Abstract
Hominin fossil evidence in the Turkana Basin in Kenya from ca. 4.1 to 1.4 Ma samples two archaic early hominin genera and records some of the early evolutionary history of Paranthropus and Homo. Stable carbon isotopes in fossil tooth enamel are used to estimate the fraction of diet derived from C3 or C4 resources in these hominin taxa. The earliest hominin species in the Turkana Basin, Australopithecus anamensis, derived nearly all of its diet from C3 resources. Subsequently, by ca. 3.3 Ma, the later Kenyanthropus platyops had a very wide dietary range—from virtually a purely C3 resource-based diet to one dominated by C4 resources. By ca. 2 Ma, hominins in the Turkana Basin had split into two distinct groups: specimens attributable to the genus Homo provide evidence for a diet with a ca. 65/35 ratio of C3- to C4-based resources, whereas P. boisei had a higher fraction of C4-based diet (ca. 25/75 ratio). Homo sp. increased the fraction of C4-based resources in the diet through ca. 1.5 Ma, whereas P. boisei maintained its high dependency on C4-derived resources.
Keywords: Theropithecus, hominid
Many approaches have been used to reconstruct the diet of early hominins. Some of the methods focus on the functional morphology of the masticatory system, others focus on tooth wear (both macroscopic and microscopic), and yet others focus on the physicochemical signatures that an animal’s diet leaves within its hard tissues (1, 2). Chemical methods include the use of strontium/calcium and barium/calcium ratios (3, 4), but this study focuses on the analysis of stable isotopes of carbon (5–9).
Modern tropical ecosystems differ from those ecosystems that predate the late Miocene. Tropical grasses were rare until the late Miocene, when they greatly expanded in abundance; therefore, by the latest Miocene and Pliocene, many mammals had changed their diets, and some had become dependent on this relatively new dietary resource (10, 11). The study of this dietary evolution is based on the difference in carbon isotope ratios of plants that use either the C3 or C4 photosynthetic pathway (12). Plants using the C3 pathway have δ13C values that range between ca. −24‰ and −32‰ (13); the more positive values are associated with xeric environments, intermediate values are associated with mesic environments, and the most negative values are associated with closed canopy environments (14, 15). Plants using the C4 pathway have δ13C values that range from about −10‰ to −14‰, with more positive values associated with mesic environments and more negative values associated with more xeric environments (16). In the tropics, C3 plants are primarily trees, shrubs, and nongrassy herbs and forbs; C4 plants are primarily grasses and sedges, with some rare dicots. A third photosynthetic pathway, Crassulacean acid metabolism, has δ13C values similar to C4 plants in the tropics; Crassulacean acid metabolism plants are mostly succulents in the African tropics and make up a minor but potentially important dietary resource in some circumstances. Carbon isotope values of animal tissues (e.g., bioapatite) are enriched in 13C compared with the diet; for large herbivorous mammals, bioapatite is enriched ca. 12–14‰ relative to dietary materials (5, 17, 18).
Thus, the δ13C of fossil tooth enamel can distinguish between diets that are predominantly based on C3 resources (leaves and fruits from trees and shrubs along with nongrassy forbs and herbs and their fruits) and diets that are predominantly based on C4 resources (primarily grasses or sedges). Meat and most other organic tissues are only slightly enriched in 13C compared with the plant-derived diet (19). Stable carbon isotopes in tooth enamel are unable to distinguish between plant- and meat-based (or insect-based) diet, but they can be used to trace the diet back to the ultimate resource: C3 or C4 plants.
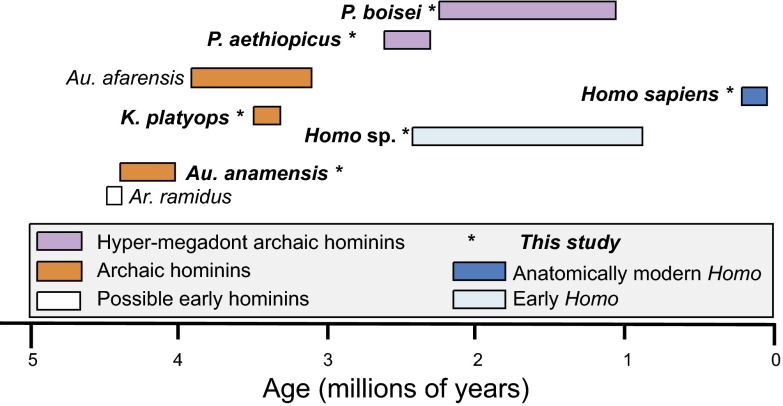
The Turkana Basin has an excellent, well-dated record (20–23) of hominin fossils from ca. 4 Ma to the present. Thus, the diets of the hominin taxa represented at sites within the Turkana Basin (Fig. 1) can be used to study dietary preferences within the hominin clade across this time interval. All samples come from collections held at the National Museums of Kenya in Nairobi. We analyzed 110 teeth from 94 different individual hominins for their stable carbon isotopes. For practical reasons, we could not always sample specimens with unambiguous taxonomic assignments, and in some cases, we could sample only associated material. Therefore, we discuss the results in the context of generic rather than specific taxonomic attributions. The genera that we discuss include Australopithecus (ca. 4 Ma), Kenyanthropus (ca. 3–3.6 Ma), Paranthropus (ca. 2.5–1.4 Ma), and Homo (ca. 2.3–0.01 Ma). We use the taxonomy favored by Wood (24) and Wood and Leakey (25), although we make no distinction among earlier Homo species (e.g., H. habilis and H. rudolfensis) because of the limitations of the size and quality of the sample. We then compare the results of our analysis of hominins from sites in the Turkana Basin with data from hominins recovered at other locations in eastern and southern Africa.
Fig. 1.
Age distribution of hominins from East Africa. Isotopic values of lineages in bold are reported in the text.
Results
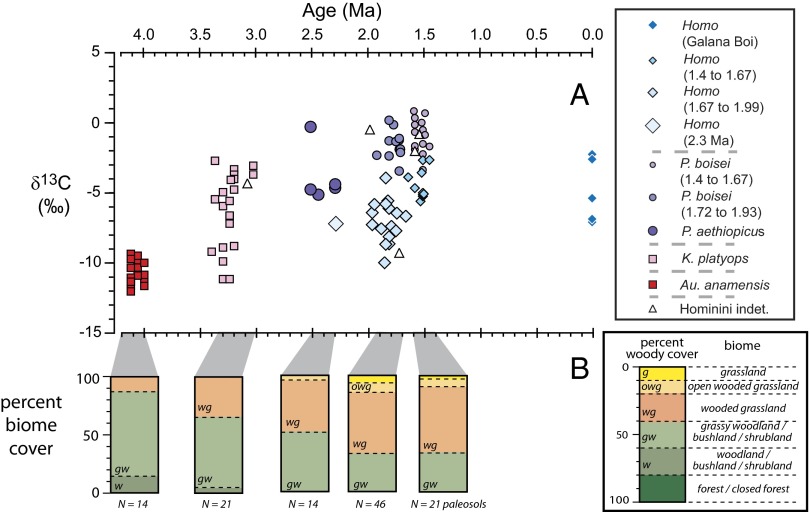
In this section, we present the results of the stable isotope analyses. We group the Turkana Basin hominin specimens by their geological age (Fig. 2 and Table S1) and discuss the taxa represented in each of the major age groupings.
Fig. 2.
(A) δ13C values of tooth enamel with respect to age for major hominin groups in the Turkana Basin, Kenya. (B) Relative proportions of biomes based on δ13C from paleosols (52), where the boundaries between biome types are as described in the text.
Intraindividual Variation.
For 10 individuals, stable isotope ratios were measured on two to five postcanine teeth. Comparison of the results (Table S1) shows that these individuals have a narrow range of δ13C and δ18O values among the teeth sampled, with average ranges of 1.0‰ and 0.7‰, respectively. Such a narrow range indicates that these individuals had a fairly homogeneous diet in terms of C3- vs. C4-derived resources across the time period represented by the development of the teeth sampled for each of the 10 individuals (i.e., based on timescales appropriate to isotope attenuation during enamel maturation). Comparison with other large mammals suggests that molar enamel in early hominins has an isotope maturation interval on the order of 1 or 2 y. Tooth enamel maturation involves an initial stage of bioapatite formation followed by a long period, the isotope maturation interval, wherein the enamel continues to increase in density and incorporate stable isotopes into the bioapatite structure (26). Therefore, the δ13C values of individual teeth, as discussed below, seem to provide a reliable but time-integrated signal reflecting the diet of each of the individuals analyzed.
Temporal Samples.
4.2–4.0 Ma.
Fossil evidence of Au. anamensis is found in ca. 4.0- to 4.2-Ma-old strata in the Turkana Basin; 17 teeth from 12 different individuals were analyzed (Table S1). Au. anamensis has a relatively narrow range of δ13C values, indicating a diet that is C3-based. The average δ13C value of −10.7 ± 0.8‰ corresponds to a δ13C diet value of about −25‰ based on an estimated isotopic enrichment (diet bioapatite) for primates of 14‰ (Methods). Such a δ13C value is compatible with either a 100% C3 diet in a mesic to xeric environment or a diet that has both C3- and C4-derived foods but with the latter making up only ca. 10% of the diet. For comparison, modern browsers (Giraffa camelopardalis) (9) from the semiarid region of Tsavo, Kenya, have δ13C1750 values of −11.2 ± 1.1‰ (δ13C1750 refers to isotope values corrected for the anthropogenic addition of 13C-depleted CO2 to Earth's atmosphere) (Methods), whereas gorillas (Gorilla beringei) from densely forested environments in eastern Democratic Republic of Congo have δ13C1750 values of −13.5 ± 1.2‰ (n = 1) (Table S1). Tooth enamel from modern baboons (Papio) from Kenya and Ethiopia has δ13C1750 values that average −9.1 ± 3.1‰ (n = 19) and range from ca. −13‰ to ca. −2‰; baboons from forested regions in Democratic Republic of Congo have δ13C1750 values that average −12.2 ± 2.3‰ (n = 5) (Methods and Table S1).
Thus, the δ13C results for Au. anamensis suggest either a C3-dominated diet or a diet with a small C4 component. Published δ13C values for Ardipithecus ramidus are similar: −10.2 ± 1.0‰ (n = 5) (27). Intertaxon comparison using ANOVA shows that the diets of Au. anamensis, Ar. ramidus, and modern G. camelopardalis (Tsavo) are indistinguishable in δ13C space, but the diets of all three taxa are significantly different (P < 0.0001) from G. beringei from forested habitats (Fig. S1 and Table S1). As is seen below, the diet of Au. anamensis differs from the diet of all later hominins from the Turkana Basin.
3.4–3.0 Ma.
K. platyops is found in the Turkana Basin between ca. 3.0 and 3.4 Ma (28). The only hominin recovered from deposits of similar age in the Awash region of Ethiopia is Au. afarensis (29); 21 teeth from 18 different individuals assigned to K. platyops were analyzed. The observed range in δ13C of this sample (average = −6.2 ± 2.7, n = 20; maximum = −2.7, minimum = −11.1) is broader than any other hominin included in this study. The only other hominins with such a large range of values are Au. afarensis (average = −7.5 ± 2.6, n = 20; maximum = −2.9, minimum = −13.0) (30) and Au. africanus (average = −6.5 ± 2.3, n = 23; maximum = −1.8, minimum = −11.3) (data in refs. 6, 8, and 31). Compared with modern taxa with a similar sample size, the range and SD for K. platyops is broad and large, respectively (14, 32). The δ13C values for the 18 K. platyops individuals are normally distributed, and the Akaike Information Criterion (33, 34) does not support a bimodal distribution for this population.
One hominin individual (KNM-ER 5431 F) in this time range is assigned to Homininae indet. It is of about the same age as the K. platyops samples discussed above, and its δ13C value (−4.3‰) is within the range of K. platyops.
Modern Papio in East Africa has a similarly wide range of δ13C1750 values; Papio from the Laikipia region of Kenya have values similar to the higher observed values.
2.5–2.3 Ma.
Six teeth from five individual hominins in this time range were analyzed in this study. Four individuals attributed to P. aethiopicus had δ13C values ranging between −0.3‰ and −5.1‰. Three of these individuals (KNM-WT 16005, KNM-WT 38351, and KNM-WT 38353) have a very narrow range of δ13C values—from −4.4‰ to −5.1‰. The fourth individual attributed to P. aethiopicus, KNM-WT 17000, is an outlier compared with the other three Paranthropus of this age range. Taken together, P. aethiopicus has a diet with a consistently high C4 component (ca. 50% or greater) in this time interval.
One specimen has been assigned to Homo sp. indet. (KNM-WT 42718) (35), and it gave a δ13C value of −7.2‰, which is outside the range of the P. aethiopicus specimens of the same age, although the sample size of P. aethiopicus from this time interval (n = 4 individuals) is small.
1.99–1.67 Ma.
The sample from this temporal interval includes two morphologically distinctive hominin taxa, P. boisei and Homo sp. indet.; 13 teeth from 13 different individual P. boisei specimens have an average δ13C value of −1.6 ± 1.0‰ ranging from 0.2‰ to −3.4‰. These values represent a diet dominated by C4 resources (i.e., a C3/C4-based resources ratio of ca. 25/75). The 16 Homo sp. indet. specimens have δ13C values significantly different (P < 0.001, ANOVA, Tukey posthoc) from the P. boisei individuals in the same age range (−7.0 ± 1.5‰, n = 16; i.e., a C3/C4-based resources ratio of ca. 65/35).
Two specimens in this time interval have proven difficult to classify. One of these specimens, KNM-ER 1482 (a taxonomically enigmatic mandible) (24, 36, 37), has a δ13C value of −0.4‰. The other, KNM-ER 2607 (a taxonomically enigmatic lower molar fragment) (24, 36, 38), has a δ13C value of −9.2‰.
1.65–1.45 Ma.
Both P. boisei and Homo sp. indet. are represented among the specimens from this time interval. The P. boisei individuals (n = 14) have an average δ13C value (−0.9 ± 1.2‰) that does not differ statistically from the P. boisei individuals in the 1.99–1.67 Ma time range. The −0.9 ± 1.2‰ value corresponds to a C3/C4-based resources ratio of ca. 20/80.
Ten Homo sp. indet. specimens in this age range have an average δ13C value of −4.3 ± 1.1‰ (i.e., a C3/C4-based resource ratio of ca. 45/55). The Homo sp. indet. individuals in this age range differ significantly from the coeval P. boisei sample (ANOVA, Tukey posthoc test, P < 0.001), and they also differ from Homo sp. indet. individuals from the earlier (1.99–1.67 Ma) time range in that there is a ca. 20% increase in the C4 diet component (ANOVA, Tukey posthoc test, P < 0.001).
Two specimens that cannot be easily assigned to either Homo sp. indet. or Paranthropus, KNM-ER 2593 and KNM-ER 42705, were both found in the Area 6/6A region near Ileret, where many P. boisei specimens have been recovered. They have δ13C values similar to the values of Paranthropus from Area 6/6A and may well be attributable to this genus.
0.01 Ma.
Five teeth from four individual hominins from the Galana Boi Formation (Holocene) have an average δ13C value of −4.8 ± 2.3‰ (i.e., a C3/C4-based resources ratio of ca. 50/50). These values are not significantly different from the earlier Homo sp. indet. samples.
Oxygen isotopes.
The range of variation of the stable oxygen isotopes (δ18O) among the groups discussed above is between 0.7‰ and −1.8‰ (the individual range is from ca. +4‰ to −4‰); SDs within each group are ca. ±1.5‰. These values and ranges of variation are equivalent to water-dependent species such as suids and elephantids (39) as well as carnivores and omnivores. δ18O as a function of time comparing the different hominins is shown in Fig. S2.
Discussion
Diets of Early Hominins: C3- and C4-Based Resources.
We use the terms C3- and C4-based resources throughout our discussion, because our isotopic method cannot distinguish between a plant-based diet, a meat-based diet, and an omnivorous diet. Thus, based on isotopes alone, we consider that the diets of the early hominins that we have investigated could be primarily herbaceous (C3 and C4 plants), or they could be a secondary C3- or C4-based diet, an apparent C3- or C4-based diet, or an omnivorous diet. A secondary C3- or C4-based diet could be a meat- or insect-based diet (in which the δ13C values are derived from the basal herbaceous diet of the prey). An apparent C4-based diet is one based on aquatic resources in which algae have elevated δ13C values because of bicarbonate uptake during photosynthesis (40); for this example, algae or fish then have δ13C values with an apparent C4 component (41, 42). Lastly, an omnivorous diet is a combination of the above resources: primary herbaceous diet along with secondary C3- or C4-derived components (i.e., meat or insects) or apparent components (i.e., aquatic).
The stable carbon isotope signature of a meat-based diet depends on the nature of the prey: small bovid herbivores less than ca. 10 kg (e.g., dik-dik and other neotragines) tend to be browsers and have C3-based diets (14, 32), whereas large herbivores can have diets that are C3-based (browsers such as most tragelephines, black rhinos, and giraffes), C4-based (grazers such as warthogs, zebra, alcelaphines, reducines, and bovines), or mixed (e.g., impala, and some gazelles). Thus, the size of prey may be important in considering possible secondary diet C3 or C4 resources. Other small mammals (e.g., hyrax, lagomorphs, or rodents) could have been an important dietary resource and would contribute to isotope mixing lines between C3- and C4-based end member values.
Evolution of Hominin Diets Between 4 and 1.4 Ma in Eastern Africa.
The earliest hominin taxon sampled in this study, the ca. 4 Ma Au. anamensis, has a diet comprised primarily of C3-based resources (an average ca. 90/10 ratio of C3/C4 diet resources with a range from 100/0 to 80/20 for C3/C4-based resources). This finding is not entirely unexpected, because the diets of the obvious outgroups for hominins, Pan and Gorilla, are both predominantly C3-based (43–45). It is impossible to refute the hypothesis that some C4 resources contributed to the diet of these Au. anamensis individuals, and we do not attempt to do so. The observed δ13C range of Au. anamensis is narrow, and it is similar to the slightly older Ar. ramidus found in the Awash region of Ethiopia (27). A diet with this carbon stable isotope signature is likely dominated by plant foods. The only alternative is a diet based on meat/insect resources based on animals that themselves consume almost entirely C3 resources. However, note that initial surveys of fossil mammals from the Turkana region show that, by 7 Ma and on, most herbivores in the Turkana Basin had C4-based diets (10, 11, 46, 47). Thus, Au. anamensis would have to have been a very specialized hunter if meat were a significant portion of the diet, because the prey would have to have been exclusively C3 consumers.
By 3.5 Ma, the diet of the hominins that we sampled had expanded to include significant C4 resources. The diet of K. platyops shows a broad range, with some individuals having strongly C3-based diets and others having C4-dominated diets. The range of values for 18 K. platyops individuals corresponds to δ13C diet average values of ca. −25‰ to −17‰; the average K. platyops diet corresponds to a C3/C4-based resources ratio of ca. 60/40, but the range of the C3/C4-based resources ratio, 95/5 to 35/65, is wide. This wide range of dietary C3 vs. C4 resources suggests that K. platyops expanded into a dietary niche hitherto unexploited by hominins in the Turkana Basin. δ13C values of Au. afarensis (30), another hominin taxon in East Africa of similar age, and Au. africanus in southern Africa of less certain age (6, 8, 31) similarly have a wide range of δ13C values. The work by Sponheimer et al. (48) discusses these similarities and differences in more detail.
One individual in the small sample of P. aethiopicus, the KNM-WT 17000 cranium (49), deserves special comment. Its left M2 has a δ13C value of −0.3‰, indicating a C4-dominated diet (ca. 15/85 for the C3/C4 diet ratio), which is a diet similar to the P. boisei sample in the later time range. The dietary breadth within Paranthropus by ca. 2.3 Ma needs to be investigated with additional samples and analyses.
Between 2.0 and 1.4 Ma, numerous P. boisei and Homo sp. indet. specimens show that the diets of the two genera were distinct, with Paranthropus having a diet comprised of a 20/80 C3/C4 diet ratio, whereas Homo sp. indet. shows C3/C4-derived ratio values that range from 25/75 to 45/55. Previous comparisons with southern African fossils suggest that P. boisei from East Africa had a diet that was much narrower in terms of C3/C4 resources than P. robustus from southern Africa (9), with a wide range of δ13C values that indicates a much broader dietary niche.
The taxonomically enigmatic mandible KNM-ER 1482 (50) has a δ13C value of −0.4‰, which is intriguing. It has shifted even farther in the direction of being dependent on C4 resources than most P. boisei. Its mandibular and dental morphology offers little or no evidence to assign it to P. boisei. Indeed, some have suggested that it may belong to the same taxon as the KNM-ER 1470 cranium (50) and the KNM-ER 62000 maxilla (51). If this proves to be the case, then within the same region, there may be at least two hominin taxa, almost certainly in different lineages, that have shifted to a diet dominated by C4 resources.
Paleoecology of the Koobi Fora and Nachukui Formations.
Precessional climatic cycles of ca. 20,000 y duration are widely recognized in marine and lacustrine sequences in tropical latitudes. Between insolation maxima and minima (separated by ca. 10,000 y), there are significant changes in rainfall and ecology. Fluvial strata present challenges for quantitative paleoenvironmental interpretation at timescales less than 20,000 y because of their abrupt and discontinuous mode of deposition. A discrete sedimentary package associated with a particular insolation cycle still does not reveal whether a fossil was deposited at maximum, minimum, or intermediate insolation. Furthermore, matching a fossil with the paleoenvironment in which it lived requires knowing the part of a cycle that a paleoenvironmental indicator (e.g., paleosol carbonate, mineralogy, indicator fossil, or biomarker) records. A vertebrate fossil from the base of a 20,000-y fining upward sequence may have lived during a climatic milieu different from the climate under which carbonate formed in a paleosol at the top of the same sequence. In most situations, it is not feasible to relate a fossil from fluvial sediments to a particular part of a precessional cycle, and it is certainly not possible using legacy collections or surface finds. Nonetheless, long-term ecological changes can be discerned through the 3-Ma record considered here.
Stable isotopes also provide important constraints on the paleoecology of the Turkana Basin. The earliest hominins reported here, from ca. 3 to 4 Ma, lived in an environment that was predominantly ca. 40–60% woody cover, which was determined from paleosol δ13C values (52, 53). For this period, the soil carbon contribution from C3 woody plants, C3 forbs and herbs, and C4 grasses would be ca. 60–40%, 15–20%, and 25–40%, respectively (Fig. 2B and Fig. S3). Such a habitat would be a grassy woodland, grassy shrubland, or grassy bushland (54). Sedimentological evidence (55) shows that the proto-Omo river was present throughout this period; this river likely had a riparian forest (>80 woody cover) that may have been hundreds of meters wide; Δ47 measurements on paleosols indicate that the region had soil temperatures between 30 °C and 40 °C (56), indicating a regional temperature regime similar to the temperature regime of today. Thus, this region had a riparian corridor with cooler temperature and little to no C4 resource availability, but close by, the woodland/shrubland/bushland was a more open habitat with significant C4 resources and much higher daily temperatures than in the riparian corridor. These paleoecological conditions suggest that, based on dietary considerations, Au. anamensis may have been restricted to a narrow riparian corridor, whereas K. platyops must have ventured into open habitats to obtain C4 dietary resources. Thus far, fossils of K. platyops are associated with alluvial fans of a large lateral stream on the western basin margin interfingering with deposits of the ancestral Omo River.
Woody cover diminished in the region over time; between ca. 2.0 and 1.4 Ma, woody cover was 20–40% based on δ13C in paleosols (52). Such an ecosystem is equivalent to wooded grassland with soil contributions of C3 woody plants, C3 forbs and herbs, and C4 grasses of ca. 40–20%, 20–30%, and 40–50%, respectively (Fig. 2B); areas of true grassland [<10% woody cover in the United Nations Educational, Scientific, and Cultural Organization terminology (54)] were uncommon, at least on the timescale of paleosol formation (>1,000 y). This change represents a great increase in the availability of C4 plants for Homo and Paranthropus compared with the earlier Australopithecus and Kenyanthropus and a significant opening of the landscape. The 2.0- to 1.4-Ma interval had intermittent lakes fed by the proto-Omo River; however, it may have been diverted to the Nile drainage for some periods during this interval (55). Geochemical and mineralogic evidence shows that some of these lakes were alkaline (56); Δ47 evidence from paleosols indicates high mean annual temperatures, similar to the temperatures of today (57).
Thus, from 4.1 to 1.4 Ma, the region had abundant C4 resources available in the 30-km broad grassy woodland/shrubland to wooded grasslands that bordered narrow (hundreds of meters wide) riparian forests or woodlands associated with the proto-Omo River.
Comparison of Hominin Diets to Theropithecus.
Theropithecus was another large-bodied primate in the Turkana Basin at this time. Stable isotope measurements of tooth enamel show that Theropithecus was a heavy C4 consumer by 4 Ma, with ca. 65% C4 resources contributing to the diet (58). Throughout the period from 4 to 1 Ma, Theropithecus had a diet that was as much or more C4-based than any hominin. Paranthropus, from 1.4 to 2.0 Ma, had a diet that was ca. 75% C4-based, whereas the coeval Theropithecus had a diet that was ca. 75–85% C4-based (58).
Modern Primates as Analogs for Hominin Diets.
Modern gorilla and chimpanzees have diets that are entirely or almost entirely C3-based (data in Table S2 and refs. 43–45). Only the earliest hominin in this study could be interpreted as having had a C3-based diet with minimal (if any) C4 components; the δ13C of Au. anamensis is more positive than gorillas or chimpanzees from closed canopy forests, but it is compatible with a pure C3 diet from riparian forests or open habitats.
Modern baboons (P. anubis and P. hamadryas) have a wide range of δ13C1750 values, showing diet strategies that range from essentially pure C3-based (e.g., from Neshisar NP) (Table S2) to dominated by C3-based resources but with measureable C4-based resources (Gona, Olorgesailie, Tsavo, and Turkana) (Table S2) to subequal with respect to C3- and C4-based resources (e.g., Laikipia region) (Table S2). Baboons have been suggested as an important study analog for early hominins (59, 60); thus future studies of baboon diets, coupled with stable isotope analyses, will be a fruitful avenue of research.
Conclusions
Within the past 4 Ma, the earliest dietary isotope evidence from hominins in the Turkana Basin comes from a single species, Au. anamensis, with a diet dominated by C3 resources but possibly with a small component of C4-derived resources. By ca. 3.5 Ma, at least one hominin taxon, Kenyanthropus, in the Turkana Basin had a diet with a broad range of C3/C4-based resources. By the 1.99- to 1.67-Ma time period, at least two morphologically highly distinctive hominin taxa, P. boisei and Homo sp. indet., had shifted in the direction of consuming higher but different proportions of C4 resources. We cannot determine from the stable isotopes by themselves what the C4 resources were that caused this shift in diet.
Methods
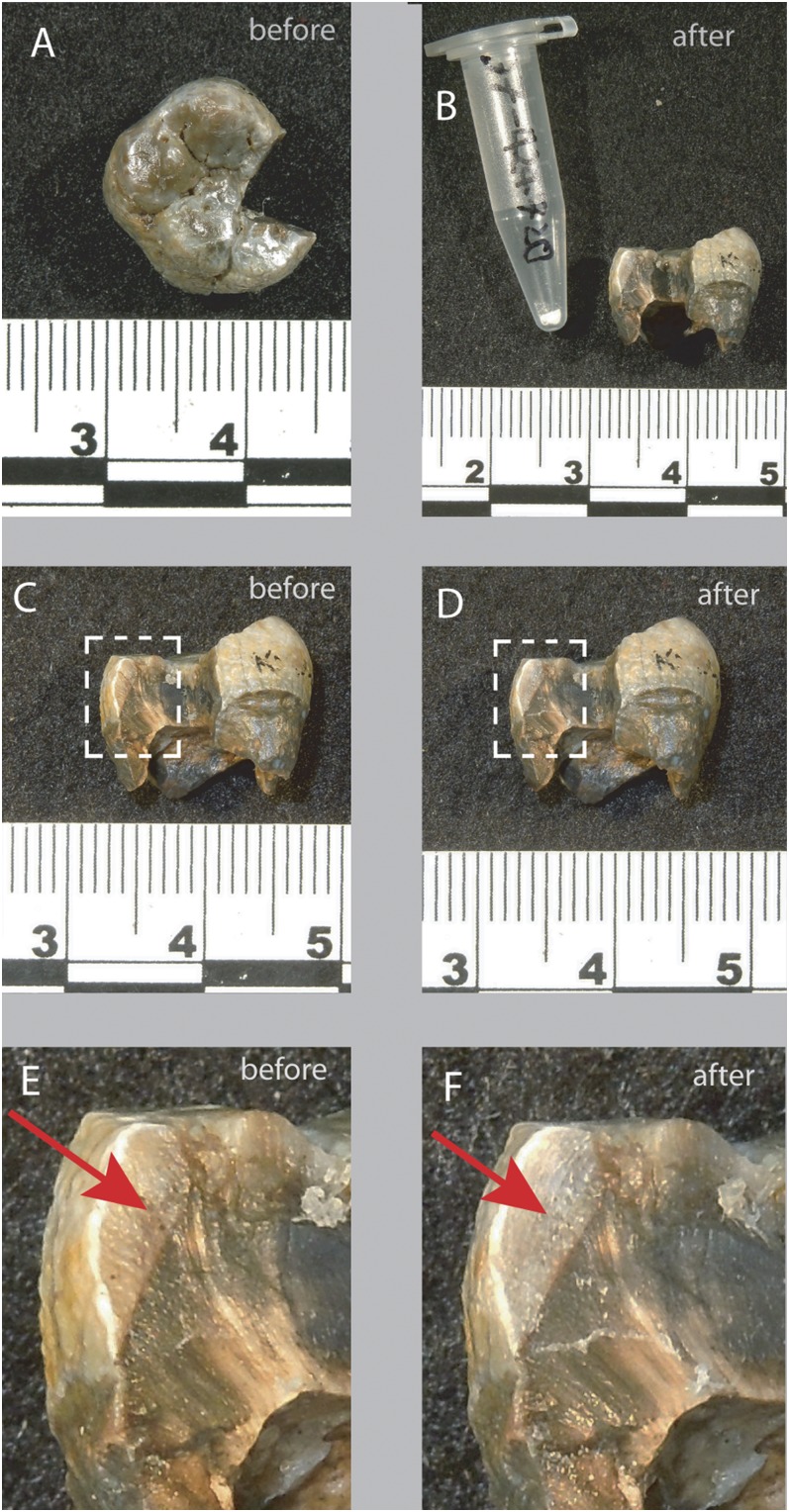
Hominin teeth from the National Museums of Kenya were sampled using a high-speed rotary drill to obtain powder (ca. 2–5 mg) from each sample. Only hominin teeth with broken surfaces were sampled; sampling was from the exposed broken enamel (Fig. 3). Enamel powder from modern gorilla (G. beringei) was obtained from archived samples in Kahuzi-Biega National Park and the Centre de Research National en Sciences Naturelles (CRNS)-Lwiro, Democratic Republic of Congo; most gorilla samples were young adults that had been killed by poachers. Other modern primates were from Democratic Republic of Congo and Kenya. All samples were treated with 0.1 M buffered acetic acid for 30 min to remove secondary carbonates; we had about 50% recovery during this treatment.
Fig. 3.
Images of KNM-ER 45502 before and after sampling. (A) Top view of tooth before sampling. (B) View of the tooth with sample powder (1.8 mg) after sampling. (C) Sample before sampling; the box shows the area chosen for sampling (E). (D) Sample after sampling (same view as C); the boxed area is the close-up view shown in F. (E) Close-up view of the area sampled for stable isotope analysis before sampling. The red arrow shows the broken enamel surface to be sampled. (F) The same area as E but after sampling. The red arrow points to the surface sampled.
Fossil samples (ca. 500 μg) were reacted with 105% phosphoric acid at 90 °C in silver capsules on an isotope ratio mass spectrometer after cryogenic separation of CO2 at the University of Utah Stable Isotope Ratio Facility for Environmental Research (SIRFER). Modern samples from Democratic Republic of Congo were analyzed in the Archaeology Department at the University of Cape Town using a Kiel device coupled to an isotope ratio mass spectrometer. Results are reported using the standard per million (‰) notation, where
and Rsample and Rstandard are the 13C/12C ratios in the sample and standard, respectively (Vienna–Pee Dee Belemnite is the standard for carbon isotope measurements). Corrections for temperature-dependent isotope fractionation in oxygen were made using modern and fossil internal reference materials that had been reacted at 25 °C (61). For comparative purposes, modern mammals have had their δ13C values adjusted to compensate for recent changes in atmospheric δ13C values (62, 63); these values are referred to in the text as δ13C1750. We use the year of death to calculate the δ13C1750 value; this date will result in a maximum correction, because tooth enamel likely formed ca. 10 y before death for these individuals.
Age estimates for each hominin use the correlations and chronology of the >350 volcanic ashes in the basin (20–23, 64); taxonomic assignments are in refs. 24 and 25.
SI Methods has additional information on isotope enrichment factors in mammals, stratigraphic information and age estimates for individual hominins (Table S3), statistical treatment, taxonomic assignments, and biome classifications used in this work.
Supplementary Material
Acknowledgments
We thank the governments of Kenya and Democratic Republic of Congo for permission to do this research. We thank the field crew of the Koobi Fora Research Project (1969–2012), whose members discovered most of the specimens analyzed in this study. We also thank John Lanham for assistance at the University of Cape Town and Sandrine Prat and anonymous reviewers for PNAS for helpful comments. This project was initiated by the National Museums of Kenya. This material is based on work supported by National Science Foundation Grant BCS-0621542, National Geographic Society Grant 7767-04, and the Fulbright Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 10470.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222568110/-/DCSupplemental.
References
- 1.Grine FE, Sponheimer M, Ungar PS, Lee-Thorp JA, Teaford MF. Dental microwear and stable isotopes inform the paleoecology of extinct hominins. Am J Phys Anthropol. 2012;148(2):285–317. doi: 10.1002/ajpa.22086. [DOI] [PubMed] [Google Scholar]
- 2.Wood BA, Schoer K. Reconstructing the diet of an extinct hominin taxon: The role of extant primate models. Int J Primatol. 2012;33:716–742. [Google Scholar]
- 3.Copeland SR, et al. Strontium isotope evidence for landscape use by early hominins. Nature. 2011;474(7349):76–78. doi: 10.1038/nature10149. [DOI] [PubMed] [Google Scholar]
- 4.Balter V, Braga J, Télouk P, Thackeray JF. Evidence for dietary change but not landscape use in South African early hominins. Nature. 2012;489(7417):558–560. doi: 10.1038/nature11349. [DOI] [PubMed] [Google Scholar]
- 5.Lee-Thorp JA, van der Merwe NJ. Carbon isotope analysis of fossil bone apatite. S Afr J Sci. 1987;83:712–715. [Google Scholar]
- 6.Lee-Thorp JA, van der Merwe NJ, Brain CK. Diet of Australopithecus robustus at Swartkrans from stable carbon isotopic analysis. J Hum Evol. 1994;27:361–372. [Google Scholar]
- 7.Sponheimer M, Lee-Thorp JA. Isotopic evidence for the diet of an early hominid, Australopithecus africanus. Science. 1999;283(5400):368–370. doi: 10.1126/science.283.5400.368. [DOI] [PubMed] [Google Scholar]
- 8.Sponheimer M, et al. Hominins, sedges, and termites: New carbon isotope data from the Sterkfontein valley and Kruger National Park. J Hum Evol. 2005;48(3):301–312. doi: 10.1016/j.jhevol.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Cerling TE, et al. Diet of Paranthropus boisei in the early Pleistocene of East Africa. Proc Natl Acad Sci USA. 2011;108(23):9337–9341. doi: 10.1073/pnas.1104627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerling TE, et al. Global change through the Miocene/Pliocene boundary. Nature. 1997;389:153–158. [Google Scholar]
- 11.Uno KT, et al. Late Miocene to Pliocene carbon isotope record of differential diet change among East African herbivores. Proc Natl Acad Sci USA. 2011;108(16):6509–6514. doi: 10.1073/pnas.1018435108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Leary M. Carbon isotopic fractionation in plants. Phytochemistry. 1981;20:553–567. [Google Scholar]
- 13.Deines P. The isotopic composition of reduced organic carbon. In: Fritz P, Fontes JB, editors. Handbook of Environmental Geochemistry. Vol 1. Amsterdam: Elsevier; 1980. pp. 329–406. [Google Scholar]
- 14.Cerling TE, Harris JM, Passey BH. Dietary preferences of East African Bovidae based on stable isotope analysis. J Mammal. 2003;84:456–471. [Google Scholar]
- 15.Cerling TE, Hart JA, Hart TB. Stable isotope ecology in the Ituri Forest. Oecologia. 2004;138(1):5–12. doi: 10.1007/s00442-003-1375-4. [DOI] [PubMed] [Google Scholar]
- 16.Hattersley PW. 13C values of C4 types in grasses. Aust J Plant Physiol. 1982;9:139–154. [Google Scholar]
- 17.Cerling TE, Harris JM. Carbon isotope fractionation between diet and bioapatite in ungulate mammals and implications for ecological and paleoecological studies. Oecologia. 1999;120:347–363. doi: 10.1007/s004420050868. [DOI] [PubMed] [Google Scholar]
- 18.Passey BH, et al. Carbon isotopic fractionation between diet, breath, and bioapatite in different mammals. J Archaeol Sci. 2005;32:1459–1470. [Google Scholar]
- 19.DeNiro MJ, Epstein S. Influence of diet on distribution of carbon isotopes in animals. Geochim Cosmochim Acta. 1978;42:495–506. [Google Scholar]
- 20.Brown FH, Sarna-Wojcicki AM, Meyer CE, Haileab B. Correlation of Pliocene and Quaternary tephra layers between the Turkana Basin of East Africa and the Gulf of Aden. Quat Int. 1992;13/14:55–67. [Google Scholar]
- 21.Brown FH, Haileab B, McDougall I. Sequence of tuffs between the KBS Tuff and the Chari Tuff in the Turkana Basin, Kenya and Ethiopia. J Geol Soc London. 2006;163:185–204. [Google Scholar]
- 22.McDougall I, Brown FH. Precise 40Ar/39Ar geochronology for the upper Koobi Fora Formation, Turkana Basin, northern Kenya. J Geol Soc London. 2006;163:205–220. [Google Scholar]
- 23.McDougall I, Brown FH. Geochronology of the pre-KBS Tuff sequence, Omo Group, Turkana Basin. J Geol Soc London. 2008;165:549–562. [Google Scholar]
- 24.Wood BA. Koobi Fora Research Project Volume 4: Hominid Cranial Remains. Vol 4. Oxford: Oxford Univ Press; 1991. [Google Scholar]
- 25.Wood BA, Leakey MG. The Omo-Turkana Basin fossil hominins and their contribution to our understanding of human evolution in Africa. Evol Anthropol. 2011;20(6):264–292. doi: 10.1002/evan.20335. [DOI] [PubMed] [Google Scholar]
- 26.Passey BH, Cerling TE. Tooth enamel mineralization in ungulates: Implications for recovering a primary isotopic time-series. Geochim Cosmochim Acta. 2002;18:3225–3234. [Google Scholar]
- 27.White TD, et al. Macrovertebrate paleontology and the Pliocene habitat of Ardipithecus ramidus. Science. 2009;326(5949):87–93. [PubMed] [Google Scholar]
- 28.Leakey MG, et al. New hominin genus from eastern Africa shows diverse middle Pliocene lineages. Nature. 2001;410(6827):433–440. doi: 10.1038/35068500. [DOI] [PubMed] [Google Scholar]
- 29.Kimbel WH, Delezene LK. “Lucy” redux: A review of research on Australopithecus afarensis. Am J Phys Anthropol. 2009;140(Suppl 49):2–48. doi: 10.1002/ajpa.21183. [DOI] [PubMed] [Google Scholar]
- 30.Wynn JG, et al. Diet of Australopithecus afarensis from the Pliocene Hadar Formation, Ethiopia. Proc Natl Acad Sci USA. 2013;110:10495–10500. doi: 10.1073/pnas.1222559110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Merwe NJ, Thackeray JF, Lee-Thorp JA, Luyt J. The carbon isotope ecology and diet of Australopithecus africanus at Sterkfontein, South Africa. J Hum Evol. 2003;44(5):581–597. doi: 10.1016/s0047-2484(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 32.Sponheimer M, et al. Diets of southern African bovidae: The stable isotope evidence. J Mammal. 2003;84:471–479. [Google Scholar]
- 33.Hurvich CM, Tsai CL. Regression and time series model selection in small samples. Biometrika. 1989;76:297–307. [Google Scholar]
- 34.Burnham KP, Anderson DR. Multimodel interference: Understanding AIC and BIC in model selection. Social Methods Res. 2004;33:261–304. [Google Scholar]
- 35.Prat S, et al. First occurrence of early Homo in the Nachukui Formation (West Turkana, Kenya) at 2.3-2.4 Myr. J Hum Evol. 2005;49(2):230–240. doi: 10.1016/j.jhevol.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Leakey RE, Wood BA. A hominid mandible from East Rudolf, Kenya. Am J Phys Anthropol. 1974;41(2):245–249. doi: 10.1002/ajpa.1330410207. [DOI] [PubMed] [Google Scholar]
- 37.Beynon AD, Wood BA. Variations in enamel thickness and structure in East African hominids. Am J Phys Anthropol. 1986;70(2):177–193. doi: 10.1002/ajpa.1330700205. [DOI] [PubMed] [Google Scholar]
- 38.Leakey RE. New hominid fossils from the Koobi Fora formation in northern Kenya. Nature. 1976;261(5561):574–576. doi: 10.1038/261574a0. [DOI] [PubMed] [Google Scholar]
- 39.Levin NE, Cerling TE, Passey BH, Harris JM, Ehleringer JR. Stable isotopes as a proxy for paleoaridity. Proc Natl Acad Sci USA. 2006;103:11201–11205. doi: 10.1073/pnas.0604719103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Popp BN, et al. Effect of phytoplankton cell geometry on carbon isotopic fractionation. Geochim Cosmochim Acta. 1998;62:69–77. [Google Scholar]
- 41.Chisholm BS, Nelson ED, Schwarcz HP. Marine and terrestrial protein in prehistoric diets on the Britsh Columbia coast. Curr Anthropol. 1983;24:396–398. [Google Scholar]
- 42.Schoeninger MJ, DeNiro MJ, Tauber H. Stable nitrogen isotope ratios of bone collagen reflect marine and terrestrial components of prehistoric human diet. Science. 1983;220(4604):1381–1383. doi: 10.1126/science.6344217. [DOI] [PubMed] [Google Scholar]
- 43.Schoeninger MJ, Moore J, Sept JM. Subsistence strategies of two “savanna” chimpanzee populations: The stable isotope evidence. Am J Primatol. 1999;49(4):297–314. doi: 10.1002/(SICI)1098-2345(199912)49:4<297::AID-AJP2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 44.Sponheimer M, et al. Do “savanna” chimpanzees consume C4 resources? J Hum Evol. 2006;51(2):128–133. doi: 10.1016/j.jhevol.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Smith CC, Morgan ME, Pilbeam D. Isotopic ecology and dietary profiles of Liberian chimpanzees. J Hum Evol. 2010;58(1):43–55. doi: 10.1016/j.jhevol.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Harris JM, Cerling TE. Dietary adaptations of extant and Neogene African suids. J Zool (1987) 2002;256:45–54. [Google Scholar]
- 47.Cerling TE, Harris JM, Leakey MG. Isotope paleoecology of the Nawata and Apak Formations at Lothagam, Turkana Basin, Kenya. In: Leakey MG, Harris JM, editors. Lothagam: The Dawn of Humanity in Africa. New York: Columbia Univ Press; 2003. pp. 605–624. [Google Scholar]
- 48.Sponheimer M, et al. Isotopic evidence of early hominin diets: Past, present, and future. Proc Natl Acad Sci USA. 2013;110:10513–10518. [Google Scholar]
- 49.Walker A, Leakey RE, Harris JM, Brown FH. 2.5 Mr Australopithecus boisei from west of Lake Turkana, Kenya. Nature. 1986;322:517–522. [Google Scholar]
- 50.Leakey RE. Evidence for an advanced plio-pleistocene hominid from East Rudolf, Kenya. Nature. 1973;242(5398):447–450. doi: 10.1038/242447a0. [DOI] [PubMed] [Google Scholar]
- 51.Leakey MG, et al. New fossils from Koobi Fora in northern Kenya confirm taxonomic diversity in early Homo. Nature. 2012;488(7410):201–204. doi: 10.1038/nature11322. [DOI] [PubMed] [Google Scholar]
- 52.Cerling TE, et al. Woody cover and hominin environments in the past 6 million years. Nature. 2011;476(7358):51–56. doi: 10.1038/nature10306. [DOI] [PubMed] [Google Scholar]
- 53.Levin NE, Brown FH, Behrensmeyer AK, Bobe R, Cerling TE. Paleosol carbonates from the Omo Group: Isotopic records of local and regional environmental change in East Africa. Palaeogeogr Palaeoecol Palaeoclim. 2011;307:75–89. [Google Scholar]
- 54.White F. The Vegetation of Africa. Paris: United Nations Scientific and Cultural Organization; 1983. [Google Scholar]
- 55.Feibel CS, Harris JM, Brown FH. Paleoenvironmental context for the Late Neogene of the Turkana Basin. In: Harris JM, editor. Koobi Fora Research Project. Vol 3. Oxford: Clarendon; 1991. pp. 321–370. [Google Scholar]
- 56.Cerling TE. Paleochemistry of Plio-Pleistocene Lake Turkana, Kenya. Palaeogeogr Palaeoclimatol Palaeoecol. 1979;27:247–285. [Google Scholar]
- 57.Passey BH, Levin NE, Cerling TE, Brown FH, Eiler JM. High-temperature environments of human evolution in East Africa based on bond ordering in paleosol carbonates. Proc Natl Acad Sci USA. 2010;107(25):11245–11249. doi: 10.1073/pnas.1001824107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cerling TE, Chritz KL, Jablonski NG, Leakey MG, Manthi FK. Diet of Theropithecus from 4 to 1 Ma in Kenya. Proc Natl Acad Sci USA. 2013;110:10507–10512. doi: 10.1073/pnas.1222571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Codron D, Lee-Thorp JA, Sponheimer M, de Ruiter D, Codron J. Inter- and intrahabitat dietary variability of chacma baboons (Papio ursinus) in South African savannas based on fecal δ13C, δ15N, and% N. Am J Phys Anthropol. 2005;129:204–214. doi: 10.1002/ajpa.20253. [DOI] [PubMed] [Google Scholar]
- 60.Codron D, Lee-Thorp JA, Sponheimer M, deRuiter D, Codron J. What insights can baboon feeding ecology provide for early hominin niche differentiation? Int J Primatol. 2008;29:757–772. [Google Scholar]
- 61.Passey BH, Cerling TE, Levin NE. Temperature dependence of oxygen isotope acid fractionation for modern and fossil tooth enamels. Rapid Commun Mass Spectrom. 2007;21(17):2853–2859. doi: 10.1002/rcm.3149. [DOI] [PubMed] [Google Scholar]
- 62.Francey RJ, et al. A 1000-year high precision record of d13C in atmospheric CO2. Tellus B Chem Phys Meteorol. 1999;51:170–193. [Google Scholar]
- 63.Keeling RF, Piper SC, Bollenbacher AF, Walker SJ. Trends: A Compendium of Data on Global Change. Oak Ridge, TN: Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy; 2010. Monthly atmospheric 13C/12C isotopic ratios for 11 SIO stations. [Google Scholar]
- 64.McDougall I, et al. New single crystal 40Ar/39Ar ages improve time scale for deposition of the Omo Group, Omo–Turkana Basin, East Africa. J Geol Soc London. 2012;169:213–226. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.