Abstract
μ-Opioid receptors are among the most studied G protein-coupled receptors because of the therapeutic value of agonists, such as morphine, that are used to treat chronic pain. However, these drugs have significant side effects, such as respiratory suppression, constipation, allodynia, tolerance, and dependence, as well as abuse potential. Efforts to fine tune pain control while alleviating the side effects of drugs, both physiological and psychological, have led to the development of a wide variety of structurally diverse agonist ligands for the μ-opioid receptor, as well as compounds that target κ- and δ-opioid receptors. In recent years, the identification of allosteric ligands for some G protein-coupled receptors has provided breakthroughs in obtaining receptor subtype-selectivity that can reduce the overall side effect profiles of a potential drug. However, positive allosteric modulators (PAMs) can also have the specific advantage of only modulating the activity of the receptor when the orthosteric agonist occupies the receptor, thus maintaining spatial and temporal control of receptor signaling in vivo. This second advantage of allosteric modulators may yield breakthroughs in opioid receptor research and could lead to drugs with improved side-effect profiles or fewer tolerance and dependence issues compared with orthosteric opioid receptor agonists. Here, we describe the discovery and characterization of μ-opioid receptor PAMs and silent allosteric modulators, identified from high-throughput screening using a β-arrestin–recruitment assay.
Keywords: analgesia, DAMGO, enkephalins, endorphins, endomorphin
Significance of Opioid Receptors
G protein-coupled receptors (GPCRs) are plasma membrane-spanning proteins that transduce signals via heterotrimeric G proteins on the inner surface of the plasma membrane, leading to intracellular signaling cascades involved in many aspects of cellular function (1). The cell surface location, tissue distribution, and diversity of these GPCRs make them ideal targets for drug intervention. Indeed, about 30% of marketed drugs target specific GPCR activity (1, 2).
Opioid receptors are members of the class A family of GPCRs. Four opioid receptor types exist [μ, δ, κ, and opioid receptor-like 1 (ORL1)], which share about 60% amino acid identity (mainly in the transmembrane domains) and signal through the Gi/o family of heterotrimeric G proteins, resulting in inhibition of adenylyl cyclase, modulation of ion channel activity, and transcriptional changes in the cell (2). Opioid receptors (and many other GPCRs) can also signal through non-G protein-mediated pathways, one of which is initiated by β-arrestin recruitment to the receptor. β-Arrestin is involved in receptor desensitization and internalization/recycling (3, 4).
Opioid receptors are key targets in the management of pain and morphine and its derivatives induce pain relief by acting as agonists at opioid receptors, especially the μ-opioid receptor (5, 6). Opioid receptors have been extensively studied because of the need for better pain control while trying to reduce or eliminate adverse side effects. These side effects include tolerance, respiratory suppression, constipation, allodynia, and dependence (3, 7).
To overcome these side effects, studies have focused on developing ligands with defined selectivity profiles between the various opioid receptors (μ, κ, δ), or partial agonists (which have reduced efficacy compared with full agonists), or agonists used together in combination therapy (7, 8). However, these diverse approaches have a single commonality in that they target the orthosteric (endogenous) agonist-binding site of the receptor. A different approach that has been used successfully with other GPCRs is the discovery and development of allosteric ligands, which can have specific advantages over their orthosteric counterparts.
Allosteric Ligands
Allosteric ligands for a GPCR bind to a site on the receptor that is distinct from the site that binds the orthosteric (or endogenous) agonist (9). An allosteric modulator can exhibit a range of activities at the target protein. Positive allosteric modulators (PAMs) may have no intrinsic efficacy but, when they bind to the receptor, enhance the binding affinity and/or efficacy of the orthosteric agonist. Negative allosteric modulators (NAMs) have no intrinsic agonist efficacy but, when they bind to the receptor, inhibit the binding affinity and/or efficacy of the orthosteric agonist. Silent allosteric modulators (SAMs), also known as neutral allosteric ligands, bind to the receptor but have no effect on orthosteric agonist affinity or efficacy. However, SAMs can act as competitive antagonists at the same allosteric site, blocking PAM or NAM activity. Although not particularly useful from a therapeutic standpoint, SAMs can be effective tools to show that presumed PAM or NAM effects are receptor-mediated. Finally, allosteric agonists can bind and produce direct agonist activation of the receptor even in the absence of orthosteric agonist.
Because allosteric sites are less evolutionarily conserved than orthosteric agonist-binding sites, allosteric ligands have the potential to exhibit greater selectivity between subtypes of GPCRs in the same family compared with orthosteric ligands. This has been demonstrated for some GPCRs, including metabotropic glutamate receptors, adenosine receptors, and muscarinic receptors (10–14). Although highly selective orthosteric agonist ligands exist for opioid receptors, opioid receptor PAMs could potentially provide benefits over existing medications. PAMs, unlike allosteric agonists, may have no effect when they bind to the receptor in the absence of orthosteric agonist. Therefore, the modulation occurs only when an orthosteric agonist is bound to the receptor. In vivo, this leads to preservation of the temporal and spatial characteristics of cell signaling; this is important, especially for signaling in the complex neuronal networks in the brain and enteric nervous system. Additionally, by preserving the temporal aspects of native receptor signaling, PAMs may avoid receptor down-regulation and other compensatory mechanisms that are triggered by sustained receptor activation produced by exogenous orthosteric agonists. Therefore, one can speculate that opioid receptor PAMs could produce less tolerance and dependence than exogenous orthosteric agonists. Here, we describe the discovery and characterization of μ-opioid receptor PAMs and SAMs. A high-throughput screen (HTS) was developed and executed using a β-arrestin–recruitment assay. μ-Selective PAMs resulting from the HTS were shown to be active in β-arrestin–recruitment assays and in G protein-mediated signaling assays (inhibition of adenylyl cyclase activity and [35S]GTPγS binding). These studies describe the existence of μ-selective PAMs and SAMs, implicating positive allostery as a potential avenue for the discovery of tightly regulated pain therapeutics.
Results and Discussion
Identification of μ-Opioid Receptor PAMs.
Potential opioid receptor ligands were identified from an HTS campaign using the PathHunter enzyme complementation assay technology (DiscoveRx) (15). In this system, an N-terminal deletion mutant of β-galactosidase is fused to the C terminus of stably expressed β-arrestin 2 in human osteosarcoma (U2OS) cells. A mutated amino-terminal fragment of β-galactosidase, termed ProLink/enzyme donor (PK), is fused to the carboxyl terminus of the μ-opioid receptor (OPRM1) expressed in these cells (U2OS-OPRM1). Binding of arrestin to the activated μ-opioid receptor results in a complementation of the enzyme and reconstitution of enzyme activity, thus providing a measure of the recruitment of arrestin to the receptor. The HTS campaign was performed in the presence of a low (20 nM; ∼EC10) concentration of the μ-selective orthosteric agonist, endomorphin-I, to identify both agonists and positive allosteric modulators (PAMs). Two compounds were identified as potential μ-opioid receptor PAMs (μ-PAMs). These compounds have been designated as BMS-986121 and BMS-986122 (Fig. 1 A and B). Neither BMS-986121 nor BMS-986122 produced significant β-arrestin recruitment on their own (agonist-detection mode), but both compounds significantly augmented the β-arrestin–recruitment response produced by a low concentration of endomorphin-I (PAM-detection mode) (Fig. 1 C and D). In PAM-detection mode, BMS-986121 increased β-arrestin recruitment by 20 nM endomorphin-I to a maximal effect (Emax) of 76% [95% confidence interval (CI): 69–83%] of the response evoked by a maximally effective (1 µM) concentration of endomorphin-I, with an EC50 of 1.0 µM (95% CI: 0.7–1.6 µM). BMS-986122 produced a similar PAM-detection mode response, increasing the effect of the low concentration of endomorphin-I to 83% (95% CI: 78–89%) of the maximal endomorphin-I response with an EC50 of 3.0 µM (95% CI: 1.9–3.9 µM).
Fig. 1.
Chemical structure and effect of μ-PAMs on β-arrestin recruitment. Two apparent μ-opioid receptor-selective μ-PAMs, BMS-986121 (A) and BMS-986122 (B), were assayed (C and D) at varying concentrations in agonist-detection mode (with compound alone) and in PAM-detection mode [compound in the presence of a low concentration (∼EC10) of orthosteric agonist, either endomorphin-I (20 nM) for μ-expressing cells (U2OS-OPRM1) or leu-enkephalin (0.4 nM) for δ-expressing cells (U20S-OPRD1)]. Data are represented as means ± SEM of three experiments. In agonist-detection mode, 0 and 100% activity represent basal activity and an Emax of endomorphin-I (in U2OS-OPRM1, a sixfold signal) or leu-enkephalin (in U2OS-OPRD1, a fourfold signal), respectively. In PAM-detection mode, 0% activity is normalized to the low concentration (∼EC10) of orthosteric agonist; 100% activity represents the response to an Emax concentration of orthosteric agonist.
We have no direct evidence that these compounds bind to the μ-opioid receptor as opposed to an associated protein. To approach this problem, the compounds were examined in a similar assay in U2OS PathHunter cells expressing PK-tagged δ-opioid receptors (U2OS-OPRD1). Neither compound had any significant effect in the absence (agonist-detection mode) or the presence (PAM-detection mode) of an ∼EC10 (0.4 nM) of the δ agonist leu-enkephalin (Fig. 1 C and D). Therefore, the compounds appear to be selective for μ- over δ-opioid receptors, which would support a direct interaction with μ receptors. However, we cannot rule out that the compounds might be SAMs at the δ-opioid receptor. SAMs bind to an allosteric site but do not produce any effect on orthosteric-agonist potency or efficacy.
Characterization of μ-PAMs.
To further assess μ-PAM activity, BMS-986121 and BMS-986122 were tested in three functional assays, β-arrestin recruitment, inhibition of adenylyl cyclase activity, and G protein activation using [35S]GTPγS binding. Compounds were also assessed in receptor-binding assays.
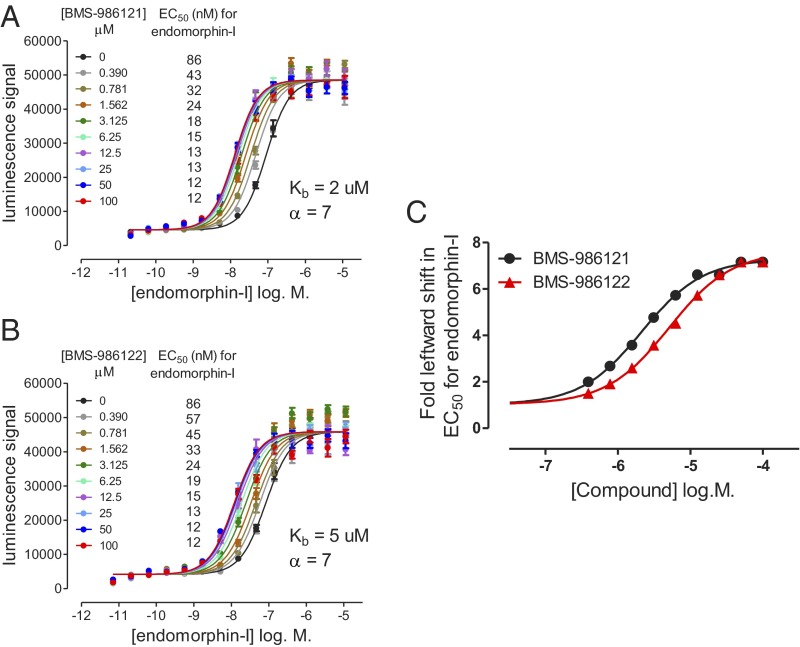
Concentration–response curves (CRCs) for endomorphin-I–mediated recruitment of β-arrestin were generated in the absence or presence of varying concentrations of each μ-PAM. BMS-986121 (Fig. 2A) or BMS-986122 (Fig. 2B) produced concentration-dependent and saturable leftward shifts in the potency of endomorphin-I. Curves were fitted globally using an allosteric ternary complex model (SI Materials and Methods). BMS-986121 produced a cooperativity factor (α) of 7 and a Kb (the equilibrium dissociation constant for the PAM binding to its allosteric site) of 2 µM. BMS-986122 produced a cooperativity factor of 7 and a Kb of 5 µM. The magnitude (fold shift) of the increase in endomorphin-I potency produced by each concentration of PAM is also shown (Fig. 2C).
Fig. 2.
Effect of μ-PAMs BMS-986121 and BMS-986122 on endomorphin-I stimulated β-arrestin recruitment in U2OS-OPRM1 cells. Both BMS-986121 (A) and BMS-986122 (B) produced concentration-dependent leftward shifts in the β-arrestin–recruitment response to the agonist endomorphin-I. The data were analyzed simultaneously using an allosteric ternary complex model to provide Kb and cooperativity factor (α) values for each compound (SI Materials and Methods). Calculated EC50 values (nanomoles per liter) for endomorphin-I at each concentration of compound are shown in the legend of each graph. The fold leftward shift in EC50 values for endomorphin-I in the presence of increasing concentrations of PAM compound is presented (C). Data are represented as means ± SEM of four experiments.
Opioid receptors inhibit adenylyl cyclase via Gαi/o proteins (16). To assess the effects of the μ-PAMs on this signaling pathway, their effects were measured in a cAMP-accumulation assay. The cAMP-inhibition responses produced by opioid agonists in the U20S PathHunter cells were small and inadequate for robust measurement. Therefore, a Chinese hamster ovary (CHO) cell line recombinantly expressing human μ-opioid receptors (CHO-μ) was used. In this cell line, endomorphin-I produced a 17-fold reduction in forskolin-stimulated (1 µM) cAMP accumulation with an EC50 of 76 pM (95% CI: 60–96 pM) (Fig. S1). BMS-986121 and BMS-986122 significantly increased the inhibition of forskolin-stimulated adenylyl cyclase activity produced by an ∼EC10 (30 pM) concentration of endomorphin-I in CHO-μ cells (Fig. 3 A and B). BMS-986121 and BMS-986122 both afforded potentiation with EC50 values of 3.1 µM (95% CI: 2.0–4.8 µM) and 8.9 µM (95% CI: 6.1–13.1 µM), respectively. These values are slightly higher than EC50 values observed in the β-arrestin assay but are within threefold. The maximal inhibition produced by endomorphin-I in the presence of the PAMs was similar to that of a maximal concentration (10 nM) of endomorphin-I alone. In this assay, both μ-PAMs also exhibited some intrinsic agonist activity causing inhibition of cAMP accumulation in the absence of any orthosteric agonist (Fig. 3 A and B). The low-efficacy agonist activity of BMS-986121 [EC50 of 13 µM (95% CI: 4–51 µM); Emax of 36% (95% CI: 21–52%)] was not always reproducible and, on some occasions, was too low to determine a fit of the concentration response data. BMS-986122 agonist activity [EC50 of 41 µM (95% CI: 20–86 µM); Emax of 60% (95% CI: 24–95%)] was more apparent. The agonist activity of BMS-986121 and BMS-986122 was seen only at concentrations above those required to produce significant potentiation of an endomorphin-I response, and both agonist responses failed to reach the Emax of endomorphin-I. The EC50 values for BMS-986121 and BMS-986122 in the absence of orthosteric agonist may more closely reflect the affinity of these compounds for the receptor because of the lack of reciprocal affinity modulation by the orthosteric agonist. The discrepancy between the agonist activity of the PAMs seen in this assay and the lack of agonist activity seen in the β-arrestin–recruitment assay in U2OS-OPRM1 cells may be attributable to differences in receptor reserve for the two assays and/or cell lines. It has been shown previously that PAMs can exhibit agonist activity in recombinant cells expressing high levels of GPCR protein (17).
Fig. 3.
Effect of μ-PAMs on inhibition of forskolin-stimulated cAMP accumulation in CHO-μ cells. Both BMS-986121 (A) and BMS-986122 (B) increased the effect of a low (∼EC10; 30 pM) concentration of endomorphin-I (PAM-detection mode) in a concentration-dependent manner. The compounds also showed some agonist activity when added alone (agonist-detection mode). For agonist-detection mode, 0% activity represents vehicle (basal) activity. For PAM-detection mode, 0% is normalized to the response to an ∼EC10 (30 pM) concentration of endomorphin-I. The 100% response represents the response to an Emax concentration of endomorphin-I (10 nM) in both agonist and PAM-detection modes. Data are represented as means ± SEM of three experiments.
Next, the μ-PAMs were characterized in G protein activation [35S]GTPγS-binding studies in membranes from C6 glioma cells stably expressing recombinant μ-opioid receptors (C6μ) (18). Agonist-stimulated [35S]GTPγS binding was determined after 5 min of incubation to capture the initial rate of G protein activation which can differentiate partial agonists from full agonists. The μ-opioid receptor agonist [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) at 10 µM produced a 250% stimulation of [35S]GTPγS binding above basal activity, with an EC50 of 222 nM (95% CI: 179–274 nM). Addition of BMS-986122 (10 µM) resulted in DAMGO potency shifting leftwards by sevenfold [EC50 of 32 nM (95% CI: 25–40 nM)] (Fig. 4A). BMS-986121 (10 µM) produced a fourfold leftward shift in the DAMGO CRC [EC50 of 57 nM (95% CI: 37–89 nM)] (Fig. S2A). No significant agonist activity was detected for either of the PAM compounds in this assay.
Fig. 4.
Effect of μ-PAM, BMS-986122, on μ-opioid agonist-stimulated [35S]GTPγS binding in membranes from C6μ cells and mouse brain and DAMGO binding affinity in C6μ cell membranes. [35S]GTPγS binding in C6μ membranes was determined as described in Methods and Materials. The EC50 of DAMGO to stimulate [35S]GTPγS binding was shifted to the left sevenfold in the presence of 10 µM BMS-986122 (A). The maximal stimulation by DAMGO was not affected by BMS-986122. The potency of morphine to stimulate [35S]GTPγS binding was shifted to the left threefold in the presence of 10 µM BMS-986122, and the Emax of morphine compared with DAMGO was increased by BMS-986122 (B). BMS-986122 (10 µM) also produced a sixfold leftward shift in DAMGO affinity in DAMGO competition-binding studies with [3H]diprenorphine (C) but had no effect on [3H]diprenorphine-binding affinity (Fig. S3A and Table S1). The EC50 of DAMGO to stimulate [35S]GTPγS binding in membranes from mouse brain was shifted to the left 4.5-fold in the presence of 10 µM BMS-986122 (D). Basal [35S]GTPγS binding (femtomoles bound per milligram of protein: 3.2 ± 0.2 in C6μ cells and 4.8 ± 0.4 in mouse brain) was not affected by 10 μΜ BMS-986122. Shown are the combined means ± SEM data from three to seven separate assays, each performed in duplicate.
μ-Opioid receptor ligand binding was determined in the presence of 100 mM NaCl and 10 µM GTPγS, a buffer that favors a low agonist-affinity state of the receptor (19, 20). In saturation binding studies, the μ-PAM BMS-986122 did not affect [3H]diprenorphine-binding affinity [Kd in the presence of vehicle was 0.27 nM (95% CI: 0.21–0.32 nM); Kd in the presence of BMS-986122 (100 µM) was 0.34 nM (95% CI: 0.13–0.55 nM)] (Fig. S3A). However, BMS-986122 significantly increased the affinity of DAMGO by sixfold in competition studies with 0.2–0.3 nM [3H]diprenorphine binding [DAMGO Ki in the presence of vehicle was 340 nM (95% CI: 208–552 nM); Ki in the presence of BMS-986122 (10 µM) was 56 nM (95% CI: 41–76 nM)] (Fig. 4C, Fig. S3A, and Table S1). Binding studies were also performed in Tris⋅HCl buffer in the absence of sodium and GTPγS, a buffer that favors a high agonist-affinity state of the receptor. In this buffer, DAMGO was ∼150-fold more potent in displacing [3H]diprenorphine binding [DAMGO Ki, 2.21 nM (95% CI: 1.51–3.23 nM)]. BMS-986122 (10 µM) produced a fourfold leftward shift in the Ki value for DAMGO [Ki = 0.51 nM (95% CI: 0.36–0.72 nM)] compared with vehicle under these conditions (Table S1). The Kd of [3H]diprenorphine was again unaffected by BMS-986122 (10 µM) (Table S1). Together, these data are consistent with the hypothesis that BMS-986122 can, at least in part, enhance the affinity of the orthosteric agonist DAMGO for the μ-opioid receptor.
Enhancement of the maximal response to a partial agonist in the [35S]GTPγS-binding assay would suggest that the μ-PAMs are also able to modulate agonist efficacy. Morphine produced an Emax of 42% (95% CI: 38–45%) of the response induced by 30 µM DAMGO after 5 min of incubation with [35S]GTPγS, with an EC50 of 110 nM (95% CI: 71–171 nM) (Fig. 4B), confirming that morphine is a partial agonist in this assay system relative to DAMGO (18). The potency of morphine was shifted to the left threefold in the presence of 10 µM BMS-986122 [EC50 of 38 nM (95% CI: 24–61 nM)], and the Emax of morphine compared with DAMGO was increased [74% (95% CI: 68–81%)] (Fig. 4B). Similar effects were observed in the presence of 10 µM BMS-986121, which increased morphine potency by 2.5-fold [EC50 of 45 (95% CI: 29–68 nM)] and increased the Emax of morphine [72% (95% CI: 67–78%)] (Fig. S2B).
Although a full agonist in the β-arrestin–recruitment assay in U2OS-OPRM1 cells and in the inhibition of forskolin-stimulated cAMP assay in CHO-μ cells, endomorphin-I exhibits only partial agonist activity in the [35S]GTPγS-binding assay in C6μ cell membranes (18). In this assay, BMS-986122 (10 µM) produced similar effects on endomorphin-I potency and efficacy as those seen with morphine (Fig. S4). Together, these data confirm that BMS-986122 can positively modulate agonist efficacy, measured as an increase in maximal responses to the partial agonists morphine and endomorphin-I in this system.
μ-PAM Activity in DAMGO-Stimulated [35S]GTPγS Binding in Membranes from Mouse Brain.
The previous experiments used heterologous cell systems expressing high concentrations of receptors. To determine whether μ-PAM activity can be observed in native tissues, DAMGO-stimulated [35S]GTPγS binding in membranes from mouse brain was assessed (Fig. 4D). The potency of DAMGO to stimulate [35S]GTPγS binding [EC50 of 458 nM (95% CI: 245–856 nM)] was shifted to the left 4.5-fold in the presence of BMS-986122 [EC50 of 101 nM (95% CI: 56–183 nM)]. No agonist activity was observed with BMS-986122. Therefore, μ-PAM activity can also be observed for DAMGO-mediated G protein activation in membranes from a physiologically relevant tissue with endogenous levels of receptor and G protein.
Structure–Activity Studies and the Identification of μ-Opioid Receptor SAMs.
A number of close analogs of BMS-986122 were tested in the β-arrestin–recruitment assay to explore the structure–activity relationship (SAR) of the chemical series. Of the 15 analogs tested, 13 showed at least some PAM activity at the μ receptor. None of these compounds showed agonist activity at the μ-opioid receptor (Fig. S5). However, five of the analogs did show some weak agonist or PAM activity at the δ receptor (Fig. S5), suggesting that modifications to this chemotype may alter the selectivity for μ-opioid vs. δ-opioid receptors. It is also conceivable that other analogs tested may be SAMs at the δ-opioid receptor, which would not be detectable in this assay. Modifications to the structure of BMS-986122 affected the compound’s PAM activity in U2OS-OPRM1 cells (Fig. S5 and Table S2). For the most part, the analogs examined retain similar potencies relative to BMS-986122 (EC50 values in the low micromolar range). However, minor changes to the structure of BMS-986122 led to a pronounced reduction in the Emax values observed in PAM-detection mode. This can be inferred to correspond to a decrease in the maximum leftward shift in endomorphin-I potency that can be produced by a compound. Of the analogs tested, none exhibited greater PAM activity than the original screening hit BMS-986122.
It has been observed that allosteric modulators of GPCRs can often exhibit “activity switching” within a chemical series: minor modifications can change a compound from a PAM to a NAM or SAM (21). Two of the 15 BMS-986122 analogs tested exhibited no measurable PAM activity, which could be attributable to loss of binding affinity, or functional switching from PAMs to NAMs or SAMs. Therefore, these two analogs (designated BMS-986123 and BMS-986124) were assessed for their ability to inhibit orthosteric agonist activity (in a NAM-detection mode assay) or for their ability to inhibit BMS-986122 PAM activity (a SAM-detection mode assay) in the β-arrestin–recruitment assay in U2OS-OPRM1 cells and in the [35S]GTPγS assay in C6μ cells. Neither compound significantly inhibited an EC80 concentration of endomorphin-I (300 nM) (Fig. S6), suggesting that they are not NAMs or orthosteric antagonists. However, both compounds were able to inhibit the PAM response to 12.5 µM (∼EC80) BMS-986122 in U2OS-OPRM1 cells in the presence of 30 nM (∼EC20) endomorphin-I (Fig. 5A). Calculated Kb values (the inhibition constant for a competitive antagonist which at equilibrium would occupy 50% of the receptors in the absence of agonist) for SAM activity of BMS-986123 and BMS-986124 were 1 and 2 µM, respectively (Table S2). DAMGO potency to stimulate [35S]GTPγS binding in C6μ cell membranes [EC50 of 224 nM (95% CI: 167–300 nM)] was again enhanced eightfold in the presence of BMS-986122 (10 µM) [EC50 of 29 nM (95% CI: 22–38 nM)] (Fig. 5B and Fig. S7). Coaddition of BMS-986122 (10 µM) with BMS-986124 (50 µM) resulted in an inhibition of the PAM effect, with DAMGO potency enhanced less than twofold [EC50 of 128 nM (95% CI: 97–168 nM)] (Fig. 5B and Fig. S7). Together, these data confirm that the PAM effects of BMS-986122 can be antagonized by BMS-986124 and strongly suggest that BMS-986123 and BMS-986124 are μ-opioid receptor SAMs (μ-SAMs), competitive antagonists at the allosteric site to which BMS-986122 binds.
Fig. 5.
Characterization of functional SAMs in the β-arrestin–recruitment assay and in DAMGO-mediated [35S]GTPγS binding. (A) BMS-986123 and BMS-986124 inhibited PAM responses to an ∼EC80 concentration of BMS-986122 (12.5 µM) plus an ∼EC20 concentration of endomorphin-I (30 nM) (SAM-detection mode) in the β-arrestin–recruitment assay in U2OS-OPRM1 cells; 100% activity represents the activity of the combined BMS-986122 plus endomorphin-I, and 0% activity represents the activity of the ∼EC20 concentration of endomorphin-I alone. Graphs show the means ± SEM of three experiments. BMS-986123 and BMS-986124 showed no activity in agonist or PAM-detection modes in either U2OS-OPRM1 cells or U2OS-OPRD1 cells (Fig. S5). Similarly, the compounds showed no NAM/antagonist activity [in the presence of an ∼EC80 (300 nM) concentration of endomorphin-I] in U2OS-OPRM1 cells (Fig. S6). (B) DAMGO potency to stimulate [35S]GTPγS binding in C6μ membranes was increased eightfold in the presence of the μ-PAM BMS-986122 (10 µM). Coincubation of the SAM BMS-986124 (50 µM) with BMS-986122 (10 µM) resulted in only a twofold increase in potency for DAMGO. Shown are the combined means ± SEM data from three to seven separate assays, each performed in duplicate. EC50 values were compared by Student t test using GraphPad Prism. **P < 0.01. CRCs for DAMGO-stimulated [35S]GTPγS binding under the various conditions are shown in Fig. S7.
The μ-SAM BMS-986123 produced a small (∼twofold) but significant decrease in [3H]diprenorphine-binding affinity (Fig. S3B) but had no significant effect on DAMGO-binding affinity (Table S1). The potency of DAMGO or morphine in the [35S]GTPγS assay was not significantly increased by BMS-986123 or BMS-986124 (Figs. S8 and S9) at 10 µM, although both SAMs increased morphine Emax to a small degree (Fig. S9). It is possible that these compounds are not truly “silent” (neutral) modulators and, in fact, show some weak efficacy enhancement that is only evident with a partial agonist (morphine) as opposed to a full agonist (DAMGO), although the observed enhancement in efficacy was relatively small. No significant agonist activity was detected for either of the SAM compounds in this assay.
Probe Dependence.
Probe dependence (the ability of an allosteric modulator to modulate one orthosteric agonist differentially versus another) is a striking characteristic of some allosteric modulators (22). BMS-986121 (100 µM) produced leftward shifts in the potency of endomorphin-I (fourfold), morphine (fivefold), and leu-enkephalin (sixfold), in inhibition of forskolin-stimulated cAMP-accumulation assays in CHO-μ cells (Fig. S10). Taken together with the DAMGO and morphine datasets from the [35S]GTPγS-binding studies and the β-arrestin data, there is no current evidence to suggest marked agonist probe dependence because BMS-986121 produced similar potentiation of peptide agonist- and small molecule agonist-evoked responses.
Possible Implications of Opioid Receptor PAMs for Drug Discovery.
With traditional agonist ligands, the receptor is turned on for long periods (based on the dosing regime), often resulting in adverse effects, such as desensitization of the receptor response or receptor-mediated side effects caused by long-term stimulation. In the case of opioid receptors, long-term dosing with opiates leads to the development of tolerance and dependence, as well as other acute receptor-mediated side effects, such as respiratory suppression, constipation, and allodynia (23). We have determined that the μ-PAMs described here can positively modulate μ-opioid receptor responses to the endogenous agonists endomorphin-I and leu-enkephalin. It will be important to determine whether μ-PAMs can produce antinociceptive effects when administered alone in vivo, by potentiating responses to endogenous opioid agonists. Evidence for a basal tone of μ-opioid receptor activation does exist. For example, inhibition of enkephalinases, which break down endogenous opioid peptides, results in antinociception in animal models of inflammatory and neuropathic pain (24). In addition, the opioid receptor antagonist naloxone increased pain perception when administered to postoperative patients who were not receiving exogenous opiates, suggesting that endogenous opioid peptides produce a basal analgesic tone (25).
Another advantage of PAMs is their ability to shift the potency of orthosteric agonist to the left by a finite amount. For example, the analogs of BMS-986122 (Table S2) showed a differential ability to shift the potency of endomorphin-I to the left, resulting in different Emax values. Drug-development programs can take advantage of this finite potency shift to improve safety by designing PAMs that cannot exceed the required level of effect.
Opioid tolerance results from prolonged exposure to opiates, resulting in changes in neuronal function. Similarly, dependence occurs following chronic opioid administration. It can be hypothesized that a lower dose of morphine administered together with a μ-PAM might produce the same functional response as a higher dose of morphine alone and, so, may spare the development of tolerance and dependence. There is some precedence for this with GABAB receptors. The GABAB receptor PAM, GS39783, when combined with a low dose of agonist, produced the same level of functional response as a higher dose of GABAB agonist and yet produced less GABAB receptor desensitization (26). This may suggest that coadministration of lower doses of opiates with a μ-PAM may discriminate between the therapeutic analgesic properties of opiates and their tolerance and dependence liabilities.
It should be noted that most of the untoward side effects of opiates (e.g., respiratory depression, constipation) are mediated through μ receptors, and there is no a priori reason to assume that μ-PAMs would not potentiate these unwanted effects of opiates, as well as their desired therapeutic effects. It has recently been suggested that biased signaling by opioid agonists may represent a therapeutic strategy for minimizing side effects while maintaining analgesic efficacy (27). What effects, if any, μ-PAMs may have on signaling bias remain unknown. One can envision that a μ-PAM could potentially bias an orthosteric agonist response away from signaling pathways that mediate tolerance and dependence and other unwanted effects in favor of signaling pathways that mediate a therapeutic response. The ability of allosteric modulators to engender signaling bias has been observed in other systems (28). Although this is currently speculation, answers to these important questions should be forthcoming using tools such as those described here.
Summary.
We have characterized two μ-opioid receptor-selective PAMs. The BMS-986122 chemotype showed chemical tractability from SAR studies and also led to the identification of μ-opioid receptor SAMs. The two PAMs show potentiation of orthosteric agonist-mediated β-arrestin recruitment, adenylyl cyclase inhibition, and G protein activation. BMS-986122 potentiates DAMGO-mediated [35S]GTPγS binding in mouse brain membranes and appears to be, at least in part, a positive affinity modulator of the μ-opioid receptor for DAMGO binding. These studies provide proof-of-concept for the development of opioid allosteric modulators that may have therapeutic potential in pain management with improved side-effect profiles and reduced tolerance and dependence liabilities.
Materials and Methods
Reagents and Cells.
PathHunter β-arrestin U2OS cells engineered to express either PK-tagged OPRM1 (μ-opioid) receptors or PK-tagged OPRD1 (δ-opioid) receptors were from DiscoveRx. CHO cells (CHO-K1) expressing recombinant μ-opioid receptors (CHO-μ) were from PerkinElmer. C6 glioma cells stably expressing recombinant μ-opioid receptors (C6μ) were developed as described previously (29). Cell culture media and supplements were from Life Technologies. Homogeneous time resolved fluorescence resonance energy transfer (HTRF) cAMP detection reagents were from Cisbio. PathHunter detection reagents were from DiscoveRx. Morphine sulfate, leu-enkephalin, β-endorphin, and all other nonopioid ligand biochemical reagents were from Sigma-Aldrich. [35S]GTPγS and [3H]diprenorphine were from PerkinElmer. All other opioid ligands were from Tocris.
β-Arrestin–Recruitment Assay.
The β-arrestin–recruitment assay was performed in U2OS-OPRM1 and U2OS-OPRD1 cell suspensions, according to DiscoveRx established protocols (SI Materials and Methods).
Inhibition of Forskolin-Stimulated cAMP-Accumulation Assays.
Inhibition of forskolin-stimulated cAMP accumulation was conducted in CHO-μ cell suspensions using the CisBio HTRF cAMP detection kit with established protocols (SI Materials and Methods).
Cell Membrane Homogenates.
C6 glioma cells stably expressing rat μ-opioid receptor (MOR) (C6μ) were grown, and cell membranes were prepared as described previously (30). For mouse brain membranes, mice (strain C57/BL6) were euthanized by cervical dislocation. Whole-brain tissue, minus cerebellum, was removed and immediately chilled in ice-cold 50 mM Tris⋅HCl (pH 7.4), and membrane homogenates were prepared as described previously (31). Final membrane pellets were resuspended in 50 mM Tris⋅HCl (pH 7.4), aliquoted, and stored at −80 °C. Protein content was determined using the method of Bradford (32).
[35S]GTPγS Binding Assay.
[35S]GTPγS binding in membranes was conducted as described previously (18) (SI Materials and Methods), using morphine or DAMGO in the presence or absence of 10 µM modulator for 5 min at room temperature.
[3H]diprenorphine Saturation Binding Studies.
[3H]diprenorphine saturation binding studies were performed as described previously (33). Briefly, membranes (5 μg) were incubated with 0–4 nM [3H]diprenorphine and 10 µM modulator with or without 10 µM naloxone in 100 mM NaCl, 10 µM GTPγS, 5 mM MgCl2, and 50 mM Tris⋅HCl (pH 7.4) for 80 min at room temperature. Samples were quickly filtered through glass-fiber filter mats as described for [35S]GTPγS binding in SI Materials and Methods.
[3H]diprenorphine Competition-Binding Studies.
[3H]diprenorphine competition-binding studies were performed as described previously (34). Membranes (10 μg) were incubated with 0.2 nM [3H]diprenorphine and 0–10 µM DAMGO with or without 10 µM modulator and/or 10 µM naloxone in 100 mM NaCl, 10 µM GTPγS, 5 mM MgCl2, and 50 mM Tris⋅HCl (pH 7.4) for 60 min at room temperature. Samples were quickly filtered through glass-fiber filter mats as described for [35S]GTPγS binding in SI Materials and Methods.
Data Analysis.
Concentration response data were fit to a logistic equation (Eq. 1) using nonlinear regression analysis to provide estimates of Ymin (Bottom), Ymax (Top), potency (EC50), and slope factor (HillSlope), using GraphPad Prism Version 5.01 (sigmoidal dose–response with variable slope):
 |
Where P values are described, data were analyzed by two-way ANOVA with a Bonferroni posttest using GraphPad Prism 5.01.
The β-arrestin curve-shift assays in Fig.2 were analyzed using an allosteric ternary complex model (GraphPad Prism Version 5.01; Dose–response–Special–Allosteric EC50 shift) to determine log Kb and the cooperativity factor (α) of the PAMs (SI Materials and Methods).
Supplementary Material
Acknowledgments
We thank A. Moytl, S. Madari, J. Prentiss, Dr. S. Jayachandra, and L. Rosenthal for technical assistance and Dr. A. J. Watson for assistance with manuscript preparation. This work was supported by National Institutes of Health Grant MH083754 (to J.R.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1300393110/-/DCSupplemental.
References
- 1.Jacoby E, Bouhelal R, Gerspacher M, Seuwen K. The 7 TM G-protein-coupled receptor target family. ChemMedChem. 2006;1(8):761–782. doi: 10.1002/cmdc.200600134. [DOI] [PubMed] [Google Scholar]
- 2.Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem. 2004;73:953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- 3.Whalen EJ, Rajagopal S, Lefkowitz RJ. Therapeutic potential of β-arrestin- and G protein-biased agonists. Trends Mol Med. 2011;17(3):126–139. doi: 10.1016/j.molmed.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shukla AK, Xiao K, Lefkowitz RJ. Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling. Trends Biochem Sci. 2011;36(9):457–469. doi: 10.1016/j.tibs.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthes HW, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383(6603):819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 6.Manglik A, et al. Crystal structure of the µ-opioid receptor bound to a morphinan antagonist. Nature. 2012;485(7398):321–326. doi: 10.1038/nature10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietis N, et al. Simultaneous targeting of multiple opioid receptors: A strategy to improve side-effect profile. Br J Anaesth. 2009;103(1):38–49. doi: 10.1093/bja/aep129. [DOI] [PubMed] [Google Scholar]
- 8.Davis MP. Evidence from basic research for opioid combinations. Expert Opin Drug Discov. 2012;7(2):165–178. doi: 10.1517/17460441.2012.648611. [DOI] [PubMed] [Google Scholar]
- 9.Burford NT, Watson J, Bertekap R, Alt A. Strategies for the identification of allosteric modulators of G-protein-coupled receptors. Biochem Pharmacol. 2011;81(6):691–702. doi: 10.1016/j.bcp.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Birdsall NJ, Lazareno S. Allosterism at muscarinic receptors: Ligands and mechanisms. Mini Rev Med Chem. 2005;5(6):523–543. doi: 10.2174/1389557054023251. [DOI] [PubMed] [Google Scholar]
- 11.Bruns RF, Fergus JH. Allosteric enhancement of adenosine A1 receptor binding and function by 2-amino-3-benzoylthiophenes. Mol Pharmacol. 1990;38(6):939–949. [PubMed] [Google Scholar]
- 12.Conn PJ, Jones CK, Lindsley CW. Subtype-selective allosteric modulators of muscarinic receptors for the treatment of CNS disorders. Trends Pharmacol Sci. 2009;30(3):148–155. doi: 10.1016/j.tips.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao ZG, Kim SK, Ijzerman AP, Jacobson KA. Allosteric modulation of the adenosine family of receptors. Mini Rev Med Chem. 2005;5(6):545–553. doi: 10.2174/1389557054023242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasparini F, Kuhn R, Pin JP. Allosteric modulators of group I metabotropic glutamate receptors: Novel subtype-selective ligands and therapeutic perspectives. Curr Opin Pharmacol. 2002;2(1):43–49. doi: 10.1016/s1471-4892(01)00119-9. [DOI] [PubMed] [Google Scholar]
- 15.Bassoni DL, Raab WJ, Achacoso PL, Loh CY, Wehrman TS. Measurements of β-arrestin recruitment to activated seven transmembrane receptors using enzyme complementation. Methods Mol Biol. 2012;897:181–203. doi: 10.1007/978-1-61779-909-9_9. [DOI] [PubMed] [Google Scholar]
- 16.Clark MJ, Furman CA, Gilson TD, Traynor JR. Comparison of the relative efficacy and potency of mu-opioid agonists to activate Galpha(i/o) proteins containing a pertussis toxin-insensitive mutation. J Pharmacol Exp Ther. 2006;317(2):858–864. doi: 10.1124/jpet.105.096818. [DOI] [PubMed] [Google Scholar]
- 17.Noetzel MJ, et al. Functional impact of allosteric agonist activity of selective positive allosteric modulators of metabotropic glutamate receptor subtype 5 in regulating central nervous system function. Mol Pharmacol. 2012;81(2):120–133. doi: 10.1124/mol.111.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alt A, et al. Stimulation of guanosine-5′-O-(3-[35S]thio)triphosphate binding by endogenous opioids acting at a cloned mu receptor. J Pharmacol Exp Ther. 1998;286(1):282–288. [PubMed] [Google Scholar]
- 19.Liu W, et al. Structural basis for allosteric regulation of GPCRs by sodium ions. Science. 2012;337(6091):232–236. doi: 10.1126/science.1219218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Werling LL, Puttfarcken PS, Cox BM. Multiple agonist-affinity states of opioid receptors: Regulation of binding by guanyl nucleotides in guinea pig cortical, NG108-15, and 7315c cell membranes. Mol Pharmacol. 1988;33(4):423–431. [PubMed] [Google Scholar]
- 21.Sharma S, Rodriguez AL, Conn PJ, Lindsley CW. Synthesis and SAR of a mGluR5 allosteric partial antagonist lead: Unexpected modulation of pharmacology with slight structural modifications to a 5-(phenylethynyl)pyrimidine scaffold. Bioorg Med Chem Lett. 2008;18(14):4098–4101. doi: 10.1016/j.bmcl.2008.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koole C, et al. Allosteric ligands of the glucagon-like peptide 1 receptor (GLP-1R) differentially modulate endogenous and exogenous peptide responses in a pathway-selective manner: Implications for drug screening. Mol Pharmacol. 2010;78(3):456–465. doi: 10.1124/mol.110.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNicol E, et al. Americal Pain Society Management of opioid side effects in cancer-related and chronic noncancer pain: A systematic review. J Pain. 2003;4(5):231–256. doi: 10.1016/s1526-5900(03)00556-x. [DOI] [PubMed] [Google Scholar]
- 24.Roques BP, Fournié-Zaluski MC, Wurm M. Inhibiting the breakdown of endogenous opioids and cannabinoids to alleviate pain. Nat Rev Drug Discov. 2012;11(4):292–310. doi: 10.1038/nrd3673. [DOI] [PubMed] [Google Scholar]
- 25.Levine JD, Gordon NC, Jones RT, Fields HL. The narcotic antagonist naloxone enhances clinical pain. Nature. 1978;272(5656):826–827. doi: 10.1038/272826a0. [DOI] [PubMed] [Google Scholar]
- 26.Gjoni T, Urwyler S. Receptor activation involving positive allosteric modulation, unlike full agonism, does not result in GABAB receptor desensitization. Neuropharmacology. 2008;55(8):1293–1299. doi: 10.1016/j.neuropharm.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 27.DeWire SM, et al. A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther. 2013;344(3):708–717. doi: 10.1124/jpet.112.201616. [DOI] [PubMed] [Google Scholar]
- 28.Davis CN, et al. Differential regulation of muscarinic M1 receptors by orthosteric and allosteric ligands. BMC Pharmacol. 2009;9:14. doi: 10.1186/1471-2210-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emmerson PJ, et al. Characterization of opioid agonist efficacy in a C6 glioma cell line expressing the mu opioid receptor. J Pharmacol Exp Ther. 1996;278(3):1121–1127. [PubMed] [Google Scholar]
- 30.Clark MJ, Harrison C, Zhong H, Neubig RR, Traynor JR. Endogenous RGS protein action modulates mu-opioid signaling through Galphao. Effects on adenylyl cyclase, extracellular signal-regulated kinases, and intracellular calcium pathways. J Biol Chem. 2003;278(11):9418–9425. doi: 10.1074/jbc.M208885200. [DOI] [PubMed] [Google Scholar]
- 31.Lester PA, Traynor JR. Comparison of the in vitro efficacy of mu, delta, kappa and ORL1 receptor agonists and non-selective opioid agonists in dog brain membranes. Brain Res. 2006;1073-1074:290–296. doi: 10.1016/j.brainres.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 32.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 33.Neilan CL, Akil H, Woods JH, Traynor JR. Constitutive activity of the delta-opioid receptor expressed in C6 glioma cells: Identification of non-peptide delta-inverse agonists. Br J Pharmacol. 1999;128(3):556–562. doi: 10.1038/sj.bjp.0702816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee KO, Akil H, Woods JH, Traynor JR. Differential binding properties of oripavines at cloned mu- and delta-opioid receptors. Eur J Pharmacol. 1999;378(3):323–330. doi: 10.1016/s0014-2999(99)00460-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.