Abstract
Psoriasis is an autoinflammatory skin disease of unknown etiology. Topical application of Aldara cream containing the Toll-like receptor (TLR)7 agonist Imiquimod (IMQ) onto patients induces flares of psoriasis. Likewise, in mice IMQ triggers pathological changes closely resembling psoriatic plaque formation. Key cytokines like IL-23 and type-I IFN (IFN-I), both being produced mainly by dendritic cells (DCs), have been implicated in psoriasis. Although plasmacytoid DCs (pDCs) are the main source of IFNα and thought to initiate disease, conventional DCs (cDCs) appear to maintain the psoriatic lesions. Any role of cDCs during lesion formation remains elusive. Here, we report that selective activation of TLR7 signaling specifically in CD11c+ DCs was sufficient to induce psoriasiform skin disease in mice. Intriguingly, both pDCs and the IFN-I pathway were dispensable for the development of local skin inflammation. Selective TLR7 triggering of Langerin+ DCs resulted in attenuated disease, whereas their depletion did not alter the severity of skin lesions. Moreover, after IMQ-painting, IL-23 was exclusively produced by Langerinneg DCs in vivo. In conclusion, TLR7-activated Langerinneg cDCs trigger psoriatic plaque formation via IL-23–mediated activation of innate IL-17/IL-22–producing lymphocytes, independently of pDCs or IFN-I. These results suggest therapeutic targeting of IL-23 production by cDCs to refine current treatment strategies for psoriasis.
Psoriasis is a common chronic autoinflammatory skin disease characterized by demarcated, red and scaly plaques (1). These are the result of environmental and genetic factors triggering hyperproliferation and disturbed differentiation of keratinocytes (parakeratosis) leading to thickening of the epidermis (acanthosis). The inflammatory cell infiltrate consists mainly of dendritic cells (DCs), macrophages and T cells in the dermis and neutrophils in the epidermis. Based on the observation that topical application of Aldara cream containing the Toll-like receptor (TLR)7 ligand Imiquimod (IMQ) can elicit psoriasis (2), we developed a mouse model closely resembling plaque-type psoriasis, including abnormal keratinocyte proliferation and differentiation as well as DC, T-cell and neutrophil infiltration (3). Thus, in the human disease as well as IMQ-induced dermatitis effector cells of both the innate and adaptive immune system take part in the dysregulated immune response.
Initially, psoriasis was defined as a T helper (Th) 1-type disease based on elevated levels of IFNγ, TNFα, and IL-12. Later on, a functional role of Th17/22 cells in psoriasis was demonstrated, associated with increased secretion of IL-17A/F and IL-22 (1, 4). Whereas IL-1β, IL-6, and TNFα contribute to the priming and skewing, IL-23 plays a pivotal role in terminal differentiation and pathogenicity of Th17/22 cells. Th cell-derived IL-17/IL-22 in turn stimulate keratinocyte proliferation and innate immune defense mechanisms like release of S100-proteins, β-defensins, and neutrophil-recruiting chemokines that contribute to the psoriatic phenotype. Novel findings concerning the pathogenic role of innate immune cells, namely γδ T cells, NK cells, and NK T cells, have challenged the prevailing view regarding psoriasis as a conventional Th cell-mediated disease (5, 6). In particular, dermis infiltrating γδ T-cell subsets as well as TCRneg RORγt+ innate lymphocytes that rapidly produce IL-17/IL-22 upon stimulation with IL-23 and IL-1β, appear to be critical for the development of psoriasiform dermatitis in mice. In agreement, an increased frequency of Vγ9+ Vδ2+ T cells was described in human psoriatic skin (7). Despite accumulating evidence that activation of innate immune pathways plays a critical role in the initiation of psoriasis, it remains unclear how these pathways are triggered in vivo.
DCs comprise a heterogeneous family of professional antigen presenting cells (APCs) that orchestrate the induction of immunity and tolerance. Plasmacytoid DCs (pDCs) are a small DC subset circulating through peripheral blood and secondary lymphoid organs. They represent key innate effector cells during antiviral immune responses due to their capacity to secrete large amounts of type-I IFN (IFN-I) upon TLR7/9 stimulation (8). Moreover, pDC-derived IFN-I represents an upstream event preceding autoimmune inflammation and psoriasis development (8, 9). In patients, an increased frequency and activation status of pDCs has been documented in early psoriatic lesions, whereas blocking IFN-I production inhibited the development of lesions in symptomless prepsoriatic skin transplants in the xenotransplantation mouse model (8). In psoriatic patients, keratinocytes produce elevated levels of the antimicrobial peptide LL-37. These form complexes with self-DNA/RNA, released by stressed/damaged cells, turning them into autoinflammatory TLR7/9-dependent triggers that activate DCs (10, 11). Subsequently, pDC-derived IFN-I together with keratinocyte-derived IL-1β, IL-6, and TNFα are thought to activate conventional DCs (cDCs), which migrate to cutaneous lymph nodes (LNs) to prime differentiation of pathogenic Th17/22 cells. Whether cDCs also contribute to the activation of IL-17/IL-22 producing innate lymphocytes in not known.
The skin contains phenotypically and functionally distinct cDC subsets (12). Langerhans cells (LCs) reside in the epidermis and are characterized by expression of Langerin/CD207, which they share with a small population of Langerin+ cDCs in the dermis, whereas the majority of dermal cDCs are Langerinneg. Recent observations indicate a functional specialization of the different skin-resident cDC subsets and, in particular, LCs can exert regulatory functions (e.g., during Leishmania major infection), whereas dermal cDCs may be more immunogenic (12, 13). In this study, we sought to dissect whether and how the different skin DC populations promote or regulate psoriatic plaque formation.
Results
MyD88LSL Mice Are Resistant to IMQ-Induced Skin Inflammation.
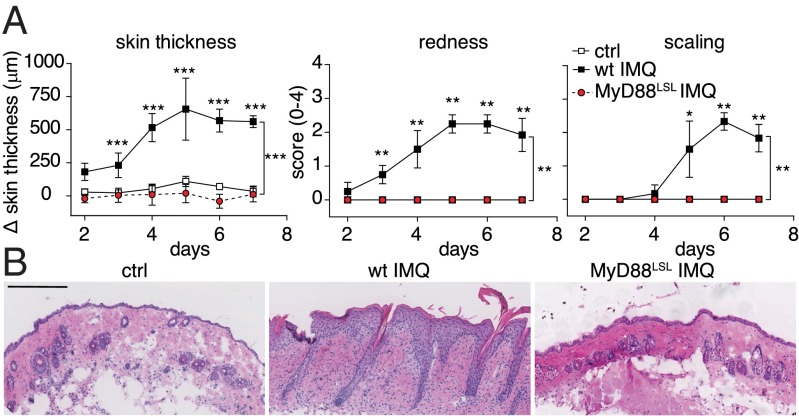
To investigate the role of DCs in IMQ-induced psoriasiform skin inflammation, we took advantage of a MyD88-inducible mouse strain (MyD88LSL), which enables Cre/loxP-mediated cell type-specific expression of the TLR-adaptor protein MyD88 on a MyD88-deficient background (14). Signaling via TLR7 is critically dependent on MyD88, and MyD88-deficient immune cells do not respond to IMQ (15). However, TLR/MyD88-independent effects of Aldara cream have been reported (16). Therefore, it was essential to exclude any TLR/MyD88-independent effects of Aldara on the formation of psoriasis-like disease in MyD88-deficient MyD88LSL mice. Repetitive application of IMQ onto wild-type mouse skin led to psoriasiform inflammation with significant thickening, redness, and scaling caused by keratinocyte hyperproliferation and leukocyte infiltration into the skin (Fig. 1). In contrast, MyD88LSL mice did not develop any signs of skin inflammation (Fig. 1A). H&E-stained back skin sections of MyD88LSL mice displayed a complete lack of infiltrating mononuclear cells (Fig. 1B). Hence, IMQ-induced psoriasiform skin disease is indeed mediated exclusively via MyD88-dependent signaling. To confirm the functional deficiency in MyD88LSL mice at the molecular level, we analyzed the expression of selected psoriasis-related transcripts in the skin (17). Topical IMQ treatment resulted in significantly increased expression of Keratin-16 (K16), S100A7, and IL-17A in back skin of wild-type but not MyD88LSL mice (Fig. S1A). Activation of the TLR7 pathway also induces a rapid increase of proinflammatory cytokines in the serum (18). To assess the impact of MyD88 deficiency on this systemic response, we analyzed serum cytokine levels 6 h after topical IMQ treatment. In contrast to wild type, the proinflammatory cytokines IL-6, TNFα, and IL-22 were not up-regulated in MyD88LSL mice (Fig. S1B). This indicates that the rapid increase of proinflammatory cytokines after IMQ application is strictly MyD88 dependent. Taken together, our data establish a functional knockout of MyD88 signaling in the MyD88LSL mouse strain and an absolute requirement of MyD88 for the development of IMQ-induced dermatitis.
Fig. 1.
MyD88LSL knockout mice are resistant to IMQ-induced skin inflammation. Wild-type and MyD88LSL mice were treated with IMQ for 6 consecutive days. (A) Increase in back skin thickness, redness, and scaling. (B) Representative H&E-stained back skin sections on day 7. Magnification 200×. (Scale bar: 200 μm.) One out of at least two representative experiments is depicted (mean ± SD of n ≥ 5 animals per group).
CD11c+ DCs Are Sufficient to Drive Psoriasis-Like Skin Inflammation Induced by IMQ.
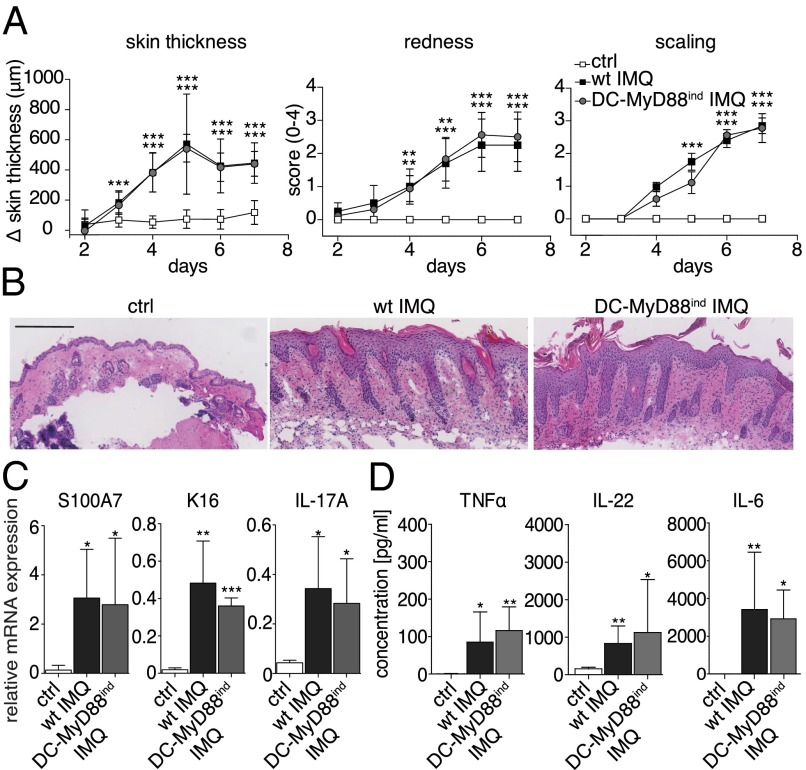
DCs are central players linking innate and adaptive immunity, because they prime and activate naïve lymphocytes in secondary lymphoid organs, but also initiate and amplify early inflammation by production of proinflammatory cytokines and chemokines in peripheral tissues. Hence, we sought to investigate the role of CD11c+ DCs to elicit psoriasiform skin inflammation. Using CD11c-Cre mice (19), we generated animals in which MyD88 was exclusively expressed in CD11c+ DCs (DC-MyD88ind), whereas all other cells remained MyD88-deficient (14). Strikingly, DC-MyD88ind and wild-type mice developed identical symptoms of psoriasiform skin disease following IMQ painting (Fig. 2A). Histological analysis of back skin of DC-MyD88ind mice revealed typical IMQ-induced pathology, indicated by acanthosis and parakeratosis (Fig. 2B). In addition, similar CD3+ T-cell infiltrates were present in the dermis of DC-MyD88ind compared with wild-type mice (Fig. S2). To confirm the psoriasis-like phenotype at the molecular level, we examined the expression of K16, S100A7, and IL-17A in the skin. Once MyD88 was selectively expressed in CD11c+ DCs, TLR7 triggering induced a comparable psoriasiform gene expression pattern in DC-MyD88ind and wild-type mice (Fig. 2C). Finally, we asked whether CD11c+ DCs were sufficient to promote an increase of proinflammatory cytokines in the serum during the onset of psoriasiform skin inflammation. As depicted in Fig. 2D, 6 h after IMQ application serum cytokine levels of TNFα, IL-6, and IL-22 were similarly elevated in DC-MyD88ind and wild-type animals. These data demonstrate that CD11c+ DCs are sufficient to induce full-blown psoriasiform skin disease in mice, including elicitation of the early systemic proinflammatory cytokine response.
Fig. 2.
CD11c+ DCs are sufficient to drive psoriasis-like skin inflammation induced by IMQ. Wild-type and DC-MyD88ind mice were treated with IMQ for 6 consecutive days. (A) Increase in back skin thickness, redness, and scaling. (B) Representative H&E-stained back skin sections on day 7. Magnification 200×. (Scale bar: 200 μm.) (C) Relative expression of Keratin-16 (K16), S100A7, and IL-17A in back skin was measured by quantitative RT-PCR. (D) Six hours after IMQ-painting of wild-type and DC-MyD88ind mice serum levels of TNFα, IL-22, and IL-6 were quantified by cytometric bead array (CBA). One out of at least two representative experiments is depicted (mean ± SD of n ≥ 6 animals per group).
pDCs Are Dispensable for Induction of the Local Skin Inflammation in IMQ-Psoriasis.
In several studies pDCs have been critically linked to initiation and early phases of developing psoriatic lesions (8, 9). Because MyD88 signaling in DC-MyD88ind mice was reconstituted in both cDCs and pDCs (19), we hypothesized that pDCs were the critical pathogenic DC subset promoting psoriasiform skin disease. To test this hypothesis, we took advantage of two different pDC-deficient transgenic mouse strains. For one, DC–E2-2−/− mice, in which the basic helix-loop-helix transcription factor (E protein) E2-2, essential for pDC development, is deleted in CD11c+ cells, constitutively lack pDCs, whereas the cDC lineage is not affected (20). Second, hBDCA-2DTR mice, that express the high-affinity diphtheria toxin receptor (DTR) under control of the human pDC-specific promoter blood dendritic cell antigen (BDCA)-2, enable efficient inducible pDC depletion by administration of DT (21).
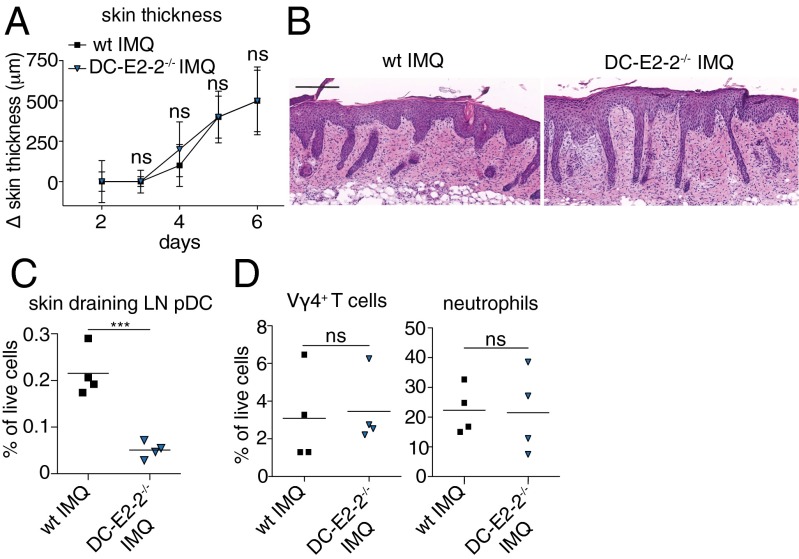
Surprisingly, in the absence of pDCs, both DC–E2-2−/− and hBDCA-2DTR mice developed a psoriasiform skin phenotype in the IMQ model that was indistinguishable from wild type (Fig. 3 A and B and Fig. S3 A and B). Efficient pDC depletion during IMQ application was confirmed by flow cytometry. In contrast to wild-type animals that showed the characteristic accumulation of pDCs in skin-draining LNs, pDCs were virtually absent in the nodes of DC–E2-2−/− and DT-treated hBDCA-2DTR mice (Fig. 3C and Fig. S3C). Because pDCs are prominent producers of large quantities of IFN-I during the initiation of psoriasis (8), we determined serum levels of IFNα 6 h after IMQ painting to prove efficient functional pDC depletion. Although topical IMQ treatment dramatically increased serum levels of IFNα in wild type, they remained low in the absence of pDCs (Fig. S3F). To investigate any impact of the pDC deficiency on the composition of the inflammatory cell infiltrate skin samples were taken on day 6 of the IMQ model and analyzed by flow cytometry. No differences were observed in the frequency of infiltrating neutrophils between wild-type and pDC-deficient mice (Fig. 3D and Fig. S3D). Moreover, the percentage of pathogenic γδ T cells was comparable in wild type and DC–E2-2−/− (Fig. 3D). Analysis of the expression of psoriasis-associated transcripts in skin lesions of wild type and pDC-deficient mice at day 6 revealed a similar up-regulation of S100A7, K16, IL-17A, and IL-22 after IMQ treatment (Figs. S3E and S4). On the other hand, serum levels of TNFα, IL-6, and IL-22 in hBDCA-2DTR mice were significantly decreased compared with IMQ-treated wild type, although they were still slightly elevated over negative controls (Fig. S3F). These findings demonstrate that pDCs are dispensable for IMQ-induced psoriatic plaque formation and local skin inflammation, whereas pDCs are required to mediate the systemic proinflammatory cytokine response typically seen after topical IMQ treatment. Consequently, psoriasiform skin disease can develop in the absence of systemic inflammation.
Fig. 3.
pDC are dispensable for local skin inflammation in IMQ-psoriasis. Wild-type and pDC-less DC–E2-2−/− mice were treated with IMQ for 5 consecutive days. (A) Increase in back skin thickness (mean ± SD, n ≥ 4). (B) Representative H&E-stained back skin sections on day 7 (n ≥ 4 mice). Magnification 200×. (Scale bar: 200 μm.) (C) FACS analysis of skin-draining LN pDC (B220+ CD11cint PDCA-1+) in IMQ-treated wild-type and DC–E2-2−/− mice. (D) Skin-infiltrating Ly6-G+ neutrophils and CD3+ Vγ4+ T cells (mean ± SD of n ≥ 4 animals per group). One out of at least two representative experiments is depicted.
IFN-I Signaling Is Not Required for the Formation of Local Psoriasiform Skin Inflammation.
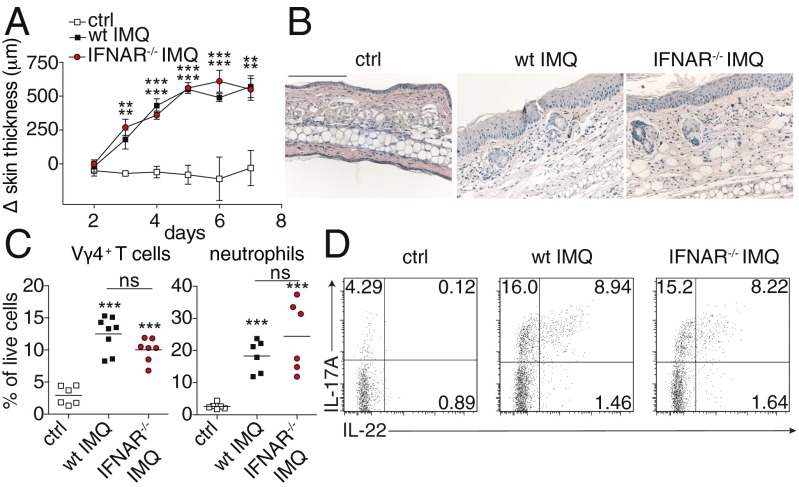
Next to pDCs, activation of the IFN-I signaling pathway has been considered as one of the primary events in the cascade initiating psoriatic inflammation (8, 22). After the unexpected finding that pDCs are dispensable for the induction of psoriasiform skin inflammation, we assessed the significance of the IFN-I signaling pathway for IMQ-induced dermatitis. To this aim, we induced psoriatic lesions in IFN-I receptor knockout mice (IFNAR−/−). Notably, IFNAR−/− also developed similar skin lesions as wild-type mice, including comparable skin thickening along with typical psoriasiform skin pathology (Fig. 4 A and B). Flow cytometric analysis of the lesional skin infiltrate revealed that neutrophil infiltration as well as recruitment of pathogenic γδ T cells was comparable to wild-type mice (Fig. 4C). Moreover, lack of IFN-I receptor signaling did not affect the production of IL-17A and IL-22 by CD3+ skin-infiltrating T cells during lesion formation (Fig. 4D). In contrast, induction of the early systemic proinflammatory cytokines IFNα, IL-22, and IL-6 was significantly lower in IFNAR−/− mice (Fig. S5). These results establish that IFN-I signaling is dispensable for local psoriatic lesion formation, thereby strongly supporting the expendability of pDCs for the development of IMQ-induced dermatitis. On the other hand, auto- and paracrine signals via IFN-I are essential to mediate the innate systemic proinflammatory cytokine response after topical IMQ application.
Fig. 4.
Type-I IFN signaling is not required for the development of local IMQ-induced skin inflammation. Wild-type and IFNAR−/− mice were treated with IMQ for 6 consecutive days. (A) Increase in back skin thickness (mean ± SD, n ≥ 5). (B) Representative H&E-stained ear skin sections on day 6. Magnification 100×. (Scale bar: 100 μm.) (C) FACS analysis of skin-infiltrating Ly6-G+ neutrophils and CD3+ Vγ4+ T cells in control, IMQ-treated wild-type and IFNAR−/− mice. (D) Production of IL-17A and IL-22 by skin CD45+ CD3+ cells of negative control and IMQ-treated mice (n ≥ 3). One out of at least two representative experiments is depicted.
Langerin+ DCs Neither Promote Nor Attenuate IMQ-Induced Psoriasis.
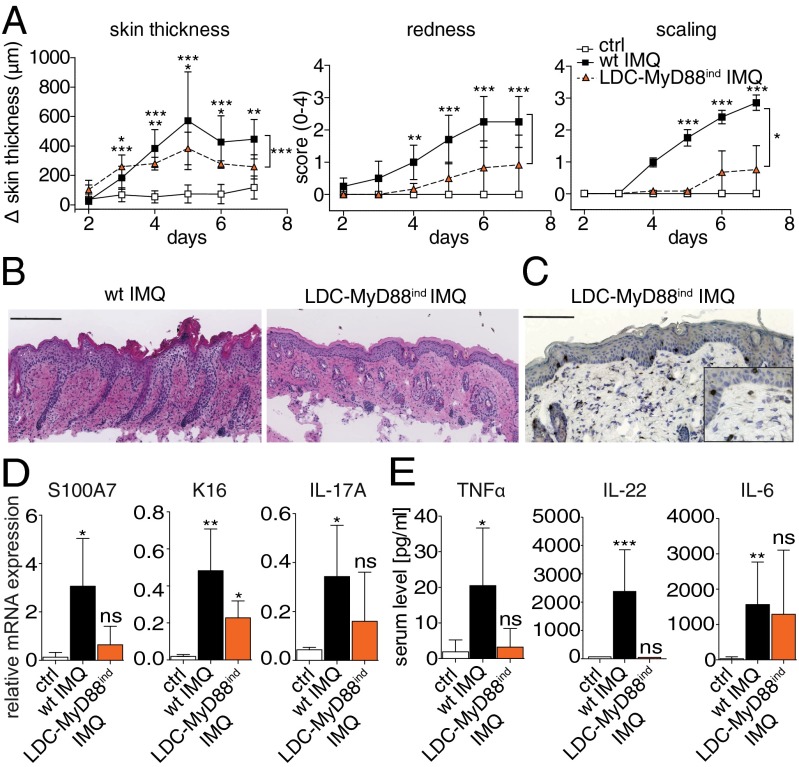
Because we demonstrated that CD11c+ DCs are sufficient, and neither pDCs nor IFN-I are required to mediate plaque formation, we sought to further dissect the responsible skin cDC subset that promotes or regulates IMQ-induced psoriasiform inflammation. To determine whether Langerin+ skin cDCs are capable to elicit psoriatic skin pathology, we generated mice in which MyD88 expression was restricted to Langerin+ DCs (LDCs) (23). Analysis of these LDC–MyD88ind mice revealed that TLR7-signaling limited to Langerin+ DC subsets, resulted in a significantly attenuated skin phenotype in comparsion to wild-type mice (Fig. 5A). Moreover, skin of LDC–MyD88ind mice displayed very mild epidermal changes and reduced leukocyte infiltration, with less abundant T-cell recruitment into the skin (Fig. 5 B and C). In addition, selective activation of Langerin+ DCs by IMQ was not sufficient to induce significant psoriasis-related gene expression indicating no true psoriatic plaque formation in the skin (Fig. 5D). To further dissect whether this inability correlated with impaired secretion of proinflammatory mediators during the onset of psoriasiform skin inflammation, we examined the systemic cytokine response induced by selective activation of TLR7 signaling in Langerin+ DCs. Although LDC–MyD88ind mice displayed an increase of IL-6 in the serum, neither TNFα nor IL-22 were significantly elevated (Fig. 5E). Thus, Langerin+ DCs alone mediate ineffective induction of both local skin inflammation and systemic proinflammatory cytokine production. Because the diminished systemic response was uncoupled from severe cutaneous inflammation and pathology in pDC– and IFN-I signaling-deficient mice (respectively, Fig. 3 and Figs. S3, and S5), these data strongly suggest that ameliorated disease in LDC–MyD88ind mice is due to inefficient activation of innate effector mechanisms by Langerin+ DCs in situ.
Fig. 5.
Langerin+ DCs do not promote nor attenuate IMQ-induced dermatitis. Wild-type and LDC–MyD88ind mice were treated with IMQ for 6 consecutive days (n ≥ 5). (A) Increase in back skin thickness, redness, and scaling (mean ± SD). (B) Representative H&E-stained back skin sections on day 7. (C) Representative staining for CD3+ T-cell infiltrates in back skin of LDC–MyD88ind mice. Magnification, 200× or 400× (Inset). (Scale bar: 200 μm.) (D) Relative expression of Keratin-16 (K16), S100A7, and IL-17A in back skin (day 7) was analyzed by quantitative RT-PCR (mean ± SD of n ≥ 5 mice). (E) Six hours after IMQ application onto wild-type and LDC–MyD88ind mice, serum levels of TNFα, IL-22, and IL-6 were quantified by CBA analysis (n ≥ 5 mice). One out of at least two representative experiments is depicted.
Alternatively, the attenuated phenotype of LDC–MyD88ind mice might be due to a regulatory function of LCs (24). To test this hypothesis, we took advantage of Langerin–DTR mice, in which Langerin+ cells can be selectively depleted by injection of diphtheria toxin (DT) (25). DT was injected 3 d before starting the IMQ treatment and then every other day throughout the experiment to ensure that the skin was devoid of Langerin+ DCs. Mice lacking Langerin+ DCs developed a similar degree and course of psoriasiform skin disease as wild type (Fig. S6). These data demonstrate that LDC–MyD88ind mice develop attenuated disease and that Langerin+ DCs are dispensable and have no regulatory function during IMQ-induced dermatitis. Hence, Langerinneg DCs, are the critical cDC subset driving IMQ-psoriasis in mice.
Langerinneg cDC-Derived IL-23 Triggers Innate IL-17/IL-22 Production.
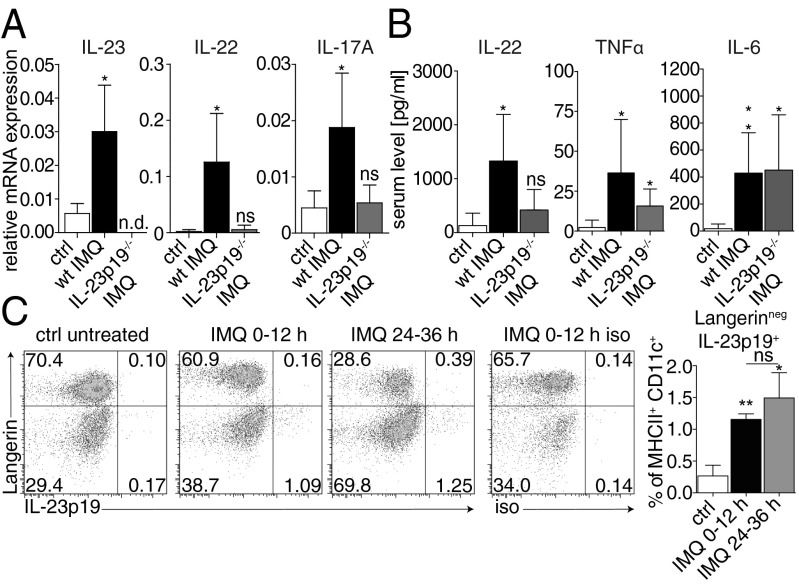
To test our hypothesis that Langerinneg cDCs are the pathogenic population promoting psoriatic plaque formation, we investigated the molecular mechanisms responsible for the onset of disease. We previously reported a pivotal role of the IL-23/IL-17 axis in IMQ-induced psoriasis (3). However, at that time, psoriasis was considered to be driven by Th17 cells, and the role of IL-23 for the induction of the early cytokine response during onset of psoriasis-like skin disease remains elusive. To examine the impact of IL-23 on local proinflammatory cytokine production in the skin, we analyzed expression levels of IL-23, IL-22, and IL-17A on day 2 of the IMQ model. Whereas expression of all three cytokines was induced in wild-type animals, neither IL-22 nor IL-17A mRNA expression was triggered in the skin in the absence of IL-23 (Fig. 6A). Concordantly, no elevated IL-22 protein could be detected in the serum of IL-23p19−/− mice 6 h after IMQ application compared with negative controls, whereas the levels of both TNFα and IL-6 were significantly increased (Fig. 6B). These data strongly suggest a direct link between cDC-derived IL-23 and innate IL-22/IL-17 in the skin and serum, whereas other systemic proinflammatory cytokines like TNFα and IL-6 are less dependent on IL-23.
Fig. 6.
cDC-derived IL-23 triggers innate IL-17/IL-22 production. IL-23p19−/− mice were painted with IMQ for 2 consecutive days. (A) Relative expression of IL-23p19, IL-17A, and IL-22 in back skin of 2-d IMQ-treated mice was analyzed by quantitative RT-PCR. (B) Serum levels of IL-6, TNFα, and IL-22 were quantified by CBA. Bars represent mean ± SD of n ≥ 4 animals per group. (C) Production of IL-23p19 by different skin DC subsets was determined during the first 12 h and between 24 and 36 h after (repetitive) IMQ-painting. Cells were gated on CD45+ MHCII+ CD11c+ DCs. Representative FACS plots out of at least two experiments (n = 3 animals per group) are depicted. Bars represent mean ± SD of n ≥ 3 animals per group.
Because our data indicated that the development of skin lesions is dependent on IL-23 in situ, we sought to determine which skin cDC subset produces IL-23 in response to TLR7 stimulation in this psoriatic setting. To this aim, we adapted a protocol using Brefeldin A for detection of in vivo cytokine production (26). Briefly, wild-type mice were treated with IMQ and simultaneously injected with Brefeldin A to inhibit cytokine secretion. In vivo production of IL-23 during the first 12 h after IMQ painting was analyzed by intracellular flow cytometry and revealed production of IL-23 exclusively by Langerinneg, but not Langerin+ skin cDCs (Fig. 6C). A comparable number of Langerinneg cDCs produced IL-23 following the second IMQ treatment combined with Brefeldin A during 24–36 h. These likely add to the initial wave of IL-23 secreting cells generated during the first 12 h after topical IMQ, however, mice cannot be treated with Brefeldin A for more than 12 h (26). Taken together, our data establish Langerinneg skin cDCs as the source of IL-23 and therefore the crucial activators of innate lymphocytes to produce IL-17 and IL-22 during the onset of IMQ-psoriasis (5, 6). Furthermore, Langerin+ skin DCs fail to secrete IL-23 required for the development of full-blown disease, which is in line with the attenuated phenotype in LDC–MyD88ind mice (Fig. 5).
Discussion
Psoriasis is considered to be a Th17-driven autoinflammatory skin disease of unknown etiology (1, 4). Previous studies reported an essential role for pDCs in the initiation of psoriasis as they transiently infiltrate early lesions and flare-ups of psoriasis upon topical application of Aldara/IMQ (2, 8). Established psoriasis lesions however, lack this marked influx of pDCs, suggesting a sequential role for different DC populations during cutaneous disease (22, 27, 28). DCs are not only crucial to maintain immune homeostasis at epithelial borders including the skin, they are also the central link between innate and adaptive immunity. Recent reports on the pathogenesis of psoriasis demonstrated a critical role for RORγt+ innate immune cells, in particular, dermis infiltrating γδ T cells that can rapidly produce IL-17/IL-22 upon stimulation with IL-1β and IL-23 (5, 6). These findings challenge the established view regarding psoriatic lesion formation as a conventional Th cell-driven disease, and prompted us to revisit the role of different DC populations in a mouse model of psoriatic plaque formation provoked by the TLR7 ligand IMQ (3).
Here, we show that MyD88-deficient mice are resistant to IMQ-induced psoriasis and that selective activation of CD11c+ DCs by IMQ is sufficient to mediate full-blown psoriasiform skin disease. Intriguingly, both pDCs and IFN-I signaling are dispensable to develop the local skin pathology. Rather, cutaneous inflammation is driven by cDCs and, in particular, Langerinneg skin DCs produce IL-23, the cytokine that is required to activate early IL-17/IL-22 production by innate lymphocytes in situ.
The fact that psoriasiform skin disease could not be induced in MyD88LSL mice, is in line with results obtained with TLR7−/− mice showing that IMQ exerts its biologic activity primarily via MyD88-dependent activation of TLR7 (6, 15). Although additional active ingredients in the Aldara cream formulation might lead to TLR7-independent effects (16), our data establish a strict MyD88-dependency of IMQ-induced psoriasis. Therefore, TLR7-independent effects observed in TLR7−/− mice might be due to activation of other signaling pathways, which are also disrupted in MyD88LSL mice.
In recent years, pDCs were considered to be the key innate effector cells initiating disease due to their transient accumulation and activation in early psoriatic skin lesions (8, 9). In contrast, our data using two different pDC-deficient mouse strains indicate that pDCs are dispensable during the onset and development of local psoriasisform skin inflammation in the IMQ model. Our finding is in line with reports on skin samples of psoriasis patients, in which activity of cDCs rather than pDCs correlated with disease progression in very early plaques (28). On the other hand, pDCs do seem to be required to amplify systemic proinflammatory cytokine expression observed in IMQ-psoriasis.
Transient activation of the IFN-I pathway was demonstrated during the early phase of disease, but not in stable plaques, and treatment of nonpsoriatic conditions with IFNα exacerbates disease pathogenesis (8, 22). These observations suggest that IFN-I represents an initial upstream event driving inflammation and psoriasis development. In contrast, our IFNAR−/− data demonstrate that signaling via the IFN-I pathway is not required for the development of the local IMQ-mediated psoriasiform skin inflammation, but a requisite for the augmented systemic response. This observation is consistent with the pDC depletion data (see above) as well as the immunomodulatory effects of IFN-I on various cell types (29). Moreover our findings provide an explanation for the failure of anti-IFNα therapy to ameliorate psoriasis lesions (30).
One explanation for the apparent incongruity between published literature and our findings may be the fact that IMQ initiates psoriasisform skin changes solely by activation of the TLR7 pathway in mice and humans, not recapitulating other triggers that may contribute to the development of the multifactorial human disease. Keratinocytes of psoriasis patients produce elevated levels of the antimicrobial peptide LL-37, which form complexes with self-nucleic acids released by stressed/damaged cells. These LL-37:self-RNA complexes serve as autoinflammatory TLR7-dependent triggers that activate myeloid cDCs and induce production of TNFα, IL-6, and potentially IL-23 (10). This pathway is likely to be active during the initiation of psoriatic plaque formation by the TLR7 ligand IMQ. On the other hand, strong activation of the TLR9 pathway via LL-37:self-DNA complexes that contributes to the activation of pDCs in psoriasis patients (11), may not be fully emulated during the IMQ mouse model. Irrespectively, our data unequivocally establish that cDCs are sufficient to promote IMQ-induced psoriasis-like pathology and skin inflammation and that pDCs and IFN-I signaling, though they may be involved in psoriasis, are not necessary for the initiation of psoriasiform plaque formation.
Because pDCs are dispensable, the relevant question remains, which cutaneous cDC population is responsible for the activation of innate lymphocytes to produce the early IL-17/IL-22 driving psoriasiform inflammation. Although conditional reconstitution of MyD88 signaling in all DCs is sufficient to elicit full-blown IMQ-psoriasis, selective activation of the MyD88 pathway in Langerin+ cDCs yields only attenuated skin disease. This observation indicates a nonessential role of Langerin+ cDCs, including LCs, during the initiation of IMQ-induced skin inflammation. Alternatively, because epicutaneous application of IMQ causes LC migration to draining LNs (31), the cells could exert a negative regulatory function by suppressing pathologic T-cell differentiation and activation (32). This hypothesis seems unlikely however, because we find that Langerin+ DC-depleted Langerin-DTR mice develop IMQ-induced psoriasis to a comparable extent as wt. Thus, LC emigration from the epidermis does not appear to be critical for the initiation or regulation of the disease. Alternatively, because the expression of TLR7 by murine LCs remains controversial (33), the attenuated skin inflammation in LDC–MyD88ind mice might be driven by the small subset of Langerin+ dermal DCs. However, taken together these data establish that Langerinneg cDCs, rather than Langerin+ cDCs, represent the critical DC population driving IMQ-psoriasis.
Further supporting this conclusion, we provide in vivo evidence that TLR7-activated dermal Langerinneg cDCs are indeed the initiators of the local skin phenotype in IMQ-induced psoriasis: Within hours after IMQ painting only Langerinneg skin DCs produce the IL-23 necessary for installing a IL-17/IL-22–dominated inflammatory cytokine milieu, i.e., by activating innate lymphocytes. In accordance with this finding, immunohistological analysis of psoriasis skin demonstrated expression of IL-23 by CD11c+ cDCs, but not CD1a+ LCs (34). Moreover, in patients an increase of dermal CD11c+ DCs marks the critical step in early stages of psoriatic lesion formation and disease development (28, 35). The pathogenicity of these infiltrating dermal CD1cneg cDCs that produce multiple inflammatory products including IL-23 is further supported by the observation that a decline of their inflammatory mediators is one of the earliest signs of effective immunotherapy (36). Conversely, relapses after treatment correlate with an increase of dermal CD1cneg inflammatory DCs. In conclusion, our data establish that Langerinneg skin DCs represent the main source of early IL-23, thereby initiating the inflammatory cascade in the IMQ model. Whether these Langerinneg cDCs belong to the resident and/or infiltrating dermal DCs that have been described in psoriasis patients needs further investigation. Although this innate function is beyond their classical role as APCs, it does not exclude that Langerinneg cDCs may also contribute to polarization of the pathogenic Th17 response at later stages of psoriasis.
Harnessing the potent immunoregulatory functions of DCs for the treatment of psoriasis critically relies on a better understanding of the distinct roles different cutaneous DC populations play during the initiation and perpetuation of chronic skin inflammation. This knowledge is essential to improve current approaches to disrupt the autoinflammatory cascade in human psoriasis. In this study, we have identified Langerinneg cDCs as the critical pathogenic DC population initiating psoriatic plaque formation in mice via production of IL-23. To this end, our data extend previous findings and provide a functional rational to interfere with IL-23 activity or production using, for example, Ustekinumab, Fumarate, or Apilimod (37–39). Moreover, our work suggests that future strategies specifically blocking IL-23 secretion by Langerinneg cDCs may provide an effective treatment for psoriasis avoiding the problems associated with the general immunosuppression seen in many therapeutic approaches today.
Materials and Methods
Mice.
MyD88LSL mice (14) were crossed to CD11c-Cre (19) or Langerin-Cre (23) to obtain DC-MyD88ind and LDC–MyD88ind animals. Langerin-DTR (25) and DC–E2-2−/− (20) mice were generated in our laboratories. IL-23p19−/− (referenced in 3) were kindly provided by E. Lubberts (Erasmus MC, Rotterdam, The Netherlands), and hBDCA-2DTR (JAX 014176) and IFNAR−/− (JAX 032045) mice were obtained from the Jackson Laboratory. Animal studies were performed at the Erasmus MC, Rockefeller University, University of Zurich, and Columbia University in compliance with national laws and approved by the respective institutional ethical committees.
IMQ-Induced Psoriasis.
The Aldara/IMQ mouse model of psoriasiform skin inflammation was performed as described (3).
In Vivo Cell Depletion and Brefeldin A Assay.
To deplete Langerin+ DCs or pDCs, Langerin-DTR, and hBDCA-2DTR animals were injected every other day i.p. with, respectively, 16 and 4.5 ng of DT (Sigma) per g of body weight 3 d before starting the IMQ treatment. To measure in vivo cytokine production, mice were injected i.v. with 0.25 mg Brefeldin A (Sigma) in PBS before application of Aldara cream. Control animals were injected with Brefeldin A and painted with vehicle cream. Eight hours after treatment, whole ear skin cell suspensions were analyzed by flow cytometry.
Cell Preparation.
Following mechanical disruption, LNs and ears were digested with 400 U/mL Collagenase IV (Worthington) and for the ears additionally 100 U/mL Hyaluronidase (Sigma) and 0.1% RNase-free DNase (Promega) in HBSS for 30–60 min at 37°. Subsequently, the cells were filtered through 70-μm cell strainers (BD Falcon) to obtain single-cell suspensions for flow cytometry.
Flow Cytometry.
Cells were preincubated with Fc-block (Biolegend) and then labeled with appropriate cell surface antibodies at 4 °C for 45 min. For intracellular cytokine staining, cells were stimulated with PMA (Applichem) and Ionomycin (Invitrogen) and treated with GolgiStop (BD Biosciences) for 3 h. For intracellular staining, cells were fixed with 2% (wt/vol) PFA, permeabilized with 0.1% Saponin, and incubated with antibodies for 60 min at 4 °C. Subsequently, samples were measured on a FACS Canto II, LSRII Fortessa, or LSRII (BD Biosciences) and analyzed using FlowJo software (Treestar).
Additional Methods.
Antibodies, cytokine detection, quantitative RT-PCR, and histology are described in SI Materials and Methods.
Statistical Analysis.
One-way ANOVA with Bonferroni-post, Kruskal–Wallis with Dunn’s post test, or unpaired Student's t test were applied as indicated, comparing IMQ versus control mice. *P < 0.05, **P < 0.01, ***P < 0.005.
Supplementary Material
Acknowledgments
We thank the members of the B.E.C. laboratory for many helpful discussions and support, Inge Brouwers-Haspels and Edwin Florencia for expert technical assistance, and Jon Laman for critical reading of the manuscript. This work was supported by grants from The Netherlands Organization for Scientific Research (NWO, VIDI 917-76-365) and the Landsteiner Foundation for Blood Transfusion Research (LSBR, 0414F) (to B.E.C.). S.H. is supported by the National Science Foundation of Switzerland, and C.C. is supported by the Canadian Institutes of Health Research (MOP-125933).
Footnotes
The authors declare no conflict of interest.
†This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307569110/-/DCSupplemental.
References
- 1.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 2.Gilliet M, et al. Psoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch Dermatol. 2004;140(12):1490–1495. doi: 10.1001/archderm.140.12.1490. [DOI] [PubMed] [Google Scholar]
- 3.van der Fits L, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182(9):5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 4.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129(6):1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 5.Cai Y, et al. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity. 2011;35(4):596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pantelyushin S, et al. Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J Clin Invest. 2012;122(6):2252–2256. doi: 10.1172/JCI61862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laggner U, et al. Identification of a novel proinflammatory human skin-homing Vγ9Vδ2 T cell subset with a potential role in psoriasis. J Immunol. 2011;187(5):2783–2793. doi: 10.4049/jimmunol.1100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nestle FO, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202(1):135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albanesi C, et al. Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment. J Exp Med. 2009;206(1):249–258. doi: 10.1084/jem.20080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganguly D, et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med. 2009;206(9):1983–1994. doi: 10.1084/jem.20090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lande R, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449(7162):564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 12.Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: Langerin-expressing dendritic cell subsets in the skin. Immunol Rev. 2010;234(1):120–141. doi: 10.1111/j.0105-2896.2009.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kautz-Neu K, et al. Langerhans cells are negative regulators of the anti-Leishmania response. J Exp Med. 2011;208(5):885–891. doi: 10.1084/jem.20102318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gais P, et al. Cutting edge: Divergent cell-specific functions of MyD88 for inflammatory responses and organ injury in septic peritonitis. J Immunol. 2012;188(12):5833–5837. doi: 10.4049/jimmunol.1200038. [DOI] [PubMed] [Google Scholar]
- 15.Hemmi H, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3(2):196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 16.Walter A, et al. Aldara activates TLR7-independent immune defence. Nat Commun. 2013;4:1560. doi: 10.1038/ncomms2566. [DOI] [PubMed] [Google Scholar]
- 17.Swindell WR, et al. Genome-wide expression profiling of five mouse models identifies similarities and differences with human psoriasis. PLoS ONE. 2011;6(4):e18266. doi: 10.1371/journal.pone.0018266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baenziger S, et al. Triggering TLR7 in mice induces immune activation and lymphoid system disruption, resembling HIV-mediated pathology. Blood. 2009;113(2):377–388. doi: 10.1182/blood-2008-04-151712. [DOI] [PubMed] [Google Scholar]
- 19.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204(7):1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cervantes-Barragan L, et al. Plasmacytoid dendritic cells control T-cell response to chronic viral infection. Proc Natl Acad Sci USA. 2012;109(8):3012–3017. doi: 10.1073/pnas.1117359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33(6):955–966. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao Y, et al. Type I interferon: Potential therapeutic target for psoriasis? PLoS ONE. 2008;3(7):e2737. doi: 10.1371/journal.pone.0002737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zahner SP, et al. Conditional deletion of TGF-βR1 using Langerin-Cre mice results in Langerhans cell deficiency and reduced contact hypersensitivity. J Immunol. 2011;187(10):5069–5076. doi: 10.4049/jimmunol.1101880. [DOI] [PubMed] [Google Scholar]
- 24.Clausen BE, Kel JM. Langerhans cells: Critical regulators of skin immunity? Immunol Cell Biol. 2010;88(4):351–360. doi: 10.1038/icb.2010.40. [DOI] [PubMed] [Google Scholar]
- 25.Bennett CL, et al. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169(4):569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu F, Whitton JL. Cutting edge: Re-evaluating the in vivo cytokine responses of CD8+ T cells during primary and secondary viral infections. J Immunol. 2005;174(10):5936–5940. doi: 10.4049/jimmunol.174.10.5936. [DOI] [PubMed] [Google Scholar]
- 27.Guttman-Yassky E, et al. Major differences in inflammatory dendritic cells and their products distinguish atopic dermatitis from psoriasis. J Allergy Clin Immunol. 2007;119(5):1210–1217. doi: 10.1016/j.jaci.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Teunissen MBM, et al. Rise in dermal CD11c+ dendritic cells associates with early-stage development of psoriatic lesions. Arch Dermatol Res. 2012;304(6):443–449. doi: 10.1007/s00403-012-1231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Connell RM, et al. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J Exp Med. 2004;200(4):437–445. doi: 10.1084/jem.20040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bissonnette R, et al. A randomized, double-blind, placebo-controlled, phase I study of MEDI-545, an anti-interferon-alfa monoclonal antibody, in subjects with chronic psoriasis. J Am Acad Dermatol. 2010;62(3):427–436. doi: 10.1016/j.jaad.2009.05.042. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki H, et al. Imiquimod, a topical immune response modifier, induces migration of Langerhans cells. J Invest Dermatol. 2000;114(1):135–141. doi: 10.1046/j.1523-1747.2000.00833.x. [DOI] [PubMed] [Google Scholar]
- 32.Romani N, Brunner PM, Stingl G. Changing views of the role of Langerhans cells. J Invest Dermatol. 2012;132(3 Pt 2):872–881. doi: 10.1038/jid.2011.437. [DOI] [PubMed] [Google Scholar]
- 33.Mitsui H, et al. Differential expression and function of Toll-like receptors in Langerhans cells: Comparison with splenic dendritic cells. J Invest Dermatol. 2004;122(1):95–102. doi: 10.1046/j.0022-202X.2003.22116.x. [DOI] [PubMed] [Google Scholar]
- 34.Yawalkar N, Tscharner GG, Hunger RE, Hassan AS. Increased expression of IL-12p70 and IL-23 by multiple dendritic cell and macrophage subsets in plaque psoriasis. J Dermatol Sci. 2009;54(2):99–105. doi: 10.1016/j.jdermsci.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Zaba LC, et al. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol. 2009;129(1):79–88. doi: 10.1038/jid.2008.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson-Huang LM, Lowes MA, Krueger JG. Putting together the psoriasis puzzle: An update on developing targeted therapies. Dis Model Mech. 2012;5(4):423–433. doi: 10.1242/dmm.009092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krueger GG, et al. CNTO 1275 Psoriasis Study Group A human interleukin-12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med. 2007;356(6):580–592. doi: 10.1056/NEJMoa062382. [DOI] [PubMed] [Google Scholar]
- 38.Ghoreschi K, et al. Fumarates improve psoriasis and multiple sclerosis by inducing type II dendritic cells. J Exp Med. 2011;208(11):2291–2303. doi: 10.1084/jem.20100977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wada Y, et al. Apilimod inhibits the production of IL-12 and IL-23 and reduces dendritic cell infiltration in psoriasis. PLoS ONE. 2012;7(4):e35069. doi: 10.1371/journal.pone.0035069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.