Abstract
Patterning of the vertebrate skeleton requires the coordinated activity of Hox genes. In particular, Hox10 proteins are essential to set the transition from thoracic to lumbar vertebrae because of their rib-repressing activity. In snakes, however, the thoracic region extends well into Hox10-expressing areas of the embryo, suggesting that these proteins are unable to block rib formation. Here, we show that this is not a result of the loss of rib-repressing properties by the snake proteins, but rather to a single base pair change in a Hox/Paired box (Pax)-responsive enhancer, which prevents the binding of Hox proteins. This polymorphism is also found in Paenungulata, such as elephants and manatees, which have extended rib cages. In vivo, this modified enhancer failed to respond to Hox10 activity, supporting its role in the extension of rib cages. In contrast, the enhancer could still interact with Hoxb6 and Pax3 to promote rib formation. These results suggest that a polymorphism in the Hox/Pax-responsive enhancer may have played a role in the evolution of the vertebrate spine by differently modulating its response to rib-suppressing and rib-promoting Hox proteins.
Keywords: axial skeleton, gene regulation, vertebrate patterning
Although the total number of vertebrae in the axial skeleton, as well as how they are distributed among the different anatomical groups, can vary widely in vertebrates, both features are remarkably stable within a given species. Hox genes are important components of the genetic networks controlling the vertebral patterns of the axial skeleton (1–3). For instance, the activity of genes of the Hox paralogue group 10 (Hox10) controls key processes in somitic patterning leading to the inhibition of rib development. The inactivation of all Hox10 functions in the mouse indeed results in the transformation of the ribless lumbar area into a posterior extension of the thorax, as defined by the presence of ectopic ribs (4). Conversely, premature expression of Hoxa10 in the paraxial mesoderm prevents rib development from the prospective thoracic area to produce completely ribless embryos (5).
Recent expression analyses of Hox10 genes in snakes challenged the incompatibility between Hox10 expression and the presence of ribs (6, 7). In particular, the expression territories of Hoxa10 and Hoxc10 in snake embryos extend anteriorly into somites that produce rib-bearing vertebrae. This suggested either that the snake Hoxa10 and Hoxc10 proteins have lost their rib-inhibiting properties or, alternatively, that the molecular network downstream of the Hox10 proteins generates a different morphological outcome. In this paper, we explore the origin of the apparent inability of Hoxa10 to block rib formation in the snake. We show that the snake Hoxa10 is able to block rib formation when tested in the mouse, indicating that rib-repressing properties are still present in the snake protein. When we analyzed the networks downstream of Hoxa10, however, we found that the snake genome contains a polymorphism in a Hox/Pax-responsive enhancer that is involved in Hox-mediated regulation of rib formation (8). Interestingly, we found that this polymorphism is also present in Paenungulata, which have extended rib cages. We performed biochemical and functional analyses of this enhancer and, altogether, our results are compatible with its involvement in the development of extended rib cages in snakes and Paenungulata.
Results and Discussion
Snake Hoxa10 Function in Mouse.
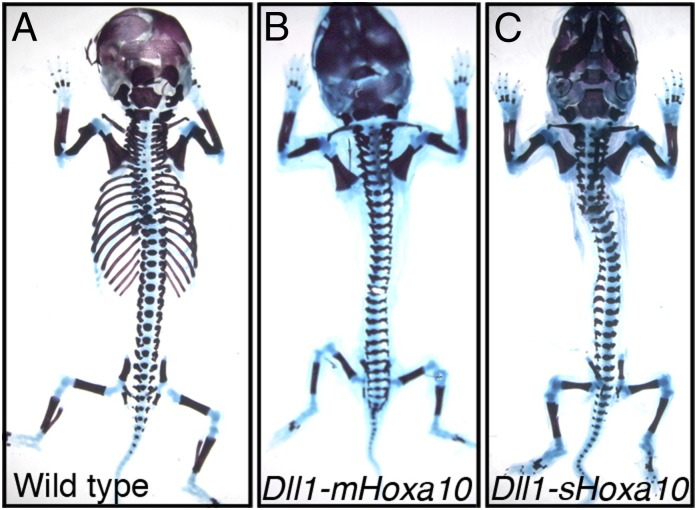
We tested the rib-repressing activity of snake Hoxa10 by using a transgenic approach in mice (5). Among nine transgenic embryos analyzed, three had rib phenotypes of various strengths. Noteworthy, one of them was completely ribless (Fig. 1C), whereas a second contained just a few remaining rib fragments, mostly comprising the cartilaginous portion. These results showed that the snake Hoxa10 protein has the intrinsic capacity of blocking rib formation, although with a slightly weaker efficiency than its mouse counterpart (5). This raised the question how Hoxa10 expression is compatible with rib development in snakes.
Fig. 1.
The snake Hoxa10 repress rib formation. The skeletons of WT (A) or transgenic fetuses expressing the mouse Hoxa10 (B) or the snake Hoxa10 (C) were analyzed at E18.5. Both versions of Hoxa10 were active in repressing rib formation from the thoracic area.
Identification of Polymorphism in Hox-Regulated Enhancer of Myf5 in Snakes.
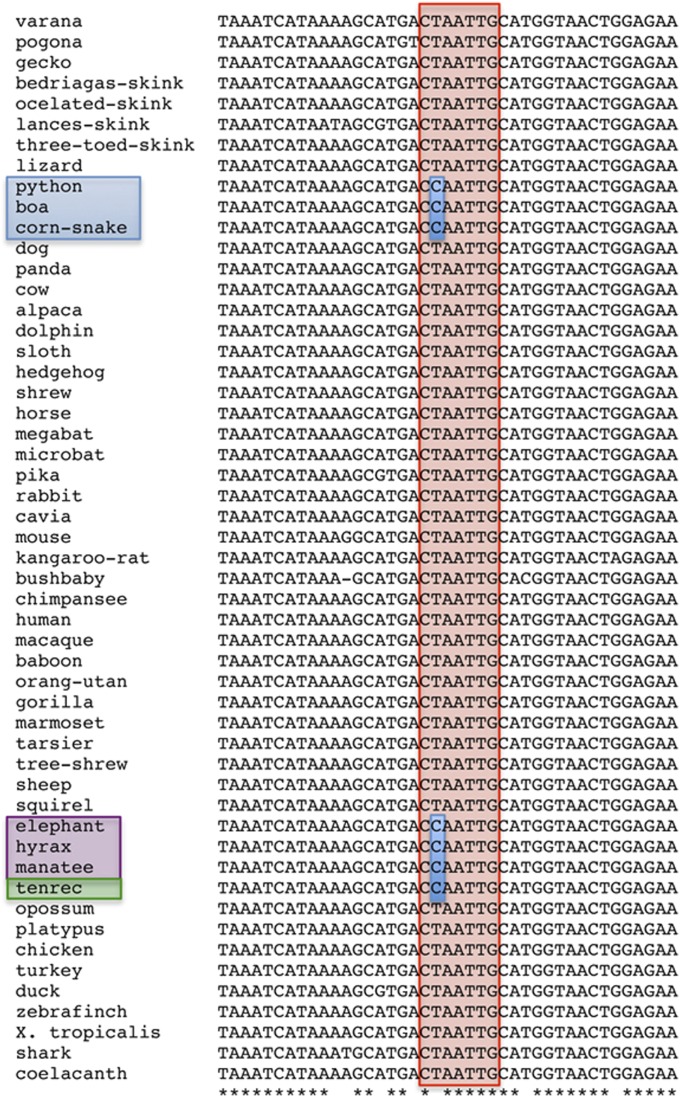
The mechanism whereby Hox genes control development of rib-bearing and ribless regions of the axial skeleton involves the coordinated regulation of Myogenic factor (Myf) 5 and Myf6 expression in the hypaxial myotome (8). This modulation is driven, at least in part, through interactions with the Homology region 1 (H1) enhancer, located 58 kb upstream of the Myf5 gene (8, 9). In the mouse, this enhancer contains a functional core with near-overlapping binding sites for Pax3, Hox6/10, and Six1/4 proteins (9–11). Pax3, Six1/4, and rib-promoting Hox proteins (like Hoxb6) activate this enhancer (8–11), whereas rib-suppressing Hox proteins (like Hoxa10) block its activity (8). To test whether the H1 enhancer is conserved in snakes, we cloned and sequenced the orthologous region from the corn snake genome (Pantherophis guttata). The snake H1 enhancer showed high overall conservation compared with the orthologous mouse sequence (Fig. S1). However, it displayed a single base pair change at the Hox binding site (CCAATTG) when aligned with the CTAATTG sequence present in the mouse (Fig. 2 and Fig. S1). To assess the relevance of this polymorphism and its potential to interfere with the binding of Hox proteins, we first compared the sequences of the H1 enhancer in a wide variety of vertebrates obtained from public databases or from amplified genomic DNA (Fig. 2). This comparison revealed that, in most vertebrates, the Hox binding site was preserved. However, all three snake species analyzed (corn snake, python, and boa) contained the CCAATTG variant at the equivalent position. Of note, we observed the same polymorphism in some afrotherian mammals, including tenrecs, elephants, hyrax, and manatees (Fig. 2). Interestingly, the latter three species belong to the Paenungulata taxon, characterized by their long ribcages composed of 19 or more thoracic vertebrae (12, 13), i.e., a higher number than what is found in most other mammals. The presence of the same polymorphism within the Hox binding site of the H1 enhancer in different vertebrates with extended thoracic regions raised the hypothesis that this variation may had played a role in the evolution of their vertebral formula.
Fig. 2.
Comparison of the central region of the H1 enhancer from a variety of vertebrate species. Indicated in red is the location of the Hox binding site. Highlighted in blue is the polymorphism observed in snakes (blue box), tenrecs (green box), and animals of the Paenungulata taxon (purple box).
Polymorphism Present in Snake-Like H1 Enhancer Affects Its Activity in Vivo.
We next analyzed the impact of this polymorphism upon the regulatory activity of the H1 enhancer. We performed transgenic bacterial artificial chromosome (BAC) reporter assays by using constructs derived from BAC-195AP/Z, which contains the β-gal reporter introduced into the mouse Myf5 locus (14). In transgenic mice, this BAC could reproduce the endogenous Myf5 expression pattern (14), including the muscular progenitors of the limbs, the epaxial myotome of all somites and the hypaxial myotome of interlimb territories, but not of limb-level somites (Fig. 3 A and A′ and Fig. S2A). We assessed whether the Hox binding site in H1, which is required to activate the enhancer when analyzed in isolation by using a conventional transgenic reporter approach (9), was also required in a more physiological Myf5/Myf6 genomic context. We thus produced the [ΔHox]-H1-BAC, in which the CTAATTG sequence was replaced by CGCGCTG. Whereas [ΔHox]-H1-BAC transgenic embryos displayed reporter expression in the epaxial myotome, they showed a significant down-regulation of β-gal activity in the hypaxial myotome of interlimb somites and in muscle precursors of the limb buds (Fig. S2B). This result indicated that, in this physiological context, the Hox binding site within H1 is also important for Myf5 expression in the hypaxial mesoderm. However, this reduction was not complete, indicating that other regions within the BAC may influence hypaxial Myf5 expression, as previously suggested (10).
Fig. 3.
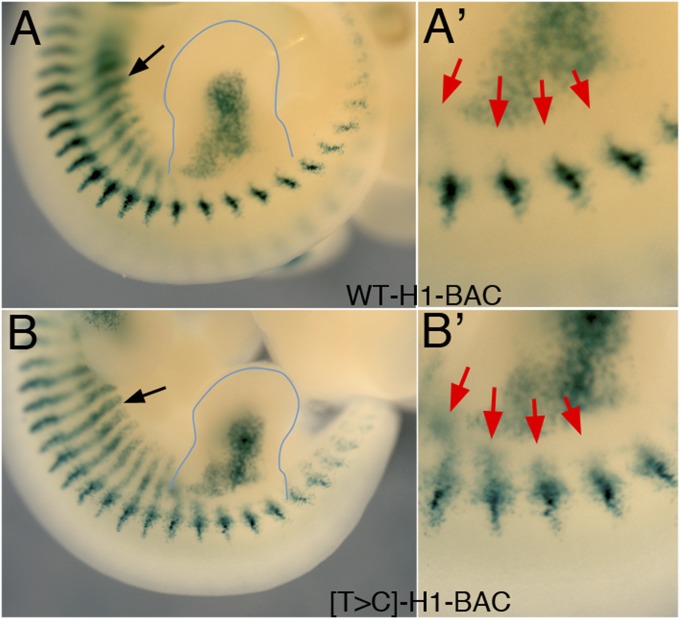
Effect of the snake-associated polymorphism on the activity of the Myf5 H1 enhancer. Images show the hindlimb area of E11.5 transgenic embryos for the WT H1-BAC (A and A′) or the [T>C]-H1-BAC (B and B′), stained for β-gal activity. The shape of the hindlimb bud is highlighted for the sake of clarity. The black arrows indicate expression in the interlimb hypaxial myotome. A′ and B′ show enlargements of proximal hindlimb area of embryos in A and B. Red arrows indicate the ventrally extended β-gal staining in the hindlimb-associated somites of the [T>C]-H1-BAC transgenic embryo, which is not observed in the control H1-BAC transgenic embryo.
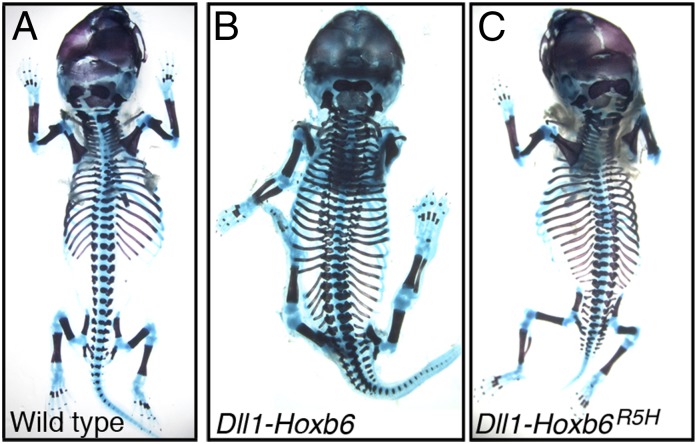
BAC transgenics carrying the snake polymorphism in H1 ([T>C]-H1-BAC) displayed β-gal activity in the hypaxial myotome and limb buds (Fig. 3B and Fig. S2C). However, hypaxial β-gal expression was not restricted to interlimb somites, but extended into the somites at the hindlimb level (Fig. 3B′), which normally develop into the lumbar vertebrae. As such, it resembled the Myf5 up-regulation associated with extra ribs in Dll1-Hoxb6 transgenics (8). This suggested that the polymorphism in H1 still allowed enhancer activation, yet it made it considerably less sensitive to its inhibition by Hox10 proteins, an observation consistent with the extended rib cages in snakes and Paenungulata species. This result also implied that the actions of rib-promoting (e.g., Hoxb6) and rib-suppressing (e.g., Hoxa10) Hox proteins over the [T>C]-H1 enhancer are mediated by distinct mechanisms.
Differential Interaction of Hoxa10 and Hoxb6 with [T>C]-H1 Enhancer.
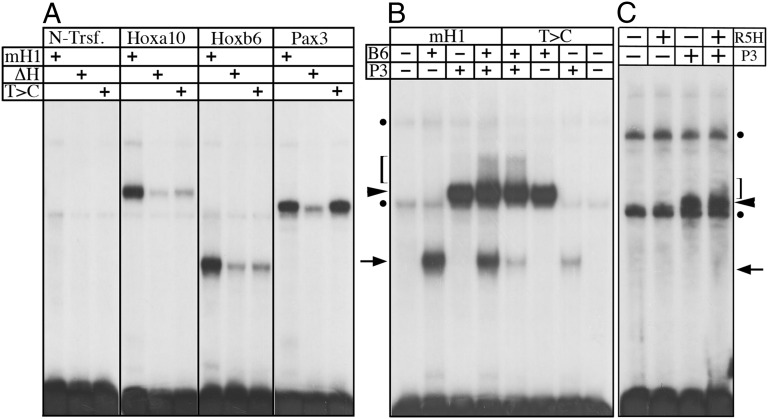
We explored this question and characterized the binding of the Hoxa10, Hoxb6, Pax3, and Six1 proteins to various versions of H1, containing the mouse sequence [mouse H1 (mH1)], the snake polymorphism, or the inactivated Hox binding site. All four proteins bound mH1 (Fig. 4A and Fig. S3A). Although [ΔHox]-H1 was also bound by Six1 (Fig. S3A), the binding of Hoxa10, Hoxb6, and Pax3 was strongly reduced (Fig. 4A). On the contrary, [T>C]-H1 was bound by Pax3 and Six1, with affinities similar to those observed for mH1 (Fig. 4A and Fig. S3A). However, the binding of Hoxb6 or Hoxa10 to this element was reduced to levels similar to those observed with [ΔHox]-H1 (Fig. 4A). These results confirmed that the snake polymorphism had destroyed the Hox binding site, which is consistent with the inability of Hoxa10 to repress expression through the snake enhancer. However, this conclusion is admittedly at odds with the rather normal hypaxial β-gal activation in the interlimb somites of [T>C]-H1-BAC transgenics, which supposedly requires Hox6 activity.
Fig. 4.
Binding of Pax3 and Hox proteins to the different Myf5 H1 enhancers. DNA binding was analyzed by EMSAs. (A) Binding of Hoxa10, Hoxb6, and Pax3 to mH1, snake (T>C), or an inactivated Hox binding site (ΔH). A control with nuclear extracts from nontransfected cells (N-Trsf.) is shown. (B) Interaction between Hoxb6 (B6) and Pax3 (P3) in the context of the mH1 and T>C enhancers. (C) Binding of the Hoxb6R5H mutant protein (R5H) to the mH1 enhancer and its interaction with Pax3 in this context. In B and C, arrows indicate positions of Hoxb6 complex, arrowheads indicate the Pax3 complex, and brackets indicate location of smeared signal corresponding to Pax3/Hoxb6 interaction. Dots indicate background bands produced by the nuclear extracts.
A possible explanation for this discrepancy is that Pax3 and Six1/4 could activate Myf5 via the H1 enhancer without any involvement of Hox proteins. Alternatively, the activity of rib-promoting and rib-suppressing Hox proteins upon this enhancer might involve distinct mechanisms, which could be affected differently by the snake-associated polymorphism. In H1, the Pax and Hox binding sites overlap (Fig. S1), and previous reports highlighted functional interactions between these two proteins (15, 16). Consequently, we looked at Hox/Pax interactions in the context of H1. When Hoxb6 and Pax3 proteins were mixed with mH1, the mobility shift pattern revealed an extra, slow-migrating smeared signal, which was not observed when either of the two factors was used in isolation (Fig. 4B). Immunodepletion of Pax3 or Hoxb6 confirmed the requirement of both proteins for the formation of this supernumerary complex (Fig. S3B). When Hoxa10 was used in a similar experiment, bands corresponding to Hoxa10 or Pax3 were observed, without any evidence for an additional complex (Fig. S4), suggesting that, at least under these conditions, Hoxb6 and Hoxa10 interact differently with the H1 enhancer, in the presence of Pax3. Interestingly, the slow-migrating complex produced by Pax3 and Hoxb6 was also present in retardation assays when using the [T>C]-H1 enhancer, with an intensity equivalent to that observed when the mH1 was used. Altogether, these data indicated that Hoxb6 may interact with the H1 enhancer in two ways, either through direct contact with the Hox binding site (leading to the Hoxb6-specific band), or indirectly via its interaction with Pax3 (producing the additional signal). Conversely, Hoxa10 may contact H1 exclusively through a direct interaction with the Hox binding site. Considering the β-gal activity observed in the [T>C]-H1-BAC transgenic animals, we hypothesized that the rib-promoting activity of Hoxb6 does not require a direct interaction with H1 but instead depends on an indirect association with the enhancer through Pax3.
Hoxb6 Can Induce Rib Formation Independently of Direct DNA Binding.
To assess whether Hoxb6 can promote rib formation in the absence of direct binding to DNA, we mutated the conserved Arg5 of the Hoxb6 homeodomain to a His (Hoxb6R5H), a mutation that interferes with DNA binding while maintaining the interactions with Pax proteins (15). In vitro, the Hoxb6R5H protein completely lost its ability to bind DNA, yet it still formed a complex with Pax3 in the context of the H1 enhancer (Fig. 4C). Interestingly, when analyzed in vivo, Hoxb6R5H was still able to induce ectopic ribs in four of eight transgenic mice (Fig. 5C). The phenotypes obtained with Hoxb6R5H were generally weaker than those produced by the native Hoxb6 (Fig. 5 B and C), which may reflect a reduced functional association of Hoxb6R5H with Pax3. Alternatively, part of the Hoxb6 activity might derive from a direct interaction with DNA. In any case, these results showed that Hoxb6 can still induce rib formation in the absence of its DNA binding properties, consistent with a Pax3-mediated interaction. In contrast, we were unable to detect any rib-repressing activity, when a similar mutant form of Hoxa10 was used (Hoxa10R5H; n = 8), suggesting that Hoxa10 requires direct binding to DNA to prevent rib formation. Taken together, these results support the notion that Hoxb6 and Hoxa10 modulate the activity of the H1 enhancer via distinct mechanisms.
Fig. 5.
Hoxb6 can induce rib formation without direct DNA binding. The skeletons of WT (A) or transgenic fetuses expressing the WT Hoxb6 (B) and the mutant Hoxb6R5H (C) in the presomitic mesoderm were analyzed at E18.5. Both versions of Hoxb6 induced rib formation in usually ribless regions.
Our results show that a polymorphism in the Pax/Hox binding site of the H1 enhancer controlling the Myf5 gene correlates with the development of an extended rib cage in snake and Paenungulata species. Strikingly, this single base pair change in the H1 enhancer has evolved twice independently, suggesting it may participate in the evolution of these convergent body plans. We argue that this parallelism is intimately associated with the duality of mechanisms whereby Hox proteins activate or repress the rib-forming program. In this view, rib promotion was stimulated from an enhancer sequence unable to respond to rib-blocking signals. The size of the ribcage, however, would not only depend upon the ability (or inability) to respond to rib-suppressing signals, but also on how active are posterior rib-promoting instructions. This is nicely illustrated by the full Hox10 mutants, in which rib formation is progressively reduced in the posterior direction despite the absence of Hox10 function (4). The observation that experimental increase of Hoxb6 levels can override the otherwise dominant rib-repressing Hox10 activity in mouse embryos (8) (Fig. 4B) suggests that quantitative aspects can be relevant to define the domain with rib-forming potential in the posterior embryo. Although rib-promoting activities within physiological ranges might be too low to prevail over a fully functional rib-repressing program, they might become relevant to determine the final size of the rib cage when the rib-blocking function is significantly reduced as it seems to happen in animals carrying the polymorphic H1 enhancer. This parameter might explain the variability in rib number among different Paenungulata, also observed within various species of manatees (13, 17, 18), as well as the shorter ribcages observed in tenrecs, compared with other Paenungulata, despite the presence of the same polymorphism in H1. In snakes, high Hox6 expression levels throughout the whole rib domain (7) might help to secure effective rib production along the animal’s trunk.
Finally, we show that the Hoxb6 rib-inducing activity is, at least to a significant extent, independent of its DNA binding ability. This conclusion deviates from the standard paradigm for Hox protein activity (19) and may point to an additional mechanism to confer Hox functional specificity. Future work on other Hox proteins in other organisms may reveal additional instances in which evolutionary changes have been facilitated or constrained by such mechanisms.
Methods
Cloning and generation of mutant versions of the Hox proteins were performed by using standard procedures (20). The snake Hoxa10 ORF was obtained by fusion of an Elaphe schrenckii partial cDNA containing exon 2 and part of exon 1, with the 5′ of exon 1 from a genomic clone of P. guttata at a unique BglII restriction site. A Kozak sequence was introduced upstream of the ATG by PCR. The mutant forms of the mouse Hoxa10 and Hoxb6 were produced by an overlapping PCR mutagenesis strategy. The introduction of the mutations into the cDNAs encoding for Hoxa10R5H and Hoxb6R5H was confirmed by direct sequencing. To express sHoxa10, Hoxa10R5H, and Hoxb6R5H in transgenic mice, the corresponding cDNAs were cloned under the control of the msd enhancer of the Delta-like 1 (Dll1) gene (21). The Dll1-Hoxa10 and Dll1-Hoxb6 constructs have been previously described (5, 8).
The 195AP/Z BAC (14) was used as a template for the modifications in the H1 enhancer. The mutations were introduced by using the recombineering technique essentially as described previously (22). To select transgenics in which BAC integration had preserved the H1 enhancer and the β-gal reporter, we first introduced a small sequence tag 5′-CATCTAGGACGAACTGCATCG-3′ 210 bp upstream of the Hox binding site of H1. We verified that this tag had no detectable effects on the β-gal staining patterns compared with those obtained with the nontagged BACs. All BACs containing different versions of the H1 enhancer were built on this tagged BAC. Transgenic embryos were identified by PCR with primers for β-gal (5′-GTTTTTCCCGATTTGGCTAC-3′ and 5′-GGACAAACCACAACTAGAATGC-3′) and for the BAC-derived H1 enhancer (5′-ACCTGACATCGCATGGTTGAG-3′ and 5′-GCCTGCCTTTAACGCAGTGTC-3′) and stained for β-gal activity at embryonic day (E) 11.5 as previously described (14).
Transgenic embryos were produced by pronuclear microinjection of these constructs according to standard protocols (23). The skeletal analyses were performed at E18.5 by staining with alcian blue and alizarin red (24).
The genomic area containing the H1 enhancer of snakes and manatee was amplified by PCR from genomic DNA using the primer pair 5′-TGTTGCAGGTTACTTAGTTATAG-3′ and 5′-TAAAATACTGCAGTGACTTCATTC-3′, cloned, and sequenced. The sequences for the same region of other vertebrates were obtained from public databases. Sequence comparisons were performed by using ClustalW2.
EMSAs were performed as previously described (25) by using nuclear extracts of 293T cells transfected with expression constructs expressing FLAG-tagged versions of the relevant proteins and 32P-labeled DNA probes for the different versions of the H1 region spanning the Pax, Hox, and Six binding sites.
Supplementary Material
Acknowledgments
We thank Jaime Carvajal and Peter Rigby for 195AP/Z BAC and help with recombineering techniques; and Elio Sucena, Patricia Beldade, Christen Mirth, and members of the M.M. laboratory for comments on the manuscript. This work was supported by Fundação para a Ciência e a Tecnologia, Grants PTDC/SAU-BID/110640/2009 and PTDC/BIA-BCM/110638/2009 (to M.M.) and by the Swiss National Research Foundation and the European Research Council (D.D.). Any use of trade, product or firm names is for descriptive purposes only and does not imply endorsement by the U.S. government.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 10473.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. JX154076–JX154080).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1300592110/-/DCSupplemental.
References
- 1.Krumlauf R. Hox genes in vertebrate development. Cell. 1994;78(2):191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- 2.Mallo M, Wellik DM, Deschamps J. Hox genes and regional patterning of the vertebrate body plan. Dev Biol. 2010;344(1):7–15. doi: 10.1016/j.ydbio.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wellik DM. Hox patterning of the vertebrate axial skeleton. Dev Dyn. 2007;236(9):2454–2463. doi: 10.1002/dvdy.21286. [DOI] [PubMed] [Google Scholar]
- 4.Wellik DM, Capecchi MR. Hox10 and Hox11 genes are required to globally pattern the mammalian skeleton. Science. 2003;301(5631):363–367. doi: 10.1126/science.1085672. [DOI] [PubMed] [Google Scholar]
- 5.Carapuço M, Nóvoa A, Bobola N, Mallo M. Hox genes specify vertebral types in the presomitic mesoderm. Genes Dev. 2005;19(18):2116–2121. doi: 10.1101/gad.338705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di-Poï N, et al. Changes in Hox genes’ structure and function during the evolution of the squamate body plan. Nature. 2010;464(7285):99–103. doi: 10.1038/nature08789. [DOI] [PubMed] [Google Scholar]
- 7.Woltering JM, et al. Axial patterning in snakes and caecilians: Evidence for an alternative interpretation of the Hox code. Dev Biol. 2009;332(1):82–89. doi: 10.1016/j.ydbio.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 8.Vinagre T, et al. Evidence for a myotomal Hox/Myf cascade governing nonautonomous control of rib specification within global vertebral domains. Dev Cell. 2010;18(4):655–661. doi: 10.1016/j.devcel.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Buchberger A, Freitag D, Arnold HH. A homeo-paired domain-binding motif directs Myf5 expression in progenitor cells of limb muscle. Development. 2007;134(6):1171–1180. doi: 10.1242/dev.02798. [DOI] [PubMed] [Google Scholar]
- 10.Bajard L, et al. A novel genetic hierarchy functions during hypaxial myogenesis: Pax3 directly activates Myf5 in muscle progenitor cells in the limb. Genes Dev. 2006;20(17):2450–2464. doi: 10.1101/gad.382806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giordani J, et al. Six proteins regulate the activation of Myf5 expression in embryonic mouse limbs. Proc Natl Acad Sci USA. 2007;104(27):11310–11315. doi: 10.1073/pnas.0611299104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shoshani J. Mammalian phylogeny: Comparison of morphological and molecular results. Mol Biol Evol. 1986;3(3):222–242. doi: 10.1093/oxfordjournals.molbev.a040389. [DOI] [PubMed] [Google Scholar]
- 13.Narita Y, Kuratani S. Evolution of the vertebral formulae in mammals: A perspective on developmental constraints. J Exp Zoolog B Mol Dev Evol. 2005;304(2):91–106. doi: 10.1002/jez.b.21029. [DOI] [PubMed] [Google Scholar]
- 14.Carvajal JJ, Cox D, Summerbell D, Rigby PWJ. A BAC transgenic analysis of the Mrf4/Myf5 locus reveals interdigitated elements that control activation and maintenance of gene expression during muscle development. Development. 2001;128(10):1857–1868. doi: 10.1242/dev.128.10.1857. [DOI] [PubMed] [Google Scholar]
- 15.Gong K-Q, Yallowitz AR, Sun H, Dressler GR, Wellik DM. A Hox-Eya-Pax complex regulates early kidney developmental gene expression. Mol Cell Biol. 2007;27(21):7661–7668. doi: 10.1128/MCB.00465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plaza S, et al. Cross-regulatory protein-protein interactions between Hox and Pax transcription factors. Proc Natl Acad Sci USA. 2008;105(36):13439–13444. doi: 10.1073/pnas.0806106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Husar SL. Trichechus inunguis. Mamm Species. 1977;72:1–4. [Google Scholar]
- 18.Husar SL. Trichechus manatus. Mamm Species. 1978;93:1–5. [Google Scholar]
- 19.Pearson JC, Lemons D, McGinnis W. Modulating Hox gene functions during animal body patterning. Nat Rev Genet. 2005;6(12):893–904. doi: 10.1038/nrg1726. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1989. [Google Scholar]
- 21.Beckers J, et al. Distinct regulatory elements direct delta1 expression in the nervous system and paraxial mesoderm of transgenic mice. Mech Dev. 2000;95(1-2):23–34. doi: 10.1016/s0925-4773(00)00322-1. [DOI] [PubMed] [Google Scholar]
- 22.Carvajal JJ, Keith A, Rigby PW. Global transcriptional regulation of the locus encoding the skeletal muscle determination genes Mrf4 and Myf5. Genes Dev. 2008;22(2):265–276. doi: 10.1101/gad.442408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hogan B, Beddington R, Constantini F, Lacy E. Manipulating The Mouse Embryo: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1994. [Google Scholar]
- 24.Mallo M, Brändlin I. Segmental identity can change independently in the hindbrain and rhombencephalic neural crest. Dev Dyn. 1997;210(2):146–156. doi: 10.1002/(SICI)1097-0177(199710)210:2<146::AID-AJA7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 25.Guerreiro I, et al. Regulatory role for a conserved motif adjacent to the homeodomain of Hox10 proteins. Development. 2012;139(15):2703–2710. doi: 10.1242/dev.081448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.