Abstract
Polycystic kidney disease (PKD), the most common genetic cause of chronic kidney failure, is characterized by the presence of numerous, progressively enlarging fluid-filled cysts in the renal parenchyma. The cysts arise from renal tubules and are lined by abnormally functioning and hyperproliferative epithelial cells. Despite recent progress, no Food and Drug Administration-approved therapy is available to retard cyst growth. MicroRNAs (miRNAs) are short noncoding RNAs that inhibit posttranscriptional gene expression. Dysregulated miRNA expression is observed in PKD, but whether miRNAs are directly involved in kidney cyst formation and growth is not known. Here, we show that miR-17∼92, an oncogenic miRNA cluster, is up-regulated in mouse models of PKD. Kidney-specific transgenic overexpression of miR-17∼92 produces kidney cysts in mice. Conversely, kidney-specific inactivation of miR-17∼92 in a mouse model of PKD retards kidney cyst growth, improves renal function, and prolongs survival. miR-17∼92 may mediate these effects by promoting proliferation and through posttranscriptional repression of PKD genes Pkd1, Pkd2, and hepatocyte nuclear factor-1β. These studies demonstrate a pathogenic role of miRNAs in mouse models of PKD and identify miR-17∼92 as a therapeutic target in PKD. Our results also provide a unique hypothesis for disease progression in PKD involving miRNAs and regulation of PKD gene dosage.
Keywords: cilia, kinesin family member 3A, autosomal dominant polycystic kidney disease
Polycystic kidney disease (PKD) is among the most common monogenic human diseases (1, 2). PKD is characterized by the presence of numerous fluid-filled cysts in the renal parenchyma. The cysts arise from renal tubules and are lined by abnormally functioning epithelial cells. The cyst epithelial cells secrete excessive fluid and display high rates of proliferation, which results in cyst expansion. The expanding cysts compress the surrounding normal nephrons, which cause renal failure. Based on the mode of inheritance, PKD is classified into autosomal dominant PKD (ADPKD) and autosomal recessive PKD (ARPKD). ADPKD is caused by mutations of PKD1 or PKD2, which encode polycystin-1 and polycystin-2, respectively. ARPKD is caused by mutations of polycystic kidney and hepatic disease 1 (PKHD1) which encodes fibrocystin (1, 3). Polycystin-1, polycystin-2, and fibrocystin localize to the primary cilium, a sensory organelle present on the apical surface of most cells in the body. Abnormalities of the primary cilium are linked to the pathogenesis of many forms of cystic kidney diseases, including PKD (1, 4, 5). Despite recent advances, no Food and Drug Administration-approved therapy is available for PKD patients.
MicroRNAs (miRNAs) are noncoding RNAs that constitute the endogenous RNA interference pathway. miRNAs are transcribed as primary miRNAs (pri-miRNAs), which are sequentially processed by the enzymes Drosha and Dicer to produce mature miRNAs (6). Watson–Crick base pairing between nucleotides 2–8 (seed sequence) at the 5′ end of the mature miRNA and sequences predominantly located within the 3′ untranslated regions (UTRs) of target mRNAs results in posttranscriptional gene silencing (6). Aberrant miRNA expression is observed in numerous diseases (7), and correcting miRNA expression has emerged as a novel therapeutic approach (8, 9). miRNAs are implicated in normal kidney development (10) and the pathogenesis of several kidney diseases (11).
Dysregulation of miRNA expression is observed in PKD (12, 13). However, whether miRNAs are directly involved in cyst formation and growth is not known. The goal of this study was to prospectively determine the contribution of miRNAs to the pathogenesis of PKD in animal models. We report that the miR-17∼92 miRNA cluster is up-regulated in mouse models of PKD. Using gain-of-function and loss-of-function alleles of miR-17∼92, we demonstrate that miR-17∼92 modulates kidney cyst growth in mice. We show that miR-17∼92 promotes cyst epithelial proliferation and inhibits the posttranscriptional expression of PKD genes Pkd1, Pkd2, and hepatocyte nuclear factor 1β (Hnf-1β) through interaction with their 3′ UTRs. These findings provide a mechanism by which miR-17∼92 promotes cyst growth and identify inhibition of miR-17∼92 as a potentially useful therapeutic approach in PKD.
Results
miR-17∼92 miRNA Cluster Is Up-Regulated in Mouse Models of PKD.
To identify miRNAs that are differentially expressed between cystic kidneys and normal kidneys, we performed miRNA microarrays using RNA from kidneys of control and kidney-specific-cadherin (Ksp)/cre;Kif3aF/F (Kif3a-KO) mice, an animal model of PKD. In Kif3a-KO mice, cre recombinase is specifically expressed in the developing renal tubules and ureters (14) where it inactivates kinesin family member 3A, a motor protein that mediates anterograde intraflagellar transport in the primary cilium. As shown in ref. 4, Ksp/cre-mediated inactivation of Kif3a resulted in the loss of primary cilia from renal tubules and the formation of kidney cysts. Loss of cilia was detected by postnatal day (P)10 (Fig. S1) and was followed by the formation of numerous cysts (Fig. S2). Cysts progressively increased in size and number, and by P28, the expanding cysts caused gross enlargement of the kidneys and produced renal failure (Fig. S2).
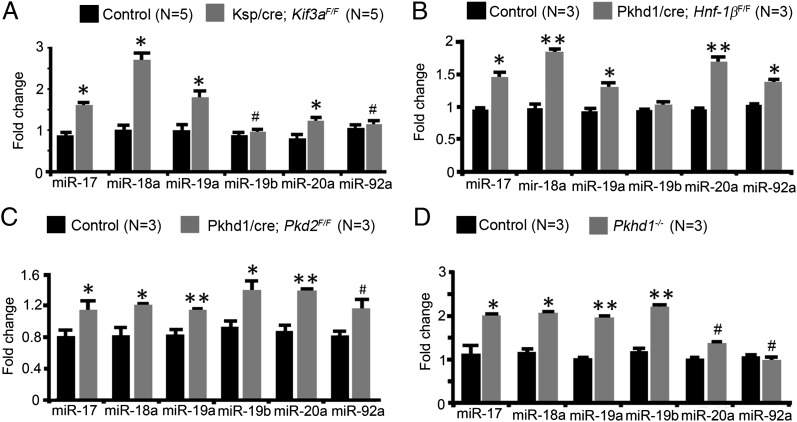
miRNA microarray analysis revealed that 64 miRNAs were differentially expressed in kidneys from 28-d-old Kif3a-KO mice and control mice (Fig. S3). Notably, members of the miR-17∼92 cluster (miR-17, miR-18, and miR-20a) were up-regulated in Kif3a-KO kidneys. miR-17 has been implicated in the pathogenesis of pronephric cysts in Xenopus (15), but its role in the metanephric kidney and mouse models of PKD is not known. Therefore, we studied the miR-17∼92 miRNA cluster in greater detail. miR-17∼92 is an evolutionarily conserved miRNA cluster that primarily encodes six miRNAs: miR-17, miR-18a, miR-19a, miR-19b-1, miR-20a, and miR-92a-1 (Fig. S4 A and B). Quantitative RT-PCR (qRT-PCR) analysis confirmed that miR-17, miR-18a, miR-19a, and miR-20a were up-regulated in kidneys from 28-d-old Kif3a-KO mice compared with control mice (Fig. 1A). To determine whether up-regulation of the miR-17∼92 cluster is present in other forms of PKD, we analyzed Pkd2, Pkhd1, and Hnf-1β mutant mice. The expression of members of the miR-17∼92 miRNA cluster was increased in cystic kidneys from Pkhd1/cre;Pkd2F/F mice (16), an orthologous model of ADPKD; Pkhd1−/− mice (17), an orthologous model of ARPKD; and Pkhd1/cre;Hnf-1βF/F mice (18), an orthologous model of renal cysts and diabetes, compared with their respective controls (Fig. 1 B–D). Thus, up-regulation of the miR-17∼92 cluster is observed in multiple mouse models of PKD.
Fig. 1.
Members of the miR-17∼92 miRNA cluster are up-regulated in mouse models of PKD. (A) qRT-PCR analysis showing increased expression of miRNAs derived from the miR-17∼92 cluster in kidneys from 28-d-old Kif3a-KO mice compared with control mice. (B–D) Expression of the miR-17∼92 cluster is also increased in kidneys from Pkhd1/cre;Hnf-1βF/F mice (B), Pkhd1/cre;Pkd2F/F mice (C), and Pkhd1−/− mice (D) compared with kidneys from their respective controls. *P < 0.05, **P < 0.01, and #P > 0.05. Error bars represent SEM.
Next, we examined whether the up-regulation of the miR-17∼92 cluster was temporally associated with cyst initiation or cyst expansion. Kidney cysts begin to form at P10 in Kif3a-KO mice and rapidly expand at P21 and P28 (Fig. S2). The expression of the miR-17∼92 miRNA cluster was increased in kidneys from 21-d-old Kif3a-KO mice compared with kidneys from age-matched control mice (Fig. S2). However, analysis at P14 showed similar levels of expression in kidneys from Kif3a-KO mice and control mice (Fig. S2). These results indicate that up-regulation of miR-17∼92 is associated with cyst expansion rather than cyst initiation in Kif3a-KO mice.
Kidney-Specific Transgenic Overexpression of miR-17∼92 Produces Kidney Cysts.
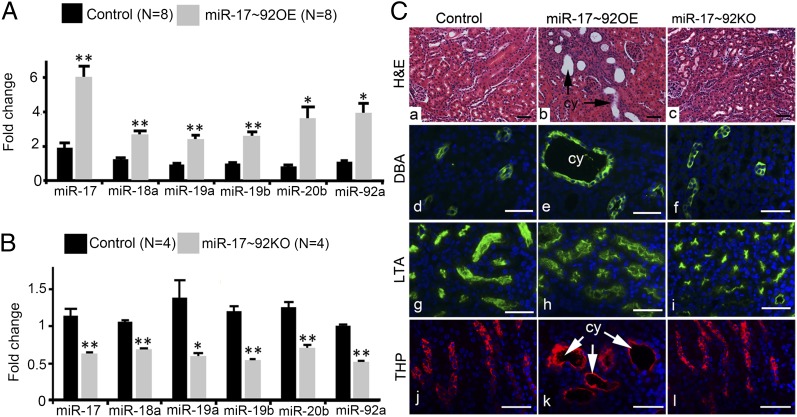
To determine whether the increased levels of miR-17∼92 in PKD are functionally significant, we produced transgenic mice that overexpress miR-17∼92 specifically in renal tubules. Kidney-specific miR-17∼92–overexpressing mice (miR-17∼92 OE mice) were produced by breeding Ksp/cre mice with mice harboring a cre-inducible miR-17∼92 transgene (19). The miR-17∼92 transgene contains (from 5′ to 3′ orientation) a CAG promoter, a loxP-flanked neomycin (Neo)-STOP cassette, genomic DNA fragment encoding the human miR-17∼92 miRNA cluster, a frt-flanked internal ribosomal entry site-enhanced green fluorescent protein (IRES-EGFP), and polyadenylation signal (Fig. S4C). In the absence of cre/loxP recombination, the Neo-STOP cassette prevents transcription of the miR-17∼92 transgene. After cre/loxP recombination, the Neo-STOP cassette is deleted, resulting in the expression of miR-17∼92 transgene. Age-matched littermates that were Ksp/cre-positive were used as controls. qRT-PCR analysis confirmed up-regulation of miRNAs derived from the miR-17∼92 cluster in kidneys from miR-17∼92 OE mice compared with kidneys from control mice (Fig. 2A). Normal kidney histology was observed in control mice (Fig. 2C). In contrast, tubular dilation, tubular cysts, and glomerular cysts were observed in kidneys of miR-17∼92 OE mice (Fig. 2C and Fig. S5). miR-17∼92 OE mice were monitored until 5 mo of age, and no lethality was observed over this time period. Staining with specific renal tubule markers showed that tubular cysts were primarily derived from collecting ducts and loops of Henle, which are the major sites of cre expression in this animal model (Fig. 2C). Collectively, these findings demonstrate that overexpression of miR-17∼92 is sufficient to produce kidney cysts in mice.
Fig. 2.
Kidney-specific transgenic overexpression of miR-17∼92 produces kidney cysts. (A) qRT-PCR analysis showing that the expression of miRNAs encoded by the miR-17∼92 miRNA cluster is up-regulated in kidneys from miR-17∼92 OE mice compared with control mice. (B) Expression of miRNAs encoded by the miR-17∼92 miRNA cluster is reduced in kidneys from miR-17∼92 KO mice compared with control mice. **P < 0.01, *P < 0.05. Error bars represent SEM. (C) H&E staining demonstrates normal kidney histology in control mice (a) and miR-17∼92KO mice (c). In contrast, kidney tubular cysts (cy, arrows) are present in 28-d-old miR-17∼92OE mice (b). Staining with Dolichos biflorus agglutinin (green, d, e, and f), a marker of collecting ducts; LTA (green, g, h, and i), a marker of proximal tubules; and THP (red, j, k, and l), a marker of loops of Henle; showed that the cysts in miR-17∼92 OE mice were derived from collecting ducts (e) and loops of Henle (k). No proximal tubular cysts were observed (h). (Scale bars: 40 μm.)
Inactivation of miR-17∼92 Retards Cyst Growth in Mouse Model of PKD.
Next, we asked whether inhibition of miR-17∼92 affected cyst growth. To test this hypothesis, we first deleted miR-17∼92 in renal tubules using cre/loxP recombination. Ksp/cre mice were crossed with miR-17∼92F/F mice (20) to generate Ksp/cre;miR-17∼92F/F (referred to as miR-17∼92 KO) mice. miR-17∼92F/F mice contain loxP sites in the genomic DNA flanking the coding sequences of the miR-17∼92 miRNA cluster (Fig. S4D). PCR analysis detected the recombined allele of miR-17∼92 in genomic DNA from kidneys of miR-17∼92 KO mice, confirming cre-mediated recombination (Fig. S4E). qRT-PCR analysis showed that the expression of the primary miR∼92 transcript was decreased by ∼60% (Fig. S4F), and the expression of mature miRNAs was decreased by 40–50% (Fig. 2B) in kidneys of miR-17∼92 KO mice compared with control mice. miR-17∼92 KO mice were phenotypically normal, fertile, and did not display any appreciable defects in kidney morphology and histology (Fig. 2C). Thus, deletion of miR-17∼92 by itself does not affect renal tubule development.
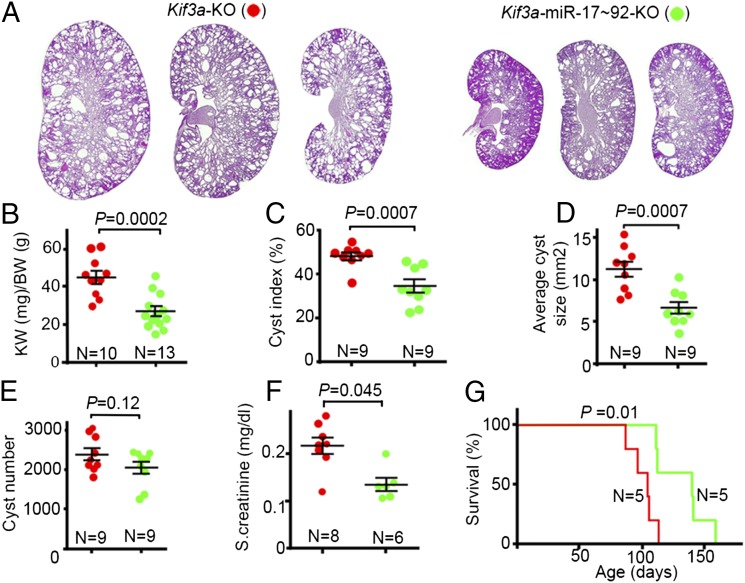
We then inactivated miR-17∼92 in renal tubules of Kif3a-KO mice (Fig. S6A). Kif3aF/F mice were bred with Ksp/cre;miR-17∼92F/F mice, and the first and second generation progeny were intercrossed to generate Ksp/cre;Kif3aF/F;miR-17∼92F/F (Kif3a-miR-17∼92 KO) mice and Ksp/cre;Kif3aF/F;miR-17∼92+/+ (Kif3a-KO) mice. Double-knockout mice (Kif3a-miR-17∼92-KO) showed reduced cyst growth compared with single Kif3a-KO mice (Fig. 3A and Fig. S6B). The kidney weight-to-body weight ratio was reduced by 41%, cyst index was reduced by 28%, and average cyst size was reduced by 41% in Kif3a-miR-17∼92-KO mice compared with Kif3a-KO mice (Fig. 3 B–D). The total number of cysts was not different between the two groups (Fig. 3E). Compared with Kif3a-KO mice, the level of serum creatinine in Kif3a-miR-17∼92-KO mice was decreased by 29%, and the median survival was increased by 36 d (Fig. 3 F and G). Thus, inactivation of miR-17∼92 attenuates cyst growth, improves renal function, and prolongs survival of Kif3a-KO mice.
Fig. 3.
Inactivation of miR-17∼92 in renal tubules of Kif3a-KO mice inhibits cyst growth. (A) H&E staining of kidneys from three different 28-d-old Kif3a-miR-17∼92-KO mice (Right) and Kif3a-KO mice (Left) is shown. Quantification revealed that kidney weight-to-body weight ratio (B), cyst index (C), and average cyst size (D) were reduced in Kif3a-miR-17∼92-KO mice (green) compared with Kif3a-KO mice (red). (E) No difference in cyst number was observed between the two groups. (F) Serum creatinine levels show improved renal function in 28-d-old Kif3a-KO mice compared with Kif3a-miR-17∼92-KO mice. (G) Kaplan–Meier survival curves of Kif3a-KO mice and Kif3a-miR-17∼92-KO. Median survival of Kif3a-KO mice was 104 d, and median survival of Kif3a-miR-17∼92-KO mice was 140 d. Error bars represent SEM.
miR-17∼92 Promotes Proliferation and Regulates the Posttranscriptional Expression of PKD Genes.
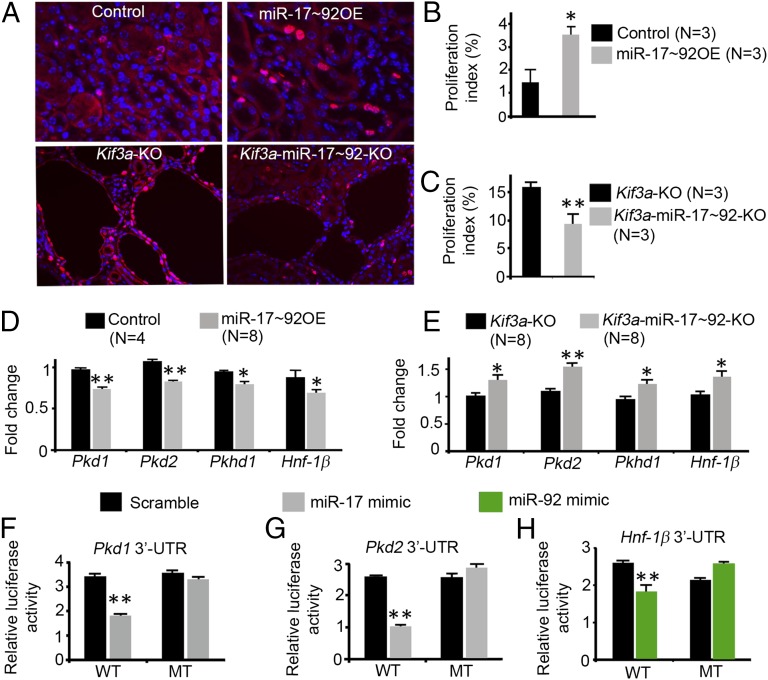
Since increased cell proliferation may underlie the growth of kidney cysts, we determined whether miR-17∼92 affects cyst growth by promoting proliferation. Kidney sections from 28-d-old control, miR-17∼92 OE, Kif3a-KO, and Kif3a-miR-17∼92-KO mice were stained with an antibody against Ki-67 to label proliferating cells (Fig. 4A). The proliferation index of renal tubular epithelial cells was increased more than twofold in miR-17∼92 OE kidneys compared with control kidneys, indicating that overexpression of miR-17∼92 results in increased proliferation of renal tubular cells (Fig. 4B). Conversely, the proliferation index of cyst epithelial cells was decreased by 42% in Kif3a-miR-17∼92-KO kidneys compared with Kif3a-KO kidneys, indicating that inhibition of miR-17∼92 results in decreased proliferation of cyst epithelial cells (Fig. 4C).
Fig. 4.
Members of the miR-17∼92 cluster promote proliferation and represses PKD genes. (A) Kidney sections from control, miR-17∼92 OE, Kif3a-KO, and Kif3a-miR-17∼92-KO mice were stained with an antibody against Ki-67 (red) to label proliferating cells. (B) Proliferation index is increased in miR-17∼92 OE mice compared with controls. (C) Proliferation index is reduced in Kif3a-miR-17∼92-KO mice compared with Kif3a-KO mice. (D) qRT-PCR analysis showed that the expression of Pkd1, Pkd2, Pkhd1, and Hnf-1β is decreased in kidneys from 28-d-old miR-17∼92 OE mice compared with control mice. (E) Expression of Pkd1, Pkd2, Pkhd1, and Hnf-1β is increased in kidneys from 28-d-old Kif3a-miR-17∼92-KO mice compared with Kif3a-KO mice. (F–H) Mouse inner medullary collecting duct (mIMCD3) cells were transfected with luciferase reporter plasmids containing the WT 3′ UTRs of Pkd1, Pkd2, and Hnf-1β. Cotransfection with miR-17 mimics (F and G) or miR-92a mimics (H) decreased luciferase activity. Mutations of the miRNA binding sites (MT) abrogated the repression. *P < 0.05, **P < 0.01. Error bars represent SEM.
To further understand the molecular mechanisms, we identified miR-17∼92 targets that are involved in the pathogenesis of PKD. Bioinformatic analysis (21) suggested that PKD1 and PKD2 contain evolutionarily conserved miR-17 binding sites in their 3′ UTRs (Fig. S7). miR-17 has been shown to posttranscriptionally regulate Pkd1 and Pkd2 in cultured cells (15, 22, 23). However, whether miR-17 inhibits Pkd1 or Pkd2 expression in vivo is not known. HNF-1β, mutations of which produce renal cysts and diabetes (RCAD), contains an evolutionarily conserved miR-92 binding site in its 3′ UTR (Fig. S7), but regulation by miR-92 has not been studied. HNF-1β is an epithelial-specific transcription factor that regulates the expression of cystic disease genes, including Pkd2 and Pkhd1 (18, 24, 25). Polycystin-1, polycystin-2, and HNF-1β retard cell proliferation (25–27). Thus, we hypothesized that miR-17∼92 promotes cyst growth, in part, through direct and indirect repression of a network of PKD genes.
Overexpression of miR-17∼92 in the kidney resulted in decreased expression of Pkd1, Pkd2, Hnf-1β, and Pkhd1 (Fig. 4D). Conversely, deletion of miR-17∼92 in Kif3a-KO mice resulted in increased expression of Pkd1, Pkd2, Hnf-1β, and Pkhd1 (Fig. 4E). Next, we tested whether miR-17∼92 directly repressed Pkd1, Pkd2, and Hnf-1β expression in renal epithelial cells. Luciferase reporter assays revealed that miR-17 repressed the 3′ UTRs of Pkd1 and Pkd2, and miR-92a repressed the 3′ UTR of Hnf-1β (Fig. 4 F–H). Mutations of the miR-17 binding sites abrogated the repression of Pkd1 and Pkd2, and mutation of the miR-92a binding site abrogated the repression of Hnf-1β (Fig. 4 F–H). Taken together, these results indicate that members of the miR-17∼92 cluster repress Pkd1, Pkd2, and Hnf-1β in vivo through interaction with binding sites in their 3′ UTRs.
Discussion
We provide three lines of evidence implicating miR-17∼92 in the pathogenesis of kidney cyst growth. First, the miR-17∼92 miRNA cluster is up-regulated in cystic kidneys from four different mouse models of PKD, suggesting that increased levels of miR-17∼92 may be a frequent feature of PKD. miRNAs encoded by the miR-17∼92 cluster are classified into four different families. Because members of each family have identical seed sequences, they are predicted to target the same mRNA transcripts. Up-regulation of members belonging to the same miRNA families was observed (e.g., miR-17 and miR-20a), which suggests that individual miR-17∼92 miRNAs cooperate in reducing the expression of target genes in cystic kidneys. miRNAs can be up-regulated because of increased transcription or enhanced posttrancriptional processing of primary miRNA transcripts. We found that the expression of pri-miR-17∼92 was increased in 21-d-old and 28-d-old, but not in 14-d-old, Kif3a-KO mice (Fig. S8). These results are consistent with the pattern of expression of the mature miRNAs and indicate that increased transcription is responsible for miR-17∼92 up-regulation in Kif3a-KO kidneys. The oncogene c-Myc binds to the promoter of the miR-17∼92 cluster and activates its transcription (28). c-Myc is a target of wingless (wnt)-β-catenin signaling. We have shown that canonical wnt-β-catenin signaling is up-regulated and the expression of c-Myc is increased in cystic kidneys from Kif3a-KO mice (4). In this study, we found that the expression of c-Myc correlated with the expression of pri-miR-17 in Kif3a-KO kidneys (Fig. S8). Expression of pri-miR-17 and c-Myc was also increased in kidneys from Pkhd1/cre;Pkd2F/F mice and Pkhd1/cre;Hnf-1βF/F mice compared with their respective controls (Fig. S8). These results suggest that c-Myc–dependent transcriptional activation may also underlie up-regulation of miR-17∼92 in Pkd2 and Hnf-1β mutant mice.
As a second line of evidence, we have shown that kidney-specific transgenic overexpression of miR-17∼92 produces cysts in mice. These results directly link miR-17∼92 to cyst formation. The kidney cysts that develop in miR-17∼92 OE mice are similar to the cysts that are observed in Pkd1 knockout mice (29), but the disease progression is milder. Unlike kidney-specific Pkd1 null mice that develop rapidly fatal PKD soon after birth (generated with the same cre deleter strain), the development of cysts in miR-17∼92 OE mice is slower and less severe. The renal phenotype of miR-17∼92 OE mice is similar to mice that are homozygous for a hypomorphic allele of Pkd1 (30). Both Pkd1 hypomorphic mice and miR-17∼92 OE mice develop slowly progressive cystic kidney disease and survive long term. In both cases, the cysts are lined by hyperproliferative epithelial cells and originate primarily from the distal nephron. Glomerular cysts are also observed in both mice. The phenotypic similarity to Pkd1 hypomorphic mice supports the hypothesis that reduced PKD gene dosage contributes to cyst formation in miR-17∼92 OE mice (see below).
Third, we show that inactivation of miR-17∼92 retards cyst growth in Kif3a-KO mice. These studies demonstrate a pathogenic role of miRNAs in a mouse model of PKD and provide genetic proof-of-principle for inhibition of miR-17∼92 as a unique therapeutic approach in PKD. Whether inactivation of miR-17∼92 will also retard cyst growth in orthologous models of PKD needs to be verified. miR-17∼92 is amplified in numerous cancers where it promotes cell proliferation, and increased proliferation may also underlie the growth of kidney cysts in PKD. We found that overexpression of miR-17∼92 resulted in increased proliferation of renal tubule epithelial cells, whereas inactivation of miR-17∼92 resulted in decreased proliferation of cyst epithelial cells, providing one potential mechanism by which miR-17∼92 may promote cyst growth.
miRNAs exert their biological effects by fine-tuning the expression of multiple target genes, which often encode proteins that regulate the same biological process (8). Although the effects of miRNAs on individual mRNA targets are typically modest, the cumulative effects of targeting multiple genes in the same pathway may be significant. Consistent with this notion, we found that miR-17∼92 modestly inhibited the expression of multiple PKD genes in vivo. The effects of miR-17∼92 on Pkd1, Pkd2, and HNF-1β appear to be direct, because functional miRNA binding sites were identified in the 3′ UTRs. The effects on Pkhd1 may be indirect, possibly mediated by down-regulation of its transcriptional activator HNF-1β (31). Accumulating evidence indicates that reduced dosage of PKD genes promotes cyst growth in humans and mice (30, 32–35). Whereas haploinsufficiency of individual PKD genes in mice causes the formation of only late-onset cysts (Pkd1+/−, Pkd2+/−) or no kidney cysts (Pkhd1+/−, HNF-1β+/−), concomitant reduction in the dosage of multiple PKD genes can exacerbate cyst formation. Genetic interaction studies have shown that mice with combined mutations of Pkd1 and Pkd2, Pkd1 and Pkhd1, or Pkd2 and Pkhd1 have more severe cystic phenotypes than mice with single gene mutations (16, 36–38). Likewise, studies of human families with PKD have shown that individuals with combined mutations of PKD1 and PKD2 or PKD2 and PKHD1 have more severe disease than siblings with single gene mutations (35, 39). A recent study has shown that humans with ADPKD who also are heterozygous for mutations of HNF-1β have more severe kidney abnormalities (35). The role of reduced gene dosage is further supported by studies of mice harboring hypomorphic alleles of PKD genes (30, 34). As discussed above, Pkd1 hypomorphic mice and miR-17∼92 OE mice have similar cystic phenotypes, suggesting that reduced Pkd1 gene dosage contributes to cyst formation in miR-17∼92 OE mice. Taken together, these findings support the hypothesis that concomitant reduction in the expression of multiple PKD genes, e.g., due to up-regulation of miR-17∼92, can promote cyst formation. Conversely, inhibiting miR-17∼92 may provide clinical benefit in PKD by increasing the expression of PKD1, PKD2, and HNF-1β. miR-17∼92 is known to regulate other signaling pathways, such as mTOR and TGF-β pathways, which are also implicated in the pathogenesis of cyst growth (28, 40–42). Bioinformatics algorithms predict that members of the miR-17∼92 cluster target thousands of mRNAs for posttranscriptional silencing. Therefore, it is likely that miR-17∼92 modulates cyst growth through repression of many other genes besides the PKD genes.
In conclusion, we have shown that the miR-17∼92 miRNA cluster regulates kidney cyst growth. Up-regulation of miR-17∼92 promotes cyst growth, in part, by stimulating proliferation and inhibiting the posttranscriptional expression of cystic disease genes (Fig. S9). Our results provide a unique mechanism involving miRNAs and regulation of PKD gene dosage in the pathogenesis of kidney cyst growth and suggest that inhibition of miR-17∼92 may represent a useful therapeutic approach in PKD.
Methods
Mice.
Ksp/cre mice (14), Pkhd1/cre mice (5), Kif3aF/F mice (4), Pkd2F/F mice (43), Pkhd1−/− mice (17), Hnf-1βF/F mice (18), miR-17∼92F/F mice (20) (stock no. 008458; Jackson Laboratory), and miR-17∼92 transgenic mice (19) (stock no. 008517; Jackson Laboratory) were used in the current study. Pkhd1/cre;Pkd2 F/F mice and Pkhd1/cre;Hnf-1β F/F mice were generated by breeding Pkhd1/cre mice with Pkd2F/F and Hnf-1βF/F mice, respectively. Pkhd1/cre mice express cre recombinase in collecting ducts (5). All experiments involving animals were approved by the University of Texas Southwestern Institutional Animal Care and Research Advisory Committee.
Microarray and Real-Time PCR.
Microarray was performed by LC Sciences. See SI Methods for more information.
Primers.
Locked nucleic acid (LNA)-enhanced PCR primers for mature miRNAs were obtained from Exiqon. The primers used were as follows: miR-17 (catalog no. 204771), miR-18a (catalog no. 204207), miR-19a (catalog no. 04781), miR-19b (catalog no. 204450), miR-20a (catalog no. 204292), and miR-92a (catalog no. 204258). PCR primers used to determine the expression pri-miR-17∼92 transcript were obtained from Life Technologies (catalog no. Mm04228426_pri). Additional primers are listed in SI Methods.
Antibodies and Immunofluorescence.
See SI Methods for more information.
Histologic Preparation, Cyst Parameters, and Proliferative Index.
Mice were anesthetized according to approved protocols and perfused with ice-cold PBS and 4% (wt/vol) paraformaldehyde. Kidneys were harvested, fixed with 4% paraformaldehyde at 4 °C for 2–4 h, and embedded in paraffin. Sagittal kidney sections were stained with hematoxylin and eosin (H&E) and examined by light microscopy. All kidneys were photographed under the same magnification. ImageJ analysis software was used to calculate the cyst index, cyst size, and count the number of cysts. Cyst index refers to the cumulative area of cysts within the total area of the kidney. The entire sagittal kidney section was used to calculate cyst burden. To calculate proliferation index, kidney sections were stained with an antibody against Ki-67, a marker of proliferating cells. Multiple fields of each kidney section were photographed by using 20× objective. AxioVision sofware (Zeiss) was used to count the percentage of Ki-67–positive cells in a kidney section. For the data shown in Fig. 4B, 80,459 tubule epithelial cells from kidneys of three control mice, and 78,642 tubule epithelial cells from kidneys of three miR-17∼92OE mice were counted. For the data shown in Fig. 4B, 4,378 cyst epithelial cells from kidneys of three Kif3a-KO mice and 6,739 cyst epithelial cells from kidneys of three Kif3a-miR-17∼92-KO mice were counted.
miRNA Mimics.
The following miRNA mimics were purchased from Dharmacon (Thermo Fischer Scientific): miR-17 (catalog no. C-310561-07-0005), miR-92a (catalog no. C-310526-03-0005), and negative control (catalog no. CN-001000-01-05).
3′ UTR Plasmids.
The construction of the pLS-Pkd1-3′-UTR and pLS-Hnf-1β-3′-UTR plasmid has been described (10). Site-directed mutagenesis was performed to mutate the miR-17 binding site in the pLS-Pkd1-3′-UTR construct. See SI Methods for more details.
Luciferase Assay.
See SI Methods for more information.
Statistical Analysis.
Data are shown as mean ± SEM. The significance of differences between the means was calculated by using Student’s t test. ANOVA followed by Dunnett’s test was used for multiple comparisons. Mantel–Cox test was used to compare differences in survival between Kif3a-KO mice and Kif3a-miR-17∼92-KO mice. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Patricia Cobo-Stark for helpful discussions. We thank the University of Texas (UT) Southwestern O'Brien Kidney Research Core Center through Grant P30DK079328 for providing critical reagents and services. Work from the authors’ laboratory is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants K08DK084311 (to V.P.), RC1DK086887 and R37DK042921 (to P.I.), and R01DK54053 (to S.S.); a Pilot and Feasibility grant from the UT Southwestern O'Brien Kidney Research Core Center (to V.P.); and by “Fondation pour la recherche médicale” (équipe FRM), European Community's Seventh Framework Programmme FP7/2009 (agreement no. 241955, SYCILIA), and Agence Nationale pour le Recherche (M.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1301693110/-/DCSupplemental.
References
- 1.Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2002;13(9):2384–2398. doi: 10.1097/01.asn.0000028643.17901.42. [DOI] [PubMed] [Google Scholar]
- 2.Harris PC, Torres VE. Polycystic kidney disease. Annu Rev Med. 2009;60:321–337. doi: 10.1146/annurev.med.60.101707.125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel V, Chowdhury R, Igarashi P. Advances in the pathogenesis and treatment of polycystic kidney disease. Curr Opin Nephrol Hypertens. 2009;18(2):99–106. doi: 10.1097/MNH.0b013e3283262ab0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin F, et al. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA. 2003;100(9):5286–5291. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel V, et al. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet. 2008;17(11):1578–1590. doi: 10.1093/hmg/ddn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell. 2012;148(6):1172–1187. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011;469(7330):336–342. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Rooij E, Marshall WS, Olson EN. Toward microRNA-based therapeutics for heart disease: The sense in antisense. Circ Res. 2008;103(9):919–928. doi: 10.1161/CIRCRESAHA.108.183426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel V, et al. MicroRNAs regulate renal tubule maturation through modulation of Pkd1. J Am Soc Nephrol. 2012;23(12):1941–1948. doi: 10.1681/ASN.2012030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel V, Noureddine L. MicroRNAs and fibrosis. Curr Opin Nephrol Hypertens. 2012;21(4):410–416. doi: 10.1097/MNH.0b013e328354e559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandey P, Qin S, Ho J, Zhou J, Kreidberg JA. Systems biology approach to identify transcriptome reprogramming and candidate microRNA targets during the progression of polycystic kidney disease. BMC Syst Biol. 2011;5:56. doi: 10.1186/1752-0509-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandey P, et al. Microarray-based approach identifies microRNAs and their target functional patterns in polycystic kidney disease. BMC Genomics. 2008;9:624. doi: 10.1186/1471-2164-9-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao X, Somlo S, Igarashi P. Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J Am Soc Nephrol. 2002;13(7):1837–1846. doi: 10.1097/01.asn.0000016444.90348.50. [DOI] [PubMed] [Google Scholar]
- 15.Tran U, et al. The RNA-binding protein bicaudal C regulates polycystin 2 in the kidney by antagonizing miR-17 activity. Development. 2010;137(7):1107–1116. doi: 10.1242/dev.046045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fedeles SV, et al. A genetic interaction network of five genes for human polycystic kidney and liver diseases defines polycystin-1 as the central determinant of cyst formation. Nat Genet. 2011;43(7):639–647. doi: 10.1038/ng.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams SS, Cobo-Stark P, James LR, Somlo S, Igarashi P. Kidney cysts, pancreatic cysts, and biliary disease in a mouse model of autosomal recessive polycystic kidney disease. Pediatr Nephrol. 2008;23(5):733–741. doi: 10.1007/s00467-007-0735-4. [DOI] [PubMed] [Google Scholar]
- 18.Gresh L, et al. A transcriptional network in polycystic kidney disease. EMBO J. 2004;23(7):1657–1668. doi: 10.1038/sj.emboj.7600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao C, et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat Immunol. 2008;9(4):405–414. doi: 10.1038/ni1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ventura A, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132(5):875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 22.Cloonan N, et al. The miR-17-5p microRNA is a key regulator of the G1/S phase cell cycle transition. Genome Biol. 2008;9(8):R127. doi: 10.1186/gb-2008-9-8-r127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun H, et al. MicroRNA-17 post-transcriptionally regulates polycystic kidney disease-2 gene and promotes cell proliferation. Mol Biol Rep. 2010;37(6):2951–2958. doi: 10.1007/s11033-009-9861-3. [DOI] [PubMed] [Google Scholar]
- 24.Ma Z, et al. Mutations of HNF-1beta inhibit epithelial morphogenesis through dysregulation of SOCS-3. Proc Natl Acad Sci USA. 2007;104(51):20386–20391. doi: 10.1073/pnas.0705957104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verdeguer F, et al. A mitotic transcriptional switch in polycystic kidney disease. Nat Med. 2010;16(1):106–110. doi: 10.1038/nm.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhunia AK, et al. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell. 2002;109(2):157–168. doi: 10.1016/s0092-8674(02)00716-x. [DOI] [PubMed] [Google Scholar]
- 27.Li X, et al. Polycystin-1 and polycystin-2 regulate the cell cycle through the helix-loop-helix inhibitor Id2. Nat Cell Biol. 2005;7(12):1202–1212. doi: 10.1038/ncb1326. [DOI] [PubMed] [Google Scholar]
- 28.O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435(7043):839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 29.Shibazaki S, et al. Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum Mol Genet. 2008;17(11):1505–1516. doi: 10.1093/hmg/ddn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hopp K, et al. Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J Clin Invest. 2012;122(11):4257–4273. doi: 10.1172/JCI64313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiesberger T, et al. Mutation of hepatocyte nuclear factor-1beta inhibits Pkhd1 gene expression and produces renal cysts in mice. J Clin Invest. 2004;113(6):814–825. doi: 10.1172/JCI20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossetti S, et al. Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease. Kidney Int. 2009;75(8):848–855. doi: 10.1038/ki.2008.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pei Y, et al. A missense mutation in PKD1 attenuates the severity of renal disease. Kidney Int. 2012;81(4):412–417. doi: 10.1038/ki.2011.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lantinga-van Leeuwen IS, et al. Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Hum Mol Genet. 2004;13(24):3069–3077. doi: 10.1093/hmg/ddh336. [DOI] [PubMed] [Google Scholar]
- 35.Bergmann C, et al. Mutations in multiple PKD genes may explain early and severe polycystic kidney disease. J Am Soc Nephrol. 2011;22(11):2047–2056. doi: 10.1681/ASN.2010101080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu G, et al. Trans-heterozygous Pkd1 and Pkd2 mutations modify expression of polycystic kidney disease. Hum Mol Genet. 2002;11(16):1845–1854. doi: 10.1093/hmg/11.16.1845. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Gonzalez MA, et al. Genetic interaction studies link autosomal dominant and recessive polycystic kidney disease in a common pathway. Hum Mol Genet. 2007;16(16):1940–1950. doi: 10.1093/hmg/ddm141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim I, et al. Fibrocystin/polyductin modulates renal tubular formation by regulating polycystin-2 expression and function. J Am Soc Nephrol. 2008;19(3):455–468. doi: 10.1681/ASN.2007070770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pei Y, et al. Bilineal disease and trans-heterozygotes in autosomal dominant polycystic kidney disease. Am J Hum Genet. 2001;68(2):355–363. doi: 10.1086/318188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendell JT. miRiad roles for the miR-17-92 cluster in development and disease. Cell. 2008;133(2):217–222. doi: 10.1016/j.cell.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nittner D, et al. Synthetic lethality between Rb, p53 and Dicer or miR-17-92 in retinal progenitors suppresses retinoblastoma formation. Nat Cell Biol. 2012;14(9):958–965. doi: 10.1038/ncb2556. [DOI] [PubMed] [Google Scholar]
- 42.Conkrite K, et al. miR-17∼92 cooperates with RB pathway mutations to promote retinoblastoma. Genes Dev. 2011;25(16):1734–1745. doi: 10.1101/gad.17027411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishio S, et al. Loss of oriented cell division does not initiate cyst formation. J Am Soc Nephrol. 2010;21(2):295–302. doi: 10.1681/ASN.2009060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.