Abstract
The base excision repair pathway is largely responsible for the repair of oxidative stress-induced DNA damage. However, it remains unclear how the DNA damage checkpoint is activated by oxidative stress at the molecular level. Here, we provide evidence showing that hydrogen peroxide (H2O2) triggers checkpoint kinase 1 (Chk1) phosphorylation in an ATR [ataxia-telangiectasia mutated (ATM) and Rad3-related]-dependent but ATM-independent manner in Xenopus egg extracts. A base excision repair protein, Apurinic/apyrimidinic (AP) endonuclease 2 (APE2, APN2, or APEX2), is required for the generation of replication protein A (RPA)-bound single-stranded DNA, the recruitment of a checkpoint protein complex [ATR, ATR-interacting protein (ATRIP), and Rad9] to damage sites, and H2O2-induced Chk1 phosphorylation. A conserved proliferating cell nuclear antigen interaction protein box of APE2 is important for the recruitment of APE2 to H2O2-damaged chromatin. APE2 3′-phosphodiesterase and 3′-5′ exonuclease activity is essential for single-stranded DNA generation in the 3′–5′ direction from single-stranded breaks, referred to as single-stranded break end resection. In addition, APE2 associates with Chk1, and a serine residue (S86) in the Chk1-binding motif of APE2 is essential for Chk1 phosphorylation, indicating a Claspin-like but distinct role for APE2 in ATR-Chk1 signaling. Our data indicate that APE2 plays a vital and previously unexpected role in ATR-Chk1 checkpoint signaling in response to oxidative stress. Thus, our findings shed light on a distinct mechanism of how an ATR-Chk1–dependent DNA damage checkpoint is mediated by APE2 in the oxidative stress response.
Cells are constantly challenged by exogenous and endogenous insults that threaten genomic integrity. Excess accumulation of reactive oxygen species leads to oxidative DNA damage, such as DNA strand breaks with 3′-modified termini, which is often the underlying pathology in a variety of diseases including neurodegenerative diseases and cancer (1–6). Cellular responses to DNA damage are mainly coordinated by two distinct DNA damage checkpoint signaling cascades: ATM (ataxia-telangiectasia mutated)-checkpoint kinase 2 (Chk2) and ATR (ATM and Rad3-related)-checkpoint kinase 1 (Chk1) pathways (7–10). ATM is activated by intermolecular autophosphorylation and dimer dissociation in response to double-stranded beaks (DSBs) (11–13). ATR is activated by primed single-stranded DNA (ssDNA) in response to a variety of DNA damage or replication stresses (14, 15). Oxidative stress has been demonstrated to activate an ATM-dependent DNA damage checkpoint (16–18). However, in previous studies, hyperoxic conditions resulted in the phosphorylation of Chk1 and p53 in an ATR-dependent but ATM-independent fashion (19). Furthermore, it remains unclear which specific DNA structures trigger checkpoint signaling during oxidative stress.
To eliminate oxidative DNA damage, base excision repair (BER) has evolved as a major DNA damage repair mechanism (20). In the initial step of BER, oxidatively damaged bases are excised by DNA glycosylases, generating apurinic/apyrimidinic (AP) sites. An incision at the AP site by AP endonuclease or AP lyase generates a single-stranded break (SSB) (21). Subsequently, the SSB is fixed via collaboration between proliferating cell nuclear antigen (PCNA), DNA polymerase β, replication factor C, flap endonuclease 1 (FEN1), and DNA ligase I (22). APE1 (AP endonuclease 1) and APE2 (AP endonuclease 2) are the two characterized AP endonucleases (23, 24). APE2, which has weak AP endonuclease activity and strong 3′-phosphodiesterase and 3′-5′ exonuclease activities, is a key player in PCNA-dependent repair of hydrogen peroxide (H2O2)-induced oxidative DNA damage (25–27). However, the biological significance of APE2 in the DNA damage response has not been elucidated. As far as we know, it remains elusive whether the DNA damage checkpoint and BER pathways are coordinated in cellular responses to oxidative stress.
We performed a series of studies in checkpoint signaling using Xenopus egg extracts, a well-characterized cell-free model system (28–32). Here, we provide evidence that suggests APE2 is required for ATR-Chk1 checkpoint activation in response to oxidative stress. Molecular characterization of the underlying mechanisms of APE2 has shed light on two distinct roles for APE2 in the ATR-Chk1 checkpoint: ssDNA generation via 3′-5′ SSB end resection and Claspin-like Chk1 binding. These roles of APE2 in the ATR-Chk1 checkpoint will help us better understand the DNA damage response after oxidative stress and provide a clear connection between the BER pathway and DNA damage checkpoint signaling.
Results
Hydrogen Peroxide Induces ATR-Dependent but ATM-Independent Chk1 Phosphorylation in Xenopus Egg Extracts.
To study oxidative stress, we first tested whether H2O2 triggers a checkpoint response in Xenopus egg extracts. Chk1 phosphorylation at serine 344 (Chk1 P-S344) is an indicator of ATR activation (33, 34). Although lower concentrations of H2O2 (1 and 10 mM) triggered relatively weak Chk1 phosphorylation (Fig. 1A), we consistently observed Chk1 P-S344 at 100 mM H2O2. Chk1 phosphorylation occurred at 40, 60, and 80 min, but not at 20 min (Fig. 1B). These data suggest that H2O2 induces a checkpoint response in a dose- and time-dependent manner. ATM specific inhibitor KU55933 inhibited H2O2-induced ATM phosphorylation at serine 1981 (ATM P-S1981), but not Chk1 phosphorylation (Fig. 1C). In addition, both ATM phosphorylation and Chk1 phosphorylation were compromised by caffeine addition. This evidence suggests that H2O2-induced Chk1 phosphorylation is ATR-dependent, but ATM-independent. To directly test whether ATR is required for H2O2-induced Chk1 phosphorylation, we removed ATR-interacting protein (ATRIP) from egg extract by immunodepletion (Fig. 1D). ATRIP antibodies codepleted ATR, consistent with a previous study (35). H2O2-induced Chk1 phosphorylation was compromised in ATRIP-depleted egg extract (Fig. 1D). Both ATR and ATRIP were recruited to H2O2-damaged chromatin in mock-depleted egg extracts, and as expected, ATR and ATRIP recruitment to H2O2-damaged chromatin was compromised in ATRIP-depleted egg extracts (Fig. 1D). In addition, H2O2 induced replication protein A 32 (RPA32) phosphorylation at serine 33 (RPA32 P-S33) (Fig. S1), which also indicates ATR activation (36). Taken together, these data suggest that H2O2 triggers Chk1 phosphorylation in Xenopus egg extracts in an ATR-dependent but ATM-independent fashion.
Fig. 1.
Hydrogen peroxide triggers an ATR-dependent, but ATM-independent, Chk1 phosphorylation in Xenopus egg extracts. (A) Sperm chromatin was added to Xenopus egg extracts supplemented with different concentrations of H2O2, as indicated. Chk1 phosphorylation at S344 and total Chk1 were examined via immunoblotting. (B) Sperm chromatin was added to egg extracts with the presence or absence of H2O2 (100 mM). At different times, as indicated, samples were collected and examined as in A. (C) KU55933 or caffeine was incubated with egg extracts, followed by the addition of sperm chromatin and H2O2. Samples were examined for the indicated proteins. (D) Sperm chromatin was added to mock-depleted or ATRIP-depleted egg extracts supplemented with H2O2. Chromatin-bound fractions (“chromatin” panel) and total egg extracts (“extract” panel) were analyzed for the indicated proteins via immunoblotting.
APE2 Is Required for Chk1 Phosphorylation Induced by Hydrogen Peroxide but Not by Stalled Replication Forks.
Domain dissection of APE2 shows three highly conserved domains: an AP endonuclease domain for its enzymatic activities on the N terminus (aa 2–307), a zinc finger domain on the C terminus (aa 461–508), and a PCNA interacting protein (PIP) box (aa 395–402) (Fig. 2A). To study the role of APE2, we expressed and purified recombinant GST-APE2 protein from Escherichia coli, which was used for custom antibody production in rabbits (Fig. S2 A and B). Our anti-APE2 antibodies recognized endogenous APE2 (∼65 kD) and recombinant Myc-tagged-APE2 (Myc-APE2) (∼75 kD) and efficiently removed endogenous APE2 by immunodepletion from egg extracts (Fig. S2C). Notably, APE2 was preferentially recruited to H2O2-damaged chromatin at 40 min (Fig. 2B), suggesting a possible hit-and-run mechanism of APE2 recruitment to damage sites. It was also noticed that tiny APE2 was recruited to chromatin at 20 and 80 min in unperturbed samples, which may suggest that a low amount of possible endogenous DNA damage can be recognized by APE2 under “normal” conditions. However, this tiny recruitment of APE2 did not trigger Chk1 phosphorylation (Fig. 1B), suggesting that a possible threshold of accumulated DNA damage may be needed for a robust checkpoint signal. Similarly, recombinant Myc-APE2 also showed the preferential binding to H2O2-damaged chromatin (Fig. 2C).
Fig. 2.
APE2 preferentially binds to hydrogen peroxide-damaged chromatin and is required for hydrogen peroxide-induced Chk1 phosphorylation. (A) Schematic diagram of APE2. (B) Sperm chromatin was added to egg extracts supplemented with H2O2. At different times, chromatin-bound fractions and total egg extracts were examined for the indicated proteins. (C) Recombinant Myc-APE2 was added to egg extracts supplemented with sperm chromatin and H2O2. Chromatin-bound fractions and total egg extracts were analyzed at 40 min via immunoblotting. (D) Sperm chromatin was added to mock- or APE2-depleted egg extracts supplemented with recombinant Myc-APE2 and H2O2 or aphidicolin (APX), as indicated. *, nonspecific bands in lanes 1–6 and an overlapping band of the nonspecific band and Myc-APE2 in lanes 7–9. Endo. APE2, endogenous APE2 in egg extracts. (E) Sperm chromatin was added to mock- or APE2-depleted egg extracts supplemented with recombinant Myc-APE2 and H2O2, as indicated. Samples were examined for the indicated proteins from chromatin fraction or extract.
To directly test the role of APE2 in DNA damage checkpoint signaling, we added sperm chromatin to either mock- or APE2-depleted egg extracts supplemented with H2O2. H2O2-induced Chk1 phosphorylation was compromised in APE2-depleted egg extract (Fig. 2D). Adding back recombinant Myc-APE2 to APE2-depleted egg extracts rescued H2O2-induced Chk1 phosphorylation (Fig. 2D). Therefore, we conclude that APE2 is required for H2O2-induced Chk1 phosphorylation. To test whether APE2 is required for Chk1 phosphorylation induced by stalled DNA replication forks, we investigated the effect of aphidicolin as a DNA replication stressor. As shown in Fig. 2D, APE2 was dispensable for aphidicolin-induced Chk1 phosphorylation, suggesting that the molecular mechanism of APE2 for the ATR-Chk1 checkpoint in oxidative stress is not tied to the uncoupling of minichromosome maintenance (MCM) helicase and DNA polymerase activities.
The assembly of DNA damage checkpoint proteins at DNA breaks is vital to triggering checkpoint signaling (37). To better understand the underlying molecular mechanism, we reasoned that APE2 might be required for the recruitment of ATR and ATRIP to damaged chromatin. H2O2-induced recruitment of ATR and ATRIP was indeed compromised in APE2-depleted egg extracts (Fig. 2E). Replication protein A (RPA) binds to ssDNA and is required for ATRIP-mediated ATR recruitment to stalled replication forks or damage sites (38–40). RPA hyperloading to damaged chromatin indirectly indicates the generation of ssDNA (41, 42). RPA32, the second largest subunit of the heterotrimeric RPA complex, was hyperloaded to H2O2-damaged chromatin, indicating ssDNA generation after H2O2 treatment (Fig. 2E and Fig. S1). More important, H2O2-induced hyperloading of RPA32 was compromised in APE2-depleted egg extracts (Fig. 2E), suggesting APE2 is responsible for ssDNA generation. To activate ATR kinase, the 9-1-1 (Rad9-Rad1-Hus1) complex must be recruited to damage sites (43, 44). The Rad9 recruitment to H2O2-damaged chromatin was also compromised in APE2-depleted egg extracts (Fig. 2E). Adding back recombinant Myc-APE2 to APE2-depleted egg extracts rescued the recruitment of ATR, ATRIP, Rad9, and RPA32 to H2O2-damaged chromatin and H2O2-induced Chk1 phosphorylation (Fig. 2E). These data suggest that APE2 is essential for the generation of RPA-bound ssDNA on damaged chromatin, thereby contributing to a checkpoint protein complex assembly onto H2O2-damaged sites. This finding reveals a distinct molecular mechanism of how APE2 contributes to ATR activation.
PCNA Association and Enzymatic Activities of APE2 Are Required for Chk1 Phosphorylation in Response to Hydrogen Peroxide.
PCNA, a DNA sliding clamp complex, orchestrates a variety of crucial players to replication forks (45, 46). Previously, it was established that APE2 associates with PCNA via a PIP box of APE2 in humans and yeast (25, 26, 47). To directly assess the role of APE2’s binding to PCNA, we constructed a PIP box mutant of Myc-APE2 with mutations from phenylalanine to alanine at amino acid residues 401 and 402 (FF; Fig. 3A). The wild-type Myc-APE2 is designated as WT. A coimmunoprecipitation experiment with anti-PCNA antibodies in APE2-depleted egg extracts showed that anti-PCNA antibodies immunoprecipitated WT but not FF APE2, indicating the PIP box is important for APE2 binding to PCNA (Fig. S3B). In APE2-depleted egg extracts (Fig. S3A), exogenous WT APE2, but not FF APE2, efficiently associated with H2O2-damaged chromatin when added at a similar level (Fig. 3B). Consistent with this result, FF APE2 failed to rescue the recruitment of RPA32 and the checkpoint complex (ATR, ATRIP, and Rad9) onto H2O2-damaged chromatin (Fig. 3B). Moreover, FF APE2 did not rescue H2O2-induced Chk1 phosphorylation in APE2-depleted egg extracts (Fig. 3C). In addition, the control experiment has shown that other APE2 mutants in this study have no deficiency in PCNA association (Fig. S3C). These observations indicate that the PCNA association with APE2 via the PIP box region is important for the recruitment of APE2 to H2O2-damaged chromatin, RPA recruitment to ssDNA, assembly of the checkpoint protein complex on damaged chromatin, and subsequent Chk1 phosphorylation.
Fig. 3.
Critical role of PCNA association and enzyme activities of APE2 in response to hydrogen peroxide-induced damage. (A) Schematic diagram for WT, PIP box mutant (FF), enzyme activity mutants (EA and DA), and CKB mutant (SA) of Myc-APE2. (B) WT or FF APE2 was added to APE2-depleted egg extracts supplemented with sperm chromatin and H2O2. Chromatin fractions and total extracts were examined for the indicated proteins. (C) Chk1 phosphorylation was analyzed from the samples in B. (D) WT, EA, or DA APE2 was added to APE2-depleted egg extracts supplemented with sperm chromatin and H2O2. Chromatin fractions and total extracts were analyzed for the indicated proteins. (E) Chk1 phosphorylation was examined from the samples in D.
Previous intensive studies have demonstrated that budding yeast Apn2 (the yeast homologue of the APE2) with glutamic acid 59 mutation to alanine and human APE2 with aspartate 277 mutation to alanine are defective in its 3′-phosphodiesterase and 3′-5′ exonuclease activities (48, 49). These two critical residues in APE2 are identical in frog, budding yeast, fission yeast, mouse, and human (Fig. S4), suggesting both residues may be in the active site of APE2 enzyme and very conserved for its enzymatic activity during evolution. To test whether APE2’s enzyme activity is essential for checkpoint signaling, we constructed two mutant Xenopus Myc-APE2 with a glutamic acid to alanine mutation at residue 34 or an aspartate to alanine mutation at residue 273 (EA and DA; Fig. 3A). Consistent with the assumption that EA and DA APE2 have enzymatic activity deficiency, we found that neither of them rescued RPA32 hyperloading and the efficient ATR checkpoint protein complex assembly to H2O2-damaged chromatin in APE2-depleted egg extracts, although EA and DA APE2 could bind to H2O2-damaged chromatin efficiently (Fig. 3D). Moreover, neither EA APE2 nor DA APE2 rescued H2O2-induced Chk1 phosphorylation in APE2-depleted egg extracts (Fig. 3E). These results suggest that the end processing of the SSB 3′-termini by APE2’s 3′-phosphodiesterase and 3′-5′ exonuclease activities may generate a long stretch of ssDNA, thereby creating the structure for RPA binding followed by assembly of the checkpoint complex. We propose to refer to this phenomenon as 3′-5′ SSB end resection, borrowing a term from a similar process of DSB end resection in a 5′-3′ direction (50).
APE2 Associates with Chk1.
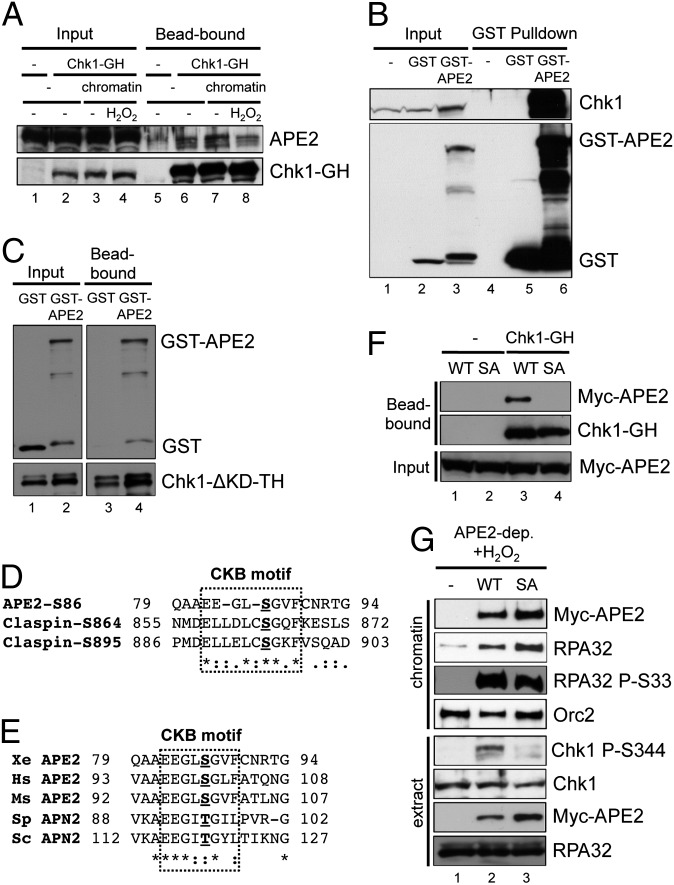
Finally, we considered a possible association between APE2 and Chk1. We tested this hypothesis through three lines of experiments. First, we added recombinant Chk1-GH protein (Chk1 with GST-tags and His-tags) to egg extracts supplemented with sperm chromatin and H2O2. After washing, we examined the bead-bound fractions for APE2 and Chk1 via immunoblotting. APE2 can bind to Chk1-coupled nickel-nitrilotriacetic acid (Ni-NTA) beads. Notably, the addition of sperm chromatin and H2O2 has no noticeable effect on the binding of APE2 to Chk1 (Fig. 4A), suggesting that the association between APE2 and Chk1 may be constitutive. The control experiment has shown that APE2 was observed in Chk1-GH-coupled bead-bound chromatin fraction, but not in “No Chk1-GH” controls (Fig. S3D). Second, we performed GST-pull-down assays and showed that GST-APE2, but not GST or no addition (−), can pull down endogenous Chk1 from egg extracts (Fig. 4B). Finally, we tested the direct interaction between APE2 and Chk1 in vitro through a protein–protein interaction assay in which we added either GST or GST-APE2 to binding buffer containing Chk1-ΔKD-TH (a kinase domain deletion mutant of Chk1 with T7-tags and His-tags). Chk1-ΔKD-TH can pull down GST-APE2, but not GST (Fig. 4C), indicating a direct interaction between APE2 and Chk1. Thus, we conclude that APE2 associates with Chk1.
Fig. 4.
Binding of Chk1 to APE2 is required for Chk1 phosphorylation by activated ATR. (A) Recombinant Chk1-GH (Chk1 with GST-tags and His-tags) was added to egg extracts supplemented with sperm chromatin and H2O2 as indicated. After incubation, extracts were mixed with nickel-nitrilotriacetic acid (Ni-NTA) beads. After washing, total egg extracts (Input, 5%) and bead-bound fractions were examined via immunoblotting. (B) GST or GST-APE2 was added to egg extracts supplemented with glutathione beads. Samples from GST pull-down and total egg extracts (Input) were examined for the indicated proteins. (C) GST or GST-APE2 was added to a binding buffer containing Ni-NTA beads coupled with Chk1-ΔKD-TH (a kinase deletion mutant of Chk1 with T7-tages and His-tags). The recombinant proteins in binding buffer (Input) and bead-bound fractions were examined via immunoblotting, as indicated. (D) Amino acid alignment of the CKB motifs in Xenopus APE2 and Claspin. In particular, S86 of APE2, S864 of Claspin, and S895 of Claspin are bolded and underlined. The CKB motifs are shown within the dashed rectangle. -, gaps in the alignment; *, identical residues; :,highly conserved residues; .,moderately conserved residues. (E) APE2 or its orthologs are aligned in Xenopus (Xe APE2), human (Hs APE2), mice (Ms APE2), fission yeast (Sp APN2), and budding yeast (Sc APN2). (F) WT or SA APE2 was added to egg extracts supplemented with Chk1-GH-coupled Ni-NTA beads. After a 1-h incubation, bead-bound and input were examined for the indicated proteins. (G) WT or SA APE2 was added to APE2-depleted egg extracts supplemented with sperm chromatin and H2O2. Chromatin fractions and total extracts were examined for the indicated proteins.
Claspin, a vital Chk1-binding protein, plays an essential role in ATR-Chk1 checkpoint signaling in response to a variety of different DNA damage and replication stress (51, 52). In Xenopus, two short conserved repeats of 10 amino acids were identified and characterized as Chk1-binding motifs (CKB motifs): ExxxLC(S/T)GxF (x represents any amino acid) (53). In Xenopus APE2, we found a Claspin-like CKB motif in the N-terminal domain: EEGLSGVF. Five amino acids in this APE2 motif are identical to the two conserved CKB motifs of Claspin (Fig. 4D). Notably, S86 of APE2 is identical to S864 and S895 of Claspin, whose phosphorylation is vital for binding to Chk1 and following Chk1 phosphorylation (Fig. 4D) (53). In addition, the CKB motif of APE2 is highly conserved in Xenopus, human, mouse, budding yeast, and fission yeast, with either serine or threonine in the fifth position of the CKB motif (Fig. 4E). We then tested whether the S86 of APE2 is essential for Chk1 binding. Our protein–protein interaction assays show that recombinant Chk1-GH associated with WT APE2, but not a mutant of APE2 from serine to alanine at residue 86 (SA; Figs. 3A and 4F). More significant, H2O2-induced Chk1 phosphorylation could be rescued by WT APE2, but not by SA APE2, in APE2-depleted extracts, although both WT and SA APE2 were efficiently recruited to H2O2-damaged chromatin (Fig. 4G). We also noticed that RPA32 was hyperloaded to H2O2-damaged chromatin and phosphorylated at serine 33 (RPA32 P-S33) after WT APE2 or SA APE2 was added back to APE2-depleted egg extracts (Fig. 4G). The control experiment has shown that other APE2 mutants including EA, DA, and FF APE2 characterized in this study are efficient in Chk1 binding (Fig. S3E). These data suggest that the CKB motif of APE2 is important for activated ATR to phosphorylate Chk1, but not other substrates of ATR, such as RPA32. Therefore, our study suggests another regulatory mechanism of the ATR-Chk1 checkpoint in oxidative stress: Claspin-like Chk1-binding of APE2 plays an indispensable role in Chk1 phosphorylation by activated ATR kinase.
Discussion
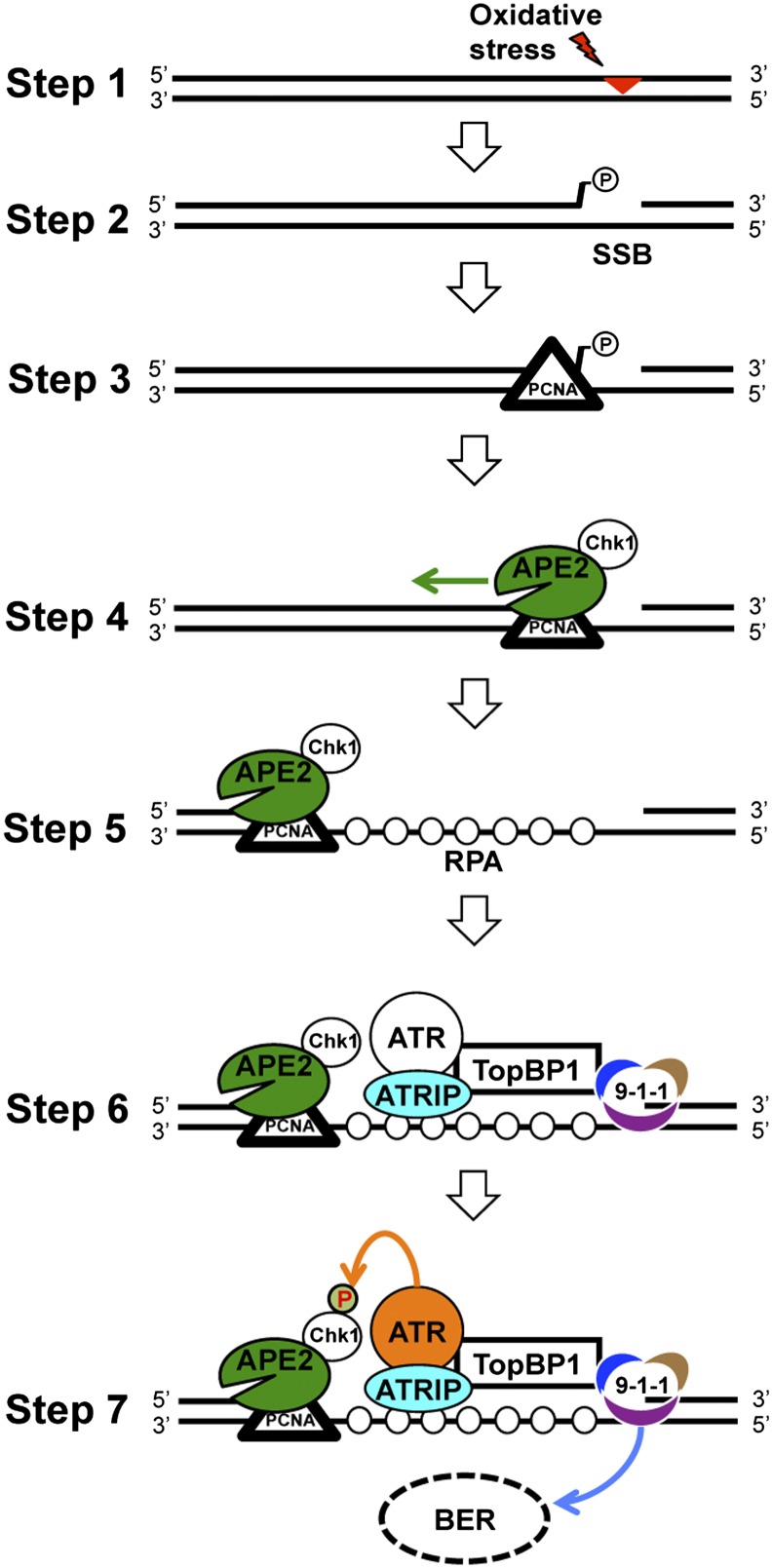
Recent studies suggest that the mismatch repair and nucleotide excision repair pathways contribute to checkpoint signaling through DNA end-processing or by direct protein–protein interactions (54–56). However, there is very little evidence that shows the interplay between the BER pathway and the ATR checkpoint. Our data suggest that APE2 links the BER pathway to checkpoint signaling via a distinct mechanism. It has been suggested that enzymes containing 3′-5′ exonuclease activities are directly involved in maintaining genome stability (57). It has also been shown that ssDNA gaps generated by DNA exonuclease III trigger an ATR- and cell division cycle 7 (Cdc7)- dependent checkpoint (58). In this report, we identify and characterize APE2 as a distinct checkpoint protein. We propose a model for APE2 in the ATR-Chk1 checkpoint in response to oxidative stress (Fig. 5): step 1, an AP site is generated by oxidative stress; step 2, the AP site is incised to generate a SSB with a damaged 3′-terminus (such as 3′-phosphate); step 3, PCNA is recruited to 3′-ssDNA/dsDNA junction; step 4, together with Chk1, APE2 is recruited to damage sites by PCNA via the PIP box; step 5, APE2 removes 3′-damaged terminus, using its 3′-phosphodiesterase activity, and continues excision by its 3′-5′ exonuclease activity, thereby generating a long stretch of ssDNA, referred as 3′-5′ SSB end resection; step 6, a checkpoint protein complex including ATR, ATRIP and the 9-1-1 complex is assembled onto the RPA-bound ssDNA and connected via topoisomerase II binding protein 1 (TopBP1), leading to ATR activation; and step 7, activated ATR phosphorylates Chk1 while the 9-1-1 complex stimulates the BER pathway as a positive feedback mechanism.
Fig. 5.
A model for APE2 in ATR-Chk1 checkpoint activation in the response to oxidative stress.
Our findings from this study provide insight into the molecular mechanisms of RPA-bound ssDNA generation in ATR-Chk1 checkpoint signaling. In response to stalled DNA replication, the RPA-bound ssDNA is generated via the functional uncoupling of MCM helicase and DNA polymerase activities (14, 59, 60). In response to DSBs, the ATR kinase is activated after ATM activation via the 5′-3′ DSB end resection by several critical factors such as C-terminal binding protein (CtBP) interacting protein (CtIP), exonuclease 1 (Exo1), and RecJ exonuclease (50, 61, 62). Our study shows a distinct mechanism for ssDNA generation: 3′-5′ SSB end resection mediated by APE2. In addition, APE2 also makes Chk1 available to convey the signal from ATR to Chk1 when ATR is activated. Further study is needed to figure out whether the S86 residue of APE2 is phosphorylated in response to oxidative stress. We speculate that the mechanism of APE2 in Chk1-binding might be distinct from Claspin, although they contain the same serine residue in the CKB motif. The 9-1-1 complex promotes BER via associating with several BER proteins including DNA glycosylases, polymerase β, APE1, FEN1, and DNA ligase I (63–69). However, it is unclear how the 9-1-1 is recruited to damage sites. Our study provides a clue: the recruitment of the 9-1-1 to H2O2-damaged sites requires APE2-mediated generation of RPA-bound ssDNA. Through positive feedback, the 9-1-1 then facilitates the BER pathway. Interestingly, APE2 has been proposed recently as a new disease candidate gene for mitochondrial DNA maintenance disorders (70). In summary, our findings demonstrate a previously unexpected but essential role of APE2 in maintaining genomic stability in cellular responses to oxidative stress in Xenopus.
Materials and Methods
The use and care of Xenopus laevis were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Charlotte. Xenopus egg extracts were prepared as described previously (28, 30). Sperm chromatin was obtained through previously described methods (71). Sperm chromatin was added to egg extracts at a concentration of ∼4,000 sperm/μL, and chromatin fractions were isolated as previously described (29). ATM inhibitor KU55933 (Calbiochem) was added to a final concentration of 120 μM, and PI3K inhibitor caffeine was added to 1 ng/μL as previously described (72, 73). Hydrogen peroxide was added at the designated final concentrations to egg extract. Aphidicolin was added to a final concentration of 100 ng/μL (29). For immunodepletions, APE2 and ATRIP antibodies were used in a similar way as the TopBP1 immunodepletion procedure (29).
Recombinant proteins, antibodies, and protein–protein interaction assays are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. W. Matthew Michael for generous gifts and critical comments on the manuscript; Drs. T. Slenn, L. Corey, and J. Weller for critical reading of the manuscript; Drs. K. Cimprich, W. G. Dunphy, H. Lindsay, and Z. You for reagents; and Drs. N. Lefebvre, C. D. Williams, and Y. M. Huet and the University of North Carolina at Charlotte Vivarium staff for maintaining the health of our frogs. This study was supported in part by funds provided by University of North Carolina at Charlotte and National Institute of General Medical Sciences/National Institutes of Health Grant R15 GM101571 (to S.Y.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1301445110/-/DCSupplemental.
References
- 1.Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol. 2010;38(1):96–109. doi: 10.1177/0192623309356453. [DOI] [PubMed] [Google Scholar]
- 2.David SS, O’Shea VL, Kundu S. Base-excision repair of oxidative DNA damage. Nature. 2007;447(7147):941–950. doi: 10.1038/nature05978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odell ID, Wallace SS, Pederson DS. Rules of engagement for base excision repair in chromatin. J Cell Physiol. 2013;228(2):258–266. doi: 10.1002/jcp.24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedberg EC. DNA damage and repair. Nature. 2003;421(6921):436–440. doi: 10.1038/nature01408. [DOI] [PubMed] [Google Scholar]
- 5.Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 6.Hamdan SM, Richardson CC. Motors, switches, and contacts in the replisome. Annu Rev Biochem. 2009;78:205–243. doi: 10.1146/annurev.biochem.78.072407.103248. [DOI] [PubMed] [Google Scholar]
- 7.Harper JW, Elledge SJ. The DNA damage response: Ten years after. Mol Cell. 2007;28(5):739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Harrison JC, Haber JE. Surviving the breakup: The DNA damage checkpoint. Annu Rev Genet. 2006;40:209–235. doi: 10.1146/annurev.genet.40.051206.105231. [DOI] [PubMed] [Google Scholar]
- 9.Branzei D, Foiani M. Maintaining genome stability at the replication fork. Nat Rev Mol Cell Biol. 2010;11(3):208–219. doi: 10.1038/nrm2852. [DOI] [PubMed] [Google Scholar]
- 10.Bartek J, Lukas C, Lukas J. Checking on DNA damage in S phase. Nat Rev Mol Cell Biol. 2004;5(10):792–804. doi: 10.1038/nrm1493. [DOI] [PubMed] [Google Scholar]
- 11.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421(6922):499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308(5721):551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- 13.Daniel JA, et al. Loss of ATM kinase activity leads to embryonic lethality in mice. J Cell Biol. 2012;198(3):295–304. doi: 10.1083/jcb.201204035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cimprich KA, Cortez D. ATR: An essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9(8):616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciccia A, Elledge SJ. The DNA damage response: Making it safe to play with knives. Mol Cell. 2010;40(2):179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie S, et al. Reactive oxygen species-induced phosphorylation of p53 on serine 20 is mediated in part by polo-like kinase-3. J Biol Chem. 2001;276(39):36194–36199. doi: 10.1074/jbc.M104157200. [DOI] [PubMed] [Google Scholar]
- 17.Shackelford RE, et al. The Ataxia telangiectasia gene product is required for oxidative stress-induced G1 and G2 checkpoint function in human fibroblasts. J Biol Chem. 2001;276(24):21951–21959. doi: 10.1074/jbc.M011303200. [DOI] [PubMed] [Google Scholar]
- 18.Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330(6003):517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 19.Kulkarni A, Das KC. Differential roles of ATR and ATM in p53, Chk1, and histone H2AX phosphorylation in response to hyperoxia: ATR-dependent ATM activation. Am J Physiol Lung Cell Mol Physiol. 2008;294(5):L998–L1006. doi: 10.1152/ajplung.00004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meira LB, Burgis NE, Samson LD. Base excision repair. Adv Exp Med Biol. 2005;570:125–173. doi: 10.1007/1-4020-3764-3_5. [DOI] [PubMed] [Google Scholar]
- 21.Kim YJ, Wilson DM., 3rd Overview of base excision repair biochemistry. Curr Mol Pharmacol. 2012;5(1):3–13. doi: 10.2174/1874467211205010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hegde ML, Hazra TK, Mitra S. Early steps in the DNA base excision/single-strand interruption repair pathway in mammalian cells. Cell Res. 2008;18(1):27–47. doi: 10.1038/cr.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tell G, Quadrifoglio F, Tiribelli C, Kelley MR. The many functions of APE1/Ref-1: Not only a DNA repair enzyme. Antioxid Redox Signal. 2009;11(3):601–620. doi: 10.1089/ars.2008.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boiteux S, Guillet M. Abasic sites in DNA: Repair and biological consequences in Saccharomyces cerevisiae. DNA Repair (Amst) 2004;3(1):1–12. doi: 10.1016/j.dnarep.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Burkovics P, Hajdú I, Szukacsov V, Unk I, Haracska L. Role of PCNA-dependent stimulation of 3′-phosphodiesterase and 3′-5′ exonuclease activities of human Ape2 in repair of oxidative DNA damage. Nucleic Acids Res. 2009;37(13):4247–4255. doi: 10.1093/nar/gkp357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unk I, et al. Stimulation of 3′—>5′ exonuclease and 3′-phosphodiesterase activities of yeast apn2 by proliferating cell nuclear antigen. Mol Cell Biol. 2002;22(18):6480–6486. doi: 10.1128/MCB.22.18.6480-6486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hadi MZ, Wilson DM., 3rd Second human protein with homology to the Escherichia coli abasic endonuclease exonuclease III. Environ Mol Mutagen. 2000;36(4):312–324. [PubMed] [Google Scholar]
- 28.Yan S, Lindsay HD, Michael WM. Direct requirement for Xmus101 in ATR-mediated phosphorylation of Claspin bound Chk1 during checkpoint signaling. J Cell Biol. 2006;173(2):181–186. doi: 10.1083/jcb.200601076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan S, Michael WM. TopBP1 and DNA polymerase-alpha directly recruit the 9-1-1 complex to stalled DNA replication forks. J Cell Biol. 2009;184(6):793–804. doi: 10.1083/jcb.200810185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willis J, DeStephanis D, Patel Y, Gowda V, Yan S. Study of the DNA damage checkpoint using Xenopus egg extracts. J Vis Exp. 2012;(69):e4449. doi: 10.3791/4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van C, Yan S, Michael WM, Waga S, Cimprich KA. Continued primer synthesis at stalled replication forks contributes to checkpoint activation. J Cell Biol. 2010;189(2):233–246. doi: 10.1083/jcb.200909105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan S, Willis J. WD40-repeat protein WDR18 collaborates with TopBP1 to facilitate DNA damage checkpoint signaling. Biochem Biophys Res Commun. 2013;431(3):466–471. doi: 10.1016/j.bbrc.2012.12.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo Z, Kumagai A, Wang SX, Dunphy WG. Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts. Genes Dev. 2000;14(21):2745–2756. doi: 10.1101/gad.842500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao H, Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol. 2001;21(13):4129–4139. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumagai A, Kim SM, Dunphy WG. Claspin and the activated form of ATR-ATRIP collaborate in the activation of Chk1. J Biol Chem. 2004;279(48):49599–49608. doi: 10.1074/jbc.M408353200. [DOI] [PubMed] [Google Scholar]
- 36.Olson E, Nievera CJ, Klimovich V, Fanning E, Wu X. RPA2 is a direct downstream target for ATR to regulate the S-phase checkpoint. J Biol Chem. 2006;281(51):39517–39533. doi: 10.1074/jbc.M605121200. [DOI] [PubMed] [Google Scholar]
- 37.Polo SE, Jackson SP. Dynamics of DNA damage response proteins at DNA breaks: A focus on protein modifications. Genes Dev. 2011;25(5):409–433. doi: 10.1101/gad.2021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300(5625):1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 39.Broderick S, Rehmet K, Concannon C, Nasheuer HP. Eukaryotic single-stranded DNA binding proteins: Central factors in genome stability. Subcell Biochem. 2010;50:143–163. doi: 10.1007/978-90-481-3471-7_8. [DOI] [PubMed] [Google Scholar]
- 40.Choi JH, et al. Reconstitution of RPA-covered single-stranded DNA-activated ATR-Chk1 signaling. Proc Natl Acad Sci USA. 2010;107(31):13660–13665. doi: 10.1073/pnas.1007856107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fanning E, Klimovich V, Nager AR. A dynamic model for replication protein A (RPA) function in DNA processing pathways. Nucleic Acids Res. 2006;34(15):4126–4137. doi: 10.1093/nar/gkl550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wold MS. Replication protein A: A heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu Rev Biochem. 1997;66:61–92. doi: 10.1146/annurev.biochem.66.1.61. [DOI] [PubMed] [Google Scholar]
- 43.Parrilla-Castellar ER, Arlander SJ, Karnitz L. Dial 9-1-1 for DNA damage: The Rad9-Hus1-Rad1 (9-1-1) clamp complex. DNA Repair (Amst) 2004;3(8-9):1009–1014. doi: 10.1016/j.dnarep.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 44.Navadgi-Patil VM, Burgers PM. A tale of two tails: Activation of DNA damage checkpoint kinase Mec1/ATR by the 9-1-1 clamp and by Dpb11/TopBP1. DNA Repair (Amst) 2009;8(9):996–1003. doi: 10.1016/j.dnarep.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129(4):665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Kelch BA, Makino DL, O’Donnell M, Kuriyan J. How a DNA polymerase clamp loader opens a sliding clamp. Science. 2011;334(6063):1675–1680. doi: 10.1126/science.1211884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsuchimoto D, et al. Human APE2 protein is mostly localized in the nuclei and to some extent in the mitochondria, while nuclear APE2 is partly associated with proliferating cell nuclear antigen. Nucleic Acids Res. 2001;29(11):2349–2360. doi: 10.1093/nar/29.11.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burkovics P, Szukacsov V, Unk I, Haracska L. Human Ape2 protein has a 3′-5′ exonuclease activity that acts preferentially on mismatched base pairs. Nucleic Acids Res. 2006;34(9):2508–2515. doi: 10.1093/nar/gkl259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Unk I, Haracska L, Prakash S, Prakash L. 3′-phosphodiesterase and 3′—>5′ exonuclease activities of yeast Apn2 protein and requirement of these activities for repair of oxidative DNA damage. Mol Cell Biol. 2001;21(5):1656–1661. doi: 10.1128/MCB.21.5.1656-1661.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Symington LS, Gautier J. Double-strand break end resection and repair pathway choice. Annu Rev Genet. 2011;45:247–271. doi: 10.1146/annurev-genet-110410-132435. [DOI] [PubMed] [Google Scholar]
- 51.Kumagai A, Dunphy WG. Claspin, a novel protein required for the activation of Chk1 during a DNA replication checkpoint response in Xenopus egg extracts. Mol Cell. 2000;6(4):839–849. doi: 10.1016/s1097-2765(05)00092-4. [DOI] [PubMed] [Google Scholar]
- 52.Yoo HY, Jeong SY, Dunphy WG. Site-specific phosphorylation of a checkpoint mediator protein controls its responses to different DNA structures. Genes Dev. 2006;20(7):772–783. doi: 10.1101/gad.1398806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumagai A, Dunphy WG. Repeated phosphopeptide motifs in Claspin mediate the regulated binding of Chk1. Nat Cell Biol. 2003;5(2):161–165. doi: 10.1038/ncb921. [DOI] [PubMed] [Google Scholar]
- 54.Brown KD, et al. The mismatch repair system is required for S-phase checkpoint activation. Nat Genet. 2003;33(1):80–84. doi: 10.1038/ng1052. [DOI] [PubMed] [Google Scholar]
- 55.Yoshioka K, Yoshioka Y, Hsieh P. ATR kinase activation mediated by MutSalpha and MutLalpha in response to cytotoxic O6-methylguanine adducts. Mol Cell. 2006;22(4):501–510. doi: 10.1016/j.molcel.2006.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giannattasio M, et al. Exo1 competes with repair synthesis, converts NER intermediates to long ssDNA gaps, and promotes checkpoint activation. Mol Cell. 2010;40(1):50–62. doi: 10.1016/j.molcel.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 57.Shevelev IV, Hübscher U. The 3′ 5′ exonucleases. Nat Rev Mol Cell Biol. 2002;3(5):364–376. doi: 10.1038/nrm804. [DOI] [PubMed] [Google Scholar]
- 58.Costanzo V, et al. An ATR- and Cdc7-dependent DNA damage checkpoint that inhibits initiation of DNA replication. Mol Cell. 2003;11(1):203–213. doi: 10.1016/s1097-2765(02)00799-2. [DOI] [PubMed] [Google Scholar]
- 59.Byun TS, Pacek M, Yee MC, Walter JC, Cimprich KA. Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint. Genes Dev. 2005;19(9):1040–1052. doi: 10.1101/gad.1301205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lyubimov AY, Costa A, Bleichert F, Botchan MR, Berger JM. ATP-dependent conformational dynamics underlie the functional asymmetry of the replicative helicase from a minimalist eukaryote. Proc Natl Acad Sci USA. 2012;109(30):11999–12004. doi: 10.1073/pnas.1209406109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shiotani B, Zou L. Single-stranded DNA orchestrates an ATM-to-ATR switch at DNA breaks. Mol Cell. 2009;33(5):547–558. doi: 10.1016/j.molcel.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Handa N, Morimatsu K, Lovett ST, Kowalczykowski SC. Reconstitution of initial steps of dsDNA break repair by the RecF pathway of E. coli. Genes Dev. 2009;23(10):1234–1245. doi: 10.1101/gad.1780709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang DY, Lu AL. Interaction of checkpoint proteins Hus1/Rad1/Rad9 with DNA base excision repair enzyme MutY homolog in fission yeast, Schizosaccharomyces pombe. J Biol Chem. 2005;280(1):408–417. doi: 10.1074/jbc.M406800200. [DOI] [PubMed] [Google Scholar]
- 64.Gembka A, et al. The checkpoint clamp, Rad9-Rad1-Hus1 complex, preferentially stimulates the activity of apurinic/apyrimidinic endonuclease 1 and DNA polymerase beta in long patch base excision repair. Nucleic Acids Res. 2007;35(8):2596–2608. doi: 10.1093/nar/gkl1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smirnova E, Toueille M, Markkanen E, Hübscher U. The human checkpoint sensor and alternative DNA clamp Rad9-Rad1-Hus1 modulates the activity of DNA ligase I, a component of the long-patch base excision repair machinery. Biochem J. 2005;389(Pt 1):13–17. doi: 10.1042/BJ20050211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friedrich-Heineken E, et al. The two DNA clamps Rad9/Rad1/Hus1 complex and proliferating cell nuclear antigen differentially regulate flap endonuclease 1 activity. J Mol Biol. 2005;353(5):980–989. doi: 10.1016/j.jmb.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 67.Wang W, Lindsey-Boltz LA, Sancar A, Bambara RA. Mechanism of stimulation of human DNA ligase I by the Rad9-rad1-Hus1 checkpoint complex. J Biol Chem. 2006;281(30):20865–20872. doi: 10.1074/jbc.M602289200. [DOI] [PubMed] [Google Scholar]
- 68.Guan X, et al. The human checkpoint sensor Rad9-Rad1-Hus1 interacts with and stimulates NEIL1 glycosylase. Nucleic Acids Res. 2007;35(8):2463–2472. doi: 10.1093/nar/gkm075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eichinger CS, Jentsch S. 9-1-1: PCNA’s specialized cousin. Trends Biochem Sci. 2011;36(11):563–568. doi: 10.1016/j.tibs.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 70.Wang W, et al. Identification of rare DNA variants in mitochondrial disorders with improved array-based sequencing. Nucleic Acids Res. 2011;39(1):44–58. doi: 10.1093/nar/gkq750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tutter AV, Walter JC. Chromosomal DNA replication in a soluble cell-free system derived from Xenopus eggs. Methods Mol Biol. 2006;322:121–137. doi: 10.1007/978-1-59745-000-3_9. [DOI] [PubMed] [Google Scholar]
- 72.Sarkaria JN, et al. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999;59(17):4375–4382. [PubMed] [Google Scholar]
- 73.Hickson I, et al. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 2004;64(24):9152–9159. doi: 10.1158/0008-5472.CAN-04-2727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.