Abstract
The enhanced dietary flexibility of early hominins to include consumption of C4/crassulacean acid metabolism (CAM) foods (i.e., foods derived from grasses, sedges, and succulents common in tropical savannas and deserts) likely represents a significant ecological and behavioral distinction from both extant great apes and the last common ancestor that we shared with great apes. Here, we use stable carbon isotopic data from 20 samples of Australopithecus afarensis from Hadar and Dikika, Ethiopia (>3.4–2.9 Ma) to show that this species consumed a diet with significant C4/CAM foods, differing from its putative ancestor Au. anamensis. Furthermore, there is no temporal trend in the amount of C4/CAM food consumption over the age of the samples analyzed, and the amount of C4/CAM food intake was highly variable, even within a single narrow stratigraphic interval. As such, Au. afarensis was a key participant in the C4/CAM dietary expansion by early australopiths of the middle Pliocene. The middle Pliocene expansion of the eastern African australopith diet to include savanna-based foods represents a shift to use of plant food resources that were already abundant in hominin environments for at least 1 million y and sets the stage for dietary differentiation and niche specialization by subsequent hominin taxa.
Keywords: stable isotope, bioapatite, carbon-13, paleodiet, human evolution
One of the traits that distinguishes humans from our closest living relatives, Pan and Gorilla, is the inclusion of significant quantities of C4/crassulacean acid metabolism (CAM) foods in the diet. C4/CAM plants include grasses and sedges (C4) common in tropical savannas and succulents (CAM) common in deserts. Therefore, the expansion of hominin diets to consume substantial amounts of C4/CAM foods signals a major ecological and adaptive divergence from the last common ancestor (LCA) that we shared with African great apes, which mostly occupy closed wooded habitats. C4/CAM food consumption is part of a general argument, based on several lines of evidence, that hominin diets diverged from the diets of the LCA during the shift to drier and more open environments in Africa during the Pliocene (1–4). This dietary transition occurred subsequent to the known fossil record of Ardipithecus ramidus from Ethiopia at 4.4 Ma (5), which shows little evidence of C4/CAM food consumption. The earliest previously published hominins to evince substantial C4/CAM food consumption are Au. africanus at ≤2.7 Ma (2, 6–8) as well as a small, poorly age-constrained (ca. 3.0–3.5 Ma) sample of Au. bahrelghazali from west-central Africa (9). Thus, current evidence places middle Pliocene Au. afarensis, a hominin with an extensive and well-defined stratigraphic range (3.7–3.0 Ma) in Ethiopia and elsewhere in eastern Africa at the crux of this hominin dietary change.
In this paper, we use stable isotope analysis to directly investigate the diet of Au. afarensis from the Hadar Formation of Ethiopia. Stable isotope analysis of mammalian tooth enamel is a paleodietary tool that allows one to determine the proportions of dietary carbon derived from plants using the C3 photosynthetic pathway (trees, shrubs, and many herbs) vs. the C4 and CAM pathways [C4 includes tropical grasses and some sedges and CAM includes succulents (6, 10–15)]. Although stable isotope studies alone do not distinguish the specific type of C4/CAM food consumed, a C4/CAM diet would imply the consumption of foods such as above- or underground portions of C4 grasses or sedges, CAM succulents, and/or animals that ate these plants. We sampled 20 teeth of Au. afarensis (SI Appendix, Table S1) from well-constrained geochronological and refined taphonomic and paleoenvironmental contexts in the Hadar Formation at the geographically and temporally adjacent sites of Hadar and Dikika, Ethiopia (16–22). This sample allows us to investigate not only what Au. afarensis ate but also, whether the diet of this species changed through time (23) in response to previously inferred shifts in local environments over a 450,000-y interval (22). We also sampled the associated macromammalian fauna to place the fossil hominin data in a broader context and address questions of diagenesis that can alter primary isotopic signatures.
Results
The median δ13C value for the sample of Au. afarensis is −7.4‰, with a range of −13.0‰ to −2.9‰ (Fig. 1). There are significant differences in the median δ13C values for Au. afarensis and presumptive C4-grazing and C3-browsing herbivores (Kruskal–Wallis, P < 0.0001). Posthoc comparisons show that the median δ13C value for Au. afarensis is statistically distinct from δ13C values of the C3-browser Giraffa (median = −10.9‰, n = 15, Mann–Whitney U, P < 0.0001) and C4-grazing alcelaphini (median = +1.4, n = 10, Mann–Whitney U, P < 0.0001) (Fig. 1), which are also found in the same Hadar Formation sediments. These end member taxa have δ13C values that validate their presumed diets, implying that diagenesis has not significantly affected the δ13C values of our samples.
Fig. 1.

Box and whisker plot showing the carbon isotopic composition (δ13C value) of tooth enamel from Au. afarensis and two end member taxa (C3 browser: Giraffa; C4 grazer: alcelaphini). The bull’s eye at center of each box represents the sample median. Open circles represent outlier values, defined at 1.5 times the interquartile range. Boxes represent 25th and 75th percentiles. Whiskers represent the range exclusive of outliers. Triangles represent the 95% confidence intervals for the medians (thus, if the areas between the triangles for two samples do not overlap, one can conclude with 95% confidence that the true medians differ). Closed circles below the box plots represent individual specimen values. Black, Australopithecus; gray, other taxa.
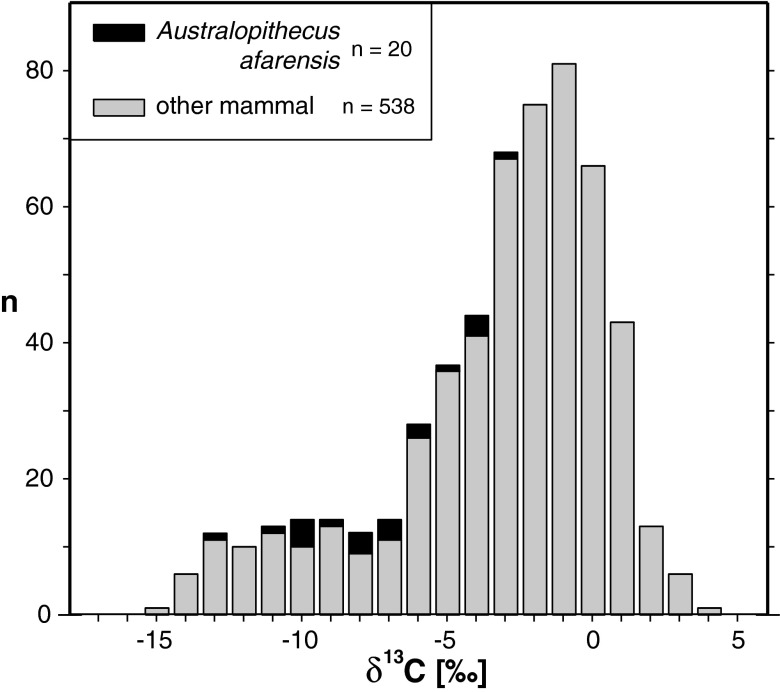
The δ13C values for Au. afarensis indicate both a significant proportion and range of C4/CAM plants in its diet. Using the median values of the two end member taxa as estimates of pure C3-browsing and pure C4-grazing diets, the nominal percentage of C4 intake for 20 Au. afarensis individuals has a median value of 22% and a range of 0–69%. Posthoc comparisons of the δ13C values from Au. afarensis also clearly show more C4/CAM consumption than inferred from the δ13C1750 values of modern Pan (24–26) (median = −10.9‰, n = 58, Mann–Whitney U, P < 0.0001) and Gorilla (27) [median δ13C1750 = −13.5‰, n = 15, Mann–Whitney U, P < 0.0001; modern values are corrected to the year 1750 for the change in the δ13C of atmospheric CO2 caused by the burning of fossil fuels (28)]. There is no statistically significant temporal trend in δ13C values through the Hadar Formation (Fig. 2) (r2 = 0.03, P = 0.40). Despite evidence for significant C4/CAM consumption, the entire range of δ13C values for Au. afarensis falls below the modal δ13C value for the Hadar Formation nonhominin mammalian sample analyzed (n = 538, median = −2.2‰, mode = −2.5‰, Mann–Whitney U, P < 0.0001) (Fig. 3).
Fig. 2.
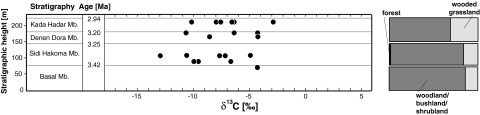
Stratigraphic distribution of the Australopithecus isotopic composition shown in Fig. 1. Specimens are divided into submembers of the Hadar Formation and plotted with stratigraphic height. Also shown are the percentages of cover of forest, woodland/bushland/shrubland, and wooded grassland estimated by samples of δ13C of pedogenic carbonates from the Hadar Formation (47).
Fig. 3.
Histogram showing the carbon isotopic composition (δ13C value) of tooth enamel from Au. afarensis and all other mammalian taxa analyzed from the Hadar Formation. Black, Au. afarensis; gray, all other mammals analyzed. Data are from this study (hominin samples are in SI Appendix, Table S1, and other samples are in SI Appendix, Table S2, and in ref. 44).
There were significant differences in δ13C values among early hominin taxa, for which a sample of at least five individuals is available (Kruskal–Wallis, P < 0.0001). Posthoc comparisons show that the δ13C values for Au. afarensis do not differ significantly from the δ13C values of slightly younger Au. africanus from South Africa [median = −6.8‰, n = 24, Mann–Whitney U, P = 0.32 (2, 6–8)]. The ranges of variation in δ13C values for these two taxa broadly overlap (Au. afarensis: −13.0‰ to −2.9‰, n = 20; Au. africanus: −11.3‰ to −1.8‰, n = 24). δ13C values for Au. afarensis show significantly more evidence of C4/CAM food consumption than in earlier Ar. ramidus [4.4 Ma, median = −10.4‰, n = 5, Mann–Whitney U, P = 0.021 (5)] and Au. anamensis (4.0 Ma, median = −11.0‰, n = 11, Mann–Whitney U, P < 0.001). Compared with later australopiths, Au. afarensis has similar δ13C values to the δ13C values of Paranthropus robustus of southern Africa [median = −7.2‰, n = 22, Mann–Whitney U, P = 0.95 (7, 13, 29, 30)], but its δ13C values differ significantly from the δ13C values of P. boisei of eastern Africa [median = −1.3‰, n = 24, Mann–Whitney U, P < 0.0001 (31, 32)]. Comparison of δ13C values of Au. afarensis with δ13C values from very small samples of Au. bahrelghazali from central Africa [median = −2.5‰, n = 3 (9)] and Au. sediba from southern Africa [median = −12.15‰, n = 2 (33)] suggests significant differences in C4/CAM food consumption among these three taxa.
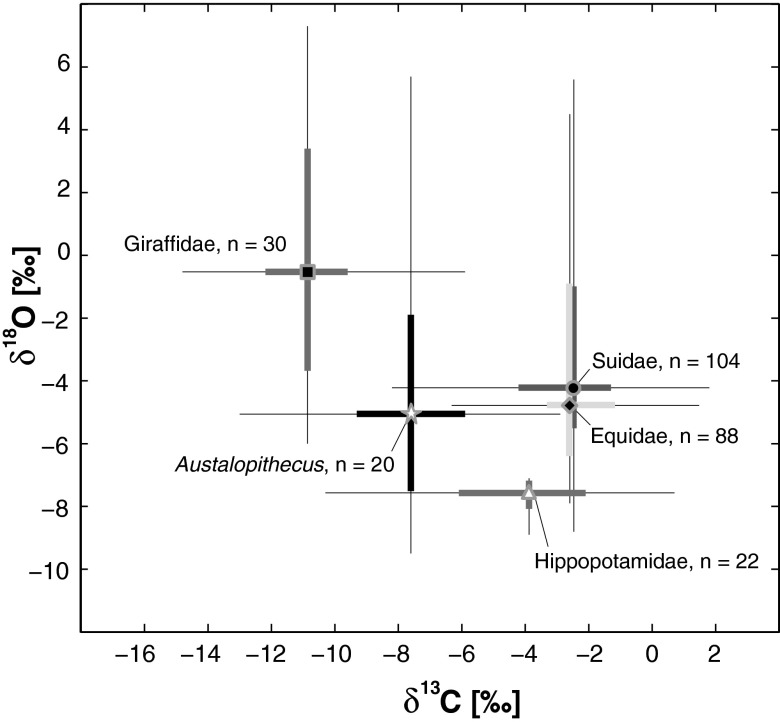
A Kruskal–Wallis test indicates strong divergence in δ18O values, which in part, reflects differences in water dependence (34) between Au. afarensis and other mammalian families from the Hadar Formation (Fig. 4) (Kruskal–Wallis, P < 0.0001). Posthoc comparisons show that the δ18O values for Au. afarensis (median = −5.6‰, n = 20) are different from the highly water-independent giraffids (median = −0.6‰, n = 30, Mann–Whitney U, P = 0.0016) as well as the very water-dependent hippopotamids (median = −7.5‰, n = 22, Mann–Whitney U, P = 0.011). Au. afarensis δ18O values are, however, statistically indistinguishable from the δ18O values of equids (median = −4.8‰, n = 88, Mann–Whitney U, P = 0.13) and suids (median = −4.2‰, n = 104, Mann–Whitney U, P = 0.10).
Fig. 4.
Cross-plot of box and whisker diagrams for carbon and oxygen isotopic composition (δ13C and δ18O values) of tooth enamel from Au. afarensis and select mammalian taxa from the Hadar Formation. Boxes represent 25th and 75th percentiles. Whiskers represent the range exclusive of outliers (defined in Fig. 1). Data are from this study (hominin samples are in SI Appendix, Table S1 and in ref. 44).
Discussion
Middle Pliocene Hominin Dietary Expansion to C4/CAM Foods.
Forest-dwelling Gorilla and Pan species have low δ13C values, reflecting pure C3 diets of plants predominantly from the forest understory, where vegetation is typically 13C-depleted by several permil compared with the upper canopy (14). Even the savanna chimpanzee (P. troglodytes verus), which frequents relatively open habitats, shows little evidence of consumption of C4/CAM foods that characterize the savanna portion of their habitat (24). The diets of these extant apes contrast sharply with the diets of our Ethiopian sample of Au. afarensis, which shows significant use of C4/CAM foods. Au. afarensis shows a greater range of C4/CAM food consumption relative to the earlier east African hominin taxa Ar. ramidus and Au. anamensis, both of which focused on C3 plant foods (5, 27). Although the lowest δ13C value in our Au. afarensis sample is the most 13C-depleted specimen of an early hominin taxon yet recorded [specimen Afar locality (A.L.) 125–11: δ13C = −13.0‰], approaching the great ape range (35), the majority of δ13C values for Au. afarensis are well above the range of δ13C1750 values for extant Pan and Gorilla (14, 27). Simple mixing calculations of pure C3 and C4 end member diets show that four individuals of Au. afarensis consumed only C3 foods, at least during the period of tooth crown mineralization, whereas the majority of sampled individuals consumed significant amounts of C4/CAM foods during this growth period, with four individuals having consumed 50% or more C4 vegetation.
The stable isotope data from Au. afarensis combined with other data from African early hominins (9, 27, 36) begin to clarify the timing of increased C4/CAM use in the hominin lineage after the LCA shared with chimpanzees. The data from Hadar and Dikika in this paper, combined with data from Kenyanthropus in the Turkana Basin (27) and Au. bahrelghazali from Koro Toro, Chad (9), show a pattern of hominin dietary expansion to include C4/CAM foods during the middle Pliocene by ca. 3.4 Ma (the age uncertainty of the first occurrence of these three taxa is within the age uncertainty of the oldest specimens from the Hadar Formation) (27, 37). Especially conspicuous is the lack of evidence for C4/CAM plant consumption in Au. anamensis, the probable anagenetic ancestor of Au. afarensis (27), despite evidence from occlusal microwear that the mechanical properties of foods were essentially the same in these two australopith species (38–41). In contrast, virtually all hominins coeval with and younger than Au. afarensis consumed substantial but variable amounts of C4/CAM foods. Regardless of whether the sampled isolated teeth attributed to Kenyanthropus (27) can ultimately be distinguished morphologically from teeth of Au. afarensis, their broad range of nominal C4/CAM intake reinforces our inference of an expansion of the hominin dietary niche by ca. 3.4 Ma. We have no data to address the C4/CAM consumption patterns of hominins between the youngest C3-focused Au. anamensis specimens and the later C4/CAM-consuming australopiths, but this gap may ultimately be filled by analysis of additional material from Laetoli, Woranso-Mille, and other localities that span this range (42, 43). Among the late Pliocene robust australopiths, P. robustus from South Africa retained a range of C4/CAM intake similar to the range in Au. afarensis, Au. africanus, and Kenyanthropus, whereas P. boisei from eastern Africa apparently specialized on a narrow range of C4/CAM foods (31). A potential exception to this late Pliocene focus on C4/CAM foods is Au. sediba from Malapa, South Africa, at ca. 2 Ma, which hints at an entirely C3 diet, but the small sample size (n = 2) dictates a cautious interpretation of these data (33).
Temporal Patterns in C4/CAM Plant Use Through the Hadar Formation.
Using high-resolution pollen data from Hadar, Bonnefille et al. (17) suggested that Au. afarensis was adapted to variable environmental conditions between 3.4 and 2.9 Ma. Our stable isotope data allow us to explore this idea by examining whether this species adjusted its plant food intake in response to environmental changes in the Hadar Formation. Increased C4/CAM use over time by Au. afarensis might be expected given evidence of faunal change and increased aridity [Kada Hadar Member level KH-2 (22, 23)]. Our data, however, reveal no shift in δ13C values over time. The entire range of C4/CAM consumption, from pure C3 to high C4, can be found within the Au. afarenis sample from any single stratigraphic member. Because a range of environments (from woodlands to bushland and edaphic grasslands) is represented by the sampled submembers (22, 44, 45), the stable isotopic variation of Au. afarensis also occurs across this range of reconstructed paleoenvironments. This observation suggests that fluctuating environmental conditions did not perceptibly influence the C4/CAM consumption patterns of Au. afarensis in the Hadar Formation and that Au. afarensis expanded its diet to exploit a range of viable resources, despite habitat variation (Fig. 2).
The lack of a temporal pattern in the isotopic composition of the diet of Au. afarensis is consistent with similar findings from occlusal microwear (39), which show little variation in Au. afarensis over time. In contrast to the homogeneity of the dental microwear pattern, however, the range of variation in the carbon isotopic composition of Au. afarensis is wide, even within the temporally brief stratigraphic subdivisions of the Hadar Formation (Fig. 2). Assuming that occlusal microwear provides direct insight into the mechanical properties of food consumed (but see ref. 41), these opposing patterns suggest use of foods that varied little in terms of these mechanical properties but differed substantially in photosynthetic pathway (for example, mixtures of C3 plants and semiaquatic C4 plants such as sedges). In any case, taken together, the dietary isotopic and microwear data imply that Au. afarensis in the Hadar Formation exploited a similar range of plant foods in the face of local environmental fluctuation over time.
Paleoecology of Au. afarensis.
Although early australopiths at about 3.4 Ma may be the earliest hominins to expand the dietary repertoire to include perceptible amounts of C4 resources in their diet, we have little insight as to what specific C4/CAM foods were consumed. Based on present data, a reasonable conclusion is that Au. afarensis was a generalist omnivore consuming isotopically varied resources at a variety of spatial (e.g., microhabitat) and temporal (e.g., inter- or intraannual) scales. C4/CAM foods consumed might have included grass seeds and roots seasonally, sedge underground storage organs at other times, termites, succulent CAM plants, and even small game or scavenged carcasses [as suggested by modified bones associated with Au. afarensis fossils from Dikika (46)]; all are possibilities consistent with the existing data. Narrowing the range of possibilities will require independent evidence from trace element chemistry, dental calculus, and other sources.
Despite this uncertainty, the stable carbon isotopic results suggest much about the ecology of Au. afarensis. Throughout its stratigraphic range, Au. afarensis has been found alongside faunal, paleobotanical, and paleosol evidence indicating a spectrum of savanna mosaic environments ranging from relatively closed woodlands to open wetlands and edaphic grasslands (17, 22, 44, 47, 48). It has been a challenge to pinpoint the species’ preferred habitats within such a broad paleoenvironmental spectrum. Stable carbon isotope data provide direct evidence that Au. afarensis consumed C4 foods and thus, likely exploited resources in areas with grassy understories and/or water-edge microhabitats, both of which provide ample opportunity for C4 food consumption. Interestingly, savanna chimpanzees, which are found in such areas today, do not measurably consume the abundant C4 resources available to them. Thus, the remarkably wide range of C3 to C4 diets of by Au. afarensis across a temporally fluctuating environmental mosaic supports the inference of a generalist hominoid primate that exploited a broad range of habitats for food resources (1, 22).
Although highly variable, the range of δ13C values for Au. afarensis does not incorporate the modal value from the other macromammalian fauna sampled from the Hadar Formation, indicating a diet that was less C4-focused than the diet of the abundant representatives of relatively open habitats from the Hadar region’s paleoenvironmental spectrum (Fig. 3). Clearly, the dietary ecology of Au. afarensis differed from the dietary ecology of specialist taxa, such as Giraffa and alcelaphini, but it is also apparent that Au. afarensis occupied a relatively underrepresented dietary niche compared with the majority of taxa in the Hadar mammalian community (Figs. 3 and 4).
Likewise, the δ18O values of Au. afarensis show that this species was distinct from many contemporaneous taxa in terms of drinking habits and/or other factors affecting oxygen isotopic composition (34). Fig. 4 suggests that Au. afarensis was intermediate in its degree of dependence on drinking water between the end members of water-independent giraffids and the water-dependent hippopotamids.
The question remains of what led Au. afarensis to consume C4/CAM foods, whereas its likely ancestor, Au. anamensis, largely avoided C4/CAM foods, despite the fact that both species inhabited similar mosaics of savanna habitats (48). Although the broad range of Au. afarensis δ13C values might reflect dietary change in response to shifting habitat distributions, these habitat shifts may have occurred at spatial and/or temporal scales too fine to be resolved with currently available data. If such a relationship does exist, then it may ultimately be possible to characterize the relationship between paleoenvironment and diet with more comprehensive sampling of both taxa from a broader temporal and/or habitat range. The slightly older sample of Au. afarensis from Laetoli may provide such an additional test, given the evidence for relatively open paleoenvironements (48). However, such a quest may also prove to be a red herring because a species’ dietary preference is not necessarily coupled to the most common habitats within the local environment. Chimpanzees offer an example of a modern analog to this potential disconnect between diet and environment: savanna chimpanzees live in relatively open habitats among abundant C4/CAM plants, whereas forest-dwelling chimpanzees do not, but both use almost exclusively C3 foods. If we were to attempt to reconcile such a paradox using only the slice of data available from the fossil record, even with extensive datasets, presently available methods would likely fail.
Denthognathic Morphology and the Advent of C4 Diet in Hominins.
Our research shows a diet for Au. afarensis that includes significant consumption of C4 resources, which has implications for our understanding of the links between hominin morphology and diet. The influential dietary hypothesis of the 1950s by Robinson (49, 50) proposed two morphologically distinct groups of early hominins from southern Africa (the subsequently branded gracile and robust australopiths) with strongly divergent dietary adaptations. Later discoveries of Au. afarensis beginning in the 1970s clarified that all Pliocene hominins morphologically expressed a “heavy chewing” signal to one degree or another (51). Au. afarensis (3.7–3.0 Ma) and its likely ancestor Au. anamensis (4.2–3.9 Ma) are the earliest hominins known to exhibit dentognathic specializations conventionally associated with mastication of mechanically resistant food items, including relatively large, thickly enameled cheek teeth, heavily built jaws, and rugose chewing muscle attachment sites (19, 52). Morphological evidence for Pliocene hominin diets suggests that the australopith lineage was marked by increasing reliance on hard and brittle food items, such as nuts and seeds, or foods with abrasive particles, such as underground storage organs (3, 4, 19, 20, 52). Meanwhile, Grine et al. (39) and Ungar et al. (53) found that Au. afarensis shows dental microwear that is dissimilar to dental microwear of known hard-object feeding primates, such as the extant mangabey (Lophocebus), and likewise, dissimilar to dental microwear of the fossil hominin P. robustus, interpreted to be a hard-object feeder. Furthermore, despite the morphological similarity of Au. afarensis and Au. anamensis, isotopic data from the latter species lack evidence of C4/CAM plant food intake (27). Although the mechanical properties of food items cannot be deciphered from isotopic data, the fact that the occlusal microwear of these two species is essentially the same, yet different from diets of known or inferred primate hard-object feeders (38, 54) implies that the links between early hominin diet (what was eaten) and the various sources of insight on dietary behavior (how food was acquired and consumed) are not as straightforward as it once seemed (55). Recent work has also underscored the importance of distinguishing the abrasion of teeth by nondietary grit from the abrasion produced by food particles (1, 41, 56). Thus, our results caution against dietary behavioral reconstructions that rely on the primacy of any single method or approach.
Conclusions
Carbon isotopic data from Au. afarensis within the Hadar Formation show that this species consumed significant amounts of relatively 13C-enriched foods (i.e., from C4/CAM plants and/or animal foods derived from such plants). Furthermore, the amount of relatively 13C-enriched food intake within the sample is highly variable, even within narrow stratigraphic intervals or paleoenvironmental settings. The two known hominin taxa antecedent to Au. afarensis with comparable stable isotopic data (Ardipithecus and Au. anamensis) are, like Au. afarensis, sampled from environments characterized by abundant C4/CAM vegetation (47), but neither apparently engaged in significant C4/CAM food consumption in their environments. In light of this newly expanded hominin stable isotopic dataset (36), Au. afarensis documents a transformational stage in our ecological history, during which hominins in eastern Africa began to expand their dietary resource base to include C4/CAM foods that had been abundant in their environments for at least 1 million y. Within the presumptive Au. anamensis–Au. afarensis lineage of eastern African australopiths (57), the C4/CAM dietary expansion is clearly demarcated by the contrast between the data from Kanapoi and Allia Bay (4.1–3.9 Ma) (27) and the data from the Hadar Formation (3.4 Ma–2.9 Ma; this work). The detailed portrait of this transition and its causes remains to be filled in with data from australopith-bearing sites in the intervening time period.
The stable isotopic data show that middle Pliocene Au. afarensis and Au. africanus consumed widely varying mixtures of C3 and C4 foods. This dietary flexibility implies unique landscape use patterns and malleable foraging behavior within a narrow time frame of a single species. Subsequent reduction of dietary isotopic variation in late Pliocene Paranthropus and early Homo (36) may be associated with terminal Pliocene aridification and concomitant narrowing of terrestrial habitats (58). The middle Pliocene C4/CAM dietary expansion may also have set the stage for dietary differentiation between these hominins, which differed markedly in their consumption of C4/CAM-derived foods.
Methods
Samples were drilled from specimens housed in the National Museum of Ethiopia using a high-speed drill and diamond-encrusted drill bits. Up to ∼2 mg powdered enamel were collected entirely from fractured surfaces of enamel, and therefore, future morphological or surface wear studies were not compromised (detailed photographs of before and after sample collection are in SI Appendix). All samples were treated to remove exogenous carbonate. Samples were soaked in 0.1 M buffered acetic acid for 30 min, rinsed with milli-Q water, and dried overnight at 60 °C. Approximately 0.6 mg of pretreated sample were reacted with 103% (vol/vol) phosphoric acid at 25 °C in 4.5-mL Labco exetainers preflushed with ultrapure helium. The isotopic composition of the CO2 resulting from this reaction was analyzed on a Thermo Finnigan Delta V isotope ratio mass spectrometer coupled to a Gasbench II preparation device. CO2 was separated from other trace gases by gas chromatography, and 10 replicate samples of each CO2 sample were analyzed. The isotopic composition of tooth enamel was normalized with respect to two isotopic reference materials (an internal sample of Carrara Marble with δ13C = 2.01‰ and δ18O = −1.79‰ and National Bureau of Standards (NBS)-18 with δ13C = −5.04‰ and δ18O = −23.05‰). Replicate measurements of 10 samples of each reference material for this sequence had errors <0.15‰ for δ13C and <0.20‰ for δ18O. A selection of other specimens (10 Giraffa and 10 alcelaphini) was analyzed in the same sequence as the hominin specimens under the same conditions. Other analyses shown here are part of a larger dataset from the Hadar Formation, and they were analyzed under slightly different conditions that allow for larger sample mass (∼2 mg sample in 12-mL exetainers). Sample yields (percent CaCO3) were calculated using the integrated peak area of voltage measured on mass 44 using the calcite standards Carrara Marble and NBS-18 as a reference of 100% CaCO3. Nominal percent C4 dietary intake was calculated using end member values of pure C4 and pure C3 diets of +1.4‰ and −10.9‰, respectively (the values of end member C4-grazing and C3-browsing taxa alcelaphini and Giraffa from this dataset). Comparisons of fossil samples with modern specimens are corrected to the year 1750 for the effect of burning of fossil fuels (28) by adding +1.5‰ to the modern δ13C value or using similarly corrected values in published data. Comparisons of δ13C values of hair and tooth enamel are made using the information in ref. 59. Statistical analyses were performed using Matlab R2008b using Mann–Whitney U and Kruskal–Wallis tests (60). Kruskal–Wallis tests were adjusted for unequal sample size and reported using P values of the χ2 statistic. A significance level of α = 0.05 was used throughout.
Supplementary Material
Acknowledgments
We thank the Authority for Research and Conservation of Cultural Heritage, the National Museum of Ethiopia, the Ethiopian Ministry of Culture and Tourism, for permission to undertake this study. Research was funded by National Science Foundation Grant BCS1064030.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 10470.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1222559110/-/DCSupplemental.
References
- 1.Grine FE, Sponheimer M, Ungar PS, Lee-Thorp JA, Teaford MF. Dental microwear and stable isotopes inform the paleoecology of extinct hominins. Am J Phys Anthropol. 2012;148(2):285–317. doi: 10.1002/ajpa.22086. [DOI] [PubMed] [Google Scholar]
- 2.Lee-Thorp JA, Sponheimer M, Passey BH, de Ruiter DJ, Cerling TE. Stable isotopes in fossil hominin tooth enamel suggest a fundamental dietary shift in the Pliocene. Philos Trans R Soc Lond B Biol Sci. 2010;365(1556):3389–3396. doi: 10.1098/rstb.2010.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strait DS, et al. The feeding biomechanics and dietary ecology of Australopithecus africanus. Proc Natl Acad Sci USA. 2009;106(7):2124–2129. doi: 10.1073/pnas.0808730106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teaford MF, Ungar PS. Diet and the evolution of the earliest human ancestors. Proc Natl Acad Sci USA. 2000;97(25):13506–13511. doi: 10.1073/pnas.260368897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White TD, et al. Macrovertebrate paleontology and the Pliocene habitat of Ardipithecus ramidus. Science. 2009;326(5949):87–93. [PubMed] [Google Scholar]
- 6.Sponheimer M, Lee-Thorp JA. Isotopic evidence for the diet of an early hominid, Australopithecus africanus. Science. 1999;283(5400):368–370. doi: 10.1126/science.283.5400.368. [DOI] [PubMed] [Google Scholar]
- 7.Sponheimer M, et al. Hominins, sedges and termites: New carbon isotope date from the Sterkfontein Valley. J Hum Evol. 2005;48:301–312. doi: 10.1016/j.jhevol.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 8.van der Merwe NJ, Thackeray JF, Lee-Thorp JA, Luyt J. The carbon isotope ecology and diet of Australopithecus africanus at Sterkfontein, South Africa. J Hum Evol. 2003;44(5):581–597. doi: 10.1016/s0047-2484(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 9.Lee-Thorp JA, et al. Isotopic evidence for an early shift to C₄ resources by Pliocene hominins in Chad. Proc Natl Acad Sci USA. 2012;109(50):20369–20372. doi: 10.1073/pnas.1204209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerling TE, et al. Global vegetation change through the Miocene/Pleistocene boundary. Nature. 1997;389(6647):153–158. [Google Scholar]
- 11.Cerling TE, Harris JM, Passey BH. Diets of east African bovidae based on stable isotope analysis. J Mammal. 2003;84(2):456–470. [Google Scholar]
- 12.Cerling TE, Harris JM, Leakey MG. Browsing and grazing in elephants: The isotope record of modern and fossil proboscideans. Oecologia. 1999;120(3):364–374. doi: 10.1007/s004420050869. [DOI] [PubMed] [Google Scholar]
- 13.Lee-Thorp JA, van der Merwe NJ, Brain CK. Diet of Australopithecus robustus at Swartkrans from stable carbon isotopic analysis. J Hum Evol. 1994;27(4):361–372. [Google Scholar]
- 14.Sponheimer M, et al. Using carbon isotopes to track dietary change in modern, historical, and ancient primates. Am J Phys Anthropol. 2009;140(4):661–670. doi: 10.1002/ajpa.21111. [DOI] [PubMed] [Google Scholar]
- 15.Sponheimer M, Lee-Thorp JA. Using carbon isotope data from fossil bovid communities to provide paleoenvironmental information. S Afr J Sci. 2003;99(5–6):273–275. [Google Scholar]
- 16.Alemseged Z, et al. A new hominin from the Basal Member of the Hadar Formation, Dikika, Ethiopia, and its geological context. J Hum Evol. 2005;49(4):499–514. doi: 10.1016/j.jhevol.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Bonnefille R, Potts R, Chalié F, Jolly D, Peyron O. High-resolution vegetation and climate change associated with Pliocene Australopithecus afarensis. Proc Natl Acad Sci USA. 2004;101(33):12125–12129. doi: 10.1073/pnas.0401709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hailemichael M, Aronson JL, Savin S, Tevesz MJS, Carter JG. δ18O in mollusk shells from Pliocene Lake Hadar and modern Ethiopian lakes: Implications for history of the Ethiopian monsoon. Palaeogeogr Palaeoclimatol Palaeoecol. 2002;186(1):81–99. [Google Scholar]
- 19.Kimbel WH, Delezene LK. “Lucy” redux: A review of research on Australopithecus afarensis. Am J Phys Anthropol. 2009;140(Suppl 49):2–48. doi: 10.1002/ajpa.21183. [DOI] [PubMed] [Google Scholar]
- 20.Kimbel WH, Rak Y, Johanson DC. The Skull of Australopithecus afarensis. New York: Oxford Univ Press; 2004. [Google Scholar]
- 21.Quade J, Wynn JG, editors. The Geological Context of Human Evolution in the Horn of Africa. Boulder, CO: Geological Society of America; 2008. [Google Scholar]
- 22.Reed KE. Paleoecological patterns at the Hadar hominin site, Afar Regional State, Ethiopia. J Hum Evol. 2008;54(6):743–768. doi: 10.1016/j.jhevol.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Lockwood CA, Kimbel WH, Johanson DC. Temporal trends and metric variation in the mandibles and dentition of Australopithecus afarensis. J Hum Evol. 2000;39(1):23–55. doi: 10.1006/jhev.2000.0401. [DOI] [PubMed] [Google Scholar]
- 24.Schoeninger MJ, Moore J, Sept JM. Subsistence strategies of two “savanna” chimpanzee populations: The stable isotope evidence. Am J Primatol. 1999;49(4):297–314. doi: 10.1002/(SICI)1098-2345(199912)49:4<297::AID-AJP2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 25.Smith CC, Morgan ME, Pilbeam D. Isotopic ecology and dietary profiles of Liberian chimpanzees. J Hum Evol. 2010;58(1):43–55. doi: 10.1016/j.jhevol.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Sponheimer M, et al. Do “savanna” chimpanzees consume C4 resources? J Hum Evol. 2006;51(2):128–133. doi: 10.1016/j.jhevol.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Cerling TE, et al. Stable isotope-based diet reconstructions of Turkana Basin hominins. Proc Natl Acad Sci USA. 2013;110:10501–10506. doi: 10.1073/pnas.1222568110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedli H, Lotscher H, Oeschger H, Siegenthaler U, Stauffer B. Ice core records of the 13C/12C ratio of atmospheric CO2 in the past two centuries. Nature. 1986;324(6094):237–238. [Google Scholar]
- 29.Lee-Thorp JA, Thackeray JF, van der Merwe NJ. The hunters and the hunted revisited. J Hum Evol. 2000;39(6):565–576. doi: 10.1006/jhev.2000.0436. [DOI] [PubMed] [Google Scholar]
- 30.Sponheimer M, et al. Isotopic evidence for dietary variability in the early hominin Paranthropus robustus. Science. 2006;314(5801):980–982. doi: 10.1126/science.1133827. [DOI] [PubMed] [Google Scholar]
- 31.Cerling TE, et al. Diet of Paranthropus boisei in the early Pleistocene of East Africa. Proc Natl Acad Sci USA. 2011;108(23):9337–9341. doi: 10.1073/pnas.1104627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Merwe NJ, Masao FT, Bamford MK. Isotopic evidence for contrasting diets of early hominins Homo habilis and Australopithecus boisei of Tanzania. S Afr J Sci. 2008;104(3–4):153–155. [Google Scholar]
- 33.Henry AG, et al. The diet of Australopithecus sediba. Nature. 2012;487(7405):90–93. doi: 10.1038/nature11185. [DOI] [PubMed] [Google Scholar]
- 34.Levin NE, Cerling TE, Passey BH, Harris JM, Ehleringer JR. A stable isotope aridity index for terrestrial environments. Proc Natl Acad Sci USA. 2006;103(30):11201–11205. doi: 10.1073/pnas.0604719103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Levin NE, et al. (2008) Herbivore enamel carbon isotopic composition and the environmental context of Ardipithecus at Gona, Ethiopia. The Geology of Early Humans in the Horn of Africa, Geological Society of America Special Paper, eds Quade J, Wynn JG (Geological Society of America, Boulder, CO), Vol 446, pp 215–234.
- 36.Sponheimer M, et al. Isotopic evidence of early hominin diets. Proc Natl Acad Sci USA. 2013;110:10513–10518. [Google Scholar]
- 37.Lebatard A-E, et al. Cosmogenic nuclide dating of Sahelanthropus tchadensis and Australopithecus bahrelghazali: Mio-Pliocene hominids from Chad. Proc Natl Acad Sci USA. 2008;105(9):3226–3231. doi: 10.1073/pnas.0708015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grine FE, Ungar PS, Teaford MF. Was the early Pliocene hominin ‘Australopithecus’ anamensis a hard object feeder? S Afr J Sci. 2006;102(7–8):301–310. [Google Scholar]
- 39.Grine FE, Ungar PS, Teaford MF, El-Zaatari S. Molar microwear in Praeanthropus afarensis: Evidence for dietary stasis through time and under diverse paleoecological conditions. J Hum Evol. 2006;51(3):297–319. doi: 10.1016/j.jhevol.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Estebaranz F, Martínez LM, Galbany J, Turbón D, Pérez-Pérez A. Testing hypotheses of dietary reconstruction from buccal dental microwear in Australopithecus afarensis. J Hum Evol. 2009;57(6):739–750. doi: 10.1016/j.jhevol.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Lucas PW, et al. Mechanisms and causes of wear in tooth enamel: Implications for hominin diets. J R Soc Interface. 2013;10(80):20120923. doi: 10.1098/rsif.2012.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haile-Selassie Y. Phylogeny of early Australopithecus: New fossil evidence from the Woranso-Mille (central Afar, Ethiopia) Philos Trans R Soc Lond B Biol Sci. 2010;365(1556):3323–3331. doi: 10.1098/rstb.2010.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrison T, editor. Paleontology and Geology of Laetoli: Human Evolution in Context. Berlin: Springer; 2011. [Google Scholar]
- 44.Bedaso ZK, Wynn JG, Alemseged Z, Geraads D. Dietary and paleoenvironmental reconstruction using stable isotopes of herbivore tooth enamel from middle Pliocene Dikika, Ethiopia: Implication for Australopithecus afarensis habitat and food resources. J Hum Evol. 2013;64(1):21–38. doi: 10.1016/j.jhevol.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 45. Campisano CJ, Feibel CS (2008) Depositional environments and stratigraphic summary of the Pliocene Hadar Formation at Hadar, Afar Depression, Ethiopia. The Geology of Early Humans in the Horn of Africa, Geological Society of America Special Paper, eds Quade J, Wynn JG (Geological Society of America, Boulder, CO), Vol 446, pp 179–201.
- 46.McPherron SP, et al. Evidence for stone-tool-assisted consumption of animal tissues before 3.39 million years ago at Dikika, Ethiopia. Nature. 2010;466(7308):857–860. doi: 10.1038/nature09248. [DOI] [PubMed] [Google Scholar]
- 47.Cerling TE, et al. Woody cover and hominin environments in the past 6 million years. Nature. 2011;476(7358):51–56. doi: 10.1038/nature10306. [DOI] [PubMed] [Google Scholar]
- 48.Su DF, Harrison T. In: The Paleoecology of the Upper Laetolil Beds at Laetoli. Hominin Environments in the East African Pliocene: An Assessment of the Faunal Evidence. Bobe R, Alemseged Z, Behrensmeyer AK, editors. Berlin: Springer; 2007. pp. 279–313. [Google Scholar]
- 49.Robinson JT. Prehominid dentition and hominid evolution. Evolution. 1954;8(4):324–334. [Google Scholar]
- 50.Robinson JT. In: The Origins and Adaptive Radiation of the Australopithecines. Evolution and Hominisation. Kurth G, editor. Stuttgart: Fischer; 1962. pp. 150–175. [Google Scholar]
- 51.Wolpoff MH. Posterior tooth size, body size, and diet in South African gracile Australopithecines. Am J Phys Anthropol. 1973;39(3):375–393. doi: 10.1002/ajpa.1330390306. [DOI] [PubMed] [Google Scholar]
- 52.White TD, et al. Asa Issie, Aramis and the origin of Australopithecus. Nature. 2006;440(7086):883–889. doi: 10.1038/nature04629. [DOI] [PubMed] [Google Scholar]
- 53.Ungar PS, Grine FE, Teaford MF. Dental microwear and diet of the Plio-Pleistocene hominin Paranthropus boisei. PLoS One. 2008;3(4):e2044. doi: 10.1371/journal.pone.0002044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ungar PS, Scott RS, Grine FE, Teaford MF. Philosophical Transactions of the Royal Society B Molar microwear textures and the diets of Australopithecus anamensis and Australopithecus afarensis. Philos Trans R Soc Lond B Biol Sci. 2010;365(1556):3345–3354. doi: 10.1098/rstb.2010.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ross CF, Iriarte-Diaz J, Nunn CL. Innovative approaches to the relationship between diet and mandibular morphology in primates. Int J Primatol. 2012;33(3):632–660. [Google Scholar]
- 56.Daegling DJ, Grine FE. Terrestrial foraging and dental microwear in Papio ursinus. Primates. 1999;40(4):559–572. [Google Scholar]
- 57.Kimbel WH, et al. Was Australopithecus anamensis ancestral to A. afarensis? A case of anagenesis in the hominin fossil record. J Hum Evol. 2006;51(2):134–152. doi: 10.1016/j.jhevol.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 58.deMenocal PB. Anthropology. Climate and human evolution. Science. 2011;331(6017):540–542. doi: 10.1126/science.1190683. [DOI] [PubMed] [Google Scholar]
- 59.Passey BH, Robinson TF, Ayliffe LK, Cerling TE. Carbon isotope fractionation between diet, breath CO2 and bioapatite in different mammals. J Archaeol Sci. 2005;32(10):1459–1470. [Google Scholar]
- 60. Trujillo-Ortiz A, Hernandez-Walls R (2003) KWtest: Kruskal–Wallis' Nonparametric Analysis of Variance. A MATLAB File. Available at www.mathworks.com/matlabcentral/fileexchange/3361-kwtest/content/kwtest.html. Accessed May 10, 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.