Significance
Traumatic events can engender persistent excessive fear responses to trauma reminders that may return even after successful treatment. In the psychotherapy of fear or anxiety disorders, patients make safety experiences that generate fear-inhibitory safety memories. Fear, however, frequently returns because safety memory retrieval fails. We find that safety memories can be strengthened and are more easily retrieved when adding a standard anti-Parkinson drug that augments brain levels of the neurotransmitter dopamine directly after a safety experience. In mice and humans, this treatment up-regulates an anti-fear area in the frontal cortex. Our findings open a unique avenue for improving psychotherapy.
Keywords: fear conditioning, psychotherapy, reinstatement, renewal, resilience
Abstract
Traumatic events can engender persistent excessive fear responses to trauma reminders that may return even after successful treatment. Extinction, the laboratory analog of behavior therapy, does not erase conditioned fear memories but generates competing, fear-inhibitory “extinction memories” that, however, are tied to the context in which extinction occurred. Accordingly, a dominance of fear over extinction memory expression—and, thus, return of fear—is often observed if extinguished fear stimuli are encountered outside the extinction (therapy) context. We show that postextinction administration of the dopamine precursor l-dopa makes extinction memories context-independent, thus strongly reducing the return of fear in both mice and humans. Reduced fear is accompanied by decreased amygdala and enhanced ventromedial prefrontal cortex activation in both species. In humans, ventromedial prefrontal cortex activity is predicted by enhanced resting-state functional coupling of the area with the dopaminergic midbrain during the postextinction consolidation phase. Our data suggest that dopamine-dependent boosting of extinction memory consolidation is a promising avenue to improving anxiety therapy.
In extinction, conditioned fear responses (CRs) are diminished by using repeated exposure to the conditioned fear stimulus (CS) in the absence of the aversive unconditioned stimulus (UCS) with which it had previously been paired (1). Most extinction protocols do not, or only partly, delete the original CS–UCS association (or fear memory) but result in the formation of an inhibitory CS–no-UCS association (extinction memory) (2, 3). At later CS exposures (test), the competition between both memory traces is thought to determine the level of conditioned responding. Dissimilarity between the test and the extinction context impairs the retrieval or expression of the extinction memory in favor of the retrieval/expression of the fear memory, which is thought to not require gating or occasion-setting by any particular context (2). In the laboratory, context dissimilarity is achieved by testing in a physically different context than extinction (renewal), by again administering UCSs shortly before testing (reinstatement) or by simply letting sufficient time pass between extinction and testing (spontaneous recovery) (2). The return of fear observed in these situations is considered a laboratory model of relapse after successful extinction-based psychotherapy of conditions such as posttraumatic stress disorder, panic disorder, or social phobia (2, 4, 5).
To generate strong and long-lasting memories, new learning must initiate cascades of molecular events that ultimately result in changes in protein expression and/or posttranslational protein modification (6–8). Animal studies have shown that release of the neurotransmitter dopamine is critically important in many such consolidation processes and specifically promotes stable forms of long-term potentiation, a cellular correlate of long-term memory (9–13). In the domain of extinction, administration of dopamine receptor antagonists before or after extinction consistently impairs animals’ capacity to later express extinction, leading to enhanced CRs at test (i.e., return of fear) (14–16). Conversely, extinction expression is improved in animals that receive the combined dopamine and norepinephrine reuptake blocker methylphenidate directly after extinction (17), suggesting that dopamine contributes to extinction memory consolidation. These findings led us to ask whether administration of the dopamine precursor l-dopa directly after extinction (i.e., during memory consolidation) would enhance extinction memory formation and thereby prevent the return of fear.
Results and Discussion
Return of Fear in Mice.
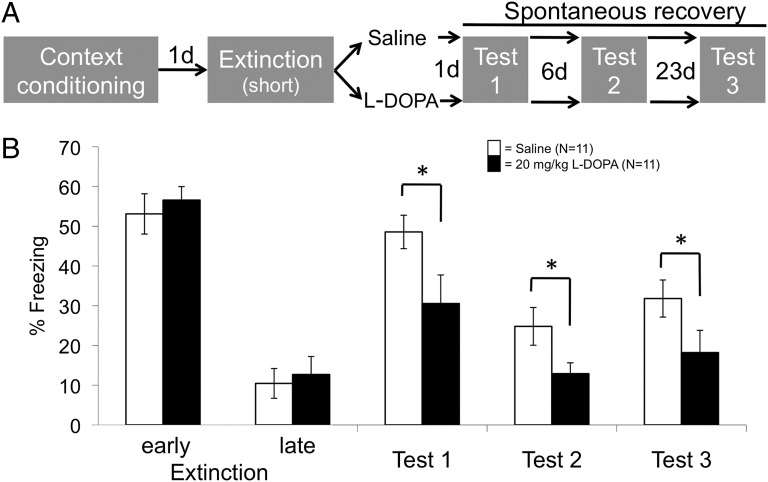
To assess spontaneous recovery, mice were context-conditioned by receiving unsignaled foot shocks while in the conditioning chamber (mouse study 1; Fig. 1A). Twelve minutes of context reexposure the next day initially induced fear memory expression (freezing-CRs in early extinction; Fig. 1B), followed by gradual extinction of CRs (late extinction in Fig. 1B). The immediately following systemic administration of 20, but not 5 or 10, mg/kg l-dopa reduced spontaneous recovery (CRs to the context) when tested 1, 7, and 30 d after extinction, compared with the group receiving saline [main effect of group: F3,37 = 3.07, P = 0.04; time by group interaction: not significant (n.s.)] (Fig. 1B, Fig. S1, and Table S1). Importantly, the short half-life of l-dopa (1.5 h; ref. 18) excludes direct effects of l-dopa during testing. This circumstance rules out the possibility that the observed long-term fear reduction was dependent on the presence of the drug at test. Hence, 20 mg/kg l-dopa attenuated spontaneous recovery of context-conditioned fear, most likely by enhancing extinction memory consolidation.
Fig. 1.
Mouse study 1: Attenuation of spontaneous recovery of contextual fear by l-dopa. Administration of 20 mg/kg l-dopa directly after extinction learning (A) results in a long-term reduction of spontaneous recovery (B), as indexed by the percentage of time spent freezing. Gray fields in A indicate the context (identical on all days). Early extinction, first 4 min; late extinction, last 4 min. The graph shows raw values (means ± SEM). For statistical testing, data from tests 1–3 were normalized by subtraction to late extinction to appropriately quantify return of fear (see Materials and Methods and Fig. S1 and Table S1, where results for doses 5 and 10 mg/kg are also given). *P < 0.05 (two-tailed planned post hoc t tests on normalized data).
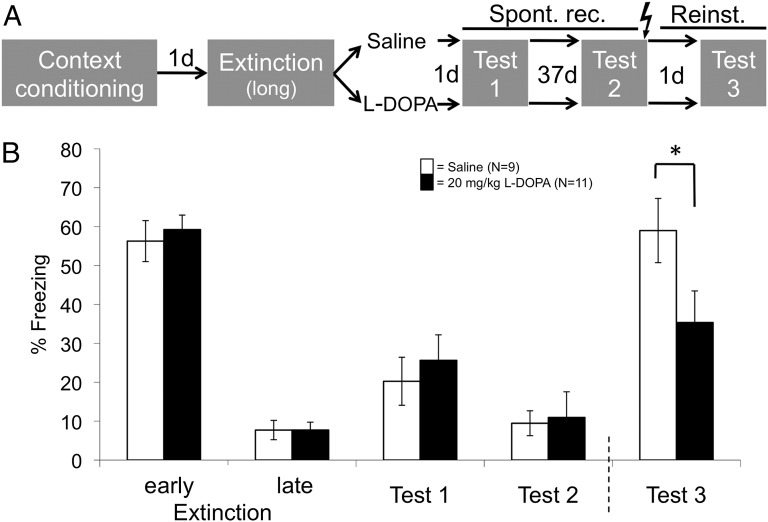
To assess reinstatement, we prolonged the context reexposure (i.e., extinction) phase on the second day to generate a stronger extinction memory trace in all groups (mouse study 2; Fig. 2A). Accordingly, spontaneous recovery of contextual CRs tested 1 and 38 d afterward was weaker than in the first experiment and no longer differed between groups (Fig. 2B, tests 1 and 2). The last recovery test was followed by administration of one unsignaled foot shock while animals were still in the chamber, in order to thereby reinstate the aversiveness of the context and thus increase its dissimilarity from the context of extinction. One day later, the reinstatement effect visible in the animals that had received saline directly after extinction was significantly reduced in the animals that had received 10 and 20 mg/kg l-dopa (main effect of group: F3,39 = 3.13, P = 0.038) (Fig. 2B and Fig. S2, test 3; Table S2). Hence, the higher doses of l-dopa also attenuated return of contextual fear by reinstatement.
Fig. 2.
Mouse study 2: Attenuation of reinstatement of contextual fear by l-dopa. Administration of 20 mg/kg l-dopa directly after extinction learning (A) results in a reduction of reinstatement 40 d later (Reinst., Test 3) (B). Gray fields in A indicate the context (identical on all days). Lightning bolt denotes UCS. The graph shows raw values (means ± SEM). For statistical testing, data from tests 1 and 2 were normalized by subtraction to late extinction (to quantify spontaneous recovery), and data from test 3 were normalized to data from test 2 (to quantify reinstatement) (see Fig. S2 and Table S2, where results for doses 5 and 10 mg/kg are also given). *P < 0.05 (two-tailed planned post hoc t tests on normalized data).
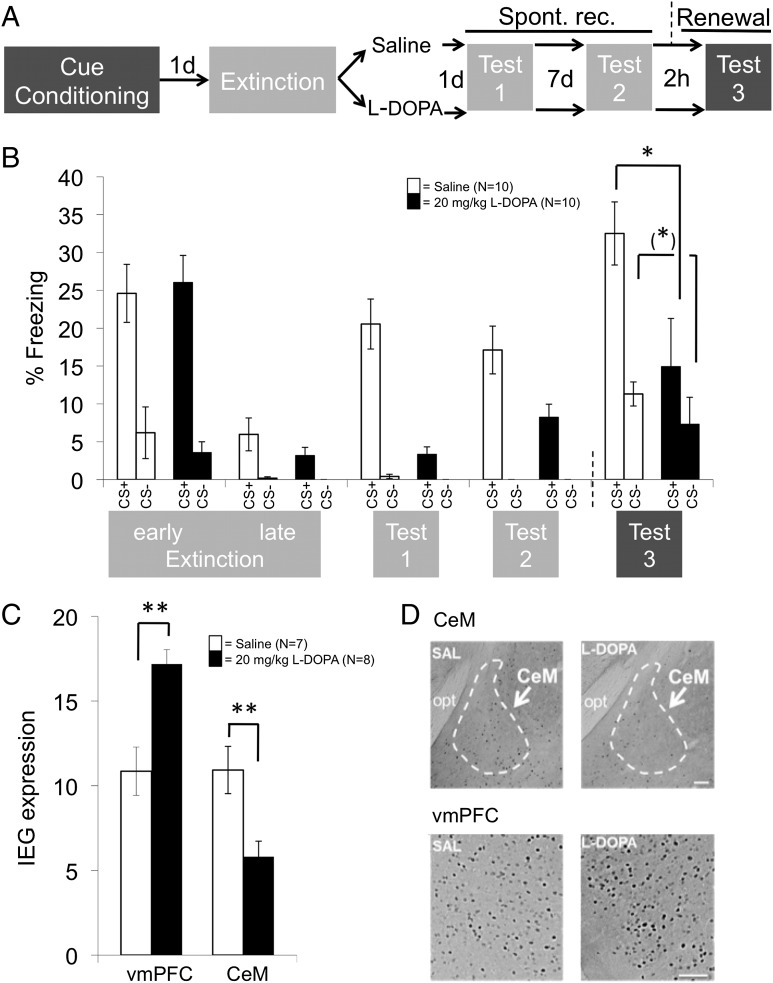
Renewal is more easily assessed within cue conditioning in which the CS is a discrete stimulus (here, a tone). Differential tone-shock conditioning with a shock-paired CS+ and an unpaired control CS− in chamber A (conditioning context) was followed the next day by extinction in a different chamber B (extinction context) and the subsequent systemic administration of either 20 mg/kg l-dopa or saline (mouse study 3; Fig. 3A). As for context conditioning in mouse study 1, l-dopa–treated mice again showed a significant reduction of spontaneous recovery (tested by CS presentation in the extinction context B 1 and 8 d later; main effect of group: F1,18 = 7.62, P = 0.012; cue by group interaction: F1,18 = 7.73, P = 0.013) (Fig. 3B and Fig. S3, tests 1 and 2; Table S3). Importantly, they also showed a significant reduction of renewal compared with saline-treated mice [tested by CS presentation in the original conditioning context A, effectively corresponding to the common ABA renewal procedure (2); see test 3 in Fig. 3B; main effect of group: F1,18 = 6.25, P = 0.022]. There was, however, no significant cue by group interaction (Table S3). Fig. 3B, test 3, seems to indicate that l-dopa treatment attenuated freezing to CSs independent of whether they had previously been associated with shock (CS+) or not (CS−). However, inspection of normalized data in Fig. S3B shows that, rather, renewal in the saline-treated mice generalized to the CS−, a phenomenon frequently observed in differential conditioning paradigms (19). Considering this finding together with the results from spontaneous recovery and reinstatement, it thus appears that l-dopa enhances extinction consolidation to the extent that extinction memories are also expressed in nonextinction contexts.
Fig. 3.
Mouse study 3: Attenuation of spontaneous recovery and renewal of cued fear by l-dopa. (A and B) Administration of 20 mg/kg l-dopa directly after extinction learning (A) results in a reduction of spontaneous recovery (Spont. rec., tests 1 and 2, in extinction context B; light gray shading) and ABA renewal (test 3, in the conditioning context A; dark gray shading) (B). The graph shows raw values (means ± SEM). For statistical testing, data from tests 1 and 2 were normalized by subtraction to late extinction (to quantify spontaneous recovery), and data from test 3 were normalized to data from test 2 (to quantify renewal) (Fig. S3 and Table S3). (C) l-dopa also enhances neural activity during test 3 [expression of the immediate early gene (IEG) c-Fos quantified as the number of c-Fos–positive cells per 0.01 mm2] in the vmPFC (infralimbic part) and reduces neural activity in the centromedial amygdala (CeM). (D) Representative stainings. SAL, saline group; opt, optical tract. (Scale bars: 100 μm.) (*)P < 0.1; *P < 0.05; **P < 0.01 (two-tailed planned post hoc t tests on normalized data).
A brain region critically involved in extinction memory expression is the ventromedial prefrontal cortex (vmPFC), in rodents mainly constituted by the infralimbic cortex (IL) (3, 20–23). IL neurons project to, and activate, intercalated GABA-ergic neurons in the amygdala, thereby inhibiting the amygdala and reducing the generation of CRs conveyed by centromedial amygdala (CeM) efferents to downstream effector regions (22, 24–28). In our mouse study 3, the reduced renewal in l-dopa–treated animals in test 3 was paralleled by enhanced immediate early gene (IEG) expression in IL (l-dopa > saline; t1,13 = 3.81, P = 0.0034; two-tailed) and reduced IEG expression in CeM (l-dopa < saline; t1,13 = 3.07, P = 0.011; two-tailed) during the test (Fig. 3C). This result is in agreement with the described roles of the two areas in fear extinction and suggests that, like the behavioral expression of extinction, neural activity typically associated with extinction expression also generalized to the nonextinction context after l-dopa treatment. Exploratory analysis of anterior and posterior prelimbic cortex (PL) as well as dorsal hippocampal CA1 and CA3 regions at a Bonferroni-corrected threshold of α = 0.0125 only found significant differences in the anterior PL (l-dopa > saline; t1,13 = 3.09, P = 0.009; two-tailed). Despite of a described role for the area in fear expression (27), the direction of the effect was opposite to the amygdala effect. Data from the ventral hippocampus could not be analyzed due to technical reasons.
Translation to Humans.
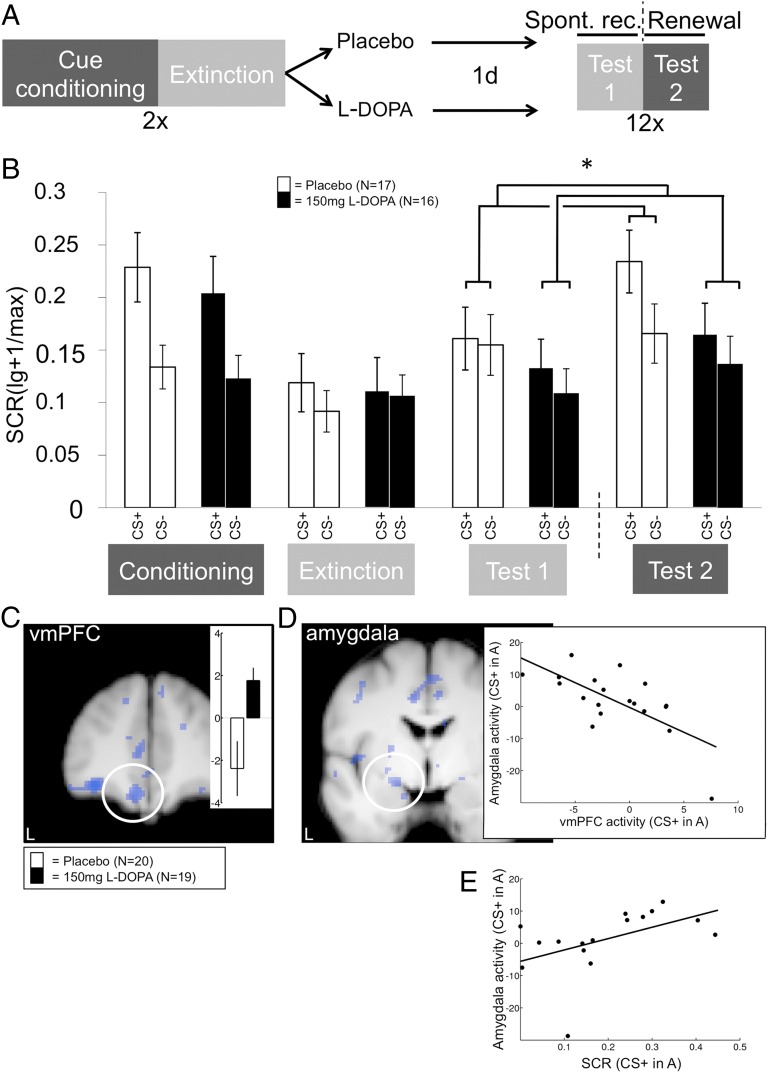
The likely human homolog for the rodent IL can be found in ventromedial aspects of the PFC (29), and studies indicate that human extinction also uses a vmPFC-based circuitry. In particular, results from functional magnetic resonance imaging (fMRI) experiments on fear/extinction memory expression are consistent with a vmPFC–amygdala interaction (e.g., refs. 30–32). In our human study, we therefore predicted a similar l-dopa–mediated attenuation of fear and concomitant modulation of the extinction circuitry as in the mouse experiments. We focused on fear renewal as the most stringent test of the context-dependency of extinction (2), choosing to modify an earlier extinction expression paradigm (31) to make participants learn, on day 1, that one cue (CS+) predicted the UCS (electrotactile pain) in one context (conditioning context, A) but not in a different context in which no UCS was given (extinction context, B). A control cue (CS−) never predicted the UCS in either context. Learning was followed by administration of either 150 mg of l-dopa or placebo. On day 2, subjects were repeatedly shown the CS+ and the CS− in both contexts, presented in alternating order, in the absence of the UCS (Fig. 4A). On the basis of our prior data (31), we expected that the conditioning context A would promote renewal of fear to CS+ cues (Fig. 4A, test 2), whereas the extinction context B would promote expression of the extinction memory that is likewise associated with the CS+, observable as relatively reduced CRs (Fig. 4A, test 1). We used skin conductance responses (SCRs) as our major outcome measure. SCRs reflect the phasic arousal associated with fear responses (33) and therefore assess fear indirectly but objectively.
Fig. 4.
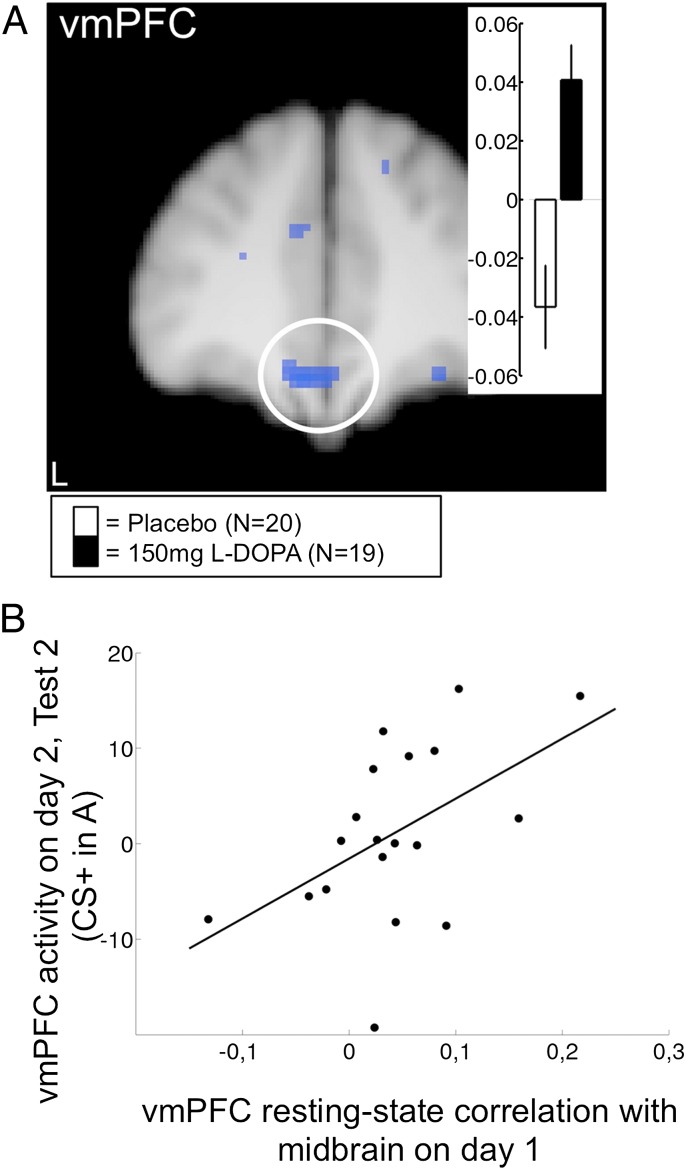
Human study: Attenuation of renewal of cued fear by l-dopa. (A) Larger differential CRs (CS+ > CS−) in the conditioning context A (dark gray shading; test 2) than in the extinction context B (light gray shading; test 1) on day 2 signify renewal [cue by context interaction (CS+ > CS−)A > (CS+ > CS−)B]. CS presentation in context B effectively assesses spontaneous recovery (test 1). (B) SCR data. Administration of 150 mg of l-dopa directly after extinction learning on day 1 abolishes renewal (test 2). The elimination of renewal is cue-specific (CS+ > CS−). As in the mouse studies, the values shown in the graph (means ± SEM) are not normalized to the preceding experimental phase. Unlike in the mouse studies, statistical analysis was also performed on nonnormalized data, to stay analogous to the fMRI data analysis (Materials and Methods). *P < 0.05 (two-tailed planned post hoc t tests on normalized data). (C) l-dopa also reverses the renewal-related deactivation [cue by context contrast (CS+ > CS−)A < (CS+ > CS−)B; see group-wise contrast estimate bars in Inset] of the vmPFC, resulting in a significant group difference (cue by context by group interaction: Montreal Neurological Institute (MNI) coordinates x, y, z: −10, 44, −20; Z = 3.9; P = 0.013; small volume correction; SVC). For separate CS+ and CS− data, see Table S5. (D) vmPFC peak activation parameter estimates during the renewal test are strongly negatively correlated to corresponding amygdala estimates in l-dopa participants (−20, −2, −12; Z = 3.25; P = 0.034 SVC; R = −0.69). (E) Extracted parameter estimates for CS+ responses in context A from this voxel in l-dopa participants correlate positively with SCRs. fMRI display threshold: P < 0.01; uncorrected. Activation superimposed on an average structural image. L, left.
Indeed, the placebo group showed significantly larger differential SCRs (CS+ > CS−) in context A (test 2) than in context B (test 1) on day 2 (cue by context interaction in placebo: F1,16 = 6.65, P = 0.02) (Fig. 4B, open bars). This pronounced renewal effect was significantly attenuated in the l-dopa group (cue by context by group interaction: F1,31 = 5.23, P = 0.029) (Fig. 4B, filled bars). Further inspection suggested that the interaction was mainly due to relatively decreased responding to the CS+ in context A (post hoc t test: t1,31 = 1.81, P = 0.082; two-tailed; Fig. 4B and Fig. S4, test 2; Table S4). Like in the mouse studies, direct l-dopa effects on renewal testing itself can again be excluded because of the short half-life of l-dopa in humans, too (18).
Stronger renewal in the placebo participants was accompanied by deactivation of the left vmPFC to CS+ in conditioning context A, an effect that was cancelled in the l-dopa participants (Fig. 4C). This finding is of particular interest because deactivation of extinction areas during return-of-fear situations has been observed before in both rodents and humans (30, 34), and reduction of this deactivation has been linked to improved extinction expression (35, 36). vmPFC responses to the CS+ in context A showed a negative correlation with corresponding responses in the left dorsal amygdala in the l-dopa group (Fig. 4D). The dorsal and medial locations of this effect are in line with the described anatomical position of the CeM in humans (37). A trend-like positive correlation between activity in the identified amygdala peak effect voxel and SCRs to the CS+ in context A fit its presumed role in CR generation [R = 0.48, P = 0.06; two-tailed; after exclusion of one outlier (more than 2 SD < mean): R = 0.59, P = 0.02] (Fig. 4E). Hence, within a model in which down-regulation of the amygdala results from enhanced vmPFC activity (see above), l-dopa–mediated generalization of extinction appears to involve generalization of vmPFC activity. In exploratory analyses, we also observed an effect similar to the vmPFC result in the anterior hippocampus (l-dopa > placebo; −30, −14, −22; Z = 3.19, P < 0.001; uncorrected), a region that we have previously implicated in extinction memory expression in a similar paradigm (31). Anterior hippocampus responses, however, did not correlate with amygdala responses, suggesting a less direct role in extinction expression for this area compared with the vmPFC. There was an inverse group difference (l-dopa < placebo) in the posterior hippocampus (36, −28, −14; Z = 3.28, P < 0.001; uncorrected), an area linked with fear memory expression (31), but not in any other region of the fear network (e.g., dorsal anterior cingulate cortex).
Potential Mechanism.
In rodents, extinction training induces spontaneous neural activity in the vmPFC (IL) in the hours following extinction, which predicts successful extinction expression 24 h later (38). Extinction training also lastingly elevates dopamine levels in the vmPFC (39). In the vmPFC, dopamine receptor expression is high and mainly confined to output layers (40), which are thought to convey fear inhibition after extinction (22, 23). Finally, responsiveness of vmPFC neurons to extinguished CSs is lastingly reduced in animals that have received a dopamine antagonist after extinction (16). Together, these findings lead to the hypothesis that dopamine-dependent spontaneous consolidation processes in the vmPFC determine later extinction expression by shaping vmPFC responding to CSs. Dopamine in the vmPFC probably derives from neurons originating in the ventral tegmental area (VTA) (41), and l-dopa enhances dopamine, but not noradrenaline, release in the frontal cortex (42). Like in the dopamine-dependent formation of long-term fear (13) and other memories (12), the dopaminergic midbrain is thus a likely candidate for participating in the boosting of extinction memory consolidation by l-dopa. To gain insight into these underlying mechanisms, we measured participants’ resting-state fMRI activity following extinction training on day 1, asking whether spontaneous activity in a dopaminergic-midbrain seed region (43) would correlate with spontaneous activity in the vmPFC. The 10-min scan was placed 45 min after drug administration when l-dopa should reach its maximum concentration in plasma and already well elevated levels in the brain (18, 44). l-dopa participants showed a significantly higher midbrain–vmPFC coupling than placebo participants (Fig. 5A). The amount of coupling predicted vmPFC responses to CS+ in context A 1 d later during the renewal test (test 2) in the l-dopa participants (R = 0.51, P = 0.027; two-tailed; Fig. 5B). There was no such relationship between the dopaminergic midbrain and the anterior hippocampus, suggesting that the vmPFC is the key area for l-dopa–mediated boosting of extinction consolidation.
Fig. 5.
Human study: Dopaminergic midbrain to vmPFC resting-state coupling during consolidation. (A) Spontaneous resting-state activity was measured 45 min after extinction and drug administration on day 1 (compare Fig. 4). vmPFC correlation with dopaminergic–midbrain seed region (−6, 40, −18; Z = 3.51; P = 0.043 SVC; R = 0.51; and −4, 36, −18; Z = 3.47; P = 0.048 SVC). Inset shows parameter estimates. (B) Prediction of renewal-related vmPFC activity on day 2 by midbrain–vmPFC resting-state coupling during consolidation on day 1 in l-dopa participants.
On this basis, we suggest that the improved dopamine availability to midbrain dopamine neurons during consolidation in the presence of l-dopa translates into higher spontaneous dopamine transmission and concomitant spontaneous neural activity in the vmPFC, which in turn improves extinction memory expression in that area at later time points. The mechanism might involve dopamine-mediated enhancement of the local activity-dependent release or expression of proteins such as brain-derived neurotrophic factor (BDNF) (45), known to be critical for long-term memory storage generally (46) and for vmPFC-dependent extinction mechanisms in particular (47, 48). A potential source of BDNF in the vmPFC is the hippocampus (47), where long-term memory-promoting dopamine–BDNF interactions have been demonstrated (45, 46). In any case, our human data further support the conclusion from our rodent experiments that l-dopa administration directly after extinction makes extinction memories context-independent and protects from return of fear when a CS is encountered in a context that differs from the extinction context. Context-independency is not produced by, or has not been investigated in, other drugs tested in clinical studies for their ability to boost extinction-based therapy (49–51).
Perspectives for Therapy.
Relapse after successful extinction-based treatment is considered to result from an inability to retrieve or express the extinction memory, a process for which gating or occasion-setting by the extinction context is a limiting factor (2). Therapists have responded to this challenge by increasingly conducting exposure in multiple and varied everyday contexts (5), thereby aiming to broaden extinction context representations and to extend the situations in which extinction can be expressed. An interesting opposite strategy recently tested in animals is to pharmacologically disrupt hippocampus-dependent contextual processing during extinction, to thus make the presence of contextual cues an irrelevant factor in the retrieval of extinction memories (52). Both strategies target the contextual gating of extinction (broadening or destroying it). Our present findings suggest that strengthening of the extinction memory itself, with the help of a potent consolidation booster, can untie it from any specific extinction context and broaden its expression. This method can be an alternative route to relapse prevention in extinction-based therapy which obviates the need for context-targeted manipulations and may have a number of potential advantages. First, context-targeted manipulations are necessarily laborious and time-consuming because they either require many varied exposures (5) or, presumably, the presence of a hippocampus disruptor during every exposure session (to exclude the development of contextual gating; ref. 52). Augmentation of therapy by l-dopa, by contrast, might not necessarily require drug administration with all exposure sessions and should, in fact, reduce the number of sessions needed to achieve stable remission. Second, our data suggest that l-dopa might be effective even when given after a treatment session. This property would allow the therapist to restrict administration to successful sessions and thereby reduce the risk that potential newly learned or reactivated fear memories may also be strengthened by the manipulation.
A complementary experimental approach to relapse prevention that is being intensely investigated consists of erasing reactivated fear memories by interfering with their reconsolidation (53, 54). Memory erasure, if practically feasible in a therapeutic context, may be desirable in severe conditions that cannot be treated otherwise. We would argue, however, that, under most other circumstances, personal empowerment by strengthening of individual coping resources (here, the ability to remember the safety of a situation) is perhaps a preferable strategy. In this sense, neurobiological research in the service of empowerment can not only help improve the treatment of pathological anxiety but may also be an answer to concerns and acceptance problems surrounding psychopharmacology.
Materials and Methods
Summary of Statistical Procedures.
Statistical analysis of behavioral data was performed by using SPSS. Repeated-measures analysis of variance (ANOVA) was used for multivariate analysis, testing for pretreatment differences between the experimental groups (all n.s.) and for return of fear. P < 0.05 was considered statistically significant, and Greenhouse–Geisser correction was used when appropriate. Significant effects were further characterized by two-tailed Student’s t tests.
For statistical analysis of return of fear in the mouse studies, freezing-CRs from the test phases were normalized by subtraction to an appropriate baseline phase (as explained in the legends of Figs. 1–3), and normalized data are displayed in Figs. S1–S3. In the human study, normalization of fMRI scores (parameter estimates) from test 1 on day 2 (spontaneous recovery) to the appropriate day-1 baseline (extinction) was not feasible because of the different task structures and correspondingly different model-based parameter estimations performed on both days. We therefore also performed the analysis of the SCR data from this experiment on nonnormalized scores. Note, however, that the repeated-measures ANOVA used to calculate the critical renewal test is equivalent to normalizing test 2 to test 1 data.
Mouse Studies 1 and 2.
Animals and husbandry.
C57BL/6J mice were bred at the University Medical Center Hamburg–Eppendorf. The male mice used in the experiments were between 4 and 8 mo old and were kept in a vivarium with an inverted 12:12-h light:dark cycle (light off at 0700) under standard housing conditions (23 ± 1 °C, 40–50% humidity, food and water ad libitum). Mice were kept in groups of two to four siblings per cage (36.5 × 20.7 cm and 14 cm high). Experiments were performed in a room adjacent to the vivarium and illuminated by red light. All mice were handled daily for 5 d a week starting 2 wk before experiments. One week before the conditioning experiments, mice underwent an open field test as described (55). Results were not used in the present study. Care was taken to minimize pain or discomfort for the animals. Experiments were conducted in accordance with the German and European Union laws on protection of experimental animals and were approved by the local authorities of the City of Hamburg, the committee for Lebensmittelsicherheit und Veterinärwesen at the authority of Soziales, Familie, Gesundheit und Verbraucherschutz Hamburg, Germany.
Paradigms.
The conditioning context was a chamber (23.5 × 23.5 cm and 19.5 cm high) with Plexiglas walls and ceiling and a stainless grid floor from which an electric shock could be elicited. The chamber was illuminated by white light (10 lux). For context conditioning, mice were placed in the center of the chamber and received three unsignaled electric foot shocks (0.25 mA, 1 s; UCS) at 120, 160, and 200 s. At 220 s, the mouse was immediately returned into its home cage. At 24 h after conditioning, the mice were again placed in the context for either 12 (short extinction protocol; mouse study 1) or 30 (long extinction protocol; mouse study 2) min. Immediately after termination of the extinction protocol, mice were injected i.p. with either saline or l-dopa (Sigma-Aldrich; 5, 10, or 20 mg/kg). Group assignment was randomized. In mouse study 1, spontaneous recovery was tested 1, 7, and 30 d after extinction (tests 1–3) by placing the mouse in the chamber for 240 s. In mouse study 2, spontaneous recovery was tested as described above 1 d after extinction (test 1). The second spontaneous recovery test 38 d after extinction (test 2) lasted only 180 s, after which the mouse received a foot shock (0.25 mA, 1 s) and remained in the chamber for another 20 s. At 24 h later, reinstatement was tested (test 3) by again placing the mouse in the chamber for 240 s. Freezing was defined as complete immobilization except respiratory movements for at least 1 s and was automatically detected by using five infrared sensors (Infra-e-motion) (56).
Mouse Study 3.
Animals and husbandry.
Experiments were carried out in adult male C57BL/6J mice (8 wk; Charles River). Animals were kept in groups of four under standard housing conditions in a 12:12-h light:dark cycle (light on at 0700) and provided with food and water ad libitum. Experiments were conducted in accordance with the German and European Union laws on protection of experimental animals and approved by the local authorities (Bezirksregierung Münster). All experimental protocols were performed in accordance with the European Community's Council Directive of 24 November 1986 (86/609/EEC) and approved by the Landesamt fuer Natur Umwelt und Verbraucherschutz Nordrhein-Westfalen, Germany (8.87-51.04.20.09.334).
Paradigm.
Mice were cue-conditioned by using a validated paradigm (57). The paradigm was adjusted to match the renewal testing procedure described by Karpova et al. (58). Animals first received two sessions of six CS− presentations each (2.5-kHz tone, 85 dB, stimulus duration 10 s, interstimulus interval 20 s; intersession interval 6 h) in chamber A (conditioning box; TSE; comprising a 16 × 32 × 20-cm acrylic glass arena and a stainless grid floor from which an electric shock could be elicited). At 24 h later, conditioning was performed in the same chamber in two sessions of three CS+ presentations each (10-kHz tone, 85 dB, stimulus duration 10 s, randomized interstimulus interval 10–30 s; intersession interval 6 h) in which each CS+ coterminated with a UCS (scrambled foot shock; 0.4 mA, duration 1 s). At 24 h later, single mice were transferred into a new context (chamber B; Makrolon Type II cage; 22 × 16 × 14 cm without bedding) for extinction. Mice first habituated to the chamber over a period of 30 min before being exposed to six extinction sessions (intersession interval: 30 min). Session 1 is equivalent to “early extinction” in Fig. 3 and Fig. S3 and session 6 is equivalent to “late extinction” in these figures. Each session consisted of four CS− and (40 s later) four CS+ presentations (stimulus duration 10 s, interstimulus interval 20 s). Immediately after termination of the extinction protocol, mice were injected i.p. with either saline or l-dopa (20 mg/kg; Sigma-Aldrich). Group assignment was randomized. At 1 and 8 d later, spontaneous recovery was tested in chamber B in a fashion corresponding to one extinction session (tests 1 and 2). At 2 h after test 2, mice were placed in the original conditioning chamber A and tested for renewal (test 3), using the same protocol as for spontaneous recovery. Freezing was defined as described above and was manually scored by an observer blind to the paradigm.
IEG expression mapping.
At 2 h after the renewal test, mice were deeply anesthetized with an overdose of sodium thiopental and transcardially perfused with 20 mL of 0.9% saline followed by 20 mL of 4% (wt/vol) paraformaldehyde in 0.1 mol/L phosphate buffer, pH 7.4. Brains were then removed and postfixed at 4 °C overnight in 4% (wt/vol) paraformaldehyde in phosphate buffer. Subsequently, the brains were sectioned in the coronal plane at 50-µm thickness on a vibratome (VT1000S; Leica Microsystems), and the sections were collected in phosphate buffer. For immunostaining (59), sections were preincubated for 30 min in normal goat serum. Thereafter, the sections were incubated with a polyclonal rabbit anti–c-Fos polyclonal primary antibody (1:10,000; sc-189; Santa Cruz Biotechnology) and a biotinylated goat anti-rabbit secondary antibody (1:200; Vector Laboratories). An avidin–biotin–horseradish peroxidase procedure (Vectastatin ABC Kit; Vector Laboratories) with 3,3′-diaminobenzidine (Sigma) as chromogen was used to visualize c-Fos–positive cells as described (59). Cells containing a nuclear brown-black reaction product were considered to be c-Fos–positive, and their number was counted bilaterally in a representative tissue area of 0.01 mm2 with the help of a light microscope (Olympus BX-40) equipped with an ocular grid, as described (59). Anatomical localization of c-Fos–positive cells was aided by using the illustrations in a stereotaxic atlas (60). Results and Discussion reports results from CeM (bregma: −1.58 mm) and an anterior IL area (bregma: −1.94 mm); results from a posterior IL area (bregma: −1.7 mm) were identical.
Human Study.
Participants.
Forty right-handed healthy male volunteers (placebo group: n = 20; l-dopa group: n = 20) participated in the study. Participation was restricted to male participants because of the described effects of female hormones and the estrous cycle on conditioning and extinction (61–64) and the well-known interactions between the dopamine and gonadal hormone systems (e.g., ref. 65), as well as to better align mouse and human experiments. One participant in the l-dopa group had to be excluded because of alcohol intake between day 1 and 2. The remaining 39 volunteers were 25–46 y of age (mean 30 ± 1.4 SEM; no difference between groups; P > 0.9); none reported any past or present psychiatric or neurological illness or any other disease affecting major organs. Abuse of illegal drugs was tested by using a urine drug screen (M-10/3-DT; Diagnostik Nord). Trait anxiety scores (66) ranged from 24 to 52 (34.1 ± 2.7; no difference between groups, P > 0.9), in agreement with values from a German norm population (67). The study was conducted in accordance with the relevant German and European laws. Written informed consent was obtained from all participants in accordance with the requirements of the local Ethics Committee of the Medical Board in Hamburg and the federal institute for pharmaceutical and medical products in Germany (Bundesinstitut für Arzneimittel und Medizinprodukte).
Drug treatment.
Participants were told not to eat in the 2 h before drug intake. Before the experiment, a third person randomly assigned participants to the groups receiving either l-dopa or placebo after extinction. This person never obtained any experimental data or had any contact with the participants. Both the participant and the experimenter were blinded. Drugs were administered orally as capsules of 150 mg of l-dopa with 37.5 mg benserazide (Madopar 125; Roche) or vitamin E (placebo).
Stimulus material.
Two black geometric symbols (a triangle and a circle) presented in the center of the screen served as CS+ and CS−; a yellow and a blue screen background served as contexts. A gray screen with a black fixation cross was presented between conditioning and extinction (test) phases. The visual stimulus material was projected onto a screen at the back of the magnet’s bore, and participants could see the screen via a mirror mounted over their heads. Stimuli were presented by using Presentation Software (NeuroBehavioral Systems). A painful electrotactile stimulus consisting of a train of three square-wave pulses of 2 ms each served as the UCS. Pain stimuli were generated by using a DS7A electrical stimulator (Digitimer) and delivered on the right dorsal hand through a surface electrode with platinum pin (Specialty Developments).
Procedure.
Day 1 (learning).
Before the experiment, UCS intensity was individually adjusted to reach maximum tolerable pain (mean 12.7 ± 3.9 mA; range 2–76 mA; no group differences; P > 0.2). Accordingly, pain unpleasantness ratings [from 0 (“I feel nothing”) to 10 (“maximally unpleasant”)] did not differ between groups (mean 8.5 ± 0.4; range 7–10; P > 0.9).
Participants were then familiarized with all stimuli (all symbols and backgrounds without any eletrotactile stimuli) and the rating procedures (see below). Participants were not informed about any contingencies or the learning element in this experiment. Instead, they were told that this was an experiment that tested how some people can maintain attention to a cognitive task despite strong occasional distraction (by pain). Throughout the experiment, the participants were asked to indicate the identity of the presented symbol (triangle or circle) by pressing the corresponding key on a button box as fast and accurately as they could. There were no group differences in reaction times or accuracy.
The experiment itself consisted of two conditioning and two extinction phases in an ABAB design. During each phase, 12 CS+ and 12 CS− [stimulus duration 3 s; intertrial interval (ITI): mean 3.6 s, range 2.5–7 s] were repeatedly presented in pseudorandomized order while the screen background was kept constant. During conditioning only, the CS+ was paired in 50% of presentations with a UCS (starting 2.5 s into CS+ presentation). The CS− was never paired with a UCS. Conditioning (A) and extinction contexts (B) were distinguished by screen color. Phases were separated by a fixation screen (mean duration 3.6 s; range 2.5–7 s). The whole experiment lasted 35 min. The assignment of symbols to the CS+ or CS− and screen colors to the conditioning or extinction context was counterbalanced between participants and groups.
Immediately after the experiment, drugs were administered, and participants stayed under medical observation for at least 60 min thereafter. At 45 min after the experiment, a 10-min resting-state fMRI scan was performed (see below). This step was followed by asking participants to report potential side effects of the drug treatment, using a questionnaire that listed the eight most common l-dopa side effects and had space for reporting any other perceived changes. Two l-dopa participants reported headache, one drowsiness, and one fatigue. One placebo participant reported headache.
Day 2 (expression test).
Participants were first asked to again report potential side effects still present. No participant reported any effect. Individual UCS intensity recalibration again revealed no group differences in chosen intensity (mean 15.2 ± 4.1 mA; range 4–80 mA; P > 0.2) or perceived unpleasantness (mean 8.4 ± 0.4; range 7–10; P > 0.7).
In the experiment, no UCS was actually administered; that is, the expression test was conducted in extinction. There were 12 presentations of the conditioning (A) and extinction (B) context each, which alternated in an ABAB… fashion. Each context presentation involved 2 CS+ and 2 CS− shown in pseudorandomized order (CS duration 3 s; ITI mean 5.2 s; range 2.5–7 s). In analogy to the mouse studies, CS presentation in the extinction context B could also be understood as corresponding to a test of spontaneous recovery (Fig. 4, test 1). By comparing CRs in the original conditioning context A (test 2) to CRs in B, one can then infer renewal of responding as a result of testing outside the extinction context. Participants again indicated stimulus identity. There were again no group differences in the task. The whole experiment lasted 45 min.
Subjective ratings.
Participants were intermittently asked to give explicit ratings about each CS on a visual analog scale (VAS). These ratings consisted of two categories: one for CS-evoked stress/fear/tension (0 = no stress/fear/tension, 100 = high stress/fear/tension) and one for the UCS expectancy induced by a CS (0 = no expectancy, 100 = high expectancy). On day 1, ratings were given after every eighth trial (four CS+ and four CS− presentations). On day 2, ratings occurred after every phase, i.e., every fourth trial (two CS+ and two CS− presentations). Ratings of zero in all trials on one of the VASs led to the exclusion of one participant (l-dopa group) from the analysis of the rating data.
Skin conductance.
SCRs were recorded by using a CED2502-SA skin conductance unit with Spike 2 software (Cambridge Electronic Design). Data were down-sampled to 10 Hz, and phasic responses were manually scored off-line by using a custom-made computer program. SCR amplitudes (in microsiemens) were scored as the largest response occurring from 1 to 3.5 s after stimulus onset. Logarithms were computed for all values, to normalize the distribution (68), and these log values were range-corrected (1 + log SCR/max) to account for interindividual variability (69). SCR measurements that showed recording artifacts or excessive baseline activity were discarded. This process led to the exclusion of 10 participants (6 placebo) from the SCR analysis of day 1 and of 6 participants (3 placebo) from the analysis of day 2.
Imaging.
As described (31), fMRI was performed on both days to keep any additional contextual factors that might occur on top of the critical change of screen backgrounds as constant as possible. However, the experimental design on day 1 mainly served to induce contextualized extinction and was not optimized for fMRI analysis. As before (31), we therefore only report the critical test data from day 2. In addition, we report data from the resting-state scan on day 1.
MR data were obtained with a 3-T MR scanner (MAGNETOM trio; Siemens) by using a 12-channel head coil. For fMRI, 34 continuous axial slices (2 mm thick, 1 mm gap) were acquired by using a T2*-sensitive gradient echo-planar imaging sequence [repetition time: 2.23 s; echo time (TE): 30 ms; field of view: 220 × 220 mm; 2 × 2 mm in-plane resolution]. TE was minimized by using a parallel acquisition technique (generalized autocalibrating partially parallel acquisitions) with an acceleration factor of 2 and 24 reference lines. The position of the slice package was individually adjusted to optimally cover the temporal lobes and the ventral prefrontal cortex. A high-resolution T1-weighted structural image (1 × 1 × 1mm) was also acquired.
Imaging data analysis.
To account for T1 equilibrium effects, the first five volumes of each time series were discarded. Both task-related and resting-state functional data were preprocessed with SPM8 running on Matlab R2009b (MathWorks). Preprocessing involved realignment, unwarping, and normalization to a cohort-specific template, using DARTEL (70). Normalized data series were spatially smoothed with a 6-mm FWHM isotropic Gaussian kernel and manually inspected for excessive head movement. Further processing of the task-related fMRI data included temporal high-pass filtering (cutoff 128 s) and correction for temporal autocorrelations using first-order autoregressive modeling.
Statistical analysis of day-2 task-related data were performed by using a standard approach for fMRI, involving a general linear convolution model (GLM) at the single-subject level and a random-effects analysis at the group level within the SPM software (see ref. 71 for details). First, for each participant, regressors were defined that modeled the predicted time courses of experimentally induced brain activation changes. Each of the four experimental conditions (CS+ in A, CS− in A, CS+ in B, and CS− in B) was modeled as a zero-duration event (that is, a series of delta functions). Additionally, these categorical regressors were modulated parametrically by multiplication with a linear decaying function to be able to assess a potential habituation of amygdala activation over trials (72, 73). Nuisance regressors were included to factor out experimental effects of no interest: phases (conditioning and extinction contexts) were modeled as “box-car” (on–off) block-type regressors, rating onsets and each button-box response were modeled as events. Each regressor was convolved with a canonical hemodynamic response function (HRF). In addition to analyzing the entire experiment on day 2, we separated the experiment into first and second half (early, late) to account for ongoing extinction (e.g., ref. 30).
Using these regressors in a GLM (multiple regression) of brain activation at each voxel yields parameter estimates of the contribution of each regressor to the fMRI signal measured in each voxel. The subject- and regressor-specific parameter estimate images of interest were entered into a random-effects group analysis using SPM’s “full factorial” model, which permits correction for possible nonsphericity of the error term (here, dependence of conditions). Factors were cue (CS+, CS−), context (A, B), and group (placebo, l-dopa). The effect of main interest was the comparison of renewal [(CS+ > CS−) in A > (CS+ > CS−) in B] between groups. Significance of effects was tested by using voxel-wise one-tailed t tests.
To analyze correlations between day-2 vmPFC responses and day-2 amygdala responses (Fig. 4), the first-level vmPFC estimates from each participant (taken from the peak voxel identified in the corresponding group-level analysis) were entered into a group-level multiple regression analysis, including intercepts and centering on the mean. Correlation between day-1 midbrain–vmPFC resting-state coupling and day-2 vmPFC responses (Fig. 5; also see below) was calculated outside of SPM on the estimates taken from the peak voxels identified in the corresponding group-level analyses.
For statistical analysis of day-1 resting-state fMRI data, the time course of average intensity within a published substantia nigra/VTA mask (43, 74) was used as a regressor in a GLM on whole-brain resting-state activity. To correct for possible effects of movement, breathing, vascular changes, or other systemic effects, the GLM also included the six movement parameters resulting from realignment and time courses of average intensity within individual white matter and cerebrospinal fluid masks. There was no convolution with the HRF, no high-pass filtering, and no autoregressive modeling. Significance of group differences was assessed by using voxel-wise unpaired t tests (unequal variance).
In all analyses, correction for multiple comparisons at an alpha threshold of P = 0.05 was restricted to predefined regions of interest in amygdala and vmPFC (small volume correction; SVC). For the amygdala, we used spheres of 6-mm radius around MNI coordinates −15, −1, −14 (left) and 24, −1, −20 (right), which were derived from the only published study of return of fear by a pure renewal manipulation (75). For the vmPFC, we referred to our own study of context-dependent extinction memory expression (31). The coordinate found there (−2, 42, −22) was midline centered (x = 0) and used as the center of a box with dimensions 20 × 16 × 16 mm that equally covered both hemispheres, in analogy to earlier work on the mPFC (76, 77).
Supplementary Material
Acknowledgments
We thank S. Kiesling, E. Boening, and U. Wolters for expert animal handling; K. S. L. Yuen, N. Bunzeck, and M. Berker for help with data analysis; and C. Büchel and B. Lutz for comments on an earlier version of the manuscript. This work was supported by Deutsche Forschungsgemeinschaft Grants KA1623/3-1, KA1623/4-1, and SFB-TR58 A03; the State of Hamburg excellence initiative (neurodapt! consortium); the Austrian Science Fund (Fonds zur Förderung der Wissenschaftlichen Forschung); Signal Processing in Neurons Grant W1206-B18); and Sonderforschungsbereich F4410 grants.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1303061110/-/DCSupplemental.
References
- 1.Pavlov IP. Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex. London: Oxford Univ Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11(5):485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- 3.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12(2):120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 4.Rachman S. The return of fear: Review and prospect. Clin Psychol Rev. 1989;9:147–168. [Google Scholar]
- 5.Craske MG, et al. Optimizing inhibitory learning during exposure therapy. Behav Res Ther. 2008;46(1):5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 6.McGaugh JL. Memory—A century of consolidation. Science. 2000;287(5451):248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 7.Izquierdo I, et al. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci. 2006;29(9):496–505. doi: 10.1016/j.tins.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147(3):509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang YY, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc Natl Acad Sci USA. 1995;92(7):2446–2450. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bissière S, Humeau Y, Lüthi A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat Neurosci. 2003;6(6):587–592. doi: 10.1038/nn1058. [DOI] [PubMed] [Google Scholar]
- 11.Sajikumar S, Frey JU. Late-associativity, synaptic tagging, and the role of dopamine during LTP and LTD. Neurobiol Learn Mem. 2004;82(1):12–25. doi: 10.1016/j.nlm.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Lisman J, Grace AA, Duzel E. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends Neurosci. 2011;34(10):536–547. doi: 10.1016/j.tins.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fadok JP, Dickerson TMK, Palmiter RD. Dopamine is necessary for cue-dependent fear conditioning. J Neurosci. 2009;29(36):11089–11097. doi: 10.1523/JNEUROSCI.1616-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hikind N, Maroun M. Microinfusion of the D1 receptor antagonist, SCH23390 into the IL but not the BLA impairs consolidation of extinction of auditory fear conditioning. Neurobiol Learn Mem. 2008;90(1):217–222. doi: 10.1016/j.nlm.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Holtzman-Assif O, Laurent V, Westbrook RF. Blockade of dopamine activity in the nucleus accumbens impairs learning extinction of conditioned fear. Learn Mem. 2010;17(2):71–75. doi: 10.1101/lm.1668310. [DOI] [PubMed] [Google Scholar]
- 16.Mueller D, Bravo-Rivera C, Quirk GJ. Infralimbic D2 receptors are necessary for fear extinction and extinction-related tone responses. Biol Psychiatry. 2010;68(11):1055–1060. doi: 10.1016/j.biopsych.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abraham AD, Cunningham CL, Lattal KM. Methylphenidate enhances extinction of contextual fear. Learn Mem. 2012;19(2):67–72. doi: 10.1101/lm.024752.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunton L, Blumenthal D, Buxton I, Parker K. Goodman and Gilman’s Manual of Pharmacology and Therapeutics. New York: McGraw-Hill Professional; 2007. [Google Scholar]
- 19.Vervliet B, Baeyens F, Van den Bergh O, Hermans D. Extinction, generalization, and return of fear: A critical review of renewal research in humans. Biol Psychol. 2013;92(1):51–58. doi: 10.1016/j.biopsycho.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109(4):681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- 21.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 22.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: Ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maren S. Seeking a spotless mind: Extinction, deconsolidation, and erasure of fear memory. Neuron. 2011;70(5):830–845. doi: 10.1016/j.neuron.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quirk GJ, Likhtik E, Pelletier JG, Paré D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23(25):8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23(35):11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454(7204):642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knapska E, et al. Functional anatomy of neural circuits regulating fear and extinction. Proc Natl Acad Sci USA. 2012;109(42):17093–17098. doi: 10.1073/pnas.1202087109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehrlich I, et al. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62(6):757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 29.Nieuwenhuis IL, Takashima A. The role of the ventromedial prefrontal cortex in memory consolidation. Behav Brain Res. 2011;218(2):325–334. doi: 10.1016/j.bbr.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 31.Kalisch R, et al. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci. 2006;26(37):9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milad MR, et al. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62(5):446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Öhman A, Hamm A, Hugdahl K. Cognition and the autonomous nervous system: Orienting, anticipation and conditioning. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 2nd Ed. Cambridge, UK: Cambridge Univ Press; 2000. pp. 533–575. [Google Scholar]
- 34.Herry C, Mons N. Resistance to extinction is associated with impaired immediate early gene induction in medial prefrontal cortex and amygdala. Eur J Neurosci. 2004;20(3):781–790. doi: 10.1111/j.1460-9568.2004.03542.x. [DOI] [PubMed] [Google Scholar]
- 35.Herry C, Vouimba RM, Garcia R. Plasticity in the mediodorsal thalamo-prefrontal cortical transmission in behaving mice. J Neurophysiol. 1999;82(5):2827–2832. doi: 10.1152/jn.1999.82.5.2827. [DOI] [PubMed] [Google Scholar]
- 36.Whittle N, Hauschild M, Lubec G, Holmes A, Singewald N. Rescue of impaired fear extinction and normalization of cortico-amygdala circuit dysfunction in a genetic mouse model by dietary zinc restriction. J Neurosci. 2010;30(41):13586–13596. doi: 10.1523/JNEUROSCI.0849-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heimer L, et al. The Primate Nervous System: Part III. Amsterdam: Elsevier; 1999. pp. 57–226. [Google Scholar]
- 38.Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53(6):871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 39.Hugues S, Garcia R, Léna I. Time course of extracellular catecholamine and glutamate levels in the rat medial prefrontal cortex during and after extinction of conditioned fear. Synapse. 2007;61(11):933–937. doi: 10.1002/syn.20448. [DOI] [PubMed] [Google Scholar]
- 40.Vincent SL, Khan Y, Benes FM. Cellular distribution of dopamine D1 and D2 receptors in rat medial prefrontal cortex. J Neurosci. 1993;13(6):2551–2564. doi: 10.1523/JNEUROSCI.13-06-02551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swanson LW. The projections of the ventral tegmental area and adjacent regions: A combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9(1-6):321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 42.Dayan L, Finberg JPM. L-DOPA increases noradrenaline turnover in central and peripheral nervous systems. Neuropharmacology. 2003;45(4):524–533. doi: 10.1016/s0028-3908(03)00190-4. [DOI] [PubMed] [Google Scholar]
- 43.Bunzeck N, Düzel E. Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron. 2006;51(3):369–379. doi: 10.1016/j.neuron.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 44.Benetello P, Furlanut M, Fortunato M, Pea F, Baraldo M. Levodopa and 3-O-methyldopa in cerebrospinal fluid after levodopa-carbidopa association. Pharmacol Res. 1997;35(4):313–315. doi: 10.1006/phrs.1997.0145. [DOI] [PubMed] [Google Scholar]
- 45.Rossato JI, Bevilaqua LRM, Izquierdo I, Medina JH, Cammarota M. Dopamine controls persistence of long-term memory storage. Science. 2009;325(5943):1017–1020. doi: 10.1126/science.1172545. [DOI] [PubMed] [Google Scholar]
- 46.Bekinschtein P, et al. Persistence of long-term memory storage requires a late protein synthesis- and BDNF- dependent phase in the hippocampus. Neuron. 2007;53(2):261–277. doi: 10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 47.Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328(5983):1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soliman F, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327(5967):863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ressler KJ, et al. Cognitive enhancers as adjuncts to psychotherapy: Use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61(11):1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 50.Bouton ME, Vurbic D, Woods AM. D-cycloserine facilitates context-specific fear extinction learning. Neurobiol Learn Mem. 2008;90(3):504–510. doi: 10.1016/j.nlm.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Quervain DJ, et al. Quervain DJ-F de et al Glucocorticoids enhance extinction-based psychotherapy. Proc Natl Acad Sci USA. 2011;108(16):6621–6625. doi: 10.1073/pnas.1018214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zelikowsky M, et al. Cholinergic blockade frees fear extinction from its contextual dependency. Biol Psychiatry. 2013;73(4):345–352. doi: 10.1016/j.biopsych.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kindt M, Soeter M, Vervliet B. Beyond extinction: Erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12(3):256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- 54.Schiller D, et al. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463(7277):49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Freitag S, Schachner M, Morellini F. Behavioral alterations in mice deficient for the extracellular matrix glycoprotein tenascin-R. Behav Brain Res. 2003;145(1-2):189–207. doi: 10.1016/s0166-4328(03)00109-8. [DOI] [PubMed] [Google Scholar]
- 56.Sawallisch C, et al. The insulin receptor substrate of 53 kDa (IRSp53) limits hippocampal synaptic plasticity. J Biol Chem. 2009;284(14):9225–9236. doi: 10.1074/jbc.M808425200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lesting J, et al. Patterns of coupled theta activity in amygdala-hippocampal-prefrontal cortical circuits during fear extinction. PLoS ONE. 2011;6(6):e21714. doi: 10.1371/journal.pone.0021714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karpova NN, et al. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science. 2011;334(6063):1731–1734. doi: 10.1126/science.1214592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muigg P, et al. Differential stress-induced neuronal activation patterns in mouse lines selectively bred for high, normal or low anxiety. PLoS ONE. 2009;4(4):e5346. doi: 10.1371/journal.pone.0005346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paxinos K, Franklin G. The Mouse Brain in Stereotaxic Coordinates. London: Academic; 2001. [Google Scholar]
- 61.Milad MR, et al. Fear conditioning and extinction: Influence of sex and menstrual cycle in healthy humans. Behav Neurosci. 2006;120(6):1196–1203. doi: 10.1037/0735-7044.120.5.1196. [DOI] [PubMed] [Google Scholar]
- 62.Milad MR, et al. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168(3):652–658. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stark R, et al. Influence of the stress hormone cortisol on fear conditioning in humans: Evidence for sex differences in the response of the prefrontal cortex. Neuroimage. 2006;32(3):1290–1298. doi: 10.1016/j.neuroimage.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 64.Merz CJ, et al. Neuronal correlates of extinction learning are modulated by sex hormones. Soc Cogn Affect Neurosci. 2012;7(7):819–830. doi: 10.1093/scan/nsr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sánchez MG, Morissette M, Di Paolo T. Effect of a chronic treatment with 17β-estradiol on striatal dopamine neurotransmission and the Akt/GSK3 signaling pathway in the brain of ovariectomized monkeys. Psychoneuroendocrinology. 2012;37(2):280–291. doi: 10.1016/j.psyneuen.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 66.Spielberger CD. Assessment of state and trait anxiety: Conceptual and methodological issues. Southern Psychol. 1985;2:6–16. [Google Scholar]
- 67.Laux L, Glanzmann P, Schaffner P, Spielberger CD. Das State-Trait-Angstinventar. Weinheim, Germany: Beltz; 1981. [Google Scholar]
- 68.Venables PH, Christie MJ. Techniques in Psychophysiology. Chichester, UK: Wiley; 1980. [Google Scholar]
- 69.Lykken DT, Venables PH. Direct measurement of skin conductance: A proposal for standardization. Psychophysiology. 1971;8(5):656–672. doi: 10.1111/j.1469-8986.1971.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 70.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 71.Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE, Penny WD. Statistical Parametric Mapping: The Analysis of Functional Brain Images. London: Academic; 2006. [Google Scholar]
- 72.Büchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: An event-related fMRI study. Neuron. 1998;20(5):947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- 73.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron. 1998;20(5):937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 74.Düzel E, et al. Functional imaging of the human dopaminergic midbrain. Trends Neurosci. 2009;32(6):321–328. doi: 10.1016/j.tins.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 75.Agren T, et al. Disruption of reconsolidation erases a fear memory trace in the human amygdala. Science. 2012;337(6101):1550–1552. doi: 10.1126/science.1223006. [DOI] [PubMed] [Google Scholar]
- 76.Raczka KA, et al. A neuropeptide S receptor variant associated with overinterpretation of fear reactions: A potential neurogenetic basis for catastrophizing. Mol Psychiatry. 2010;15(11):1045, 1067–1074. doi: 10.1038/mp.2010.79. [DOI] [PubMed] [Google Scholar]
- 77.Paret C, et al. A test for the implementation-maintenance model of reappraisal. Front Psychol. 2011;2:216. doi: 10.3389/fpsyg.2011.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.