Abstract
Theropithecus was a common large-bodied primate that co-occurred with hominins in many Plio-Pleistocene deposits in East and South Africa. Stable isotope analyses of tooth enamel from T. brumpti (4.0–2.5 Ma) and T. oswaldi (2.0–1.0 Ma) in Kenya show that the earliest Theropithecus at 4 Ma had a diet dominated by C4 resources. Progressively, this genus increased the proportion of C4-derived resources in its diet and by 1.0 Ma, had a diet that was nearly 100% C4-derived. It is likely that this diet was comprised of grasses or sedges; stable isotopes cannot, by themselves, give an indication of the relative importance of leaves, seeds, or underground storage organs to the diet of this primate. Theropithecus throughout the 4- to 1-Ma time range has a diet that is more C4-based than contemporaneous hominins of the genera Australopithecus, Kenyanthropus, and Homo; however, Theropithecus and Paranthropus have similar proportions of C4-based resources in their respective diets.
Keywords: C3, East Africa, Koobi Fora, Nachukui, baboon
Today, the Old World monkey genus Theropithecus is represented by one species, T. gelada, which lives only in the highlands of central Ethiopia. This unusual, grass-eating relict is all that remains of a previously widespread radiation that extended over much of Africa during the Pliocene and Pleistocene. From the period from ∼4 to 0.25 Ma, fossils of Theropithecus are found in abundance at most of the well-known Plio-Pleistocene hominin fossil localities of Africa (1). The nature and pattern of occurrence of Theropithecus fossils attracted the attention of Clifford Jolly early in his career, and his famous 1970 paper (2) on the “seed-eater hypothesis” was one of the first to model early hominin ecology and functional morphology on the characteristics of a nonhuman primate. Most of the Theropithecus fossil record is dominated by members of the continuous and geographically widespread T. darti–T. oswaldi lineage, but during the early and middle Pliocene, the distinct T. brumpti lineage was found in the Omo-Lake Turkana Basin (1, 3). The virtual absence of geographic or temporal overlap between the two Theropithecus lineages has invited speculation as to their respective habitat preferences and diets (4–7). The association of T. brumpti fossils with presumed forest-dwelling bovid fossils and the species’ idiosyncratic pattern of dental wear led some to conclude that the species was a semiarboreal frugivore (7, 8).
Theropithecus exhibits a distinctive suite of dental, gnathic, and postcranial characteristics related to chewing and food harvesting. These characteristics include an elongated thumb and foreshortened index finger; this morphology permits precise and efficient plucking and pinching of food items, notably grasses in the case of geladas (9, 10). The combination of features associated with manual grazing along with craniodental specializations facilitating the comminution of high-fiber and/or silica-rich vegetation was highly successful. During the Pliocene, Theropithecus was thought to have occupied an ecological niche that is dominated today by ungulates, many of which are ruminants; thus, Theropithecus may have shared some of the dietary features of ungulates (such as being capable of chewing and digesting large volumes of low-quality, high-fiber, and/or highly siliceous vegetation), although Theropithecus did not have the benefit of hooves or ruminant digestion (11). Modern geladas are able to masticate grass as effectively as an equid, and they can also ferment cellular material from grass in their hindguts but less effectively than a zebra, which may have aided in this adaptation (6, 12, 13). Even with the richness of the genus’s fossil record and the many paleoecological and functional anatomical studies that have speculated on the respective habitat and dietary preferences of the T. brumpti and T. oswaldi lineages, many questions remain about their respective dietary specializations and how they may have contributed to the eventual extinction of both lineages.
The present study of the stable isotopic composition of the molars of T. brumpti and T. oswaldi through time was undertaken to shed light on this persistent and vexing set of questions. Stable isotope ratios of 13C/12C are ideally suited to test this hypothesis because of the difference in isotope ratios between C3 plants (most dicots) and C4 plants (grasses and sedges, both of which are monocots) in the tropics; the dietary distinction between C3 and C4 plant-derived foods is preserved in the fossil record of Africa for most of the past 10 Ma (14, 15). The δ13C values of tooth enamel from modern and fossil browsers are about −12‰ in open forests through grasslands, whereas grazers have δ13C values near 2‰, and mixed feeders have intermediate values (16–19). We note that mammals from closed canopy forests are even more depleted in 13C than those mammals from open forests (20). Previous studies using isotopes in fossil primates show dietary preferences from pure C3-derived to predominantly C4-derived diets (21–27). Theropithecus from Southern Africa had a high component of C4 biomass in the diet during the Plio-Pleistocene (21, 23); however, dating fossils from South African cave deposits is problematic, and a good chronology for the history of dietary evolution in this genus cannot be established.
First, to address the comparison between preadult (during molar formation and maturation) and adult diets (postmolar formation and maturation), we compare diets of modern baboons (Papio cynocephalus) using stable isotope ratios of feces from known individuals; baboons were from two groups monitored over a 3-wk period.
We then present stable isotope data for 44 Theropithecus specimens from Kenya, principally from the Lake Turkana region but also from Olorgesailie, that range in age from ca. 4 to <1 Ma. For purposes of considering average carbon isotope ratios for the two main Theropithecus species under consideration, we included one sample of T. darti; this species should be compared with T. oswaldi, because it is widely accepted that T. darti is the earliest representative of the T. oswaldi chronospecies. We discuss Theropithecus in the context of C3- and C4-derived diet resources and the overall context of isotope ecology in the Turkana Basin. The diet of Theropithecus is of interest compared with the diets of early hominins (26, 28) from the same deposits; hominins exhibit a change in the use of C4 resources over this time interval, and thus, these primates were in potential competition for dietary resources.
Results
Preadult Vs. Adult Diet.
Isotope ratios measured in tooth enamel are set by the diet of preadults; therefore, to characterize the species as a whole, it is important to establish whether the preadult diet differs from the adult diet. We measured δ13C values of fecal matter from two groups of baboons collected over a restricted time period; preadults are not significantly different from adult baboons for each group. Group 1.1 has average δ13C values of −22.0 ± 1.7‰ (n = 12) and −22.6 ± 1.1‰ (n = 7) for preadult and adult individuals, respectively; group 1.2 has average δ13C values of −24.2 ± 1.4‰ (n = 9) and −24.3 ± 1.0‰ (n = 4) for preadult and adult individuals, respectively (data in Table S1).
Theropithecus Isotope Results.
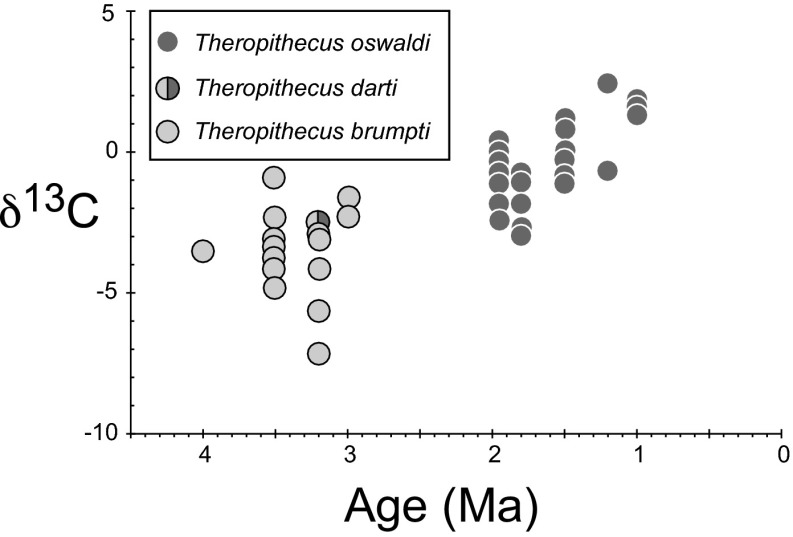
The geological age ranges of the specimens of T. brumpti and T. oswaldi in this study are ca. 4–2.5 and 2–1 Ma, respectively. Most samples are from the Turkana Basin in northern Kenya, but the later time period includes three specimens from Olorgesailie in southern Kenya. The age ranges represented are discontinuous, with an important gap between 2.5 and 2.0 Ma (Fig. 1); additional specimens from this critical time interval in the evolution of Theropithecus may be obtained in the future from the Ethiopian National Museum.
Fig. 1.
δ13C vs. age for T. brumpi, T. darti, and T. oswaldi from Kenya.
The average δ13C of T. brumpti is −3.5 ± 1.5‰ (Table S1) (n = 15 teeth from 14 individuals), corresponding to an estimated diet that is ca. 65 ± 10‰ C4-based; this result is significantly different (ANOVA; P < 0.001) (Fig. 2) than the later T. oswaldi, which has an average δ13C of −0.7 ± 1.5‰ (Table S1) (n = 29), corresponding to an estimated diet that is ca. 80 ± 10% C4-based. The single T. darti specimen, which is much older than the related T. oswaldi, has a δ13C value of −2.5‰. Thus, the δ13C values of tooth enamel from Theropithecus increase between 4 and 1 Ma (Fig. 1).
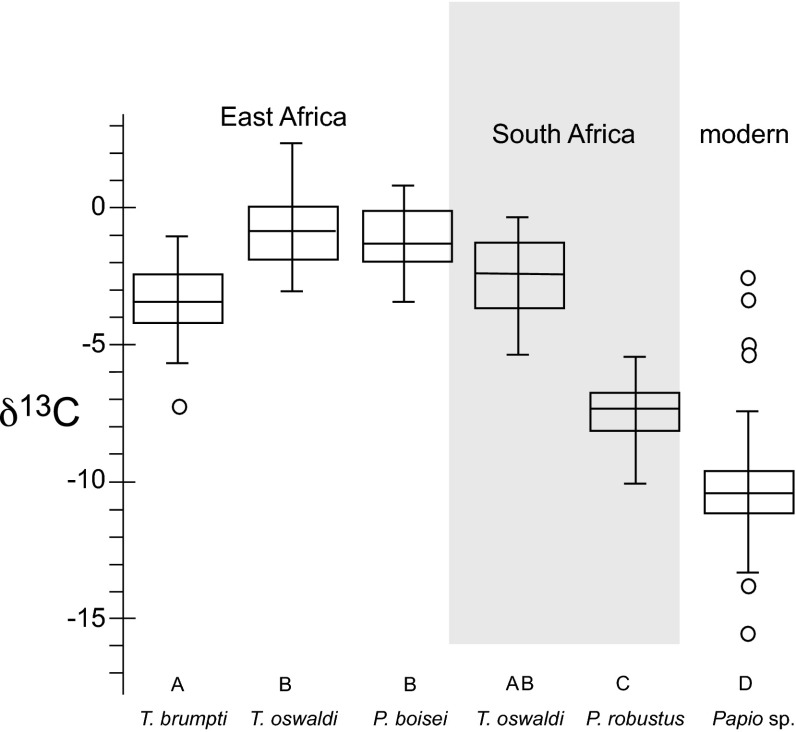
Fig. 2.
Box and whisker diagrams showing the δ13C ranges for tooth enamel from Theropithecus (this study) and Paranthropus from the Turkana Basin (26, 28), Theropithecus (17, 19) and Paranthropus from South Africa (21–23), and baboons from Eastern and Central Africa (28). Letters correspond to statistically different groups based on ANOVA analysis (Tukey posthoc; P > 0.05).
For comparison, the T. oswaldi from South Africa have a δ13C value of −2.3 ± 1.6‰ (21, 23) (n = 11), which can be distinguished from none of the East African species (Fig. 2).
Comparison with Modern Baboons.
Tooth enamel from modern baboons from Africa, including P. anubis and P. hamadryas, have an average δ13C1750 value of −10.2 ± 2.7‰ (n = 36; maximum = −2.5‰; minimum = −15.5‰) (data in ref. 28), corresponding to a ca. 15 ± 20% C4-based diet; the diets of modern baboons are very different from T. brumpti or T. oswaldi (Fig. 2), with modern baboons having a much a higher dependence on C3-based diet resources.
Discussion
Preadult Vs. Adult Diet.
Tooth enamel forms in baboons in preadults; tooth formation is complete by the time that teeth are erupted, which in Papio, is ca. 7 y (29). The results of fecal material collected from two groups of baboons over a short time interval in Amboseli, Kenya, show no significant difference in δ13C between preadults and adults, indicating that their respective diets are not distinguished at this level (data in Table S2). Thus, δ13C values from the later-formed premolars and molars are representative of long-term diets [i.e., only the earliest permanent teeth (e.g., m1/M1, p4/P4) or deciduous teeth may not fully represent adult diets related to weaning issues]. For this reason, we sampled primarily the second or third molar teeth.
C3, C4, and Crassulacean Acid Metabolism Resources in Primate Diets.
Primary dietary components are derived from the base of the food web; in this discussion, we consider terrestrial plants (but see discussion of aquatic food webs below). C3 and C4 plants provide a variety of direct dietary resources ranging from low-protein bark and wood to intermediate-protein leaves to high-protein seeds and nuts. Underground storage organs (bulbs, rhizomes, and tubers) also can be important dietary resources.
Most dicots in East Africa use the C3 pathway. Thus, most trees, shrubs, and bushes are C3 along with many of the herbaceous dicots (including many legumes, melons, fruits, and vegetables). Most C3 plants in Africa have δ13C1750 values ranging from ca. −25‰ to −28‰ (14, 17, 18); values more positive (to −23‰) are found in xeric regions, and closed canopy forests have δ13C values between ca. −30‰ and −35‰ (20). Primary forest resources are almost entirely C3; soil carbon isotopes (30) show that few, if any, C4 plants are present in forests (>80% canopy cover). Even open grasslands in Africa can have an important abundance of nonwoody C3 forbs and herbs present (30).
C4 plants are primarily tropical grasses and sedges, both of which are monocots. C4 plants in East Africa have δ13C values between ca. −10‰ and −15‰ (14, 17, 18). Tropical grasses make up >30% of the photosynthetic primary productivity (NPP) in the tropics (31, 32), and thus, tropical grasses and sedges are the most likely candidates as significant dietary resources of primates. Leaves, seeds, and underground storage organs are potential diet resources for grasses and sedges. It is important to note that a few dicots use the C4 pathway, including some known to be food resources for modern humans (33); these dicots include members of the Acanthaceae, Amananthaceae, and Boraginaceae families as well as others. Today, C4 dicots in Africa are minor parts of the regional ecosystem in terms of their contribution to total photosynthetic productivity, although C4 dicot plants may be important on a local scale.
Crassulacean Acid Metabolism (CAM) plants have δ13C values similar to the δ13C values of C4 plants, especially in Africa (34). CAM plants in Africa include many succulents and are represented in a number of families, including Agavaceae (e.g., Sansevieria), Aizoaceae, Chenopodiaceae (e.g., Salsola), Crassulaceae, Euphorbiaceae (e.g., Euphorbia), and Liliaceae (e.g., Aloe). However, none of these plants are known to be important dietary resources for primates, and CAM plants are unlikely to have been important diet resources for fossil primates.
Secondary diet components are important for omnivores and carnivores; the C3 or C4 primary isotope signal can be inherited through a diet comprised of animals that themselves consumed C3 or C4 resources (35). There is little indication in the morphology of Theropithecus that omnivory or carnivory was important in dietary considerations, and we do not consider either omnivory or carnivory to be a major potential source of C4 resources for Theropithecus. One of the remarkable aspects of the ecology of the modern gelada is its near-exclusive and year-round reliance on grasses (36, 37). Plio-Pleistocene theropiths had a more varied diet than the diet of the gelada, but there are no dental indicators of carnivory (7).
Aquatic food webs are based primarily on the primary production of algae, which primarily uses the C3 pathway; because of CO2 limitations in aquatic ecosystems, algae sometimes use bicarbonate rather than CO2 for carbon assimilation, which gives them higher δ13C values than are typical for C3 photosynthesis (38, 39). Thus, some aquatic ecosystems could have an apparent C4 component because of this effect, which would be passed along the food web to secondary consumers, such as fish. We do not consider aquatic resources as an important dietary component for Theropithecus in the discussion below.
Diet of Theropithecus in East Africa from 4 to 1 Ma.
The diet of the earliest Theropithecus, T. brumpti, in the Turkana Basin has a high component of C4-based resources. On average, this diet was between ca. 55% and 75% C4-based; even the most 13C-depleted specimen (KNM-ER 1566) has a δ13C value of −7.2‰, corresponding to a diet that is ca. 35–40‰ C4-based. Paleosol evidence from the Koobi Fora and Nachukui Formations from this time interval suggests a habitat that had 40–60% woody cover (30, 40). Stable isotope studies of modern soils in East Africa show that closed riparian forests [>80% woody cover; e.g., Tana River (30)] have negligible C4 biomass in the understory but that C4 plants are found in riparian woodlands [<80% woody cover (30)]. Paleogeographic reconstructions for this time interval (41) show that the proto-Omo River flowed through the region, and this river was likely accompanied by a narrow (hundreds of meters wide) riparian forest corridor but with grassy woodland (i.e., >40% woody cover) (definition in ref. 42) outside of the corridor. Thus, T. brumpti had a diet that was strongly skewed to C4-based resources, and Theropithecus could not have been restricted to riparian forests. Using mixing lines and mass balance relationships based on the relationship between soil carbon and woody cover (30), 60% woody cover would have soil δ13C contributions from C3 woody cover, C3 forbs and herbs, and C4 grasses or sedges of ca. 60%, 15%, and 25%, respectively. In a riparian forest, for which there is no paleosol evidence of >1% areal coverage on the timescales of paleosol formation (ca. 1,000 y for a single locality), 80% woody cover would correspond to soil δ13C contributions from C3 woody cover, C3 forbs and herbs, and C4 grasses or sedges of ca. 80%, 10%, and 10%, respectively (30). Thus, a true forest would have little of the dietary resources used by T. brumpti, and therefore, T. brumpti would have obtained its dietary resources from outside any narrow riparian forest corridor.
The later (ca. 2- to 1-Ma time interval) T. oswaldi had increasingly higher contributions of C4-based diet between 2 and 1 Ma and by 1 Ma, had a diet that was comprised essentially of 100% C4 resources. At 1 Ma, three specimens from Olorgesailie have an average δ13C value of +1.6‰; for comparison, modern warthogs (Phacochoerus aethiopicus) from Kenya have an average δ13C1750 value of 0.8 ± 1.2‰ (n = 41; values from ref. 43 corrected to 1750 as described in Methods). This difference of ca. 1‰ could be because of a difference in the isotope enrichment between the primate and suid species (i.e., a physiological difference in digestion processes), or it could be because of a real, but slight, dietary difference. The habitat in the upper part of the Koobi Fora and Nachukui Formations [Upper Burgi, Kay Behrensmeyer Site (KBS), and Okote Members] had less woody cover than the early periods: paleosol evidence suggests a woody cover between 20% and 40% for this time interval, which would be a wooded grassland using the United Nations Educational, Scientific, and Cultural Organization terminology for African vegetation (42). Using mixing lines and mass balance relationships (30), 20% woody cover would have soil δ13C contributions from C3 woody cover, C3 forbs and herbs, and C4 grasses or sedges of 20%, 30%, and 50%, respectively.
Overall, the environment throughout the 4- to 1-Ma time interval shows that the habitat became increasingly open: from grassy woodlands or shrublands to wooded grasslands or bushed grasslands. Throughout the 4- to 1-Ma period, most of the diet resources of both T. brumpti and T. oswaldi were predominantly C4-based, with average C4-based contributions of ca. 60% and 80%, respectively. The fraction of C4-based diet resources for T. brumpti is higher than previous interpretations, which implied a predominantly C3-based browsing diet for this species (5–7). The composition of the diet of T. brumpti has been a subject of speculation for decades, because the masticatory apparatus of the species is highly specialized for the ingestion of large objects and the requirements of a wide gape, especially in males (6). Underground storage organs of C4-based bulbous grasses and sedges (i.e., corms, rhizomes) may have been important to the species’ diet, which has been speculated for some contemporaneous hominins (44, 45). However, Theropithecus has higher δ13C values than modern African mole rats that feed extensively on underground storage organs (46), suggesting that underground storage organs alone were not sufficient for the extent of C4 use by Theropithecus.
Comparison with South African Theropithecus.
Theropithecus from East Africa has similar δ13C values to values previously reported for Theropithecus from South Africa (Fig. 2). Although T. brumpti does not occur outside of the Turkana Basin, members of the T. darti–T. oswaldi are represented at the South African Plio-Pleistocene cave sites of Makapansgat (T. darti), Swartkrans (T. oswaldi), and Gladysvale (T. oswaldi). Theropithecus co-occurs with diverse cercopithecoids—including several Parapapio species—and Australopithecus africanus (Makapansgat and Gladysvale), Paranthropus robustus (Swartkrans), and early Homo (Swartkrans and Gladysvale) (1, 47). Theropithecus consistently exhibits stable isotopic profiles—indicating a strongly C4-based diet composed mostly of grasses—whereas other cercopithecoids concentrated on a wide variety of C3 plant foods (21, 23, 47).
Comparison with East African Contemporary Hominins.
The earliest Theropithecus analyzed is a single M-fragment (KNM-ER 20441) that is ca. 4 Ma, and it is from the same site where Australopithecus anamensis is found. The four individual Au. anamensis have δ13C values that range from −10.0‰ to −11.6‰ (27), which represents a pure or nearly pure C3-derived diet. In contrast, this single Theropithecus individual has a δ13C value of −3.5‰, which indicates a high (>60%) reliance on C4 resources.
The later Au. afarensis and Kenyanthropus platyops, with ages between ca. 3.0 and 3.5 Ma, have a mixed C3/C4 diet, with tooth enamel δ13C values ranging from ca. −3‰ to −13‰ and averaging −7.5 ± 2.6‰ (n = 20) and −6.2 ± 2.7‰ (n = 20), respectively (26, 48). In contrast, the contemporaneous T. brumpti has a δ13C range of ca. −1‰ to −7‰, with an average of −3.5 ± 1.6‰ (n = 14), indicating a diet using a much higher fraction of C4 resources than the hominins of this age range.
P. boisei was contemporary to T. oswaldi. Both of these primates had δ13C values indicating that C4-based resources (26) were predominant in their respective diets: both had a ca. 25/75 ratio for C3- to C4-based diet resources. From the perspective of stable isotope analysis, P. boisei and T. oswaldi have similar diets (Fig. 2) and could have been competing for similar resources.
Several species of Homo overlap in time with T. oswaldi. The diet of Homo was consistently depleted in 13C compared with Theropithecus (26), with little overlap in the range of δ13C values. Although Homo consumed a mixed C3–C4 diet, its reliance on C4 resources was considerably less than Theropithecus.
In the several million years of overlap in time with hominins, Theropithecus consistently had the most positive δ13C values of any primate in East Africa except Paranthropus, where it has indistinguishable values. The earliest hominin to be compared, Au. anamensis at ca. 4 Ma, had a diet that was solely or almost solely based on C3 resources, whereas the contemporaneous Theropithecus had a C4-dominated diet. Of the hominins, only P. boisei had a diet with a reliance on C4 resources as high as a contemporaneous Theropithecus species.
Comparison with Modern Theropithecus and Papio.
Modern baboons and gelada monkeys are the closest relatives to the fossil T. brumpti and T. oswaldi. Today, a single species of Theropithecus is restricted to montane regions in Ethiopia; Papio is found throughout most of Africa. Modern baboons in Africa have δ13C1750 values that indicate a diet dominated by C3 diet resources (Fig. 2), but some baboons show a component of C4-based resources in their diets. Modern primates often have a small C4 component to their diet, which can be obtained from stripping of seeds from mature grasses or digging for grass rhizomes (Fig. 3). Modern T. gelada, now living only in the Ethiopian Highlands above ca. 3 km elevation, has an almost exclusive C3 grass diet (49, 50); C4 grasses are rarely present above 3 km elevation (51) because of the cooler temperatures. Thus, modern geladas are grazers, but their diets are very distinct from their fossil relatives: modern Theropithecus has a C3 grass diet, whereas fossil Theropithecus had a C4-derived diet, likely C4 grasses. There are significant anatomical differences between C3 and C4 grasses (e.g., proteins are protected by the bundle sheath cells in C4 grasses) (52), but it is not known if this feature makes a significant difference in digestibility of grasses by mammals.
Fig. 3.
(A) Example of C4 grass (Cynodon sp.) with seeds that are used seasonally by primates (vervet monkeys and baboons) in Samburu Reserve, Kenya. (Left) Before handling. (Right) After seeds have been stripped. (B) Common baboons (P. anubis) digging C4 grass rhizomes in Samburu Reserve, Kenya. (C) T. gelada in C3 grassland of the Simien Mountains, Ethiopia. Photograph by George Chaplin.
Paleoenvironmental Considerations.
Paleotemperature reconstructions of the fossil habitat of Theropithecus in the Turkana basin are based on the Δ47-clumped thermometer (53); the results of that study suggest that soil temperatures and therefore, mean annual temperatures in the Turkana Basin from 4 to 1 Ma were similar to the analogous temperatures of today. The modern mean annual temperature is ca. 30 °C, which is at the hot extreme of global mean annual temperatures.
Conclusions
Theropithecus was a common and ecologically significant large-bodied primate in East Africa from 4 to 1 Ma. Stable isotope evidence shows that the early T. brumpti had a diet that was dominated by C4 plants, presumably grasses or sedges, which made up ca. 65% of its diet between 4 and 2.5 Ma. This interpretation contrasts with earlier reconstructions of T. brumpti as a forest-dwelling creature that derived all, or most, of its resources from the forest. The later T. oswaldi had an even higher percentage of C4-derived resources, comprising virtually 100% C4 by 1 Ma. The overall diet trend of T. brumpti to T. oswaldi is from an earlier diet, where C4 resources were dominant, to the later diet, which was comprised almost exclusively of C4-derived resources. Theropithecus is ecologically and evolutionarily significant, because it is the only primate genus to have occupied a grass-eating niche throughout its history. During the Pliocene, Theropithecus species competed successfully with ungulates in environments increasingly dominated by C4 grasses. It is likely that several factors may have contributed to the eventual extinction of T. oswaldi (54, 55); the most fundamental of these factors was the species’ inability to survive amid hooved ruminant competitors in the grasslands of the Pleistocene (28) while competing for forage resources with highly variable nutritional qualities through the seasonal cycle. C4 grasses have undergone major expansion in tropical ecosystems over the past 10 million y (14), beginning at ca. <1% NPP and now contributing >60% NPP in tropical savannas (31, 32). The C4 clades of grasses underwent significant evolution during this time (56), although there is almost no macrofossil record of C4 grasses or their evolution. During these millions of years of evolution, C4 plants evolved defenses, and likewise, their primary consumers evolved strategies to overcome these defenses. The competition between the various ungulates and between ungulates and other grazers, such as Theropithecus, is part of that evolutionary story.
Methods
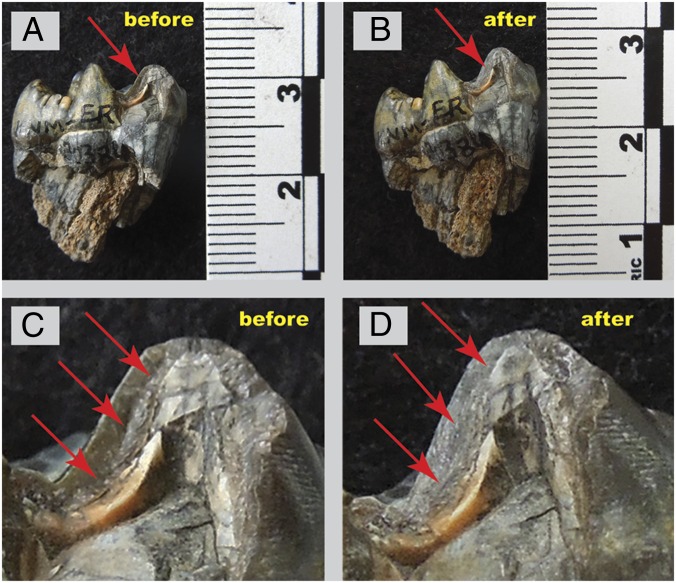
Samples were obtained from the National Museums of Kenya; 41 specimens were from the Koobi Fora and Nachukui Formations in northern Kenya, and 3 specimens were from the Olorgesailie Formation in southern Kenya. Of these specimens, two teeth were sampled from one of the specimens (KNM-ER 3775). Theropithecus enamel from broken tooth surfaces was sampled, and therefore, information concerning microwear was not compromised (Fig. 4); approximately 1–5 mg powder were obtained using a high-speed rotary drill. Powdered samples were treated with 0.1 M buffered acetic acid for 30 min to remove secondary carbonates; this process leads to a significant sample loss (ca. 0.5 to >1 mg per sample) but is needed to remove contamination (26).
Fig. 4.
Example of sampling of KNM-ER 30384. (A) Before sampling (arrow shows region to be sampled). (B) Same view as A but after the sample was collected. (C) Enlargement of A with enamel to be sampled (arrows show region to be sampled). (D) Same view as C but after the sample was collected.
Samples (200–500 μg) were reacted with phosphoric acid (57) at 90 °C in silver capsules and analyzed on an isotope ratio mass spectrometer after cryogenic separation of CO2; results are reported using the standard permil (‰) notation, with Vienna–Pee Dee Belemnite as the standard for both oxygen and carbon isotope measurements. Corrections for temperature-dependent isotope fractionation in oxygen were made using modern and fossil internal reference materials that had been reacted at 25 °C and 90 °C (58). For comparative purposes, modern mammals have had their δ13C values adjusted to compensate for recent changes in atmospheric δ13C values (17, 59, 60); such δ13C values are reported as δ13C1750.
Baboon fecal material was used to compare diets of preadult and adult primates; these data can evaluate the issue of diet recorded in tooth enamel, which forms in preadults. Final tooth eruption in baboons occurs at about 7 y (29); therefore, we compare diets of preadults (>2 and <7 y) with adult (>7 y) diet. Samples were collected over a ca. 3-wk period from two distinct baboon groups as part of the long-running Amboseli Baboon Research Project. Samples were dried at 105 °C and analyzed for δ13C after combustion in an elemental analyzer in series with an isotope ratio mass spectrometer operating in continuous-flow mode. δ13C values are reported relative to Vienna–Pee Dee Belemnite.
We use the δ13C value of −26‰ for a pure C3 diet and the δ13C value of −12‰ for a pure C4-based diet to estimate the nominal fraction of the C4 component to the diet of these primates. The isotope enrichment for diet–enamel in primates has not been established but is likely between 12‰ and 15‰ based on comparison with other large mammals (17, 61); using an isotope enrichment of 14‰, these nominal values for C3 and C4 plants give enamel values of −12.4‰ and + 1.8‰ for pure C3- and pure C4-based diets. These values are compatible with δ13C values of sympatric browsers (deinotheres and giraffes) and grazers (equids and suids) from the fossil record in the Turkana Basin and the atmosphere-corrected δ13C values of modern browsers (bovids and giraffes) and grazers (bovids, equids, and suids) from eastern Africa (17–19, 26, 43, 62).
Supplementary Material
Acknowledgments
We thank the government of Kenya for permission to do this research. We thank the field crew of the Koobi Fora Research Project (1969–2012), whose members discovered many of the specimens analyzed in this study. We also thank Frank Brown and Mbaluka Kimeu for grass identifications and discussions, Jeanne Altmann and Susan Alberts for access to the Amboseli material, and Naomi Levin for assistance in the laboratory. This material is based on work supported by National Science Foundation Grant BCS-0621542. The Amboseli Baboon Research Project data were obtained primarily with support from National Science Foundation Grants IOB-0322613, IOB-0322781, BCS-0323553, and BCS-0323596.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 10470.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222571110/-/DCSupplemental.
References
- 1.Jablonski NG, Frost S. Cercopithecoidea. In: Werdelin L, Sanders WJ, editors. Cenozoic Mammals of Africa. Berkeley, CA: University of California; 2010. pp. 393–428. [Google Scholar]
- 2.Jolly CJ. The seed-eaters: A new model of hominid differentiation based on a baboon analogy. Man (Lond) 1970;5:5–26. [Google Scholar]
- 3.Jablonski NG, Leakey MG, Anton M. Systematic paleontology of the cercopithecines. In: Jablonski NG, Leakey MG, editors. Koobi Fora Research Project: The Fossil Monkeys. Vol 6. San Francisco, CA: California Academy of Sciences; 2008. pp. 103–300. [Google Scholar]
- 4.Eck GG. Diversity and frequency distribution of Omo Group Cercopithecoidea. J Hum Evol. 1977;6:55–63. [Google Scholar]
- 5. Eck GG, Jablonski NG (1987) The skull of Theropithecus brumpti as compared with those of other species of the genus Theropithecus. Les Faunes Plio-Pleistocenes de la Vallee de l'Omo (Ethiopie), Cahiers de Paleontologie, eds Coppens Y, Beden M, Eck GG (Ed. du Centre National de la Recherche Scientifique, Paris), Vol 3, pp 12–122.
- 6.Jablonski NG. The evolution of the masticatory apparatus in Theropithecus. In: Jablonski NG, editor. Theropithecus: The Rise and Fall of a Primate Genus. Cambridge, United Kingdom: Cambridge Univ Press; 1993. pp. 299–329. [Google Scholar]
- 7.Benefit BR, McCrossin ML. Diet, species diversity and distribution of African fossil baboons. Pap Kroeber Anthropol Soc. 1990;71–72:77–93. [Google Scholar]
- 8.Bobe R, Behrensmeyer AK. The expansion of grassland ecosystems in Africa in relation to mammalian evolution and the origin of the genus Homo. Palaeogeogr Palaeoclimatol Palaeoecol. 2004;207:399–420. [Google Scholar]
- 9.Jablonski NG. The hand of Theropithecus brumpti. In: Else JG, Lee PC, editors. Primate Evolution: Selected Proceedings of the Tenth Congress of the International Primatological Society. Vol 1. Cambridge, United Kingdom: Cambridge Univ Press; 1986. pp. 173–182. [Google Scholar]
- 10.Jablonski NG, Leakey MG, Kiarie C, Antón M. A new skeleton of Theropithecus brumpti (Primates: Cercopithecidae) from Lomekwi, West Turkana, Kenya. J Hum Evol. 2002;43(6):887–923. doi: 10.1006/jhev.2002.0607. [DOI] [PubMed] [Google Scholar]
- 11.Jablonski NG, Leakey MG. The importance of the Cercopithicoidea from the Koobi Fora formation in the context of primate and mammalian evolution. In: Jablonski NG, Leakey MG, editors. Koobi Fora Research Project: The Fossil Monkeys. Vol 6. San Francisco, CA: California Academy of Sciences; 2008. pp. 397–416. [Google Scholar]
- 12.Jablonski NG. Convergent evolution in the dentitions of grazing macropodine marsupials and the grass-eating cercopithecine primate Theropithecus gelada. J R Soc West Aust. 1994;77:37–43. [Google Scholar]
- 13.Mau M, Johann A, Sliwa A, Hummel J, Südekum KH. Morphological and physiological aspects of digestive processes in the graminivorous primate Theropithecus gelada-a preliminary study. Am J Primatol. 2011;73(5):449–457. doi: 10.1002/ajp.20921. [DOI] [PubMed] [Google Scholar]
- 14.Cerling TE, et al. Global change through the Miocene/Pliocene boundary. Nature. 1997;389:153–158. [Google Scholar]
- 15.Uno KT, et al. Late Miocene to Pliocene carbon isotope record of differential diet change among East African herbivores. Proc Natl Acad Sci USA. 2011;108(16):6509–6514. doi: 10.1073/pnas.1018435108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee-Thorp JA, van der Merwe NJ. Carbon isotope analysis of fossil bone apatite. S Afr J Sci. 1987;83:712–715. [Google Scholar]
- 17.Cerling TE, Harris JM. Carbon isotope fractionation between diet and bioapatite in ungulate mammals and implications for ecological and paleoecological studies. Oecologia. 1999;120:347–363. doi: 10.1007/s004420050868. [DOI] [PubMed] [Google Scholar]
- 18.Cerling TE, Harris JM, Passey BH. Dietary preferences of East African Bovidae based on stable isotope analysis. J Mammal. 2003;84:456–471. [Google Scholar]
- 19.Sponheimer M, et al. Diets of Southern African bovidae: Stable isotope evidence. J Mammal. 2003;84:471–479. [Google Scholar]
- 20.Cerling TE, Hart JA, Hart TB. Stable isotope ecology in the Ituri Forest. Oecologia. 2004;138(1):5–12. doi: 10.1007/s00442-003-1375-4. [DOI] [PubMed] [Google Scholar]
- 21.Lee-Thorp JA, van der Merwe NJ, Brain CK. Isotopic evidence for dietary differences between two extinct baboon species from Swartkrans. J Hum Evol. 1989;18:183–189. [Google Scholar]
- 22.Sponheimer M, Lee-Thorp JA. Isotopic evidence for the diet of an early hominid, Australopithecus africanus. Science. 1999;283(5400):368–370. doi: 10.1126/science.283.5400.368. [DOI] [PubMed] [Google Scholar]
- 23.Codron D, et al. Utilization of savanna-based resources by Plio-Pleistocene baboons. S Afr J Sci. 2005;101:245–248. [Google Scholar]
- 24.van der Merwe NJ, Masao FT, Bamford MK. Isotopic evidence for contrasting diets of early hominins Homo habilis and Australopithecus boisei of Tanzania. S Afr J Sci. 2008;104:153–155. [Google Scholar]
- 25.White TD, et al. Macrovertebrate paleontology and the Pliocene habitat of Ardipithecus ramidus. Science. 2009;326(5949):87–93. [PubMed] [Google Scholar]
- 26.Cerling TE, et al. Diet of Paranthropus boisei in the early Pleistocene of East Africa. Proc Natl Acad Sci USA. 2011;108(23):9337–9341. doi: 10.1073/pnas.1104627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao L-X, Zhang L-Z, Zhang F-S, Wu X-Z. Enamel carbon isotope evidence of diet and habitat of Gigantopithecus blacki and associated mammalian megafauna in the Early Pleistocene of South China. Chin Sci Bull. 2011;56:3590–3595. [Google Scholar]
- 28.Cerling TE, et al. Stable isotope-based diet reconstructions of Turkana Basin hominins. Proc Natl Acad Sci USA. 2013;110:10501–10506. doi: 10.1073/pnas.1222568110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galbany J, Altmann J, Pérez-Pérez A, Alberts SC. Age and individual foraging behavior predict tooth wear in Amboseli baboons. Am J Phys Anthropol. 2011;144(1):51–59. doi: 10.1002/ajpa.21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cerling TE, et al. Woody cover and hominin environments in the past 6 million years. Nature. 2011;476(7358):51–56. doi: 10.1038/nature10306. [DOI] [PubMed] [Google Scholar]
- 31.Still CJ, Berry JA, Collatz GJ, DeFries RS. Global distribution of C3 and C4 vegetation: Carbon cycle implications. Global Biogeochem Cycles. 2003;17(1):1006. [Google Scholar]
- 32.Lloyd J, et al. Contributions of woody and herbaceous vegetation to tropical savanna ecosystem productivity: A quasi-global estimate. Tree Physiol. 2008;28(3):451–468. doi: 10.1093/treephys/28.3.451. [DOI] [PubMed] [Google Scholar]
- 33.Peters CR, Vogel JC. Africa’s wild C4 plant foods and possible early hominid diets. J Hum Evol. 2005;48(3):219–236. doi: 10.1016/j.jhevol.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Mooney HA, Troughton JH, Berry JA. Carbon isotope ratio measurements of succulent plant in Southern Africa. Oecologia. 1977;30:295–305. doi: 10.1007/BF00399762. [DOI] [PubMed] [Google Scholar]
- 35.DeNiro MJ, Epstein S. Influence of diet on distribution of carbon isotopes in animals. Geochim Cosmochim Acta. 1978;42:495–506. [Google Scholar]
- 36.Dunbar RIM. Feeding ecology of gelada baboons: A preliminary report. In: Clutton-Brock TH, editor. Primate Ecology: Studies of Feeding and Ranging Behavior in Lemurs, Monkeys and Apes. London: Academic; 1977. pp. 251–273. [Google Scholar]
- 37.Iwamoto T. Studies of the Gelada Society (III). Contemp Primatol 5th Int Congr Primat (Karger, Basel, Switzerland) 1974. Food resource and the feeding activity; pp. 475–480. [Google Scholar]
- 38.Deines P. The isotopic composition of reduced organic carbon. In: Fritz P, Fontes JB, editors. Handbook of Environmental Geochemistry. Vol 1. Amsterdam: Elsevier; 1980. pp. 329–406. [Google Scholar]
- 39.Popp BN, et al. Effect of phytoplankton cell geometry on carbon isotopic fractionation. Geochim Cosmochim Acta. 1998;62:69–77. [Google Scholar]
- 40.Levin NE, Brown FH, Behrensmeyer AK, Bobe R, Cerling TE. Paleosol carbonates from the Omo Group: Isotopic records of local and regional environmental change in East Africa. Palaeogeogr Palaeoecol Palaeoclim. 2011;307:75–89. [Google Scholar]
- 41.Feibel CS, Harris JM, Brown FH. Paleoenvironmental context for the Late Neogene of the Turkana Basin. In: Harris JM, editor. Koobi Fora Research Project. Vol 3. Oxford: Clarendon; 1991. pp. 321–370. [Google Scholar]
- 42.White F. The Vegetation of Africa. Vol 20. Paris: United Nations Scientific and Cultural Organization; 1983. [Google Scholar]
- 43.Harris JM, Cerling TE. Dietary adaptations of extant and Neogene African suids. J Zool. 2002;256:45–54. [Google Scholar]
- 44.Lee-Thorp JA, Sponheimer M, van der Merwe NJ. What do stable isotopes tell us about hominid dietary and ecological niches in the pliocene? Int J Osteoarchaeol. 2003;13(1-2):104–113. [Google Scholar]
- 45.Ungar PS, Sponheimer M. The diets of early hominins. Science. 2011;334(6053):190–193. doi: 10.1126/science.1207701. [DOI] [PubMed] [Google Scholar]
- 46.Yeakel JD, Bennett NC, Koch PL, Dominy NJ. The isotopic ecology of African mole rats informs hypotheses on the evolution of human diet. Proc Biol Sci. 2007;274(1619):1723–1730. doi: 10.1098/rspb.2007.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Zaatari S, Grine FE, Teaford MF, Smith HF. Molar microwear and dietary reconstructions of fossil cercopithecoidea from the Plio-Pleistocene deposits of South Africa. J Hum Evol. 2005;49(2):180–205. doi: 10.1016/j.jhevol.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Wynn JG, et al. Diet of Australopithecus afarensis from the Pliocene Hadar Formation, Ethiopia. Proc Natl Acad Sci USA. 2013;110:10495–10500. doi: 10.1073/pnas.1222559110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dunbar RIM. Australopithecuine diet based on a baboon analogy. J Hum Evol. 1976;5:161–167. [Google Scholar]
- 50.Dunbar RIM, Bose U. Adaptatin to grass-eating in gelada baboons. Primates. 1991;32:1–7. [Google Scholar]
- 51.Tieszen LL, Senyimba MM, Imbama SK, Troughton JH. Distribution of C3-grass and C4-grass and carbon isotope discrimination along an altitudinal and moisture gradient in Kenya. Oecologia. 1979;37:337–350. doi: 10.1007/BF00347910. [DOI] [PubMed] [Google Scholar]
- 52.Ehleringer JR, Cerling TE, Helliker B. C4 photosynthesis, atmospheric CO2, and climate. Oecologia. 1997;112:285–299. doi: 10.1007/s004420050311. [DOI] [PubMed] [Google Scholar]
- 53.Passey BH, Levin NE, Cerling TE, Brown FH, Eiler JM. High-temperature environments of human evolution in East Africa based on bond ordering in paleosol carbonates. Proc Natl Acad Sci USA. 2010;107(25):11245–11249. doi: 10.1073/pnas.1001824107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dunbar RIM. Socioecology of the extinct theropiths: A modelling approach. In: Jablonski NG, editor. Theropithecus: The Rise and Fall of a Primate Genus. Cambridge, United Kingdom: Cambridge Univ Press; 1993. pp. 465–486. [Google Scholar]
- 55.Lee PC, Foley RA. Ecological energetics and extinction of giant gelada baboons. In: Jablonski NG, editor. Theropithecus: The Rise and Fall of a Primate Genus. Cambridge, United Kingdom: Cambridge Univ Press; 1993. pp. 487–498. [Google Scholar]
- 56.Edwards EJ, et al. The origins of C4 grasslands: Integrating evolutionary and ecosystem science. Science. 2010;328(5978):587–591. doi: 10.1126/science.1177216. [DOI] [PubMed] [Google Scholar]
- 57.McCrea JM. On the isotopic chemistry of carbonates and a paleotemperature scale. J Chem Phys. 1950;18:849–853. [Google Scholar]
- 58.Passey BH, Cerling TE, Levin NE. Temperature dependence of oxygen isotope acid fractionation for modern and fossil tooth enamels. Rapid Commun Mass Spectrom. 2007;21(17):2853–2859. doi: 10.1002/rcm.3149. [DOI] [PubMed] [Google Scholar]
- 59.Francey RJ, et al. A 1000-year high precision record of d13C in atmospheric CO2. Tellus B Chem Phys Meteorol. 1999;51:170–193. [Google Scholar]
- 60.Keeling RF, Piper SC, Bollenbacher AF, Walker SJ. Trends: A Compendium of Data on Global Change. Oak Ridge, TN: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy; 2010. Monthly atmospheric 13C/12C isotopic ratios for 11 SIO stations. [Google Scholar]
- 61.Passey BH, et al. Carbon isotopic fractionation between diet, breath, and bioapatite in different mammals. J Archaeol Sci. 2005;32:1459–1470. [Google Scholar]
- 62.Levin NE, et al. Herbivore enamel carbon isotopic composition and the environmental context of Ardipithecus at Gona, Ethiopia. Spec Pap Geol Soc Am. 2008;446:215–234. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.