Abstract
Background and Objectives
The emergence of extended-spectrum β-lactamase (ESBL)-producing Shigella spp. is of increasing clinical concern specially in children worldwide. The aim of this study was to investigate the occurrence of extended-spectrum β-lactamase producing Shigella spp. in Tehran, Iran
Materials and Methods
The study included all Shigella isolates recovered from pediatric patients aged less than 12 years admitted to a major pediatric hospital in Tehran, Iran, from 2008 to 2010. Bacterial identification, antimicrobial susceptibility testing, extended spectrum β-lactamases (ESBLs) screening and confirmatory tests were performed according to the standard guidelines. Conjugal transfer experiments and plasmid analysis were also carried out. Polymerase chain reaction and sequencing were used to identify the genetic determinants responsible for ESBL production.
Results
Four out of 55 Shigella isolates, including three S. sonnei and one S. flexneri, showed an ESBL-positive phenotype. Plasmid transfer of the ESBL phenotype was successful for the S. flexneri isolate only. By PCR and sequencing, one S. sonnei isolate tested positive for the CMY-59 gene, while the other two S. sonnei and the S. flexneri isolates tested positive for the bla TEM-1 and bla CTX-M-15 genes.
Conclusion
We found the prevalence of ESBL producing Shigella isolates was higher than detection rates observed in many other countries. Our finding raise concerns about the dissemination of ESBL among the strains of endemic S. sonnei throughout the country, because this species is now the most frequently isolated Shigella species in Iran and shigellosis by such strains in the community can pose a significant threat to patients and presents a challenge for disease management.
Keywords: ESBLs, Shigella spp, Antibiotic resistance
INTRODUCTION
Infections caused by Shigella species remain a major cause of diarrheal disease associated with high morbidity and mortality in developing countries, such as Iran (1). Among the four Shigella spp., S. sonnei, is the most commonly isolated species particularly in industrialized countries (2).
Adequate antibiotic therapy is helpful in shigellosis because it may shorten the clinical course of illness, significantly reduce the risk of transmission and prevent also potentially lethal complications. However, high frequencies of resistance to commonly used antimicrobial agents have been reported worldwide in Shigella spp. Moreover, the emergence of resistance to third-generation cephalosporins (3GC) in Shigella spp. is a matter of great public health concern, mainly in developing countries (3).
Since the initial report of SHV-11–type β-lactamase in S. dysenteriae from India in 1999, Shigella species producing CTX-M, SHV, and TEM-type extended spectrum β-lactamases (ESBLs) have been identified worldwide from different regions (4). Indeed, an increasing number of countries, including United States, France, Turkey, Lebanon, Bangladesh, Hong Kong and China have recently notified the isolation of ESBL-producing Shigella isolates (5–11).
There are several reports showing that the frequency of resistance against different antibiotic groups other than third-generation cephalosporins is increasing among the Shigella strains isolated in Iran (12–15).
Here we report the identification and characterization of ESBL producing Shigella strains in Tehran, Iran.
MATERIALS AND METHODS
Bacterial isolates and antimicrobial susceptibility
The study included all Shigella isolates recovered from pediatric patients aged less than 12 years and admitted to Children's Medical Center in Tehran, Iran, from November 2008 to May 2010. A single specimen was obtained from each patient, and rectal swabs were collected from patients on the day of admission at the hospital. All strains were identified at a genus level by conventional methods according to previously described procedures (16). Serotyping of the isolates was performed by slide agglutination (Shigella Antisera, Denka Seiken, Tokyo, Japan).
Antibiotic susceptibility testing was performed according to CLSI guidelines (17) using the following antibiotic disks (Oxoid, Basingstoke, Hampshire, UK): ampicillin (AM, 10 µg), amoxicillin–clavulanic acid (AMC, 20–10 µg), cephalotin (CF, 30 µg), cefixime (CFM, 5 µg), cefotaxime (CTX, 30 µg), ceftizoxime (CT, 30 µg), ceftriaxone (CRO, 30 µg), ceftazidime (CAZ, 30 µg), ciprofioxacin (CIP, 5 µg), chloramphenicol (C, 30 µg), doxycycline (D, 30 µg), gentamicin (GN, 10 mg), kanamycin (K, 10 µg), nalidixic acid (NA, 30 µg), neomycin (N, 30 µg), nitrofurantoin (FD, 300 µg), piperacillin (PIP, 100 µg), streptomycin (S, 10 µg), tetracycline (TE, 30 µg), ticarcillin (TIC, 75 µg), tobramycin (TOB, 10 µg), trimethoprim–sulfamethoxazole (SXT, 1.25-23.75 µg).
Detection and characterization of ESBLs
Screening of 3rd generation cephalosporins (3GC) resistant Shigella isolates was performed by disk diffusion method using ceftazidime, cefotaxime, ceftriaxone and cefepime disks. According to CLSI criteria for detection of ESBL producers, each isolate with inhibition zone diameter ≤22 mm for ceftazidime or ≤27 mm for cefotaxime was considered as a potential ESBL-producer. Phenotypic confirmation of ESBL production was done by double disk synergy and combination disk tests (18).
Escherichia coli ATCC 25922 and previously characterized ESBL-positive E. coli, Klebsiella pneumoniae and AmpC-positive Enterobacter cloacae strains were used as controls for antibiotic susceptibility testing and the following ESBL analysis. Phenotypic detection of AmpC enzymes was performed by using cefoxitin supplemented with cloxacillin and E. coli ATCC 25922 according to the previously described method (19). In brief, the 30 µg cefoxitin disk was supplemented with 200 µg of cloxacillin (Sigma-Aldrich, Singapore). A strain was considered to be an AmpC producer when it showed an increase in diameter around the antibiotic disk with added cloxacillin compared to that with the cefoxitin disk alone.
A multiplex PCR assay approach was used for a rapid screening of frequently encountered β-lactamases according procedure previously described by Dallenne et al. (20). DNA of the isolates under study was subjected to the multiplex PCR reactions I to III in 50 µL reaction mixtures containing variable concentrations of specific-group primers and 1 U of Taq polymerase (20). Amplification was carried out as follows: initial denaturation at 94°C for 10 min; 30 cycles of 94°C for 40 s, 60°C for 40 s and 72°C for 1 min; and a final extension step at 72°C for 7 min. Amplicons were visualized on 2% agarose gels.
To identify the β-lactamase genes detected in the multiplex PCR assays, amplicons were purified using a QIA quick PCR Purification Kit (Qiagen, GmbH, Germany) and bidirectional sequencing was performed. The nucleotide sequences and the deduced protein sequences were analyzed with the BLAST and Clustal W programs (multiple sequence alignment, pairwise comparisons of sequences and dendrograms).
Resistance transfer experiments were performed for all four isolates as previously described with the rifampicin resistant Escherichia coli K12J5 as the recipient. Briefly, mixed cultures of the donors and the rifampicin resistant recipient E. coli strain were incubated at 37°C overnight. The transconjugants were selected on MacConkey agar plates supplemented with ceftazidime or cefotaxime- 30 µg/ml - and rifampicin - 250 µg/ml.
A confirmatory test for ESBL and AmpC phenotypes and PCR detection for ESBL genes were performed on transconjugant colonies by the above mentioned procedures. Plasmids from Shigella isolates and E. coli transconjugants were extracted by an alkaline lysis extraction method (21).
RESULTS
Fifty five Shigella isolates were isolated during the study period. Of theses, 51 strains (92.7%) were susceptible to 3GC. As shown in Table 1, four strains (7.3%), including three S. sonnei and one S. flexneri showed concomitant resistance to these 3GC antibiotics and were selected for ESBL production.
Table 1.
Characteristics of the ESBL-producing Shigella strains under study.
| Strain No. | Time of isolation | Serotype | Antibiotic resistance pattern* | Type of β-lactamase |
|---|---|---|---|---|
| 1 | June 12, 2009 | S. sonnei | AMC-AM-NA-CAZ-TIC-CTX-CRO-CT-PIP-D-TE-S-SXT-CF | CMY-59 |
| 2 | July 23, 2009 | S. flexneri | AMC-AM-NA-CAZ-TIC-CTX-CRO-D-TE-S-SXT-C-CF | TEM-1, CTX-M-15 |
| 3 | September 3, 2009 | S. sonnei | AMC-AM-NA-CAZ-TOB-TIC-CTX-CRO-PIP-D-TE-S-SXT | TEM-1, CTX-M-15 |
| 4 | November 10, 2009 | S. sonnei | AMC-AM-CZA-TOB-TIC-CTX-CRO-D-TE-S-SXT-CF | TEM-1, CTX-M-15 |
AM: ampicillin, AMC: amoxycillin-clavulanic acid, C: chloramphenicol, CAZ: ceftazidime, CF: cephalothin, CRO: ceftriaxone, CT: ceftizoxime, CTX: cefotaxime, D: doxycycline, NA: nalidixic acid, PIP: piperacillin, S: streptomycin, SXT: trimethoprim-sulfamethoxazole, TE: tetracycline, TIC: ticarcillin, TOB: tobramycin
Double disk synergy and combination disk tests confirmed that these four strains had ESBL-positive phenotype (Table 1). bla TEM and bla CTX-M sequences were detected in the S. flexneri and two S. sonnei isolates, whereas the third S. sonnei isolate showed a bla CMY sequence. DNA sequencing revealed that the bla TEM and bla CTX-M sequenceswere bla TEM-1 and bla CTX-M-15, respectively, whilst the CMY ESBL was identified as CMY-59.
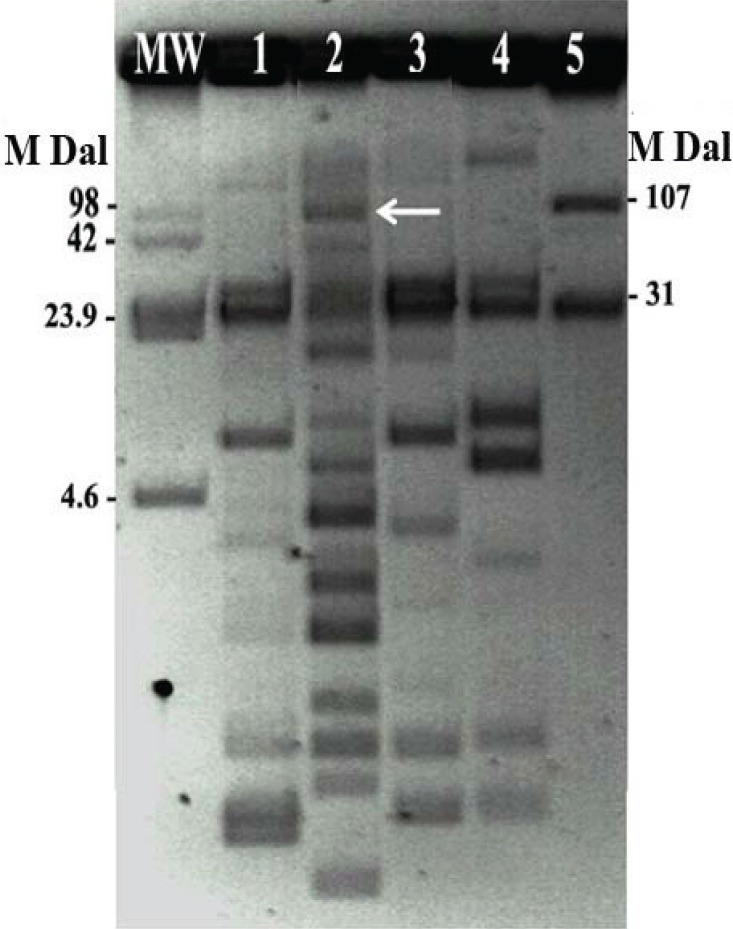
Based on the conjugation assay, only the S. flexneri isolate was able to transfer the bla gene to transconjugant, confirming that it was mediated by a plasmid of approximately 107MDa (Fig. 1). Only resistance to trimethoprim was co-transferred along with the ESBL encoding sequence.
Fig. 1.
Plasmid patterns of the Shigella clinical isolates and the transconjugant Escherichia coli from the S. flexneri isolate.
Lanes: MW, molecular weight, Escherichia coli 39R861; 1, S. sonnei n.1; 2, S. flexneri n.2; 3, S. sonnei n. 3; 4, S. sonnei n.4; 5, E. coli K12J5 transconjugant from the S. flexneri isolate n.2.
DISCUSSION
Ampicillin and sulfamethoxazole-trimethoprim are generally indicated as the first-line drugs for therapy of shigellosis. However, in the event of disease caused by a multidrug resistant strain, second-line drugs including 3GC and fluoroquinolones are used for treatment of children and adults, respectively (7). Unfortunately, it has been recently reported that the prevalence of resistance to some commonly used antibiotics is increasing in Shigella species in Iran (22). Class 2 integrons have proved to be at least in part responsible for the antibiotic resistance spectrum (12).
Beta-lactams are the most frequently used antimicrobial agents in Iran and the ESBL producer's organisms have been reported frequently in several bacterial species in this country (23). Recently Tajbakhsh and her colleagues have documented the presence of bla CTX-M-15 and bla CMY-2 in six Shigella strains isolated from patients admitted to Milad Hospital, Tehran, Iran (24).
An increase in the occurrence of antimicrobial resistance, including resistance to 3GC and fluoroquinolones among Shigella spp. has been observed in several countries. To date, bla CTX-M-15, bla CTX-M-14, bla CTX-M-3 and bla CMY-2 genes have been reported in Shigella spp. isolates (7, 25, 26).
In our study, the prevalence of ESBL producing Shigella isolates, accounting for 7.3% of all Shigella isolates identified in the period under study, is higher than detection rates observed in many other countries (5, 7, 9). Moreover, CMY-59 has never been reported in Shigella, whereas CMY-2-type AmpC ß-lactamases has been previously detected, but infrequently. Recently, the detection of AmpC β-lactamase producer strains in Shigella spp. has been reported in Iran (24).
Among all ESBL producing strains, only the S. flexneri isolate was able to transfer the bla gene to transconjugant, indicating it was mediated by a plasmid however we did not make further attempts to obtain the transfer of resistance determinants. This could have resulted from the presence of chromosomally-mediated β-lactamase genes in the parental strains that could not be transferred to the recipients. Alternatively, they can be located on large low-copy number plasmids with a low transfer frequency.
A ceftriaxone resistant Shigella strain with a plasmid mediated CMY-2 AmpC type ESBL was also responsible for a dysentery outbreak in Taiwan (26). On the contrary, CTX-M-15 are the most common types of cefotaximases identified among Shigella isolates (3, 5, 6, 27, 28). Of special concern, plasmid mediated production of this β-lactamase is widely disseminated in Enterobacteriaceae, particularly among strains isolated from nosocomial infections, but more and more frequently from community-acquired cases.
Our findings raise concerns about the dissemination of ESBL among the strains of endemic S. sonnei throughout the country, because S. sonnei is now the most frequently isolated Shigella species in Iran and shigellosis by such strains in the community can pose a significant threat to patients and presents a challenge for disease management. Thus, active and continued surveillance of antibacterial drug resistance appears to be needed as the first step, along with a judicious use of antibiotics, to minimize the spread of ESBL producing Shigella isolates. This is especially critical for the pediatric patients for whom antibiotic therapeutic options are inherently limited.
ACKNOWLEDGMENTS
This research was supported in part by a grant from Iranian Ministry of Health and Medical Education, Deputy of Research and Technology. The authors would like to thank Dr. Haghi- Ashtiani and Mrs. Mina Abedini from Microbiology Laboratory of the Children's Medical Center for their kind Cooperation.
REFERENCES
- 1.Ranjbar R, Hosseini MJ, Kaffashian AR, Farshad S. An outbreak of shigellosis due to Shigella flexneri serotype 3a in a prison in Iran. Arch Iran Med. 2010;13:413–416. [PubMed] [Google Scholar]
- 2.Filliol-Toutain I, Chiou CS, Mammina C, Gerner-Smidt P, Thong KL, Phung DC, et al. Global distribution of Shigella sonnei clones. Emerg Infect Dis. 2011;17:1910–2. doi: 10.3201/eid1710.101486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang R, Zhou HW, Cai JC, Zhang J, Chen GX, Nasu M, Xie XY. Serotypes and extended-spectrum β-lactamase types of clinical isolates of Shigella spp. from the Zhejiang province of China. Diagn Microbiol Infect Dis. 2011;69:98–104. doi: 10.1016/j.diagmicrobio.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 4.Ahamed J, Kundu M. Molecular characterization of the SHV-11 β-lactamase of Shigella dysenteriae . Antimicrob Agents Chemother. 1999;43:2081–2083. doi: 10.1128/aac.43.8.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folster JP, Pecic G, Krueger A, Rickert R, Burger K, Carattoli A, Whichard JM. Identification and characterization of CTX-M-producing Shigella isolates in the United States. Antimicrob Agents Chemother. 2010;54:2269–2270. doi: 10.1128/AAC.00039-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lartigue MF, Poirel L, Decousser JW, Nordmann P. Multidrug-Resistant Shigella sonnei and Salmonella enterica serotype Typhimurium isolates producing CTX-M β-lactamases as causes of community-acquired infection in France. Clin Infect Dis. 2005;40:1069–1071. doi: 10.1086/428667. [DOI] [PubMed] [Google Scholar]
- 7.Acikgoz ZC, Gulay Z, Bicmen M, Gocer S, Gamberzade S. CTX-M-3 extended-spectrum beta-lactamase in a Shigella sonnei clinical isolate: first report from Turkey. Scand J Infect Dis. 2008;35:503–505. doi: 10.1080/00365540310013270. [DOI] [PubMed] [Google Scholar]
- 8.Matar GM, Jaafar R, Sabra A, Hart CA, Corkill JE, Dbaibo GS, Araj GF. First detection and sequence analysis of the bla-CTX-M-15 gene in Lebanese isolates of extended-spectrum-lactamase-producing Shigella sonnei . Ann Trop Med Parasitol. 2007;101:511–517. doi: 10.1179/136485907X193860. [DOI] [PubMed] [Google Scholar]
- 9.Rahman M, Shoma S, Rashid H, Siddique AK, Nair GB, Sack DA. Extended-spectrum beta-lactamase-mediated third-generation cephalosporin resistance in Shigella isolates in Bangladesh. J Antimicrob Chemother. 2004;54:846–847. doi: 10.1093/jac/dkh413. [DOI] [PubMed] [Google Scholar]
- 10.Cheung TK, Chu YW, Tsang GK, Ngan JY, Hui IS, Kam KM. Emergence of CTX-M-type beta-lactam resistance in Shigella spp. in Hong Kong. Int J Antimicrob Agents. 2005;25:350–352. doi: 10.1016/j.ijantimicag.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Xiong Z, Li T, Xu Y, Li J. Detection of CTX-M-14 extended-spectrum beta-lactamase in Shigella sonnei isolates from China. J Infect. 2007;55:e125–128. doi: 10.1016/j.jinf.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Ranjbar R, Aleo A, Giammanco GM, Dionisi AM, Sadeghifard N, Mammina C. Genetic relatedness among isolates of Shigella sonnei carrying class 2 integrons in Tehran, Iran, 2002 – 2003. BMC Infect Dis. 2007;22:62. doi: 10.1186/1471-2334-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ranjbar R, Soltan-Dallal MM, Pourshafie MR. Antibiotic resistance among Shigella serogroups isolated in Tehran, Iran (2002-2004) J Infect Dev Ctries. 2009;3:647–648. doi: 10.3855/jidc.560. [DOI] [PubMed] [Google Scholar]
- 14.Hosseini MJ, Ranjbar R, Ghasemi H, Jalalian HR. The prevalence and antibiotic resistance of Shigella spp. recovered from patients admitted to Bouali Hospital, Tehran, Iran during 1999-2000. Pak J Biol Sci. 2007;10:2778–2780. doi: 10.3923/pjbs.2007.2778.2780. [DOI] [PubMed] [Google Scholar]
- 15.Soltan-Dallal MM, Ranjbar R, Pourshafie MR. The study of antimicrobial resistance among Shigella flexneri strains isolated in Tehran, Iran. J Pediatr Infect Dis. 2011;6:125–129. [Google Scholar]
- 16.Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken FC, editors. Manual of clinical microbiology. 6th ed. Washington, DC: American Society for Microbiology; 1995. p. 1, 482. [Google Scholar]
- 17.Clinical and Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing: 17th informational supplement (M100-S17); Wayne, PA: CLSI; 2008. [Google Scholar]
- 18.Clinical and Laboratory Standard Institute. Performance standards for antimicrobial susceptibility testing: sixteenth informational supplement. CLSI document M100-S16; Wayne, Pa: CLSI; 2006. [Google Scholar]
- 19.Tan TY, Ng LS, He J, Koh TH, Hsu LY. Evaluation of screening methods to detect plasmid-mediated AmpC in Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis . Antimicrob Agents Chemother. 2009;53:146–149. doi: 10.1128/AAC.00862-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dallenne C, Da Costa A, Decré D, Favier C, Arlet G. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae . J Antimicrob Chemother. 2010;65:490–495. doi: 10.1093/jac/dkp498. [DOI] [PubMed] [Google Scholar]
- 21.Birnboim HC, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–23. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranjbar R, SoltanDallal MM, Talebi M, Pourshafie MR. Increased isolation and characterization of Shigella sonnei obtained from hospitalized children in Tehran, Iran. J Health Popul Nutr. 2008;26:426–430. doi: 10.3329/jhpn.v26i4.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohammadi-Mehr M, Feizabadi MM. Antimicrobial resistance pattern of Gram-negative bacilli isolated from patients at ICUs of Army hospitals in Iran. Iran J Microbiol. 2011;3:26–30. [PMC free article] [PubMed] [Google Scholar]
- 24.Tajbakhsh M, GarcíaMigura L, Rahbar M, Svendsen CA, Mohammadzadeh M, Zali MR, Aarestrup FM, Hendriksen RS. Antimicrobial-resistant Shigella infections from Iran: an overlooked problem? J Antimicrob Chemother. 2012;67:1128–33. doi: 10.1093/jac/dks023. [DOI] [PubMed] [Google Scholar]
- 25.Hong SJ, Lee CH, Wang JH, Song W, Jung SH. Clinical characteristics of extended-spectrum β-lactamase producing Shigella sonnei infection outbreaked in Chungju area. Korean J Lab Med. 2006;26:168–73. doi: 10.3343/kjlm.2006.26.3.168. [DOI] [PubMed] [Google Scholar]
- 26.Huang IF, Chiu CH, Wang MH, Wu CY, Hsieh KS, Chiou CC. Outbreak of dysentery associated with ceftriaxone-resistant Shigella sonnei: first report of plasmid-mediated CMY-2-type AmpC-lactamase resistance in S. sonnei . J Clin Microbiol. 2005;43:2608–2612. doi: 10.1128/JCM.43.6.2608-2612.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabra AH, Araj GF, Kattar MM, Abi-Rached RY, Khairallah MT, Klena JD, Matar GM. Molecular characterization of ESBL-producing Shigella sonnei isolates from patients with bacilliary dysentery in Lebanon. J Infect Dev Ctries. 2009;3:300–5. doi: 10.3855/jidc.128. [DOI] [PubMed] [Google Scholar]
- 28.Upton A, Mohiuddin J, Bathgate T, Taylor S, Simmons G, Woodhouse R, Heffernan H. High prevalence of CTX-M-15 extended-spectrum β-lactamase among contacts of patients with shigellosis due to Shigella flexneri carrying CTX-M-15. J Antimicrob Chemother. 2007;60:906–908. doi: 10.1093/jac/dkm277. [DOI] [PubMed] [Google Scholar]