Abstract
Here we describe the phenomenon of symmetry breaking within a series of M4L6 container molecules. These containers were synthesized using planar rigid bis-bidentate ligands based on 2,6-substituted naphthalene, anthracene, or anthraquinone spacers and FeII ions. The planarity of the ligand spacer favors a stereochemical configuration in which each cage contains two metal centers of opposite handedness to the other two, which would ordinarily result in an S4-symmetric, achiral configuration. Reduction of symmetry from S4 to C1 is achieved by the spatial offset between each ligand’s pair of binding sites, which breaks the S4 symmetry axis. Using larger CdII or CoII ions instead of FeII resulted, in some cases, in the observation of dynamic motion of the symmetry-breaking ligands in solution. NMR spectra of these dynamic complexes thus reflected apparent S4 symmetry owing to rapid interconversion between energetically degenerate, enantiomeric C1-symmetric conformations.
Keywords: coordination chemistry, metal–organic capsules, self-assembly, supramolecular chemistry, stereochemistry
Symmetry breaking must occur before complexity can develop (1, 2). In cosmology, the perfect symmetry of the singularity at the inception of time (3) evolved under the direction of physical laws to produce the present-day universe filled anisotropically with asymmetrical pieces of matter. In biology, a zygote must break its symmetry before the functional specialization involved with cell differentiation and tissue architecture development can occur (1, 4). Studies on model systems for biological symmetry breaking reveal that the complexity on larger scales is often underpinned by asymmetry on smaller scales (4, 5), which is a consequence of dynamic interactions at the molecular level (4). Investigations of symmetry breaking during complex molecular self-assembly phenomena thus present an opportunity to shed light upon the foundations of the evolution of matter toward complexity.
To contribute to the understanding of symmetry breaking, in the present study we demonstrate a rational method of systematic symmetry breaking within a series of M4L6 metal–organic tetrahedra through the control of linker geometry.
Metal–organic polyhedra (6–13) have attracted significant attention due to their host–guest behavior that can be applied in molecular storage (14–17), separation (18), and catalysis (19–22). Platonic or Archimedean metal–organic polyhedra can be constructed by a careful choice of ligands and metal ions (8, 23–27), where the geometries of the individual building blocks define the symmetry axes of the polyhedron. Although high-symmetry architectures represent a great achievement in terms of logical molecular design, lower-symmetry ones are possibly of still greater interest. Besides the fundamental interest in symmetry breaking mentioned above (1), an asymmetric capsule could achieve recognition of asymmetric substrates (28) and possibly catalyze asymmetric transformations (29). So far all reported metal–organic capsules have high symmetry (27, 30, 31), although in some cases they are chiral (29, 32–34), and specific guest molecules are observed to stabilize lower-symmetry structures (32, 35).
M4L6 capsules are constructed from octahedrally coordinated metal ions and linear bis-bidentate ligands. Because each vertex is defined by a metal stereocenter (Δ or Λ), an M4L6 structure can have T (ΔΔΔΔ/ΛΛΛΛ, homochiral), C3 (ΔΔΔΛ/ΛΛΛΔ, heterochiral), or S4 (ΔΔΛΛ, achiral) symmetry (36), with the homochiral cage being most commonly observed (33, 37–43). When the T-symmetric configuration is adopted, all metal-to-metal distances are the same, avoiding strain potentially incurred by differences in these distances present in the C3- or S4-symmetric configurations (44). The geometries and steric properties of the ligands can nonetheless overrule the preference for T symmetry. For example, in cages constructed from pyridyl-imine ligands via subcomponent self-assembly (45), S4 symmetry, rather than T, was found to be the lowest-energy stereochemical configuration when the two terminal phenylene rings within the ligand spacer are forced to be coplanar (44), a configuration that is optimal for the four syn ligands that an S4-symmetric cage requires to connect metal centers with opposite stereochemistry.
Building upon the S4-symmetric framework, we hypothesized that the S4 symmetry axis could be broken if an offset was introduced between the two coordination sites within the same ligand. We reasoned that bis(pyridylimine) ligands derived from the planar, rigid diamines A, B, or C (Fig. 1) could be used to introduce such an offset.
Fig. 1.
Synthesis of M4L6 (where L6 = 2Lanti + 4Lsyn) structures that contain two Δ (shown in purple) and two Λ (green) metal vertices along with two anti and four syn ligands. The anti ligands are colored blue and the syn ligands red.
Results and Discussion
Synthesis and Characterization of Asymmetric FeII4L6 Cages.
As illustrated in Fig. 1, the reactions between diamines A, B, or C (3 eq), 2-formylpyridine (6 eq), and either Fe(ClO4)2 or Fe(SO3CF3)2 (−OTf, 2 eq) in acetonitrile provided a new set of M4L6 cages as the uniquely observed products in solution, as verified by 1H NMR and electrospray ionization (ESI)-MS and detailed below.
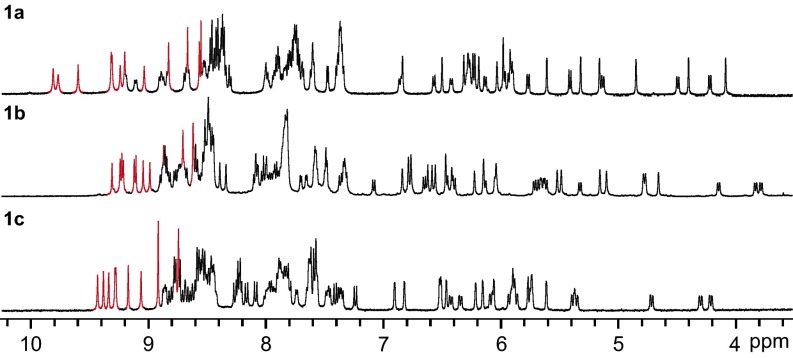
NMR spectra (Fig. 2 and SI Appendix, Figs. S4, S5, S7, S8, S13, S14, S17, and S18) for the corresponding products 1a, 1b, and 1c (Fig. 2) contained more peaks than could be attributed to any single diastereomer of T, C3, or S4 symmetry, or a mixture of all three of them. Diffusion-ordered spectroscopy (DOSY) 1H NMR spectra were consistent in each case with the presence of only a single species. Although there was significant peak overlap in both the 1H and 13C NMR spectra, it was possible to identify 12 imine carbon signals in the 13C NMR and heteronuclear multiple quantum correlation spectra (HMQC), indicating the presence of 12 ligand environments with equal populations. These observations suggest that all of the ligand hydrogen atoms are in inequivalent environments, and the cage is therefore asymmetric. A change in counter anion or in solvent (e.g., using nitromethane instead of acetonitrile) did not affect the symmetry and only resulted in slight shifts for some signals in the NMR spectra (e.g., SI Appendix, Figs. S4 and S7).
Fig. 2.
The 1H NMR spectra for FeII4L6 cages 1a·OTf, 1b·OTf, 1c·OTf, with imine protons in red.
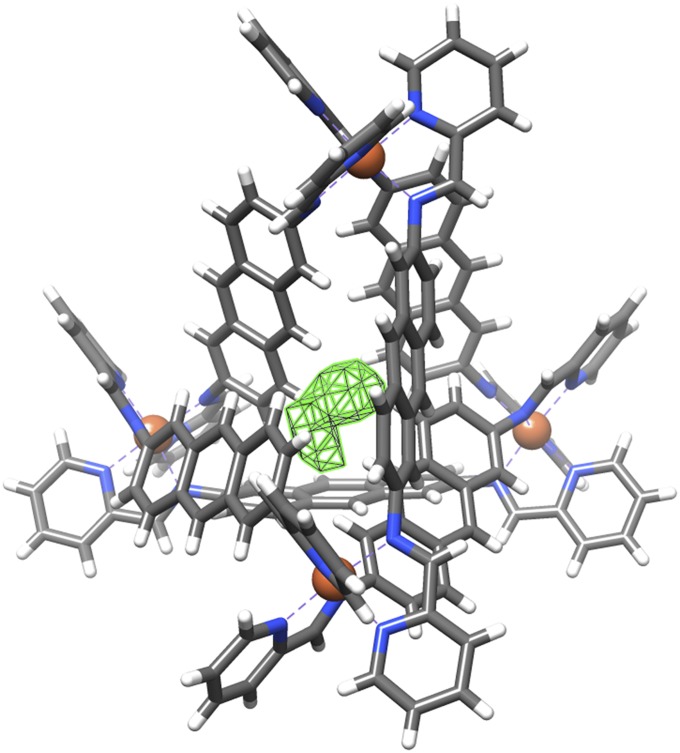
Vapor diffusion of diisopropyl ether into a nitromethane solution of cage 1a·ClO4 allowed the isolation of single crystals suitable for X-ray analysis. The crystal structure (Fig. 3) confirmed the presence of the M4L6 cage framework. The unit cell contains two cages that are enantiomers of each other. The cage is crystallographically asymmetric yet has many features in common with an S4-symmetric framework: Two FeII centers are of the same handedness whereas the other two are of the opposite handedness; each pair of FeII centers of the same stereochemistry is connected by ligands adopting anti conformations, whereas the other four ligands are syn.
Fig. 3.
(Upper) Crystal structure of FeII cage 1a·ClO4 (counter anions, solvent molecules and hydrogen atoms are omitted for clarity; only one conformation of the disordered naphthyl group is shown). (Lower) A schematic illustration of how the S4 axis of symmetry is broken. The Δ and Λ metal centers are colored purple and green, respectively. The anti ligands are colored blue and the syn ligands red. The parts of the naphthyl spacer that point out of and into the plane of the page are colored orange and maroon, respectively.
The offset geometry of the naphthyl spacers causes them to adopt an arrangement whereby within each ligand one ring is oriented inward, roughly toward the center of the cage and one ring points outward. As shown in Fig. 3, an S4 operation (consisting of a 90° rotation about the axis that bisects both anti ligands of 1a, followed by a reflection through a mirror plane perpendicular to this axis) results in a configuration in which all syn ligands are indistinguishable from their arrangement before the operation, but where the anti ligands have adopted a different arrangement. These two configurations are enantiomeric mirror images of each other. If the naphthyl groups of the anti ligands were to adopt a conformation twisted by 90° in either direction along their N-N axes (i.e., where the ligand becomes planar), the S4 operation would result in a configuration indistinguishable from the initial one, and the molecule would possess S4 symmetry. Models suggest (SI Appendix, Fig. S37), however, that such an S4-symmetric configuration would result in steric clashes between naphthyl rings.
The asymmetric configurations assumed by the ligands thus allow the naphthyl rings to avoid steric clashes with each other near the metal centers, reducing strain. The ligands pack tightly together (SI Appendix, Fig. S33), which eliminates any cavity space and provides additional stabilizing CH–π interactions between neighboring naphthalene rings.
Fe–Fe distances in 1a are in the range of 10.368(4)–10.724(4) Å; the two FeII centers linked by an anti ligand are 0.3 Å farther away from each other than those connected by a syn ligand. The FeII–N bond lengths are in the range of 1.96–2.01 Å, consistent with the low-spin configuration observed by NMR.
Either of the enantiotopic conformations of the anti ligands of 1a (Fig. 3) could be adopted with minimal energetic perturbation to the overall cage framework, as highlighted by disorder observed in the X-ray structures of both the perchlorate and the trifluoromethanesulfonate salts of 1a. Whereas the syn ligands in this structure (and all others reported herein) were observed to be well-ordered, the naphthyl groups of one (in 1a·ClO4) or both (in 1a·OTf; SI Appendix, Fig. S34) of the anti ligands were observed to be disordered across two positions.
X-ray–quality crystals of anthracene-edged cage 1b·OTf were obtained by vapor diffusion of diethyl ether into an acetonitrile solution of the cage. Cage 1b·OTf is also asymmetric in the solid state with no disorder observed (Fig. 4). The Fe–Fe distances are 12.131(3)–12.894(4) Å, a range of variation 0.3 Å wider than the naphthalene-edged cage 1a·ClO4. The longer edges also engender a discrete void space within cage 1b, whereas 1a showed no internal void. The VOIDOO program revealed the shape of this 16.8 Å3 cavity (Fig. 4), showing how the C1 symmetry of host 1b is imposed on the shape of its cavity. The small cavity size is attributed to the packing of the anthracene spacers to minimize steric clashes, as with 1a.
Fig. 4.
The X-ray crystal structure of cage 1b, superimposed upon a plot of the asymmetric cavity (16.8 Å3) found with VOIDOO (46).
The 1H NMR spectra of dissolved crystals of 1a (i.e., both 1a·ClO4 and 1a·OTf) and 1b were indistinguishable from that of the corresponding cage freshly prepared from subcomponents in acetonitrile, indicating the rotation of the anti ligands about their N-N axes is slow on the NMR time scale, and therefore C1 symmetry is the lowest energy configuration for the system in solution. For all of the three FeII cages 1a, 1b, and 1c, when the temperature was increased to 343 K (SI Appendix, Figs. S12, S16, and S20), neither decomposition nor coalescence was observed in the 1H NMR spectrum, indicating relatively high thermal stability of the complexes and limited rotational freedom of the ligands.
CoII4L6 and CdII4L6 Cages.
Co(BF4)2 (4 eq) was also observed to form M4L6 structures with both diamines A and B (6 eq) and 2-formylpyridine (12 eq); when C was used an insoluble product was obtained. These products, 2a and 2b, were characterized in solution by paramagnetic 1H NMR spectroscopy and ESI-MS. In similar fashion to the FeII cage 1a, CoII cage 2a also exhibited 12 imine signals in the 1H NMR spectrum (SI Appendix, Fig. S21), indicating that cage 2a is also asymmetrical.
The crystal structure obtained for 2a·BF4 (SI Appendix, Fig. S35) shows the same overall configuration as the iron analog 1a. The coordination bond lengths are in the range of 2.117(6)–2.197(6) Å, on average 0.15 Å longer than that for Fe–N in 1a·ClO4. The average bond length for Co–Nimine is around 0.03 Å longer than that for Co–Npy, and no significant Jahn–Teller distortion was observed, consistent with the presence of CoII ions in the high-spin state. There is a wider range of Co–Co distances in 2a·BF4 [10.339(3)–10.947(5) Å] compared with the Fe–Fe distances in 1a and correspondingly greater distortions from idealized octahedral geometry in the CoII cage.
Intriguingly, the analogous tetrahedron 2b·BF4 has a much simpler 1H NMR spectral profile (SI Appendix, Fig. S25). Only three imine signals were observed, consistent with S4 symmetry on the NMR time scale. We infer that the anthracenyl groups of the anti ligands of 2b were undergoing rapid rotation, as opposed to adopting an S4-symmetric lowest-energy state, based upon our observations of their analogs and examination of models, which suggested that for CoII, as for CdII and FeII, the S4-symmetric configuration would experience steric clashes (SI Appendix, Fig. S37).
The 1H NMR peaks for 2b·BF4 were observed to sharpen as the temperature increased, indicating fast thermal motion within the complex; these peaks broadened into the baseline below 15 °C, consistent with a slowing of the anti ligands’ rotation about their N-N axes below this temperature (SI Appendix, Fig. S26). No coalescence of the 1H NMR signals of 2a·BF4 was observed upon increasing the temperature to 70 °C (SI Appendix, Fig. S23), indicating that the anti ligands do not rotate about their N-N axes at a rate comparable to the NMR time scale at this temperature. We attribute the faster ligand rotation in 2b than in 2a to the looser geometrical requirements imposed by the longer Co–N bonds, which allow the individual ligands to rotate past each other with a lower energetic penalty and to the greater lability of these bonds, which may loosen along one N–Co–N axis by undergoing Jahn–Teller distortion during ligand rotation.
CdII was not observed to template the formation of a single product with B or C under the conditions that gave clean cage formation with FeII; instead, mixtures of soluble and insoluble products were obtained. Cage 3a was generated cleanly, however, when diamine A was used, as confirmed by its 1H NMR spectrum (SI Appendix, Fig. S27). NMR spectra of 3a were consistent with rapid rotation of the anti ligands even at −35 °C in solution (SI Appendix, Fig. S32), in contrast to its FeII or CoII analogs. As with 2b, we infer 3a to undergo interconversion between enantiomeric C1-symmetric configurations rapidly on the NMR time scale, such as to exhibit apparent S4 symmetry, as opposed to adopting an S4-symmetric lowest-energy state. We hypothesize that steric clashes between ligands during these interconversions would incur a lower energetic penalty owing to the larger ionic radius of CdII, resulting in the more rapid rate of rotation observed in the case of this metal ion. We infer that the mechanism of ligand rotation may involve distortion of the coordination sphere of CdII, but not breaking of the Cd–Nimine linkage on the NMR time scale, because satellite peaks were associated with the imine 1H NMR resonances of 3a, attributable to J-coupling with the two spin-1/2 isotopes of cadmium (SI Appendix, Fig. S27).
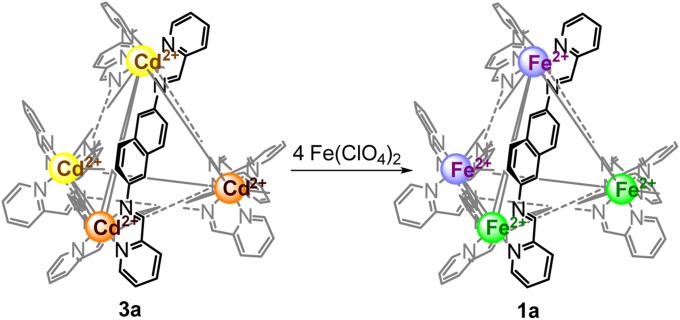
Because CdII has a d10 electron configuration, cage 3a does not benefit from crystal field stabilization, and its metal–ligand bonds are thus weaker than in complex 1a. When Fe(ClO4)2 (4 eq per cage) was added to an acetonitrile solution of cage 3a·ClO4, the orange solution turned dark purple immediately, suggesting rapid formation of a FeII complex. Cage 1a·ClO4 was the only observed complex in both the 1H NMR spectrum (SI Appendix, Fig. S36) and ESI-MS of the reaction mixture, with no evidence for the presence of 3a or any mixed-metal species, consistent with a clean metal displacement where FeII substituted all CdII to transform cage 3a·ClO4 into cage 1a·ClO4 (Fig. 5), halting the anti ligands’ rapid rotation. Although transmetallation has been demonstrated in macrocyclic complexes (47) and helicates (48), to our knowledge this is a unique example of the conversion of one metal–organic polyhedron into another via metal exchange.
Fig. 5.
Transformation of 1c·ClO4 into 1a·ClO4 via metal exchange.
Conclusions
We have demonstrated that by introducing an offset between the two metal binding sites within a linear bis-bidentate ligand an asymmetric M4L6 cage structure could be generated. This symmetry breaking was achieved as a consequence of three features of the system. First, this class of cages (44) is known, when both ends of the diamine spacer are forced to be coplanar, to adopt a configuration where two metal centers have the opposite handedness to the other two, resulting ordinarily in an S4-symmetric structure. Second, the diamine spacer’s offset geometry breaks the erstwhile S4 symmetry axis, due to steric interactions between the naphthyl groups. Third, racemization of these asymmetric cages, via rotation of the spacer groups of the anti ligands, can be slowed through the use of FeII as the metal ion. The shorter FeII–N bonds led to higher energetic barriers to ligand torsion, locking the cage’s stereochemistry.
The phenomenon of asymmetric cage formation was observed to be general across three metal ions and three offset spacer groups; other metal ions and spacers also thus show promise because the steric effects giving rise to symmetry breaking operate next to the metal centers (SI Appendix, Fig. S37), independently of ligand length. Although the cavity of the largest cage structurally characterized herein, 1b (Fig. 4), is too small for even the smallest chiral guest, the methods outlined here demonstrate how larger asymmetrical cages, imprinted with stereochemical information (29, 32–34), might selectively encapsulate and asymmetrically transform low-symmetry, information-rich guest species.
Materials and Methods
Syntheses and characterization (i.e., 1H NMR, 13C NMR, mass spectra, elemental analysis, and crystallography) of all new compounds are described in the text of SI Appendix. Details of experimental instrumentation are summarized in the text of SI Appendix. Further discussion on the mechanism of symmetry breaking is available in SI Appendix.
Supplementary Material
Acknowledgments
We thank the NMR service team at the Cambridge Chemistry Department for carrying out some of the NMR spectroscopy and Diamond Light Source (United Kingdom) for synchrotron beam time on I19 (MT7114 and MT7569). This work was supported by the European Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Crystallography, atomic coordinates, and structure factors have been deposited in the Cambridge Structural Database, Cambridge Crystallographic Data Centre, Cambridge CB2 1EZ, United Kingdom (CSD reference nos. 901947–901951).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1302683110/-/DCSupplemental.
References
- 1.Anderson PW. More is different. Science. 1972;177(4047):393–396. doi: 10.1126/science.177.4047.393. [DOI] [PubMed] [Google Scholar]
- 2.Livio M. Physics: Why symmetry matters. Nature. 2012;490(7421):472–473. doi: 10.1038/490472a. [DOI] [PubMed] [Google Scholar]
- 3.Lemaître G. Un Univers homogène de masse constante et de rayon croissant rendant compte de la vitesse radiale des nébuleuses extra-galactiques. Ann. Soc. Sci. Brux. A. 1927;47:49–59. [Google Scholar]
- 4.Li R, Bowerman B. Symmetry breaking in biology. Cold Spring Harb Perspect Biol. 2010;2(3):a003475. doi: 10.1101/cshperspect.a003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullins RD. Cytoskeletal mechanisms for breaking cellular symmetry. Cold Spring Harb Perspect Biol. 2010;2(1):a003392. doi: 10.1101/cshperspect.a003392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward MD. Polynuclear coordination cages. Chem Commun (Camb) 2009;(30):4487–4499. doi: 10.1039/b906726b. [DOI] [PubMed] [Google Scholar]
- 7.Dalgarno SJ, Power NP, Atwood JL. Metallo-supramolecular capsules. Coord Chem Rev. 2008;252(8-9):825–841. [Google Scholar]
- 8.Fujita M, Tominaga M, Hori A, Therrien B. Coordination assemblies from a Pd(II)-cornered square complex. Acc Chem Res. 2005;38(4):369–378. doi: 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]
- 9.Saalfrank RW, Maid H, Scheurer A. Supramolecular coordination chemistry: The synergistic effect of serendipity and rational design. Angew Chem Int Ed Engl. 2008;47(46):8794–8824. doi: 10.1002/anie.200702075. [DOI] [PubMed] [Google Scholar]
- 10.Tranchemontagne DJ, Ni Z, O’Keeffe M, Yaghi OM. Reticular chemistry of metal-organic polyhedra. Angew Chem Int Ed Engl. 2008;47(28):5136–5147. doi: 10.1002/anie.200705008. [DOI] [PubMed] [Google Scholar]
- 11.Seidel SR, Stang PJ. High-symmetry coordination cages via self-assembly. Acc Chem Res. 2002;35(11):972–983. doi: 10.1021/ar010142d. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh K, Hu J, White HS, Stang PJ. Construction of multifunctional cuboctahedra via coordination-driven self-assembly. J Am Chem Soc. 2009;131(19):6695–6697. doi: 10.1021/ja902045q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng Y-R, et al. Coordination-driven self-assembly of truncated tetrahedra capable of encapsulating 1,3,5-triphenylbenzene. Inorg Chem. 2010;49(22):10238–10240. doi: 10.1021/ic1018373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong VM, Fiedler D, Carl B, Bergman RG, Raymond KN. Molecular recognition and stabilization of iminium ions in water. J Am Chem Soc. 2006;128(45):14464–14465. doi: 10.1021/ja0657915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mal P, Breiner B, Rissanen K, Nitschke JR. White phosphorus is air-stable within a self-assembled tetrahedral capsule. Science. 2009;324(5935):1697–1699. doi: 10.1126/science.1175313. [DOI] [PubMed] [Google Scholar]
- 16.Sawada T, Yoshizawa M, Sato S, Fujita M. Minimal nucleotide duplex formation in water through enclathration in self-assembled hosts. Nat Chem. 2009;1(1):53–56. doi: 10.1038/nchem.100. [DOI] [PubMed] [Google Scholar]
- 17.Yoshizawa M, Tamura M, Fujita M. Chirality enrichment through the heterorecognition of enantiomers in an achiral coordination host. Angew Chem Int Ed Engl. 2007;46(21):3874–3876. doi: 10.1002/anie.200700103. [DOI] [PubMed] [Google Scholar]
- 18.Riddell IA, Smulders MMJ, Clegg JK, Nitschke JR. Encapsulation, storage and controlled release of sulfur hexafluoride from a metal-organic capsule. Chem Commun (Camb) 2011;47(1):457–459. doi: 10.1039/c0cc02573a. [DOI] [PubMed] [Google Scholar]
- 19.Yoshizawa M, Tamura M, Fujita M. Diels-alder in aqueous molecular hosts: unusual regioselectivity and efficient catalysis. Science. 2006;312(5771):251–254. doi: 10.1126/science.1124985. [DOI] [PubMed] [Google Scholar]
- 20.Pluth MD, Bergman RG, Raymond KN. Acid catalysis in basic solution: a supramolecular host promotes orthoformate hydrolysis. Science. 2007;316(5821):85–88. doi: 10.1126/science.1138748. [DOI] [PubMed] [Google Scholar]
- 21.Hastings CJ, Fiedler D, Bergman RG, Raymond KN. Aza Cope rearrangement of propargyl enammonium cations catalyzed by a self-assembled “nanozyme.”. J Am Chem Soc. 2008;130(33):10977–10983. doi: 10.1021/ja8013055. [DOI] [PubMed] [Google Scholar]
- 22.Kuil M, Soltner T, van Leeuwen PWNM, Reek JNH. High-precision catalysts: regioselective hydroformylation of internal alkenes by encapsulated rhodium complexes. J Am Chem Soc. 2006;128(35):11344–11345. doi: 10.1021/ja063294i. [DOI] [PubMed] [Google Scholar]
- 23.Albrecht M, Janser I, Frohlich R. Catechol imine ligands: From helicates to supramolecular tetrahedra. Chem Commun (Camb) 2005;(2):157–165. doi: 10.1039/b410828k. [DOI] [PubMed] [Google Scholar]
- 24.Caulder DL, Raymond KN. Supermolecules by design. Acc Chem Res. 1999;32(11):975–982. [Google Scholar]
- 25.Liu Y, et al. Directed assembly of metal-organic cubes from deliberately predesigned molecular building blocks. Chem Commun (Camb) 2004;(24):2806–2807. doi: 10.1039/b409459j. [DOI] [PubMed] [Google Scholar]
- 26.Sun Q-F, Sato S, Fujita M. An M18L24 stellated cuboctahedron through post-stellation of an M12L24 core. Nat Chem. 2012;4(4):330–333. doi: 10.1038/nchem.1285. [DOI] [PubMed] [Google Scholar]
- 27.Meng W, et al. A self-assembled M8L6 cubic cage that selectively encapsulates large aromatic guests. Angew Chem Int Ed Engl. 2011;50(15):3479–3483. doi: 10.1002/anie.201100193. [DOI] [PubMed] [Google Scholar]
- 28.Fiedler D, Leung DH, Bergman RG, Raymond KN. Selective molecular recognition, C-H bond activation, and catalysis in nanoscale reaction vessels. Acc Chem Res. 2005;38(4):349–358. doi: 10.1021/ar040152p. [DOI] [PubMed] [Google Scholar]
- 29.Lee SJ, Hu A, Lin W. The first chiral organometallic triangle for asymmetric catalysis. J Am Chem Soc. 2002;124(44):12948–12949. doi: 10.1021/ja028099s. [DOI] [PubMed] [Google Scholar]
- 30.Olenyuk B, Levin MD, Whiteford JA, Shield JE, Stang PJ. Self-assembly of nanoscopic dodecahedra from 50 predesigned components. J Am Chem Soc. 1999;121(44):10434–10435. [Google Scholar]
- 31.Tominaga M, et al. Finite, spherical coordination networks that self-organize from 36 small components. Angew Chem Int Ed Engl. 2004;43(42):5621–5625. doi: 10.1002/anie.200461422. [DOI] [PubMed] [Google Scholar]
- 32.Ziegler M, Davis AV, Johnson DW, Raymond KN. Supramolecular chirality: A reporter of structural memory. Angew Chem Int Ed Engl. 2003;42(6):665–668. doi: 10.1002/anie.200390183. [DOI] [PubMed] [Google Scholar]
- 33.Argent SP, Riis-Johannessen T, Jeffery JC, Harding LP, Ward MD. Diastereoselective formation and optical activity of an M4L6 cage complex. Chem Commun (Camb) 2005;(37):4647–4649. doi: 10.1039/b509239f. [DOI] [PubMed] [Google Scholar]
- 34.Ousaka N, Clegg JK, Nitschke JR. Nonlinear enhancement of chiroptical response through subcomponent substitution in M4L6 cages. Angew Chem Int Ed Engl. 2012;51(6):1464–1468. doi: 10.1002/anie.201107532. [DOI] [PubMed] [Google Scholar]
- 35.Jiang W, Rebek J., Jr Guest-induced, selective formation of isomeric capsules with imperfect walls. J Am Chem Soc. 2012;134(42):17498–17501. doi: 10.1021/ja3090737. [DOI] [PubMed] [Google Scholar]
- 36.Beissel T, Powers RE, Parac TN, Raymond KN. Dynamic isomerization of a supramolecular tetrahedral M4L6 cluster. J Am Chem Soc. 1999;121(17):4200–4206. [Google Scholar]
- 37.Stang PJ, Olenyuk B, Muddiman DC, Smith RD. Transition-metal-mediated rational design and self-assembly of chiral, nanoscale supramolecular polyhedra with unique T symmetry. Organometallics. 1997;16(14):3094–3096. [Google Scholar]
- 38.Mal P, Schultz D, Beyeh K, Rissanen K, Nitschke JR. An unlockable-relockable iron cage by subcomponent self-assembly. Angew Chem Int Ed Engl. 2008;47(43):8297–8301. doi: 10.1002/anie.200803066. [DOI] [PubMed] [Google Scholar]
- 39.Caulder DL, Powers RE, Parac TN, Raymond KN. The self-assembly of a predesigned tetrahedral M4L6 supramolecular cluster. Angew Chem Int Ed Engl. 1998;37(13-14):1840–1843. [Google Scholar]
- 40.Tidmarsh IS, et al. Further investigations into tetrahedral M4L6 cage complexes containing guest anions: New structures and NMR spectroscopic studies. New J Chem. 2009;33(2):366–375. [Google Scholar]
- 41.Albrecht M, Burk S, Weis P. Chiral confined space: Induction of stereochemistry in a M4L4 metallosupramolecular container. Synthesis. 2008;(18):2963–2967. [Google Scholar]
- 42.Saalfrank RW, et al. Enantiomerisation of tetrahedral homochiral [M4L6] clusters: Synchronised four Bailar twists and six atropenantiomerisation processes monitored by temperature-dependent dynamic 1H NMR spectroscopy. Chemistry. 2002;8(12):2679–2683. doi: 10.1002/1521-3765(20020617)8:12<2679::AID-CHEM2679>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 43.Cotton FA, Murillo CA, Yu R. Deliberate synthesis of the preselected enantiomer of an enantiorigid molecule with pure rotational symmetry T. Dalton Trans. 2005;(19):3161–3165. doi: 10.1039/b507998e. [DOI] [PubMed] [Google Scholar]
- 44.Meng W, Clegg JK, Thoburn JD, Nitschke JR. Controlling the transmission of stereochemical information through space in terphenyl-edged Fe4L6 cages. J Am Chem Soc. 2011;133(34):13652–13660. doi: 10.1021/ja205254s. [DOI] [PubMed] [Google Scholar]
- 45.Nitschke J, Ronson T, Zarra S, Black SP. Metal-organic container molecules through subcomponent self-assembly. Chem Commun (Camb) 2012;(49):2476–2490. doi: 10.1039/c2cc36363a. [DOI] [PubMed] [Google Scholar]
- 46.Kleywegt GJ, Jones TA. Detection, delineation, measurement and display of cavities in macromolecular structures. Acta Crystallogr D Biol Crystallogr. 1994;50(Pt 2):178–185. doi: 10.1107/S0907444993011333. [DOI] [PubMed] [Google Scholar]
- 47.Beckmann U, et al. Dicobalt(II) complexes of a triazolate-containing Schiff-base macrocycle: Synthesis, structure and magnetism. Dalton Trans. 2003;(7):1308–1313. [Google Scholar]
- 48.Dömer J, Slootweg JC, Hupka F, Lammertsma K, Hahn FE. Subcomponent assembly and transmetalation of dinuclear helicates. Angew Chem Int Ed Engl. 2010;49(36):6430–6433. doi: 10.1002/anie.201002776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.