ± 0.0 | 0.6 ± 0.0 | 0.391 |
| Arabinan | 0.5 ± 0.0 | 0.4 ± 0.0 | 0.6 ± 0.1 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 | 0.765 |
| Xylan | 16.8 ± 0.1 | 15.8 ± 0.0 | 17.9 ± 0.9 | 18.1 ± 0.0 | 17.2 ± 0.2 | 17.3 ± 0.3 | 0.043 |
| Mannan | 2.9 ± 0.2 | 3.1 ± 0.3 | 2.8 ± 0.3 | 2.6 ± 0.0 | 2.6 ± 0.0 | 2.6 ± 0.1 | 0.023 |
| Galactan | 1.5 ± 0.3 | 1.7 ± 0.3 | 1.2 ± 0.2 | 1.4 ± 0.0 | 0.8 ± 0.0 | 1.0 ± 0.0 | 0.070 |
| Glucan | 38.3 ± 0.9 | 42.2 ± 1.5 | 43.2 ± 1.7 | 41.1 ± 0.6 | 43.8 ± 0.1 | 44.7 ± 1.2 | 0.149 |
| Uronic acid | 10.9 ± 0.1 | 11.2 ± 0.7 | 8.2 ± 0.7 | 8.6 ± 0.9 | 8.7 ± 0.4 | 9.2 ± 0.8 | 0.002 |
Values are means ± SE (n = 2 for lignin and polysaccharide analysis). Prob > |t| value in bold shows significant changes using JMP analysis (P < 0.05).
Values are expressed as weight percent based on vacuum-dried extractive free wood weight.
Lignin Composition and Structure.
To examine whether lignin composition was altered in plants overexpressing Ptr-MIR397a, woody stem tissues were analyzed using nitrobenzene oxidation (Table 2). P. trichocarpa lignin is rich in syringyl (S) subunits, represented by the oxidation products syringaldehyde and syringic acid. The guaiacyl (G) lignin, represented by vanillin and vanillic acid, was about half of the S content, whereas the content of p-hydroxyphenyl (H) subunits in lignin, represented by p-hydroxybenzaldehyde, was low (0.2%), as expected. Overexpression transgenics showed an S/G ratio of 2.2 compared with 2.1 in WT. The difference was not significant.
Table 2.
Lignin composition by nitrobenzene oxidation
| Mol %* | WT1 | WT2 | 397-1 | 397-6 | 397-9 | 397-10 |
| p-Hydroxybenzaldehyde | 0.5 ± 0.0 | 0.4 ± 0.1 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 |
| p-Hydroxybenzoic acid | — | — | — | — | — | — |
| Vanillin | 17.3 ± 0.9 | 16.5 ± 2.2 | 16.7 ± 0.2 | 17.0 ± 0.9 | 16.3 ± 0.2 | 17.2 ± 0.0 |
| Vanillic acid | 0.5 ± 0.0 | 0.3 ± 0.1 | 0.2 ± 0.2 | — | 0.6 ± 0.0 | 0.6 ± 0.0 |
| Syringaldehyde | 34.7 ± 0.5 | 34.1 ± 3.0 | 36.5 ± 0.6 | 37.2 ± 2.2 | 34.9 ± 1.1 | 36.8 ± 0.4 |
| Syringic acid | 2.0 ± 0.2 | 1.5 ± 0.1 | 1.4 ± 0.6 | 1.1 ± 0.1 | 2.3 ± 0.4 | 2.7 ± 0.1 |
| H† | 0.5 ± 0.0 | 0.4 ± 0.1 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 |
| V‡ | 17.8 ± 0.9 | 16.9 ± 2.2 | 16.9 ± 0.1 | 17.0 ± 0.9 | 16.9 ± 0.2 | 17.8 ± 0.0 |
| S§ | 36.7 ± 0.3 | 35.6 ± 3.1 | 37.8 ± 1.2 | 38.3 ± 2.3 | 37.3 ± 1.5 | 39.4 ± 0.3 |
| S/V ratio | 2.1 ± 0.1 | 2.1 ± 0.1 | 2.2 ± 0.1 | 2.2 ± 0.0 | 2.2 ± 0.1 | 2.2 ± 0.0 |
| H/V ratio | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 |
—, below detection limits.
Results are means ± SE (n = 2), assuming that lignin’s C9 molecular mass is 210 g/mol.
Sum of p-hydroxybenzaldehyde and p-hydroxybenzoic acid.
Sum of vanillin and vanillic acid.
Sum of syringaldehyde and syringic acid.
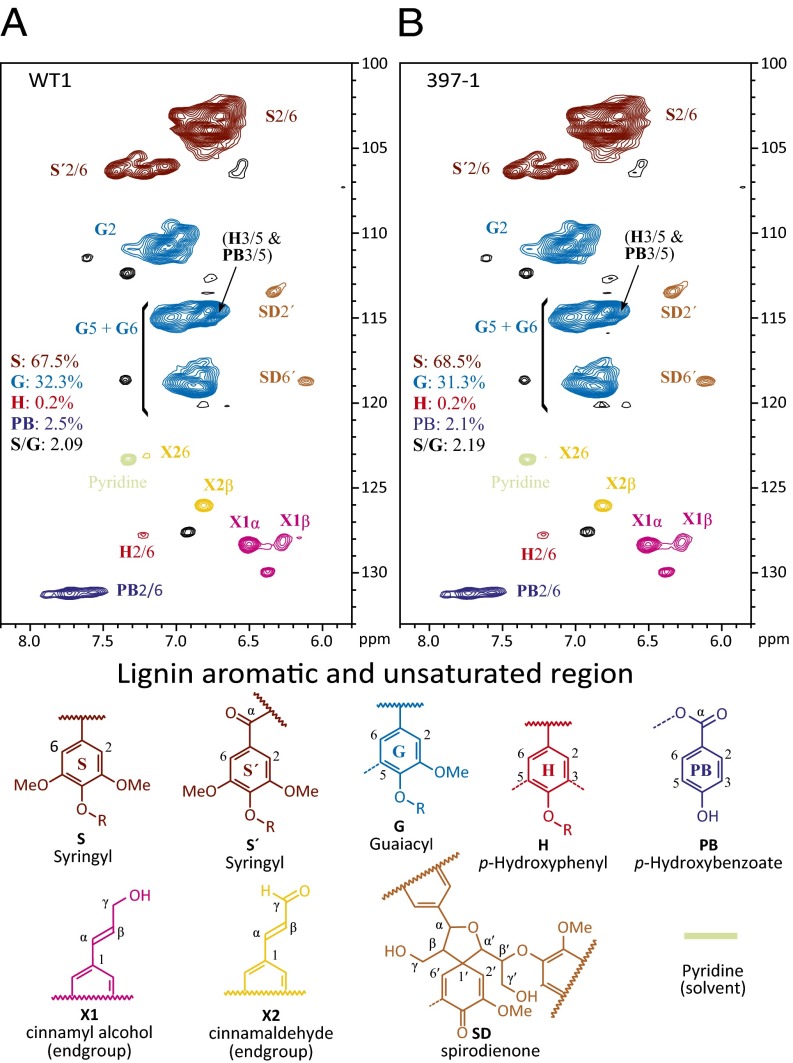
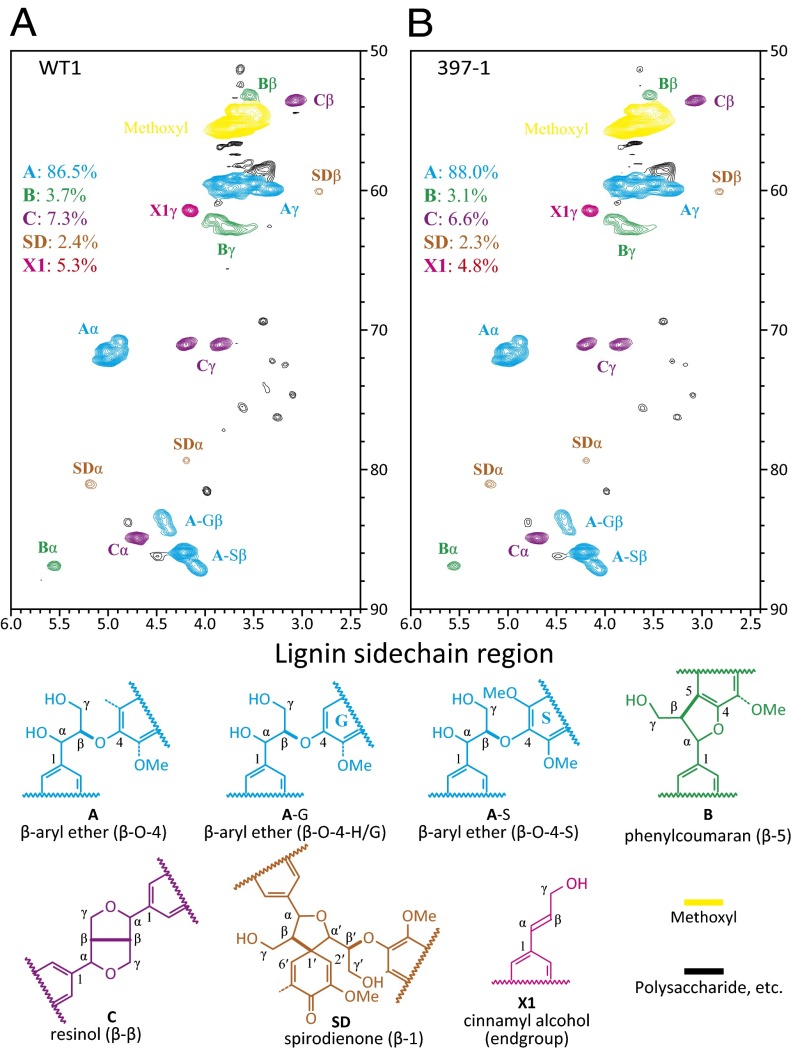
Lignin structural components and linkages were determined using NMR spectroscopy on lignins remaining after cellulolytic enzyme digestion (24). Three transgenic lines, 397a-1, 397a-9, and 397a-10, covering the range of the observed lignin reduction were analyzed and compared with WT. Consistent with the results from nitrobenzene oxidation, the stem wood was rich in S, lower in G, and very low in H (Fig. 3 A and B; Table S2). S/G ratios in the aromatic region of 397a-1, 397a-9, and 397a-10 lignin were 2.19, 3.03, and 2.56, respectively, compared with 2.09 in WT (Table S2). We examined the side chain region (Fig. 4 A and B), which generally substantiates the H/G/S ratio changes in the aromatic region and provides details regarding the bonding types and distribution of interunit linkages in the lignin structure (25, 26). Most of the lignin linkage types were nicely resolved in the 2D heteronuclear single-quantum correlation (HSQC) spectrum for all samples (Table S2). The compositional ratios of the side chains of lignin in the transgenics were almost identical to those in WT plants. Only minor structural changes were noticed from the relative intensity estimation (Table S2). The β-aryl ether units A in transgenics were slightly increased 3.4% (Table S2), consistent with the marginally increased S levels revealed by the correlations in the aromatic region. Syringyl units have a higher probability of β-aryl ether coupling in the growing lignin polymer (7, 26). Other structures, such as phenylcoumarans (B), resinols (C), and spirodienones (SD), were relatively decreased in the transgenics; phenylcoumarans require participation by coniferyl alcohol and are therefore likely lower due to higher S/G. Overexpression of PtrMIR397a caused only slight changes in lignin composition and structure.
Fig. 3.
HSQC NMR spectra of CELs from Ptr-MIR397a–overexpressing transgenic wood. Lignin aromatic region is shown for (A) WT1 and (B) 397-1. Correlations from the various aromatic ring unit types are well dispersed and can be categorized as the core lignin units (S, syringyl; G, guaiacyl; H, p-hydroxyphenyl), as well as from the p-hydroxybenzoate PB units known to acylate lignin side chains.
Fig. 4.
HSQC NMR spectra of CELs from Ptr-MIR397a–overexpressing transgenic wood. The lignin side chain region is shown for WT1 (A) and 397-1 (B). The major interunit structural units A, B, C, SD, as well as end-units X1 and X2, are also shown, color coded by their indicated structures.
Down-Regulation of PtrLAC Transcript Abundance by Overexpression of Ptr-MIR397a.
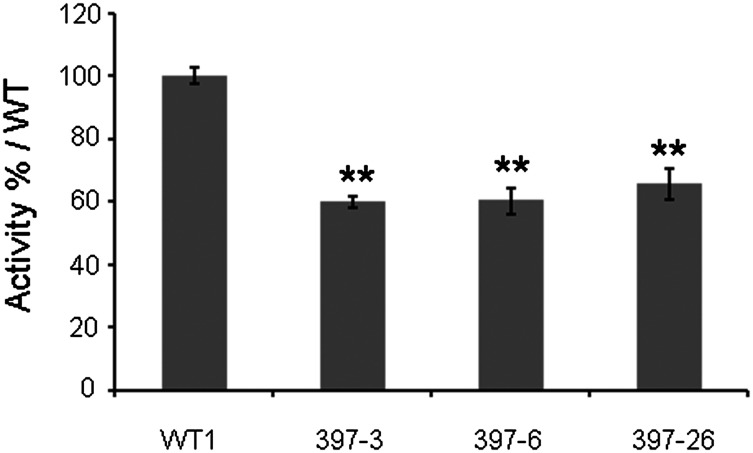
RNA-seq of the SDX from nine transgenic lines and three WT trees was carried out to examine the changes in the transcriptome caused by Ptr-MIR397a overexpression. Differentially expressed genes (DEGs) were identified using edgeR (27) by comparing the transcript abundance of each gene in the transgenic lines and the WT trees. We identified a total of 459 DEGs, with 289 down-regulated and 170 up-regulated. Thirty-four laccase genes of the 49 laccase gene models in P. trichocarpa were expressed in the WT SDX (Table S3), consistent with our qRT-PCR analysis (Fig. 1). The expression of 17 of the 34 SDX-expressed laccase genes was down-regulated by 32–86% compared with WT (Table S3), of which 5 are homologs of AtLAC4 and 6 are homologs of AtLAC17. Consistent with the reduction of laccase gene expression, the total laccase activity of SDX proteins in 397-3, 397-6, and 397-26 was reduced by ∼40% compared with WT (Fig. 5). No change in transcript abundance was observed for all 20 SDX monolignol genes (28), indicating that the lignin content reduction is specific and caused by down-regulation of laccases.
Fig. 5.
Quantification of laccase activity of purified SDX proteins from one WT (WT1) and three transgenic lines (397-3, 397-6, and 397-26). Data represent means ± SD (n = 5). **P < 0.01.
Specific Domain Families in the Xylem Transcriptome Respond to Ptr-MIR397a Overexpression.
To identify overrepresented gene families among the DEGs, we performed a domain enrichment analysis (29). Several protein families were overrepresented, including 20 laccases (types 1, 2, and 3 multicopper oxidases), 7 peroxidases, and 4 chalcone/stilbene synthases (Table S4).
Overexpression of ptr-miR397a Affects Expression of Network Genes.
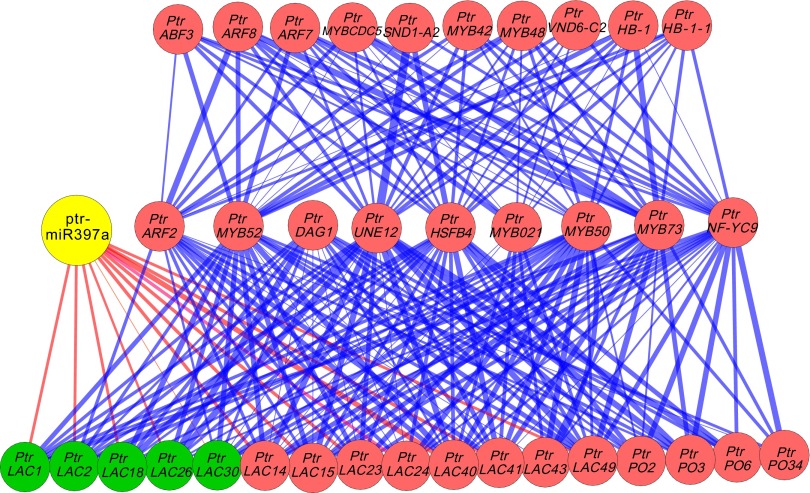
Ptr-MIR397a is part of a hierarchical network controlling wood formation. Overexpression of Ptr-MIR397a combined with RNA-seq may identify downstream components of a network. We developed and used a unique bottom-up graphic Gaussian model (GGM) algorithm to construct a causal hierarchical genetic regulatory network (GRN) from DEGs. The bottom-up algorithm constructs a multilayer GRN starting from the enzyme level and building up. Using these 19 significantly down- and up-regulated laccase genes, identified by DEG, domain-enrichment, and all transcription factors including ptr-miR397a as input for the bottom up algorithm, a GRN of three layers was obtained (Fig. 6). Ptr-miR397 is at the second layer and directly controls the laccase (bottom). The specific causal relationships of ptr-miR397a and their target laccase genes were accurately predicted when 19 laccase genes and 15 others (encoding chalcone/stilbene synthase and peroxidase protein domains) were used as the bottom/first layer in an implementation of the GGM. The regulatory genes in the second and third layers were recognized by the GGM bottom-up algorithm from 1,208 TFs and ptr-miR397a. The GRN indicates the control of 13 laccases and 4 peroxidases by ptr-miR397a and TFs. No direct regulatory relationships between ptr-miR397a and the other genes used as the implementation in the bottom layer were identified. Among the 13 PtrLACs identified in the bottom layer, 12 PtrLACs are directly regulated by ptr-miR397a, and these 12 PtrLACs belong to clades 1, 2, and 5 (Fig. S1). PtrLAC43, belonging to clade 6 (Fig. S1), is not directly regulated by ptr-miR397a but is regulated by other TFs in the second layer. These results are consistent with the computational inference that no predicted targets were found in clades 3 and 6. Adding 44 genes involved in lignocellulosic biosynthesis in the input failed to identify any causal relationships between ptr-miR397 and these genes, indicating that the regulatory relationships between ptr-miR397 and the 12 laccase genes are specific.
Fig. 6.
A three-layer genetic regulatory network (GRN) indicating the control of 13 laccase genes and 4 peroxidase genes. On the bottom layer, 12 laccase genes are shown as direct targets (red lines) of ptr-miR397a (yellow). Five of the laccases genes (green) have been validated as targets for ptr-miR397a by 5′ RACE. One laccase PtrLAC43 is implicated in the network by putative interactions with several TFs in layer 2. Four peroxidases are included in the GRN also because of proposed interaction with TFs in layer 2. The thickness of the connecting lines reflects the calculated relative strength of the proposed interaction. The remainder of layer 2 is composed of TFs selected by the GGM algorithm based on putative interactions with genes in the bottom layer. Similarly, layer 3 (top layer) represents TFs derived by GGM based on interactions with the TFs in layer 2 as described in SI Materials and Methods. See Tables S1 and S5 for gene models.
In the GRN, we identified several wood formation–related TFs present in the upper layers. Two MYB genes, PtrMYB021 (30) and PtrMYB52 (Table S5), are present in second layer, and two NAC genes, PtrSND1-A2 and PtrVND6-C2 (30), are in the third layer. PtrMYB021 is the poplar ortholog of Arabidopsis AtMYB46, known to up-regulate AtLAC10 and other laccase genes through an eight-nucleotide core motif (RKTWGGTR) (31, 32), further validating the GRN. We also found some genes with unknown functions in the GRN. For example, PtrUNE12 (Table S5), encoding a basic helix–loop–helix (bHLH) TF, has an inferred interaction with PtrSND1-A2, 10 laccase genes, and 4 peroxidase genes (Table S5), suggesting it is an important regulator in lignin biosynthesis. All of the proposed interactions (Fig. 6) need experimental validation.
Discussion
The involvement of laccases in lignin polymerization was proposed more than five decades ago based on the ability of a cambial extract containing laccase activities from spruce to produce a lignin-like polymer (6). Peroxidase activities were also found in the cambial sap, and therefore peroxidases were also implicated in lignin polymerization (6). Since 1959, evidence has implicated both laccases and peroxidases in lignin polymerization. Genetic data to demonstrate an essential role for either laccases or peroxidases in lignin biosynthesis have been difficult to obtain due to the large number of functionally redundant genes in these gene families (13, 33). Transgenic suppression of peroxidases in tobacco and aspen has shown effects on lignin content (34, 35). Transgenic suppression of laccases in poplar (12) showed effects on phenolic metabolites but no effect on lignin content. In a recent publication, a double mutant of two laccase genes affected lignin content. Each single mutant had slightly reduced levels of lignin, but the double mutant reduced lignin content as much as 40%, providing clear genetic evidence to support the role of laccases (13).
Transgenic P. trichocarpa plants overexpressing Ptr-MIR397a resulted in a reduction in Klason lignin content up to 22% (Table 1), consistent with the results from the Arabidopsis mutants (13). Nitrobenzene oxidation and NMR showed few if any changes in lignin composition and structure. The transcript levels of all of the monolignol pathway genes (not predicted targets of ptr-miR397a) were not significantly affected, providing evidence for the specificity of the laccase effect. The supply of monolignols in the Ptr-MIR397a transgenics can be expected to be essentially the same as WT. These results verify the involvement of laccases in lignin polymerization and suggest ptr-miR397a to be a master regulator in the process of lignin polymerization. Ptr-miR397a is the first example of a miRNA regulating lignin biosynthesis for wood formation.
Laccase is a blue copper oxidase, a metalloenzyme with four copper ions required for utilization of dioxygen as a substrate (36). Cu+ is highly toxic to living cells (37). Organisms need to maintain copper homeostasis for many processes such as energy transduction, iron mobilization, and oxidative stress responses (38). High copper levels cause lignin accumulation in plants (39, 40), and copper deficiency decreases plant lignin content (41). The underlying mechanisms are not known. If copper-binding protein laccases were reduced in Ptr-MIR397a overexpression transgenics, then copper could accumulate. To alleviate the effect of higher copper concentration, plant cells accumulate hydrogen peroxide and increase activity of antioxidant enzymes, including superoxide dismutase, catalase, ascorbate peroxidase, glutathione reductase, and particularly peroxidase (39, 40). Both DEG and domain enrichment analysis of the Ptr-MIR397a–overexpressing transgenics showed seven peroxidase genes were significantly up-regulated (Table S4). Alternatively, the relative abundance of laccases and peroxidases may be reciprocally regulated for lignin polymerization, further contributing to functional redundancy of enzymes for oxidative polymerization. Redundancy of oxidative enzymes is likely the reason for the maximum of 22% lignin content reduction when 17 laccases genes are down-regulated by 32–86%.
GRNs resulting from the bottom-up GGM algorithm confirmed that ptr-miR397a is a negative regulator of many members of the laccase gene family. The GRN indicates that overexpression of Ptr-MIR397a leads to a coordinated repression of laccases and up-regulation of peroxidases in the bottom layer of the network (Fig. 6). The TFs in the network regulating laccases and peroxidases indicate that multiple regulators are involved in overlapping control of specific gene regulation. Future studies should investigate the consequences of this regulation and the extent of its indirect effects. The network suggests several additional targets including ptr-miR397a and laccases for genetic manipulation of lignin formation, potentially leading to improved materials for pulp/paper and biofuel production.
Materials and Methods
Plant materials, qRT-PCR, computational prediction and experimental validation of ptr-miR397a targets followed those of previous publications (22, 23). Ptr-MIR397a cDNA cloning, vector construction and transformation, wood chemical composition and lignin composition analysis, NMR sample preparation and analysis, and RNA-seq analysis are described in detail in SI Materials and Methods. Primers used for qRT-PCR and validation of ptr-miR397a targets are listed in Tables S6 and S7.
Supplementary Material
Acknowledgments
This work was supported by grants from National Science Foundation Plant Genome Research Program Grant (DBI-0922391) to V.L.C.; the National Key Basic Research Program of China (973 program) (2012CB114502) to S.L.; the National Natural Science Foundation of China (31070534) to L.Y.; the DOE Great Lakes Bioenergy Research Center (DOE Office of Science BER DE-FC02-07ER64494) to J.R. and H.K., and the North Carolina State University Forest Biotechnology Industrial Research Consortium (grant no. 556051) to Q.L. and J.L.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308936110/-/DCSupplemental.
References
- 1.Sarkanen K. In: Lignin, Occurrence, Formation, Structure and Reactions. Sarkanen KV, Kudwig CH, editors. New York: Wiley-Interscience; 1971. pp. 95–163. [Google Scholar]
- 2.Chen F, Dixon RA. Lignin modification improves fermentable sugar yields for biofuel production. Nat Biotechnol. 2007;25(7):759–761. doi: 10.1038/nbt1316. [DOI] [PubMed] [Google Scholar]
- 3.Chiang VL. From rags to riches. Nat Biotechnol. 2002;20(6):557–558. doi: 10.1038/nbt0602-557. [DOI] [PubMed] [Google Scholar]
- 4.Sarkanen KV. Renewable resources for the production of fuels and chemicals. Science. 1976;191(4228):773–776. doi: 10.1126/science.191.4228.773. [DOI] [PubMed] [Google Scholar]
- 5.Higuchi T. Pathways for monolignol biosynthesis via metabolic grids: Coniferyl aldehyde 5-hydroxylase, a possible key enzyme in angiosperm syringyl lignin biosynthesis. Proc Jpn Acad Ser B. 2003;79(8):227–236. [Google Scholar]
- 6.Freudenberg K. Biosynthesis and constitution of lignin. Nature. 1959;183(4669):1152–1155. doi: 10.1038/1831152a0. [DOI] [PubMed] [Google Scholar]
- 7.Ralph J, et al. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids. Phytochem Rev. 2004;3(1–2):29–60. [Google Scholar]
- 8.Dean J, Eriksson K. Laccase and the deposition of lignin in vascular plants. Holzforschung. 1994;48(Suppl):21–33. [Google Scholar]
- 9.Sato Y, Wuli B, Sederoff Rs Whetten R. Molecular cloning and expression of eight laccase cDNAs in loblolly pine (pinus taeda) J Plant Res. 2001;114(2):147–155. [Google Scholar]
- 10.Bao W, O’malley DM, Whetten R, Sederoff RR. A laccase associated with lignification in loblolly pine xylem. Science. 1993;260(5108):672–674. doi: 10.1126/science.260.5108.672. [DOI] [PubMed] [Google Scholar]
- 11.Ranocha P, et al. Biochemical characterization, molecular cloning and expression of laccases—a divergent gene family—in poplar. Eur J Biochem. 1999;259(1–2):485–495. doi: 10.1046/j.1432-1327.1999.00061.x. [DOI] [PubMed] [Google Scholar]
- 12.Ranocha P, et al. Laccase down-regulation causes alterations in phenolic metabolism and cell wall structure in poplar. Plant Physiol. 2002;129(1):145–155. doi: 10.1104/pp.010988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berthet S, et al. Disruption of LACCASE4 and 17 results in tissue-specific alterations to lignification of Arabidopsis thaliana stems. Plant Cell. 2011;23(3):1124–1137. doi: 10.1105/tpc.110.082792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J, Lee C, Zhong R, Ye ZH. MYB58 and MYB63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in Arabidopsis. Plant Cell. 2009;21(1):248–266. doi: 10.1105/tpc.108.063321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu S, Sun YH, Chiang VL. Stress-responsive microRNAs in Populus. Plant J. 2008;55(1):131–151. doi: 10.1111/j.1365-313X.2008.03497.x. [DOI] [PubMed] [Google Scholar]
- 16.Kozomara A, Griffiths-Jones S. miRBase: Integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39(Database issue):D152–D157. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu S, Sun YH, Chiang VL. Adenylation of plant miRNAs. Nucleic Acids Res. 2009;37(6):1878–1885. doi: 10.1093/nar/gkp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu S, Yang C, Chiang VL. Conservation and diversity of microRNA-associated copper-regulatory networks in Populus trichocarpa. J Integr Plant Biol. 2011;53(11):879–891. doi: 10.1111/j.1744-7909.2011.01080.x. [DOI] [PubMed] [Google Scholar]
- 19.Jones-Rhoades MW, Bartel DP. Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol Cell. 2004;14(6):787–799. doi: 10.1016/j.molcel.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 20.Zhukhlistova NE, Zhukova YN, Lyashenko AV, Zaitsev VN, Mikhailov AM. Three-dimensional organization of three-domain copper oxidases: A review. Crystallogr Rep. 2008;53(1):92–109. [Google Scholar]
- 21.Dai X, Zhuang Z, Zhao PX. Computational analysis of miRNA targets in plants: Current status and challenges. Brief Bioinform. 2011;12(2):115–121. doi: 10.1093/bib/bbq065. [DOI] [PubMed] [Google Scholar]
- 22.Lu S, et al. Novel and mechanical stress-responsive MicroRNAs in Populus trichocarpa that are absent from Arabidopsis. Plant Cell. 2005;17(8):2186–2203. doi: 10.1105/tpc.105.033456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song J, Lu S, Chen ZZ, Lourenco R, Chiang VL. Genetic transformation of Populus trichocarpa genotype Nisqually-1: A functional genomic tool for woody plants. Plant Cell Physiol. 2006;47(11):1582–1589. doi: 10.1093/pcp/pcl018. [DOI] [PubMed] [Google Scholar]
- 24.Balakshin M, Capanema E, Gracz H, Chang HM, Jameel H. Quantification of lignin-carbohydrate linkages with high-resolution NMR spectroscopy. Planta. 2011;233(6):1097–1110. doi: 10.1007/s00425-011-1359-2. [DOI] [PubMed] [Google Scholar]
- 25.Kim H, Ralph J. Solution-state 2D NMR of ball-milled plant cell wall gels in DMSO-d(6)/pyridine-d(5) Org Biomol Chem. 2010;8(3):576–591. doi: 10.1039/b916070a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ralph J, Landucci LL. In: Lignin and Lignans: Advances in Chemistry. Heitner C, Dimmel DR, Schmidt JA, editors. CRC Press, Boca Raton, FL; 2010. pp. 137–234. [Google Scholar]
- 27.Robinson MD, McCarthy DJ, Smyth GK. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi R, et al. Towards a systems approach for lignin biosynthesis in Populus trichocarpa: transcript abundance and specificity of the monolignol biosynthetic genes. Plant Cell Physiol. 2010;51(1):144–163. doi: 10.1093/pcp/pcp175. [DOI] [PubMed] [Google Scholar]
- 29.Wei H, et al. Global transcriptomic profiling of aspen trees under elevated [CO2] to identify potential molecular mechanisms responsible for enhanced radial growth. J Plant Res. 2013;126(2):305–320. doi: 10.1007/s10265-012-0524-4. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, et al. Splice variant of the SND1 transcription factor is a dominant negative of SND1 members and their regulation in Populus trichocarpa. Proc Natl Acad Sci USA. 2012;109(36):14699–14704. doi: 10.1073/pnas.1212977109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko JH, Kim WC, Han KH. Ectopic expression of MYB46 identifies transcriptional regulatory genes involved in secondary wall biosynthesis in Arabidopsis. Plant J. 2009;60(4):649–665. doi: 10.1111/j.1365-313X.2009.03989.x. [DOI] [PubMed] [Google Scholar]
- 32.Kim WC, Ko JH, Han KH. Identification of a cis-acting regulatory motif recognized by MYB46, a master transcriptional regulator of secondary wall biosynthesis. Plant Mol Biol. 2012;78(4-5):489–501. doi: 10.1007/s11103-012-9880-7. [DOI] [PubMed] [Google Scholar]
- 33.Hiraga S, Sasaki K, Ito H, Ohashi Y, Matsui H. A large family of class III plant peroxidases. Plant Cell Physiol. 2001;42(5):462–468. doi: 10.1093/pcp/pce061. [DOI] [PubMed] [Google Scholar]
- 34.Blee KA, et al. A lignin-specific peroxidase in tobacco whose antisense suppression leads to vascular tissue modification. Phytochemistry. 2003;64(1):163–176. doi: 10.1016/s0031-9422(03)00212-7. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Kajita S, Kawai S, Katayama Y, Morohoshi N. Down-regulation of an anionic peroxidase in transgenic aspen and its effect on lignin characteristics. J Plant Res. 2003;116(3):175–182. doi: 10.1007/s10265-003-0087-5. [DOI] [PubMed] [Google Scholar]
- 36.Solomon EI, Sundaram UM, Machonkin TE. Multicopper oxidases and oxygenases. Chem Rev. 1996;96(7):2563–2606. doi: 10.1021/cr950046o. [DOI] [PubMed] [Google Scholar]
- 37.Wong M, Bradshaw A. A comparison of the toxicity of heavy metals, using root elongation of rye grass, lolium perenne. New Phytol. 1982;91(2):255–261. [Google Scholar]
- 38.Arredondo M, Núñez MT. Iron and copper metabolism. Mol Aspects Med. 2005;26(4-5):313–327. doi: 10.1016/j.mam.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H, Xia Y, Wang G, Shen Z. Excess copper induces accumulation of hydrogen peroxide and increases lipid peroxidation and total activity of copper-zinc superoxide dismutase in roots of Elsholtzia haichowensis. Planta. 2008;227(2):465–475. doi: 10.1007/s00425-007-0632-x. [DOI] [PubMed] [Google Scholar]
- 40.Chen E, Chen Y, Chen L, Liu Z. Effect of copper on peroxidase activity and lignin content in raphanus sativus. Plant Physiol Biochem. 2002;40(5):439–444. [Google Scholar]
- 41.Robson A, Hartley R, Jarvis S. Effect of copper deficiency on phenolic and other constituents of wheat cell walls. New Phytol. 1981;89(3):361–371. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.