Abstract
How females store and use sperm after remating can generate postcopulatory sexual selection on male ejaculate traits. Variation in ejaculate performance traits generally is thought to be intrinsic to males but is likely to interact with the environment in which sperm compete (e.g., the female reproductive tract). Our understanding of female contributions to competitive fertilization success is limited, however, in part because of the challenges involved in observing events within the reproductive tract of internally fertilizing species while discriminating among sperm from competing males. Here, we used females from crosses among isogenic lines of Drosophila melanogaster, each mated to two genetically standardized males (the first with green- and the second with red-tagged sperm heads) to demonstrate heritable variation in female remating interval, progeny production rate, sperm-storage organ morphology, and a number of sperm performance, storage, and handling traits. We then used multivariate analyses to examine relationships between this female-mediated variation and competitive paternity. In particular, the timing of female ejection of excess second-male and displaced first-male sperm was genetically variable and, by terminating the process of sperm displacement, significantly influenced the relative numbers of sperm from each male competing for fertilization, and consequently biased paternity. Our results demonstrate that females do not simply provide a static arena for sperm competition but rather play an active and pivotal role in postcopulatory processes. Resolving the adaptive significance of genetic variation in female-mediated mechanisms of sperm handling is critical for understanding sexual selection, sexual conflict, and the coevolution of male and female reproductive traits.
Keywords: cryptic female choice, heritability, sperm ejection
Because females of many species mate with multiple males within a reproductive cycle (1–3), sexual selection can continue after mating. When sperm from different males co-occur in the female reproductive tract, they compete for fertilization of the eggs, and females may bias sperm use to favor some males over others. Such sperm competition and cryptic female choice are regarded as the postcopulatory equivalents of premating male–male competition and female choice, respectively (4, 5). However, this characterization may be overly simplistic and belie differences between selection episodes that are critical for understanding selection dynamics.
Adaptations arising through premating versus postcopulatory sexual selection are likely to differ in phenotypic and genotypic complexity. With premating sexual selection, male armaments and ornaments tend to be complex somatic traits under the control of multiple genes (e.g., ref. 6), and female mate preferences predominantly have sensory and cognitive bases (7–9). In contrast, the principal target of postcopulatory sexual selection on males is the ejaculate. (Note: Penis and copulatory courtship traits are excluded here for the sake of argument.) Postcopulatory ornaments and armaments thus predominantly include single active molecules such as accessory gland proteins (Acps) that are controlled by single genes (10, 11) and traits borne by haploid single cells [e.g., sperm structures, membrane-bound proteins, energetics (12, 13)]. The genetics of these traits are relatively unresolved (12, 14–17). The primary targets of postcopulatory sexual selection on females will be aspects of reproductive tract biochemistry, neurophysiology, and morphology that interact with ejaculates and potentially bias paternity (5, 18–21). The genetics of cryptic female choice also are not well resolved (but see ref. 22). Because ejaculate competition and processes of female sperm selection occur within the female reproductive tract, the relative competitiveness of ejaculates is predicted to be a function of ejaculate–female compatibility. If so, then sperm competition and cryptic female choice represent more of a continuum than dichotomous processes, especially (but not exclusively; e.g., refs. 23–26) in internally fertilizing species (20, 21).
Adaptations arising through premating versus postcopulatory sexual selection also are likely to differ fundamentally in the extent to which intersexual interactions influence their expression. Sex-specific, premating traits generally are considered separate entities with distinct phenotypes and fitness consequences. In contrast, consider ejaculate processing and function within females. Seminal fluid is biochemically complex, with ∼150 Acps being inseminated into female Drosophila melanogaster (27, 28). Most Acps are believed to have unique target receptors within the female (11), although to date only one [for sex peptide (29)] has been identified. Moreover, phenotypic expression of some Acps follows modification (e.g., proteolytic cleavage) within the female, a process thought to require both male and female secretory contributions (11, 21). Likewise, sperm may complete maturation, capacitate, or otherwise undergo modification within the female. In some cases, these modifications are known to involve biochemical ejaculate–female interactions (21), with direct implications for competitive fertilization success [e.g., (30, 31)]. A major focus in the study of postcopulatory sexual selection has been to understand the evolution of ejaculate quality traits that are likely to influence competitive fertilization success, such as swimming velocity [reviewed by (32–34)]. Variation in these phenotypes has almost exclusively been assayed in vitro and interpreted as intrinsic to males. However, to the extent that ejaculate phenotypes are influenced by females and/or are the product of male-by-female interactions, ejaculate phenotypes in the narrow sense may not exist outside of the biochemically and structurally complex environment of the female reproductive tract. Rather, they may have to be considered a special case of gene-by-environment interactions (also see ref. 35).
Therefore, the use of assays conducted in vivo under competitive conditions to investigate genetically variable traits that influence competitive fertilization success and the respective contribution of the sexes to their expression would strengthen our knowledge of postcopulatory sexual selection and its role in maintaining variation and driving diversification. In a series of pioneering experiments using fixed-chromosome lines of D. melanogaster, Clark and colleagues (36–40) demonstrated male, female, and male-by-female genotypic contributions to patterns of sperm precedence (also see ref. 41). We have expanded this approach using isogenic lines [inbred lines that approximate genetic clones, henceforth referred to as “isolines” (42, 43)] of D. melanogaster expressing either GFP or RFP in their sperm heads. The fluorescently tagged sperm allow direct visualization of real-time and spatiotemporal in vivo sperm performance and fate while distinguishing between sperm from competing males (44, 45), thereby enabling the association of genotypic variation with sperm precedence traits and processes. We recently documented heritable, strictly male-mediated variation (i.e., all females derived from a single isoline) in ejaculate traits, including sperm length, velocity, and number, and showed that these traits significantly influence fertilization success at different stages following competitive matings (44). In the present paper, we examine strictly female-mediated additive and nonadditive genetic variance in remating, progeny production, and sperm performance and fate in D. melanogaster and its effects on competitive fertilization success between pairs of genetically standardized males (i.e., males derived from two isolines). Investigations of male-by-female interactions in sperm performance and competitive fertilization success are in progress and will be the subject of a future report.
Results
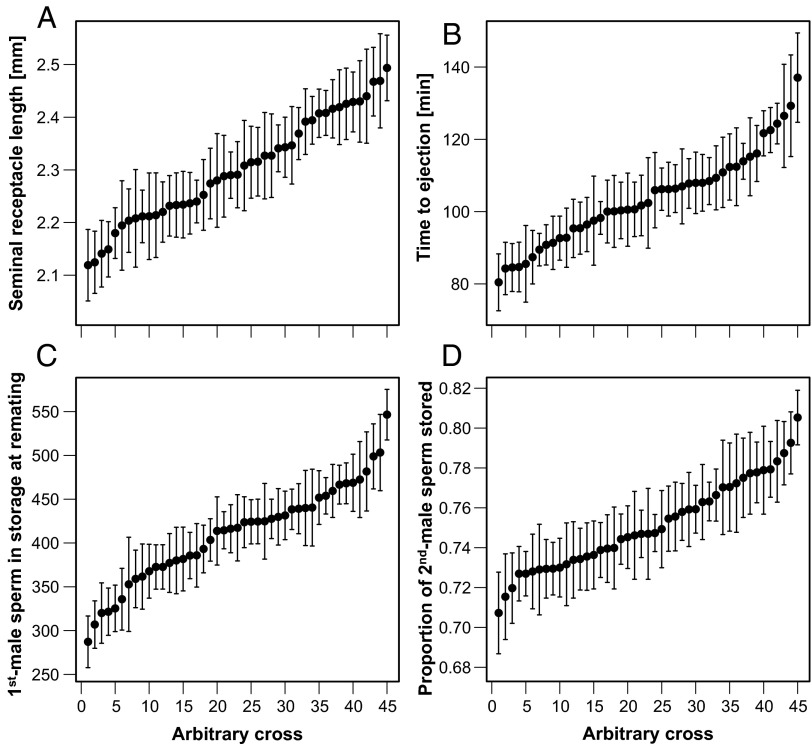
Across 90 diallel crosses (45 nuclear genotypes) controlled for female genetic background and block and vial (e.g., family) effects (Materials and Methods), we found significant heritability for seminal receptacle (SR) length, remating interval, rate of progeny production before remating, time from copulation to female sperm ejection, and numerous female sperm-handling traits (Table 1 and Fig. 1). The number of first-male sperm still in storage at the time of remating was significantly heritable (Table 1) but was not significantly associated with SR length or with the number of progeny produced before remating (|t| < 1.60, P > 0.11, conditional model R2 = 0.25). In the 72-h experiment, however, SR length covaried positively with the total number of sperm remaining in storage at the end of the 3-d oviposition period (n = 453 families, t = 4.61, P < 0.0001, R2 = 0.15) and in a heritable manner [h2 = 0.20, log-likelihood ratio (LLR) = 10.20, P = 0.037]. Females with a relatively long SR also tended to store more sperm in the SR as the main sperm storage organ (n = 1,169 females, t = 1.89, P = 0.06, R2 = 0.23) but to remate sooner (n = 1,398 females, t = −3.09, P = 0.0005, R2 = 0.15) and to produce more progeny per unit of time, albeit not significantly (n = 1,333 females, t = 1.71, P = 0.09, R2 = 0.49). In contrast to first-male sperm, the number of sperm transferred by the second male was not affected by the female genetic background (Table 1), and in a multivariate model (n = 960 females, R2 = 0.23), it was independent of copulation duration (t = 0.66, P = 0.51), female thorax length (t = 0.07, P = 0.95), and SR length (t = −0.25, P = 0.80). However, the number of sperm retained from each male after female ejection was significantly heritable (Table 1).
Table 1.
Additive (VA) and nonadditive (VD) genetic variance components, phenotypic variance (VP), and heritability (h2) of female-mediated effects on ejaculate quality and handling, controlled for block and vial effects
| Trait | N | VA | VD | VP | h2 | LLR | P |
| Thorax length* | 484 | 1.29 | 0 | 5.44 | 0.24 | 8.2 | 0.09 |
| Absolute SR length*, | 484 | 0.02 | 0.006 | 0.04 | 0.50 | 49.4 | <0.0001 |
| Relative SR length*,† | 484 | 0.02 | 0.006 | 0.04 | 0.54 | 50.3 | <0.0001 |
| Day of remating | 1,585 | 0.06 | 0.03 | 0.46 | 0.14 | 18.2 | 0.0001 |
| Progeny before remating (E) | 1,572 | 329.6 | 0 | 340.5 | 0.97 | 386.6 | <0.0001 |
| Progeny before remating (P)* | 487 | 436 | 0 | 485 | 0.90 | 46.3 | <0.0001 |
| Duration of copulation | 1,573 | 1.63 | 0 | 34.24 | 0.05 | 7.9 | 0.24 |
| Resident sperm at remating | 1,115 | 9,729 | 965 | 20,815 | 0.47 | 61.8 | 0.0003 |
| Number of sperm transferred | 1,104 | 0 | 0 | 65,502 | 0.00 | 0.0 | 1.0 |
| Time to ejection | 1,277 | 0.05 | 0 | 0.14 | 0.36 | 65.3 | <0.0001 |
| Mean sperm velocity* | 536 | 130 | 0 | 1,044 | 0.13 | 4.7 | 0.32 |
| First-male sperm stored | 1,272 | 599 | 108 | 4,853 | 0.12 | 8.1 | 0.044 |
| Second-male sperm stored | 1,272 | 1,955 | 0 | 10,395 | 0.19 | 16.1 | 0.001 |
| Total sperm stored | 1,228 | 2,737 | 0 | 13,697 | 0.20 | 28.0 | <0.0001 |
| S2 (pre-ejection) ‡ | 1,104 | 0.003 | 0 | 0.007 | 0.36 | 66.6 | <0.0001 |
| S2 (postejection) | 1,272 | 0.001 | 0 | 0.010 | 0.14 | 20.1 | 0.0005 |
| S2 in SR (postejection) | 1,241 | 0.008 | 0 | 0.025 | 0.29 | 64.2 | <0.0001 |
| Prop. first-male sperm in SR | 1,293 | 0.008 | 0.0004 | 0.020 | 0.43 | 78.9 | <0.0001 |
| Prop. second-male sperm in SR | 1,296 | 0.002 | 0 | 0.009 | 0.19 | 16.0 | 0.001 |
| Second-male paternity (P2)* | 419 | 0.005 | 0.001 | 0.028 | 0.17 | 7.8 | 0.051 |
For further details, see Materials and Methods, Statistical Analyses. E, ejection experiment; P, paternity experiment. Boldface indicates traits with significant heritability.
Based on one female per family (i.e., maximum n = 6 per isoline cross).
Controlled for female thorax length as a fixed effect (t = 2.42, P = 0.016).
Proportion of second-male sperm among all resident first-male sperm and the entire second-male ejaculate.
Fig. 1.
Within- and between-cross variation in (A) SR length, (B) time to female sperm ejection after the end of copulation, (C) the number of first-male sperm still in storage at the time of remating, and (D) the proportion of second-male sperm among all sperm stored (i.e., S2). Each point represents an individual isoline cross (for simplicity, the reciprocal crosses are combined by nuclear genotype); error bars indicate SE. For statistics on heritability, see Table 1.
Female genotypes also differed significantly in the interval between the end of copulation and the ejection of displaced first-male sperm and excess second-male sperm (Table 1). Ranging between a mean ± SEM of 55.3 ± 5.0 min and 134.0 ± 12.4 min among the 90 isoline crosses, this heritable variation played an important role in determining the relative fertilization success among the competing males. For example, controlling for SR length, first- and second-male sperm velocity, and the numbers of sperm competing for storage, a prolonged time to ejection significantly reduced the number of first-male sperm retained (n = 682 females; t = −6.11, P < 0.0001) (Table S1) and significantly increased the proportion of resident sperm that were displaced (n = 682 females, t = 5.73, P < 0.0001) (Table S2). Sperm velocity did not differ among female genotypes (Table 1) and had no significant influence on first-male sperm storage (Tables S1 and S2). The same results were obtained in multiple regression analyses based on the mean values within crosses (Tables S1 and S2).
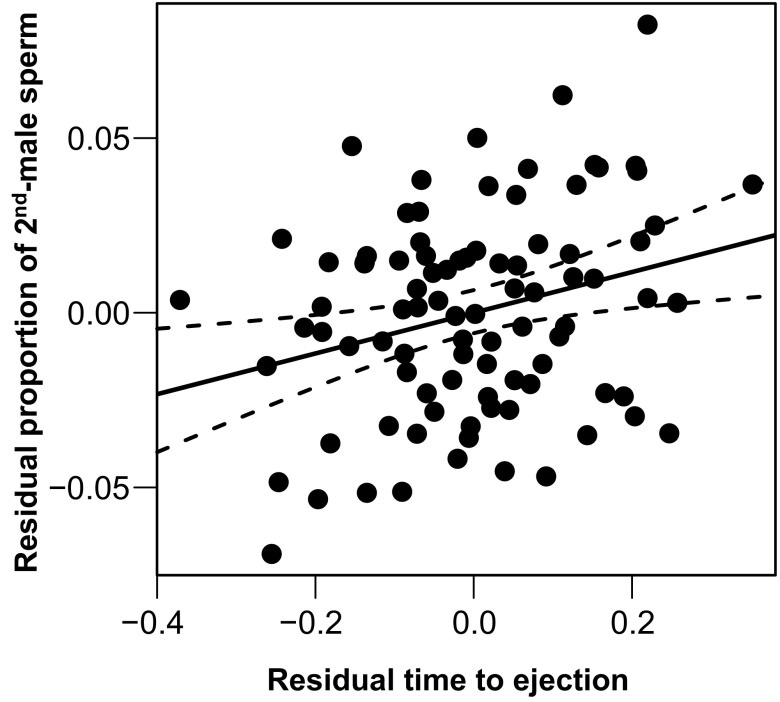
The number of second-male sperm retained was influenced by the relative sperm velocities among the competing ejaculates, with slower sperm being better at remaining in storage (Table S3), thus confirming an earlier report (44). We obtained qualitatively similar results when focusing on the proportion of all transferred second-male sperm that remained in storage (Table S4). Despite the sperm-velocity effect on second-male sperm storage described above, the proportion of second-male sperm among all retained sperm (i.e., S2) was explained by the time to ejection and the numbers of first- and second-male sperm competing for access to storage (Table 2 and Table S5). These results were consistent in a multiple regression analysis based on the mean values within crosses (n = 90 crosses; time to ejection: t = 2.68, P = 0.009; first-male sperm: t = −6.06, P < 0.0001; second-male sperm: t = 3.20, P = 0.002; model R2 = 0.32) (Fig. 2), as well as with each predictor analyzed separately (all |t| > 8.39, P < 0.0001).
Table 2.
Minimal adequate linear mixed-effects model explaining the variation in the proportion of second-male sperm (S2) among all sperm retained by the female, after sequential elimination of nonsignificant random and fixed effects (Materials and Methods)
| Fixed terms | Estimate ± SE | ddf | t | P |
| Time to ejection | 0.17 ± 0.02 | 808.2 | 5.65 | <0.0001 |
| Resident sperm (first male) | −0.62 ± 0.03 | 803.1 | −19.14 | <0.0001 |
| Sperm transferred (second male) | 0.55 ± 0.03 | 849.4 | 16.81 | <0.0001 |
Following sequential elimination, the maternal isoline (LLR = 8.11, P = 0.004) and paternal isoline (LLR = 2.73, P = 0.099) were the only random effects remaining in the minimal adequate model. Conditional model R2 = 0.38; n = 855 females from 90 diallel crosses derived from 10 isolines. For full model see Table S5. Parameter estimates are standardized. Ddf, denominator degrees of freedom estimated using Satterthwaite’s approximation.
Fig. 2.
Partial residual plot reflecting the significant relationship between the respective partial residuals of the time to female sperm ejection and the proportion of second-male sperm in storage (S2) postejection (t = 2.68, P = 0.009). Data points depict mean values for each of the 90 isoline crosses; dashed lines indicate the 95% confidence interval. Both axes are controlled for the number of first-male resident sperm at remating and the number of sperm transferred by the second male (full statistics of the multiple regression model are given in the main text).
Combining the experimental units at the family (vial) level and controlling for block effects and female genetic background, the relative numbers of sperm from each male remaining in storage after female sperm ejection significantly influenced competitive fertilization success: The paternity share of the second male, as measured by the proportion of progeny produced after remating that were sired by the second male (P2), increased with the number of second-male sperm retained (n = 389 families within 90 crosses, t = 2.95, P = 0.003), controlling for the number of first-male sperm (t = 1.52, P = 0.13) and SR length (t = −1.57, P = 0.12; model R2 = 0.11). SR length had no further significant effect on S2 among the sperm still in storage after 72 h of oviposition (n = 464 families, t = −1.74, P = 0.08, R2 = 0.09), but it increased the absolute sperm numbers still in storage after that period for both the first (n = 464 families, t = 3.36, P = 0.001, R2 = 0.11) and second males (n = 464 families, t = 3.45, P = 0.0006, R2 = 0.18). Similar results were obtained in regression analyses using mean values within each of the 90 crosses.
Discussion
Our results reveal within-population heritable variation in female SR length, remating interval, rate of progeny production, time from copulation to sperm ejection, and aspects of sperm storage. In addition, the variable female genetic background significantly affected competitive fertilization success between standardized competitor males, with functional associations established. For example after remating, sperm of the last male move into the female’s sperm-storage organs and start displacing resident sperm from the previous male back into the bursa, with displacement rates higher for the SR than the spermathecae (45). The female terminates this storage and displacement process 1–5 h after mating by ejecting all the sperm located in the bursa, which include any excess sperm from the second male and all displaced first-male sperm (45). As predicted a priori, the timing of sperm ejection had a particularly strong effect on the absolute and relative numbers of each male’s sperm remaining in storage, thereby determining the fertilization set (i.e., the sperm able to compete for egg fertilization). Females with relatively late ejection retained a disproportionate number of second-male compared with first-male sperm, presumably because the sperm of the second male had more time to achieve entry into the sperm-storage organs and to displace first-male sperm residing there. In fact, our data indicate that this bias was driven primarily by displacement of first-male sperm rather than by variation in second-male sperm storage, both in terms of absolute numbers displaced and the proportion of each male’s total sperm mass that was ejected. The potential adaptive significance of sperm ejection time is evident in its direct influence on paternity, which was determined by the relative numbers of sperm in the fertilization set (also see refs. 44–46).
Once the fertilization set is established, female D. melanogaster may not be able to bias competitive fertilization per se further, given that sperm for fertilization in this species derive primarily from the SR and in direct proportion to their representation (46). This pattern of sperm use contrasts starkly with that of Drosophila simulans, in which females may influence relative fertilization success directly even after sperm ejection. In this species, sperm for fertilization derive equally from the spermathecae and SR, and each sperm-storage organ exhibits a significant bias: favoring first-male sperm in the SR and second-male sperm in the spermathecae, with females able to shift toward one or the other storage organ depending on the mating order of males of differing quality (46, 47). Nevertheless, in the present study we also did find genetic variation in female remating intervals and progeny production rates (also see refs. 48–50), both of which can generate postcopulatory sexual selection on males.
Previous experimental evolution research with D. melanogaster found heritable variation in SR length and revealed that the evolution of longer SRs drove the evolution of longer sperm (e.g., ref. 51). This latter result was attributed to a demonstrated interaction between SR length and sperm length that influenced competitive fertilization success (51). Longer sperm were found to be superior to shorter sperm in displacing, and resisting displacement by, competing sperm (52) (also see ref. 44), and this advantage increased with SR length (51). In the absence of systematic variation in sperm length, SR length variation was unrelated to the pattern of sperm precedence (53). Here, we similarly found significant heritable variation in SR length and the lack of any relationship to the second-male paternity share (P2) in the absence of sperm-length variation. We did, however, find that females with relatively long SRs remated faster, tended to produce progeny at a higher rate during that period, stored more sperm initially, and had more sperm remaining in storage after 3 d of oviposition than females with a shorter SR; all these factors may contribute to postcopulatory sexual selection on males (53). The underlying mechanisms for these relationships currently remain unresolved. It is possible that females with longer SRs are more strongly influenced by male seminal proteins that are known to mediate various aspects of female sperm storage, receptivity, and oviposition (10, 11), because the longer organ receives or retains more seminal plasma and/or because it possesses more seminal fluid protein receptors. Alternatively, SR length may be genetically correlated with female quality and thus fecundity, with highly fecund females remating faster and more frequently than females of poor quality (e.g refs. 54–56, but see refs. 57 and 58).
In addition to sperm-ejection time, females potentially could have impacted the composition of the fertilization set, and hence P2, by influencing either the number of sperm transferred during copulation (e.g., ref. 59) or the behavior of sperm (i.e., swimming velocity). Sperm velocity has been found to be a critical determinant of fertilization success in diverse taxa, with faster sperm having an advantage in some taxa (e.g., refs. 60 and 61) and slower sperm having an advantage in others (44, 62). In D. melanogaster, slower sperm have been shown to be superior in displacing and resisting displacement by faster sperm, with sperm velocity significantly influenced by male genotype (44). However, we found no significant female genetic variation for copulation duration or the number of sperm transferred, supporting the contention that these phenomena are under male control in D. melanogaster and related species (ref. 63 and references therein). The absence of a relationship between the number of sperm transferred and female genetic background further reinforces the interpretation that the number of sperm entering or remaining in storage is attributable primarily to female effects rather than to differential male allocation relative to the female genotype (see above). Similarly, we found that neither female genetic background nor SR length significantly affected sperm velocity. This negative result is potentially important; although a few previous investigations have shown significant female and/or male-by-female interaction effects on sperm velocity (23–26), all these studies have been conducted in vitro with externally fertilizing species and were not designed to explore genetic variation.
It is important to note that variation in reproductive phenotypes attributed to female-mediated genetic variation in the present study (where competing male genotypes were held constant) and attributed to male-mediated genetic variation in a previous study (ref. 44, in which female genotypes were held constant) may be explained in part or entirely by genetic variation in male-by-female interactions (22, 37–39, 64). An investigation in progress soon will sort out these results. Such interaction between the sexes is predicted by genetic compatibility models of sexual selection (e.g., refs. 65 and 66) and is expected to be mediated often by physiological interactions between ejaculates and female reproductive tracts [e.g., via seminal fluid proteins and female receptors for them (21)]. Whatever the adaptive significance may be, genetic variation in male and female reproductive characters identified in investigations of our isolines likely represent some of the mechanisms underlying previous demonstrations of genetic male-by-male and male-by-female interactions in sperm precedence (e.g., refs. 37–39 and 41).
Cryptic female choice is defined as “nonrandom paternity biases resulting from female morphology, physiology, or behavior that occur after coupling” (67), and our results meet those criteria. Nevertheless, because our investigation was designed to reveal strictly female-mediated genetic variation in traits relevant to postcopulatory sexual selection, which necessitated standardizing the genetic contribution of competing males (18), the implications of our results for understanding directional postcopulatory sexual selection cannot yet be fully ascertained. Specifically, the demonstrated associations between female genetic variation and patterns of nonrandom reproductive success represent male mating-order biases. Unless male mating order correlates with differential male quality, the identified genetic variation will be selectively neutral (at least in the absence of male-by-female interactions; also see ref. 36). Indeed, some of the most convincing demonstrations of cryptic female choice/sperm choice have shown fertilization bias patterns based on MHC loci genotype (68, 69) or that are consistent with adaptation to avoid selfing (e.g., ref. 70) or inbreeding (e.g., refs.71 and 72), which also may fail to generate directional sexual selection (18). Notably, sperm ejection by female fowl Gallus gallus domesticus has been shown to be adaptively plastic, with the probability of ejection occurring and the proportion of the ejaculate ejected being greater for subordinate than dominant males (73). However, further investigation exploring the relationships between variation in male and female “sperm competition” phenotypes (e.g., sperm number, sperm length, sperm velocity, SR length, ejection time) is needed to clarify the adaptive significance of female-mediated variation revealed here.
Materials and Methods
Experimental Material.
To discriminate sperm from different males and quantify sperm motility in vivo, all experiments were conducted with LHm populations of D. melanogaster that express a protamine labeled with either GFP or RFP in sperm heads (backcrossed for six generations to wild type; see ref. 45 for transformation and fitness assay details). The GFP line also ubiquitously expresses GFP, thus permitting paternity assignments on progeny (e.g., P2).
All experimental flies were derived from isogenic lines (isolines; refs. 42 and 43) generated for each sperm-tag color by 15 generations of full-sibling inbreeding. The experimental males were F1 progeny from crosses among a single pair of isolines per sperm-tag color (i.e., virgin females from one and males from the other isoline in each cross). Based on isoline characterization under standardized conditions [standard female and competitor male (44)], we selected isolines with intermediate values for sperm length, sperm velocity, and ejaculate size. Our two hybrid isolines did not differ significantly in sperm length [GFP, n = 15 males: 1.86 ± 0.01 mm (mean ± SEM); RFP, n = 15 males: 1.84 ± 0.02 mm; t28 = 1.21, P = 0.24].
To vary the female genetic background, we crossed single pairs of virgin males and females of 10 different RFP isolines in all nonself combinations (i.e., 90 diallel crosses with 45 different nuclear genotypes, all independent of the RFP standard competitor male). In each of two blocks, separated by two generations, we used flies from three separate male–female pairs for each cross, and for each pair we assayed five F1 females (i.e., 90 crosses × two blocks × three families × five females = 2,700 females). Three females per family were used in the ejection experiment, and two females were used in the 72-h experiment (see below). All flies were maintained at low densities in vials with standard cornmeal-molasses-agar medium supplemented with yeast, were collected as virgins upon eclosion and were aged for 3 d before their first mating. All males were used only once; all females were mated to two males of opposite sperm-tag color.
Sperm-Competition Experiment.
We investigated reproductive outcomes at two biologically relevant time points after the second mating (45): (i) immediately after female sperm ejection (i.e., <5 h after mating and before the first egg has entered the bursa for fertilization) and (ii) after 72 h, which is the typical female remating interval and thus represents a reliable window to examine variation in paternity. We conducted both experiments using the same isoline crosses but different sets of males and females: Each female was mated with a virgin GFP male and, 2 d later, with a virgin RFP male, with additional 6-h remating opportunities on days 3–4 for any refractory females. For each mating, we recorded the copulation duration, removed the males from the mating vials immediately after the end of copulation, and dissected the females at a given time point after mating.
In the sperm-ejection experiment, we isolated females in glass three-well spot plates beneath glass coverslips immediately after mating to the second male and checked for ejection every 10 min for up to 5 h using a stereomicroscope. We recorded the time to ejection, removed females from the wells immediately, and transferred the ejected masses to saline on slides. Subsequently, we anesthetized these females under CO2, gently dissected the reproductive tract into 20 µL of enhanced Grace’s Supplemented Insect Medium (BD Biosciences) at room temperature, and captured a 10-s movie at 400× magnification using an Olympus DP71 cooled, color digital camera mounted onto an Olympus BX-60 fluorescent microscope equipped with a red–green dual filter. We analyzed sperm velocity within the SR, using the Manual Tracking plugin for ImageJ v. 1.44j (National Institutes of Health). We restricted our analyses to the SR because this is the primary sperm-storage organ (45, 74) and because tracking individual sperm for multiple frames in the spermathecae generally is not possible.
In the 72-h experiment, we transferred each female daily to a different vial until freezing it 72 h after remating for later dissection and quantification of sperm. We reared all progeny and assigned paternity based on the presence/absence of the ubiquitin GFP marker. We further measured the length of the thorax and the SR of one of the frozen females per family (i.e., six females per cross). We dissected the reproductive tract into PBS on a microscopic slide and covered it with a glass coverslip that had clay at the corners to allow the SR to be flattened to two dimensions without stretching. We measured SR length using ImageJ at 200× magnification under an Olympus BX-60 microscope with Nomarski DIC optics.
For all dissected females of both experimental units, we counted the sperm of both competitors across the different organs of the female reproductive tract (bursa copulatrix, SR, and paired spermathecae) and determined the total number of sperm for each male in all female sperm-storage organs combined, the proportion of total sperm derived from the first (S1) or second male (S2), respectively, and the proportion of each male’s total sperm represented in the female tract that reside in the SR. Combining these counts with those of the ejected masses further allowed us to calculate the number of first-male sperm still in storage at the time of remating, sperm displacement, second-male sperm transfer, and the number and proportion of each male’s sperm ejected.
Statistical Analyses.
We performed all analyses using the statistical software package R version 2.15.2 (R Development Core Team 2012), with S2 and P2 values normalized by arcsine/square-root transformations and the time to ejection log-transformed to meet the parametric requirements of the statistical models. Unless stated otherwise, we used general linear mixed-effects models (R package lmer) with restricted maximum likelihood (REML). We controlled for random block effects and for the female genetic background by including the random maternal and paternal isoline effects (i.e., general combining ability), the random isoline cross effects (i.e., specific combining ability), the random diallel reciprocal effects, and the replicate family (vial) nested within the isoline cross. Fixed effects were included as necessary and are mentioned in the text or listed in the tables.
After examining the results deriving from the full models, we performed stepwise model selection by comparing mixed models using likelihood ratio tests (maximum likelihood, ML) and refitting the final, minimum adequate models with REML (75), first removing nonsignificant random effects and then nonsignificant fixed effects. Model diagnostics revealed no evidence for overdispersion in any of our analyses based on the Pearson residuals [i.e., the sum of the squared Pearson residuals divided by the residual degrees of freedom (75); all <0.8], for serious collinearity among fixed effects given the correlation structure in the model outputs (all <0.6), or for non-Gaussian distributions of the residuals. To estimate denominator degrees of freedom and P values of the fixed effects, we used Satterthwaite’s approximation (implemented in the R package lmerTest), which resulted in P values nearly identical to those obtained with Bayesian probability estimates (function pvals.fnc in the languageR package). P values of random effects were calculated based on log-likelihood ratio tests comparing models with and without the random effect of concern. To investigate further the relationships revealed by mixed models, we performed multiple regression analyses based on the within-cross means. Most associations were stable across these different levels and thus are likely to be biologically relevant rather than statistical artifacts. Finally, for each mixed model we report the total variance explained by the fixed and random effects combined [i.e., conditional R2 (76)] and, for multiple regression analyses the multiple R2, as indicators of the model goodness-of-fit.
Supplementary Material
Acknowledgments
We thank E. Droge-Young, B. Gress, T. Pearson, N. Ali, and R. Wilk for assistance with data collection; J. Friedman and W. T. Starmer for insightful discussions; and two reviewers for helpful comments on the manuscript. This work was funded by National Science Foundation Grant DEB-1145965 (to S.P., S.L., J.M.B., and M.K.M.) and by the Swiss National Science Foundation Fellowship PA00P3_134191 (to S.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1300954110/-/DCSupplemental.
References
- 1.Arnqvist G, Rowe L. Sexual Conflict. Princeton, NJ: Princeton Univ Press; 2005. [Google Scholar]
- 2.Hosken DJ, Stockley P. Benefits of polyandry: A life history perspective. Evol Biol. 2003;33:173–194. [Google Scholar]
- 3.Jennions MD, Petrie M. Why do females mate multiply? A review of the genetic benefits. Biol Rev Camb Philos Soc. 2000;75(1):21–64. doi: 10.1017/s0006323199005423. [DOI] [PubMed] [Google Scholar]
- 4.Parker GA. Sperm competition and its evolutionary consequences in the insects. Biol Rev Camb Philos Soc. 1970;45(4):526–567. [Google Scholar]
- 5.Thornhill R. Cryptic female choice and its implications in the scorpionfly Harpobittacus nigriceps. Am Nat. 1983;122(6):765–788. [Google Scholar]
- 6.Lande R. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution. 1980;34(2):292–305. doi: 10.1111/j.1558-5646.1980.tb04817.x. [DOI] [PubMed] [Google Scholar]
- 7.Jennions MD, Petrie M. Variation in mate choice and mating preferences: A review of causes and consequences. Biol Rev Camb Philos Soc. 1997;72(2):283–327. doi: 10.1017/s0006323196005014. [DOI] [PubMed] [Google Scholar]
- 8.Kirkpatrick M, Rand AS, Ryan MJ. Mate choice rules in animals. Anim Behav. 2006;71(5):1215–1225. [Google Scholar]
- 9.Ryan MJ. Sexual selection, sensory systems and sensory exploitation. Oxf Surv Evol Biol. 1990;7:157–195. [Google Scholar]
- 10.Chapman T. Seminal fluid-mediated fitness traits in Drosophila. Heredity (Edinb) 2001;87(Pt 5):511–521. doi: 10.1046/j.1365-2540.2001.00961.x. [DOI] [PubMed] [Google Scholar]
- 11.Ravi Ram K, Wolfner MF. Seminal influences: Drosophila Acps and the molecular interplay between males and females during reproduction. Integr Comp Biol. 2007;47(3):427–445. doi: 10.1093/icb/icm046. [DOI] [PubMed] [Google Scholar]
- 12.Dorus S, Karr TL. In: Sperm Biology: An Evolutionary Perspective. Birkhead TR, Hosken DJ, Pitnick S, editors. San Diego: Academic Press; 2009. pp. 435–469. [Google Scholar]
- 13.Pitnick S, Hosken DJ, Birkhead TR. In: Sperm Biology: An Evolutionary Perspective. Birkhead TR, Hosken DJ, Pitnick S, editors. San Diego: Academic Press; 2009. pp. 69–149. [Google Scholar]
- 14.Pitnick S, Dobler R, Hosken DJ. Sperm length is not influenced by haploid gene expression in the flies Drosophila melanogaster and Scathophaga stercoraria. Proc Biol Sci. 2009;276(1675):4029–4034. doi: 10.1098/rspb.2009.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simmons LW, Moore AJ. In: Sperm Biology: An Evolutionary Perspective. Birkhead TR, Hosken DJ, Pitnick S, editors. San Diego: Academic Press; 2009. pp. 405–434. [Google Scholar]
- 16.Fiumera AC, Dumont BL, Clark AG. Sperm competitive ability in Drosophila melanogaster associated with variation in male reproductive proteins. Genetics. 2005;169(1):243–257. doi: 10.1534/genetics.104.032870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiumera AC, Dumont BL, Clark AG. Associations between sperm competition and natural variation in male reproductive genes on the third chromosome of Drosophila melanogaster. Genetics. 2007;176(2):1245–1260. doi: 10.1534/genetics.106.064915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birkhead TR. Cryptic female choice: Criteria for establishing female sperm choice. Evolution. 1998;52(4):1212–1218. doi: 10.1111/j.1558-5646.1998.tb01848.x. [DOI] [PubMed] [Google Scholar]
- 19.Birkhead TR, Pizzari T. Postcopulatory sexual selection. Nat Rev Genet. 2002;3(4):262–273. doi: 10.1038/nrg774. [DOI] [PubMed] [Google Scholar]
- 20.Eberhard WG. Female Control: Sexual Selection by Cryptic Female Choice. Princeton, New Jersey: Princeton Univ Press; 1996. [Google Scholar]
- 21.Pitnick S, Wolfner MF, Suarez SS. In: Sperm Biology: An Evolutionary Perspective. Birkhead TR, Hosken DJ, Pitnick S, editors. San Diego: Academic Press; 2009. pp. 247–304. [Google Scholar]
- 22.Giardina TJ, Beavis A, Clark AG, Fiumera AC. Female influence on pre- and post-copulatory sexual selection and its genetic basis in Drosophila melanogaster. Mol Ecol. 2011;20(19):4098–4108. doi: 10.1111/j.1365-294X.2011.05253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans JP, Marshall DJ. Male-by-female interactions influence fertilization success and mediate the benefits of polyandry in the sea urchin Heliocidaris erythrogramma. Evolution. 2005;59(1):106–112. [PubMed] [Google Scholar]
- 24.Rosengrave P, Gemmell NJ, Metcalf V, McBride K, Montgomerie R. A mechanism for cryptic female choice in chinook salmon. Behav Ecol. 2008;19(6):1179–1185. [Google Scholar]
- 25.Urbach D, Folstad I, Rudolfsen G. Effects of ovarian fluid on sperm velocity in Arctic charr (Salvelinus alpinus) Behav Ecol Sociobiol. 2005;57(5):438–444. [Google Scholar]
- 26.Simmons LW, Roberts JD, Dziminski MA. Egg jelly influences sperm motility in the externally fertilizing frog, Crinia georgiana. J Evol Biol. 2009;22(1):225–229. doi: 10.1111/j.1420-9101.2008.01628.x. [DOI] [PubMed] [Google Scholar]
- 27.Findlay GD, MacCoss MJ, Swanson WJ. Proteomic discovery of previously unannotated, rapidly evolving seminal fluid genes in Drosophila. Genome Res. 2009;19(5):886–896. doi: 10.1101/gr.089391.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Findlay GD, Yi X, Maccoss MJ, Swanson WJ. Proteomics reveals novel Drosophila seminal fluid proteins transferred at mating. PLoS Biol. 2008;6(7):e178. doi: 10.1371/journal.pbio.0060178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yapici N, Kim Y-J, Ribeiro C, Dickson BJ. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 2008;451(7174):33–37. doi: 10.1038/nature06483. [DOI] [PubMed] [Google Scholar]
- 30.Peng J, et al. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr Biol. 2005;15(3):207–213. doi: 10.1016/j.cub.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 31.Fricke C, Wigby S, Hobbs R, Chapman T. The benefits of male ejaculate sex peptide transfer in Drosophila melanogaster. J Evol Biol. 2009;22(2):275–286. doi: 10.1111/j.1420-9101.2008.01638.x. [DOI] [PubMed] [Google Scholar]
- 32.Snook RR. Sperm in competition: Not playing by the numbers. Trends Ecol Evol. 2005;20(1):46–53. doi: 10.1016/j.tree.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Pizzari T, Parker GA. In: Sperm Biology: An Evolutionary Perspective. Birkhead TR, Hosken DJ, Pitnick S, editors. San Diego: Academic; 2009. pp. 207–245. [Google Scholar]
- 34.Simmons LW, Fitzpatrick JL. Sperm wars and the evolution of male fertility. Reproduction. 2012;144(5):519–534. doi: 10.1530/REP-12-0285. [DOI] [PubMed] [Google Scholar]
- 35.Ingleby FC, Hunt J, Hosken DJ. The role of genotype-by-environment interactions in sexual selection. J Evol Biol. 2010;23(10):2031–2045. doi: 10.1111/j.1420-9101.2010.02080.x. [DOI] [PubMed] [Google Scholar]
- 36.Clark AG, Begun DJ. Female genotypes affect sperm displacement in Drosophila. Genetics. 1998;149(3):1487–1493. doi: 10.1093/genetics/149.3.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark AG, Begun DJ, Prout T. Female x male interactions in Drosophila sperm competition. Science. 1999;283(5399):217–220. doi: 10.1126/science.283.5399.217. [DOI] [PubMed] [Google Scholar]
- 38.Clark AG, Dermitzakis ET, Civetta A. Nontransitivity of sperm precedence in Drosophila. Evolution. 2000;54(3):1030–1035. doi: 10.1111/j.0014-3820.2000.tb00102.x. [DOI] [PubMed] [Google Scholar]
- 39.Chow CY, Wolfner MF, Clark AG. The genetic basis for male x female interactions underlying variation in reproductive phenotypes of Drosophila. Genetics. 2010;186(4):1355–1365. doi: 10.1534/genetics.110.123174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang R, Clark AG, Fiumera AC. Natural genetic variation in male reproductive genes contributes to nontransitivity of sperm competitive ability in Drosophila melanogaster. Mol Ecol. 2013;22(5):1400–1415. doi: 10.1111/mec.12113. [DOI] [PubMed] [Google Scholar]
- 41.Bjork A, Starmer WT, Higginson DM, Rhodes CJ, Pitnick S. Complex interactions with females and rival males limit the evolution of sperm offence and defence. Proc Biol Sci. 2007;274(1619):1779–1788. doi: 10.1098/rspb.2007.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parsons PA, Hosgood SMW. Genetic heterogeneity among the founders of laboratory populations of Drosophila. I. Scutellar chaetae. Genetica. 1968;38(3):328–339. doi: 10.1007/BF01507465. [DOI] [PubMed] [Google Scholar]
- 43.David JR, et al. Isofemale lines in Drosophila: An empirical approach to quantitative trait analysis in natural populations. Heredity (Edinb) 2005;94(1):3–12. doi: 10.1038/sj.hdy.6800562. [DOI] [PubMed] [Google Scholar]
- 44.Lüpold S, et al. How multivariate ejaculate traits determine competitive fertilization success in Drosophila melanogaster. Curr Biol. 2012;22(18):1667–1672. doi: 10.1016/j.cub.2012.06.059. [DOI] [PubMed] [Google Scholar]
- 45.Manier MK, et al. Resolving mechanisms of competitive fertilization success in Drosophila melanogaster. Science. 2010;328(5976):354–357. doi: 10.1126/science.1187096. [DOI] [PubMed] [Google Scholar]
- 46.Manier MK, et al. Rapid diversification of sperm precedence traits and processes among three sibling Drosophila species. Evolution. 2013 doi: 10.1111/evo.12117. [DOI] [PubMed] [Google Scholar]
- 47. Manier MK, Lüpold S, Pitnick S, Starmer WT (2013) An analytical framework for estimating fertilization bias from multiple sperm-storage organs during sperm competition. Am Nat, 10.1086/671782. [DOI] [PubMed]
- 48.Gromko MH, Newport MEA. Genetic basis for remating in Drosophila melanogaster. II. Response to selection based on the behavior of one sex. Behav Genet. 1988;18(5):621–632. doi: 10.1007/BF01082313. [DOI] [PubMed] [Google Scholar]
- 49.Casares P, Carracedo MC, Piñeiro R, San Miguel E, Garcia-Florez L. Genetic basis for female receptivity in Drosophila melanogaster: A diallel study. Heredity (Edinb) 1992;69(Pt 5):400–405. doi: 10.1038/hdy.1992.142. [DOI] [PubMed] [Google Scholar]
- 50.Piñeiro R, Carracedo MC, Izquierdo JI, Casares P. Bidirectional selection for female receptivity in Drosophila melanogaster. Behav Genet. 1993;23(1):77–83. doi: 10.1007/BF01067556. [DOI] [PubMed] [Google Scholar]
- 51.Miller GT, Pitnick S. Sperm-female coevolution in Drosophila. Science. 2002;298(5596):1230–1233. doi: 10.1126/science.1076968. [DOI] [PubMed] [Google Scholar]
- 52.Pattarini JM, Starmer WT, Bjork A, Pitnick S. Mechanisms underlying the sperm quality advantage in Drosophila melanogaster. Evolution. 2006;60(10):2064–2080. [PubMed] [Google Scholar]
- 53.Miller GT, Pitnick S. Functional significance of seminal receptacle length in Drosophila melanogaster. J Evol Biol. 2003;16(1):114–126. doi: 10.1046/j.1420-9101.2003.00476.x. [DOI] [PubMed] [Google Scholar]
- 54.Gage MJG. Influences of sex, size, and symmetry on ejaculate expenditure in a moth. Behav Ecol. 1998;9(6):592–597. [Google Scholar]
- 55.Wedell N, Cook PA. Butterflies tailor their ejaculate in response to sperm competition risk and intensity. Proc Biol Sci. 1999;266(1423):1033–1039. [Google Scholar]
- 56.Simmons LW, Kvarnemo C. Ejaculate expenditure by male bushcrickets decreases with sperm competition intensity. Proc Biol Sci. 1997;264(1385):1203–1208. [Google Scholar]
- 57.Pitnick S, Brown WD, Miller GT. Evolution of female remating behaviour following experimental removal of sexual selection. Proc Biol Sci. 2001;268(1467):557–563. doi: 10.1098/rspb.2000.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pitnick S, García-González F. Harm to females increases with male body size in Drosophila melanogaster. Proc Biol Sci. 2002;269(1502):1821–1828. doi: 10.1098/rspb.2002.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pilastro A, Simonato M, Bisazza A, Evans JP. Cryptic female preference for colorful males in guppies. Evolution. 2004;58(3):665–669. [PubMed] [Google Scholar]
- 60.Gage MJG, et al. Spermatozoal traits and sperm competition in Atlantic salmon: Relative sperm velocity is the primary determinant of fertilization success. Curr Biol. 2004;14(1):44–47. [PubMed] [Google Scholar]
- 61.Malo AF, et al. Male fertility in natural populations of red deer is determined by sperm velocity and the proportion of normal spermatozoa. Biol Reprod. 2005;72(4):822–829. doi: 10.1095/biolreprod.104.036368. [DOI] [PubMed] [Google Scholar]
- 62.Dziminski MA, Roberts JD, Beveridge M, Simmons LW. Sperm competitiveness in frogs: Slow and steady wins the race. Proc Biol Sci. 2009;276(1675):3955–3961. doi: 10.1098/rspb.2009.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mazzi D, Kesäniemi J, Hoikkala A, Klappert K. Sexual conflict over the duration of copulation in Drosophila montana: Why is longer better? BMC Evol Biol. 2009;9:132. doi: 10.1186/1471-2148-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nilsson T, Fricke C, Arnqvist G. The effects of male and female genotype on variance in male fertilization success in the red flour beetle (Tribolium castaneum) Behav Ecol Sociobiol. 2003;53(4):227–233. [Google Scholar]
- 65.Zeh JA, Zeh DW. The evolution of polyandry II: Post-copulatory defences against genetic incompatibility. Proc Biol Sci. 1997;264(1378):69–75. [Google Scholar]
- 66.Tregenza T, Wedell N. Genetic compatibility, mate choice and patterns of parentage: Invited review. Mol Ecol. 2000;9(8):1013–1027. doi: 10.1046/j.1365-294x.2000.00964.x. [DOI] [PubMed] [Google Scholar]
- 67.Pitnick S, Brown WD. Criteria for demonstrating female sperm choice. Evolution. 2000;54(3):1052–1056. doi: 10.1111/j.0014-3820.2000.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 68.Yeates SE, et al. Atlantic salmon eggs favour sperm in competition that have similar major histocompatibility alleles. Proc Biol Sci. 2009;276(1656):559–566. doi: 10.1098/rspb.2008.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gillingham MAF, et al. Cryptic preference for MHC-dissimilar females in male red junglefowl, Gallus gallus. Proc Biol Sci. 2009;276(1659):1083–1092. doi: 10.1098/rspb.2008.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bishop JDD, Jones CS, Noble LR. Female control of paternity in the internally fertilizing compound ascidian Diplosoma listerianum. II. Investigation of male mating success using RAPD markers. Proc Biol Sci. 1996;263(1369):401–407. [Google Scholar]
- 71.Olsson M, Shine R, Madsen T, Gullberg A, Tegelström H. Sperm selection by females. Nature. 1996;383(6601):585. [Google Scholar]
- 72.Bretman A, Wedell N, Tregenza T. Molecular evidence of post-copulatory inbreeding avoidance in the field cricket Gryllus bimaculatus. Proc Biol Sci. 2004;271(1535):159–164. doi: 10.1098/rspb.2003.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pizzari T, Birkhead TR. Female feral fowl eject sperm of subdominant males. Nature. 2000;405(6788):787–789. doi: 10.1038/35015558. [DOI] [PubMed] [Google Scholar]
- 74.Nonidez JF. The internal phenomena of reproduction in Drosophila. Biol Bull. 1920;39(4):207–230. [Google Scholar]
- 75.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GS. Mixed Effects Models and Extensions in Ecology with R. New York: Springer; 2009. [Google Scholar]
- 76.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4(2):133–142. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.