Abstract
Background and Objectives
The Escherichia coli strains are greatly important in nosocomial and community acquired infections. The aim of this study was to determine the transmission of bacterial infections using genetic analysis.
Materials and Methods
Two hundred and thirty Escherichia coli strains, isolated from different clinical samples, were characterized by enterobacterial repetitive intergenic consensus (ERIC)–PCR technique. The results and the similarity between the strains were determined on the basis of Jaccard similarity coefficient in the SAHN program of the NTSYS-pc software.
Results
The ERIC–PCR profiles allowed typing of the 230 isolates into 205 ERIC-types which were grouped into twenty main clusters (C01–C20).The first group makes up 4.34% of the total isolates. Out of the 230 isolates, 34.2% belonged to D phylogenic group which were associated with extra-intestinal samples.
Conclusion
Our results showed high diversity in E. coli isolates indicating low rate of hospital infection in our university hospitals. The majority of the isolates belonged to the D phylogenic group.
Keywords: E. coli, genetic diversity, ERIC–PCR, fingerprinting
INTRODUCTION
Bacterial genomes contain repeat sequences such as enterobacterial repetitive intergenic consensus (ERIC) sequence (1). These can be used as molecular biological tools to assess the clonal variability of many bacterial isolates including E. coli (2–4). The advent of molecular genetic techniques and their applications to microbial ecology has demonstrated that only a small proportion of natural microbial diversity has been discovered. Detailed information on the molecular genetics of previously uncharacterized micro-organisms may shed more light on the evolution of functionality of bacteria of a given species within its own habitat or in a foreign and hostile environment (5). REP-PCR is a genomic fingerprinting technique that generates specific strain patterns obtained by the amplification of repetitive DNA elements present along the bacterial genome (6–9). Enterobacterial repetitive intergenic consensus (ERIC sequences), also known as intergenic repetitive units, were initially reported in Escherichia coli and Salmonella enterica serovar Typhimurium. These sequences are now described in most bacterial species such as Enterobacteriaceae family as well as in Vibrio cholera (10, 11). The ERIC sequences have been located in intergenic regions as palindromes of 127 bps (11–14). Their copy numbers differ in different bacteria; e.g. 30 copies reported in E. coli K-12 and 150 in S. enterica serovar Typhimurium (15).
The aim of this study was to characterize the genetic diversity of E. coli isolates obtained from different clinical sources using ERIC-PCR. The degree of similarity between isolates was assessed by construction of a dendrogram which also enabled the comparison of clusters generated by analysis of samples from different sampling sites as well as assessment of the diversity of possible sources of contamination. The other specifications of the isolates were also considered for further evaluation.
MATERIALS AND METHODS
Study population and specimen types
This study was conducted at the School of Medicine, Kurdistan University of Medical science, Sanandaj, Iran. Consecutive, non-duplicate isolates of E. coli cells were collected from various specimens of patients who referred for Toohid and Beesat Hospitals. Most of the specimens were from the ICU, Infectious, Internal, Surgery, Women's and Pediatrics wards. Specimens included urine, wound, respiratory secretions, blood, cerebrospinal fluid, and others.
Microbiological methods
All samples were routinely cultured on MacConkey and blood agar plates. Blood samples were cultured in Blood culture bottles. Isolates were identified at the species level using standard biochemical tests and microbiological methods such as colony types, motility, carbohydrate fermentation of glucose, lactose and sucrose, citrate assimilation, lysine decarboxylase, indole production, oxidase test, and catalase reaction.
ERIC-PCR
E. coli isolates were fingerprinted using the enterobacterial repetitive intergenic consensus polymerase chain reaction (ERIC-PCR) (9). The primers used for the ERIC-PCR reaction were ERIC lR, 5'ATGTAAGCTCCTGGGGATTCAC3’ and ERIC2 5'AAGTAAGTGACTGGGGTGAGCG3’ (9).
DNA templates were prepared, purified and stored until needed at -20°C (16). ERIC-PCR reactions were performed in 25 µl volumes containing of 1 µl of each primer (final concentration 2 pmol/µl), 12.5 µl of Master Mix (Applied Biosystem) and 9.5 µl of deionized water. The ERIC-PCR reaction was as follows: initial denaturation at 95°C for 2min, with the next 35 cycles consisting of a denaturation step at 92°C for 30 s; annealing at 50°C for 1min; extension at 65°C for 8 min; as well as, a final extension step at 65°C for 8min and final storage at 4°C. The ERIC-PCR fragments obtained were examined by electrophoresis in 1.5% agarose gel. One-kb DNA markers (Fermentas Co.) were used and an electric field of 100 V were applied during electrophoresis for 45 minutes. The gels were subsequently stained for 30 min in a solution containing 0.5 mg of ethidium bromide per ml. (17).
Computer-Assisted ERIC-PCR DNA Fingerprint Analysis
Gel images were captured using an electronic documentation system. ERIC-PCR fingerprints of amplified DNA fragments obtained by agarose gel electrophoresis were recorded. The positions of the bands on each lane and each gel were normalized using the 1 kb DNA ladder as an external reference standard. The presence of a given band was coded as 1 and the absence of a given band was coded as 0 in a data matrix and analyzed using the NTSYS-pc software (version 2.02 K, Applied Biostatistics, Inc., NY, USA). Dendrograms of dissimilarity were constructed for each case. The similarity between the strains was determined on the basis of the Jaccard similarity. The dendrogram was constructed on the basis of the averaged similarity of the matrix with the use of the algorithm of the Unweighted Pair-Group Method (UPGMA) in the SAHN program of the NTSYS-pc software. The nearest neighbor-joining clustering method has been used to show relations between similar groups.
Phylogenetic grouping
Phylogenic groups were detected by PCR using primers for chuA, yjaA, and tspE genes (18).
RESULTS
The genomic diversity analysis of 230 strains of E. coli has been carried out using the ERIC-PCR fingerprinting method with ERIC-type primer. The electrophoretic profiles of the DNA fragments obtained after PCR amplification using specific primers for ERIC sequences were determined for the E. coli isolates. The ERIC–PCR profiles allowed differentiation of the 230 isolates into 205 ERIC-types which were grouped into twenty main clusters (C01–C20) (Table 1). Complex patterns of fingerprints have been obtained for all strains. Generally, the electrophoretic analysis of the PCR reaction products has revealed that the number of bands in particular electrophoretic paths ranged from 6-15. The sizes of the PCR products ranged from slightly less than 100 bp to about 1400 bp. Products ranging from 200-800 bp were encountered more routinely. The dendrogram has grouped the 230 strains of E. coli into ten similarity groups. Isolates of the first group make up 4.34% of the totalisolates (Fig. 1). Their property similarity is 59%, as shown in (19) (Table 1). Characterization of 18 patterns and genetic diversity of each pattern that contain more than one isolate is illustrated in Table 2. Out of the 230 isolates, 34.2% of them belonged to D phylogenic group that were associated with extra-intestinal samples.
Table 1.
Clustering and similarity of E. coli isolates and Number of strains in each cluster.
| Clusters | Dendrogram similarity in clusters | Number of strains in each cluster |
|---|---|---|
| C01 | 59% | 10 4.34 |
| C02 | 57% | 21 9.13 |
| C03 | 65% | 16 6.95 |
| C04 | 58% | 11 4.78 |
| C05 | 55% | 11 4.78 |
| C06 | 56% | 14 6.08 |
| C07 | 56% | 7 3.04 |
| C08 | 50% | 7 3.04 |
| C09 | 57% | 13 5.65 |
| C10 | 49% | 17 7.39 |
| C11 | 53% | 13 5.65 |
| C12 | 47% | 6 2.60 |
| C13 | 40% | 11 4.78 |
| C14 | 45% | 13 5.65 |
| C15 | 35% | 18 7.82 |
| C16 | 35% | 9 3.91 |
| C17 | 51% | 9 3.91 |
| C18 | 30% | 10 4.34 |
| C19 | 33% | 11 4.78 |
| C20 | 100% | 2 0.87 |
Fig. 1.
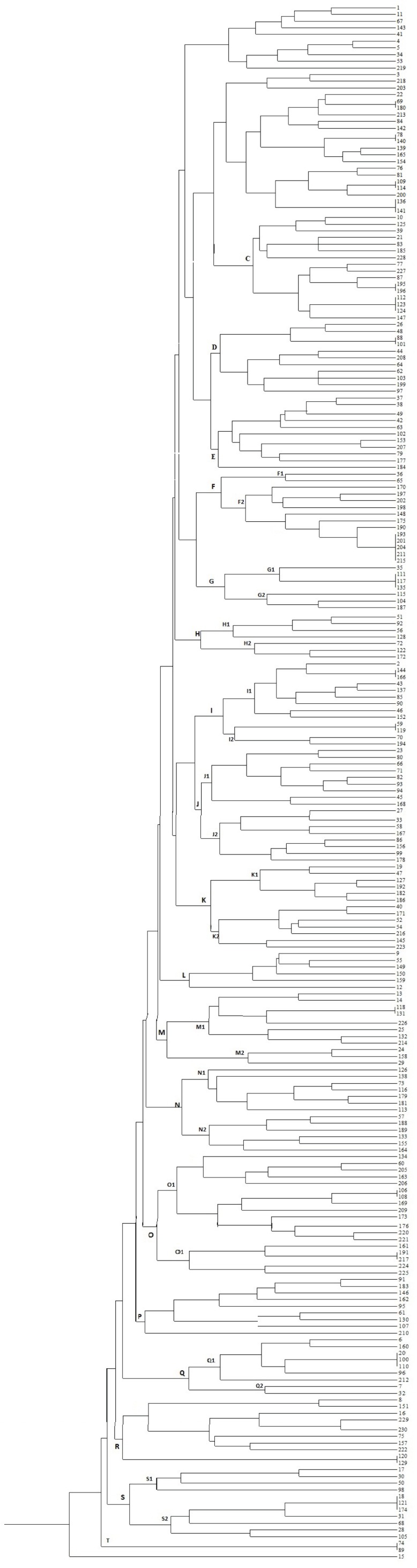
Dendrograms of genomic similarity of 230 E. coli strains in Sanandaj hospitals.
Table 2.
Genetic diversity and characterization of 18 patterns of 230 E. coli clinical isolates.
| pattern | Clusters | groups | Specimens | Hospital ward | ESBL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| No. | Blood | CSF | lung discharge | Urine | Wound | Women's | Out patients | ICU | Surgery | Pediatrics | Internal | Yes | No | |||
| SZ15 | 2 | C02 | B2 | 1 | 1 | 1 | 1 | 2 | ||||||||
| SZ19 | 2 | C02 | B2 | 1 | 1 | 1 | 1 | 2 | ||||||||
| SZ25 | 2 | C02 | D | 2 | 2 | 2 | ||||||||||
| SZ27 | 2 | C02 | B1 | 2 | 1 | 1 | 2 | |||||||||
| SZ40 | 2 | C03 | D | 1 | 1 | 1 | 1 | 2 | ||||||||
| SZ41 | 3 | C03 | A | 3 | 2 | 1 | 3 | |||||||||
| SZ45 | 2 | C04 | D | 2 | 1 | 1 | 2 | |||||||||
| SZ53 | 2 | C05 | B1 | 1 | 1 | 1 | 1 | 2 | ||||||||
| SZ72 | 5 | C06 | A | 1 | 4 | 1 | 2 | 2 | 5 | |||||||
| SZ74 | 3 | C07 | A | 1 | 2 | 1 | 1 | 1 | 2 | 1 | ||||||
| SZ86 | 2 | C09 | B2 | 1 | 1 | 1 | 1 | 2 | ||||||||
| SZ93 | 2 | C09 | B1 | 2 | 1 | 1 | 2 | |||||||||
| SZ134 | 3 | C13 | B2 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | ||||||
| SZ168 | 2 | C15 | D | 1 | 1 | 1 | 1 | 2 | ||||||||
| SZ182 | 3 | C17 | D | 1 | 2 | 2 | 1 | 3 | ||||||||
| SZ185 | 2 | C17 | D | 1 | 1 | 1 | 1 | 2 | ||||||||
| SZ194 | 2 | C18 | B1 | 2 | 2 | 2 | ||||||||||
| SZ204 | 2 | C20 | D | 2 | 1 | 1 | 2 | |||||||||
| Total | 43 | 3 | 1 | 8 | 30 | 1 | 9 | 13 | 10 | 2 | 5 | 4 | 15 | 28 | ||
DISCUSSION
This is the first study in this region to elucidate the genetic diversity of E. coli clinical isolates. There are several methods for determination of bacterial transmission trace in the community and hospitals. ERIC-PCR has been used by several studies and for different bacterial isolates including Pseudomonas aeruginosa, Heamophilus spp. (20), Vibrio cholera (21), Corynebacterium (22), Salmonella spp. (23), Streptococcus (24), and other bacterial strains (25, 26). ERIC-PCR typing method showed 205 patterns for 230 clinical isolates. One hundred eighty-seven (81.3%) of the isolates displayed a single profile; whereas, 43 (18.7%) of them showed shared patterns which is indicative of similar origin of dissemination. ERIC-PCR profiles did not demonstrate genetic relatedness between 187 E. coli strains. Therefore, infections caused by most of our university hospital isolates were not clonally spread and resulted from individual infections. Eighteen profiles each containing 2 to 5 isolates showed similar genotype which indicate clonal spreading of bacteria in hospital wards. Similar results were obtained for E. cloacae isolates of nosocomial origin by ERIC-PCR technique (27).
Molecular epidemiology using ERIC-PCR fingerprinting patterns showed that isolates in cluster exhibit similar profiles, which demonstrate the clonal transmission of bacteria in hospital environments. In addition, the bacterial patterns were further divided into twenty main clusters (C01–C20) with the C02 cluster being the major one. Clonal transmission of A. baumannii has been evidenced by ERIC-PCR DNA fingerprinting patterns in multi-drug resistant isolates (28). In another studies, E. coli and K. pneumoniae nosocomial outbreaks in Novosibirsk Regional Hospital were studied by ERIC-PCR (29).
The aim of this study was to show the genetic relationship in isolates and their hospital transmission pattern. Most of the isolates show unique patterns which indicate that the rate of nosocomial infections are very low in Sanandaj University hospitals. The remaining patterns showed similarity in most characteristics. The strains in this study presented similar antibiotic resistance patterns especially in regards to extended spectrum beta lactamase (ESBLs). We reported the rate of ESBL production in E. coli isolates in previous reports (30–36). Phylogenetic group D was the most prevalent (34.22%) in our results. Distribution of the other groups was as follows: B2 (32.89%), A (21.78%), and B1 (11.11%). As reported previously, the phylogenetic groups B2 and D of E. coli strains are mostly associated with extra-intestinal infections; whereas, groups A and B1 bacteria are considered to be commensal isolates (37, 38).
In conclusion, ERIC-PCR fingerprint types suggested considerable diversity in E. coli isolates which may be due to the low rate of hospital infection in our university hospitals. The rate of hospital infection is very low and most isolates belonged to the D phylogenetic groups that were associated with extra-intestinal samples.
ACKNOWLEDGMENTS
Authors wish to extend their gratitude to the Kurdistan University of Medical Sciences for supporting the project. We thank Dr. Amirmozafari for editing the manuscript. We also thank the Microbiology laboratory staff of the Beasat and Toohid Hospitals for their technical support.
REFERENCES
- 1.Hulton CS, Higgins CF, Sharp PM. ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli, Salmonella typhimuirum and other enterobacteria. Mol Microbiol. 1991;5:825–834. doi: 10.1111/j.1365-2958.1991.tb00755.x. [DOI] [PubMed] [Google Scholar]
- 2.Versalovic J, Koeuth T, Lupski JR. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalla-Costa LM, Irino K, Rodrigues J, Rivera ING, Trabulsi LR. Characterisation of diarrhoeagenic Escherichia coli clones by ribotyping and ERIC-PCR. J Med Microbiol. 1998;47:227–234. doi: 10.1099/00222615-47-3-227. [DOI] [PubMed] [Google Scholar]
- 4.Chansiripornchai N, Ramasoota P, Sasipreyajan J, Svenson SB. Differentiation of avian Escherichia coli (APEC) isolates by random amplified polymorphic DNA (RAPD) analysis. Vet Microbiol. 2001;80:75–83. doi: 10.1016/s0378-1135(00)00380-1. [DOI] [PubMed] [Google Scholar]
- 5.Schloter M, Lebuhn M, Heulin T, Hartmann A. Ecology and evolution of bacterial microdiversity. FEMS Microbiol Rev. 2000;24:647–660. doi: 10.1111/j.1574-6976.2000.tb00564.x. [DOI] [PubMed] [Google Scholar]
- 6.Busch U, Nitschko H. Methods for the differentiation of microorganisms. J Chromatogr. 1999;722:263–278. doi: 10.1016/s0378-4347(98)00369-7. [DOI] [PubMed] [Google Scholar]
- 7.Olive DM, Bean P. Principles and applications of methods for DNA-based typing of microbial organisms. J Clin Microbiol. 1999;37:1661–1669. doi: 10.1128/jcm.37.6.1661-1669.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lupski JR, Weinstock GM. Short, interspersed repetitive DNA sequences in prokaryotic genomes. J Bacteriol. 1992;174:4525–4529. doi: 10.1128/jb.174.14.4525-4529.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Versalovic J, Koeuth T, Lupski JR. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acid Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharples GJ, Lloyd RG. A novel repeated sequence located in the intergenic regions of bacterial chromosomes. Nucleic Acids Res. 1990:6503–6508. doi: 10.1093/nar/18.22.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hulton CSJ, Higgins CF, Sharp PM. ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli, Salmonella typhimurium and other enterobacteria. Mol Microbiol. 1991:825–834. doi: 10.1111/j.1365-2958.1991.tb00755.x. [DOI] [PubMed] [Google Scholar]
- 12.Sharp PM, Leach DRF. Palindrome-induced deletion in enterobacterial repetitive sequences. Mol Microbiol. 1996:1055–1056. doi: 10.1046/j.1365-2958.1996.01546.x. [DOI] [PubMed] [Google Scholar]
- 13.Cromie G, Collins J, Leach DRF. equence interruptions in enterobacterial repeated elements retain their ability to encode well-folded RNA secondary structure. Mol Microbiol. 1997:1311–1314. doi: 10.1046/j.1365-2958.1997.4211766.x. [DOI] [PubMed] [Google Scholar]
- 14.Sharp PM. Insertions within ERIC sequences. Mol Microbiol. 1997:1314–1315. doi: 10.1046/j.1365-2958.1997.4221767.x. [DOI] [PubMed] [Google Scholar]
- 15.Duchaud E, Rusniok C, Frangeul L. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nat Biotechnol. 2003;21:1307–1313. doi: 10.1038/nbt886. [DOI] [PubMed] [Google Scholar]
- 16.Maniatis T, Fritisch EF, Sambrook J. Molecular Clonig: A Laboratory Mannual. Cold Spring Harbor Laboratory Press ISBN. 1982:545. [Google Scholar]
- 17.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd edition. Cold Spring Harbor Laboratory Press; 1999. Bacterial diseases of potato: relevance to in vitro potato seed production. ISBN 0-87969-309-6.23. [Google Scholar]
- 18.Clermont O, Bonacorsi S, Bingen E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol. 2000;66:4555–4558. doi: 10.1128/aem.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kidd TJ, Grimwood K, Ramsay KA, Rainey PB, Bell SC. Comparison of three molecular techniques for typing Pseudomonas aeruginosa isolates in sputum samples from patients with cystic fibrosis. J Clin Microbiol. 2011;49:263–268. doi: 10.1128/JCM.01421-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macedo NR, Oliveira SR, Lage AP, Santos JL, Araujo MR, Guedes RM. ERIC-PCR genotyping of Haemophilus parasuis isolates from Brazilian pigs. Vet J. 2011;188:362–364. doi: 10.1016/j.tvjl.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 21.Shuan Ju Teh C, Thong KL, Osawa R, Heng Chua K. Comparative PCR-based fingerprinting of Vibrio cholerae isolated in Malaysia. J Gen Appl Microbiol. 2011;57:19–26. doi: 10.2323/jgam.57.19. [DOI] [PubMed] [Google Scholar]
- 22.Guimaraes Ade S, Dorneles EM, Andrade GI, Lage AP, Miyoshi A, Azevedo V, et al. Molecular characterization of Corynebacterium pseudotuberculosis isolates using ERIC-PCR. Vet Microbiol. 2011;153:299–306. doi: 10.1016/j.vetmic.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Nath G, Maurya P, Gulati AK. ERIC PCR and RAPD based fingerprinting of Salmonella Typhi strains isolated over a period of two decades. Infect Genet Evol. 2010;10:530–536. doi: 10.1016/j.meegid.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Valdes I, Jaureguiberry B, Romalde JL, Toranzo AE, Magarinos B, Avendano-Herrera R. Genetic characterization of Streptococcus phocae strains isolated from Atlantic salmon, Salmo salar L., in Chile. J Fish Dis. 2009;32:351–358. doi: 10.1111/j.1365-2761.2008.01014.x. [DOI] [PubMed] [Google Scholar]
- 25.Munoz V, Ibanez F, Tonelli ML, Valetti L, Anzuay MS, Fabra A. Phenotypic and phylogenetic characterization of native peanut Bradyrhizobium isolates obtained from Cordoba, Argentina. Syst Appl Microbiol. 2011;34:446–452. doi: 10.1016/j.syapm.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Aquino MH, Filgueiras AL, Matos R, Santos KR, Ferreira T, Ferreira MC, et al. Diversity of Campylobacter jejuni and Campylobacter coli genotypes from human and animal sources from Rio de Janeiro, Brazil. Res Vet Sci. 2010;88:214–217. doi: 10.1016/j.rvsc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Xia Y, Liang Z, Su X, Xiong Y. Characterization of carbapenemase genes in enterobacteriaceae species exhibiting decreased susceptibility to carbapenems in a university hospital in chongqing, china. Ann Lab Med. 2012;32:270–275. doi: 10.3343/alm.2012.32.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z, Liu W, Zhang Y, Li Y, Jian Z, Deng H, et al. Molecular epidemiology of carbapenem-resistant Acinetobacter spp. from XiangYa Hospital, in Hunan Province, China. J Basic Microbiol. 2012 May 14; doi: 10.1002/jobm.201100420. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Edelstein M, Pimkin M, Palagin I, Edelstein I, Stratchounski L. Prevalence and Molecular Epidemiology of CTX-M Extended-Spectrum β-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae in Russian Hospitals. Antimicrobial Agents and Chemotherapy. 2003;47:3724–3732. doi: 10.1128/AAC.47.12.3724-3732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mansouri M, Ramazanzadeh R, Norabadi P. Cefepime resistance and associated risk factors among Escherichia coli strains isolated from clinical specimens. Chemotherapy. 2011;57:134–137. doi: 10.1159/000323623. [DOI] [PubMed] [Google Scholar]
- 31.Ramazanzadeh R, Chitsaz M, Bahmani N. Prevalence and antimicrobial susceptibility of extended-spectrum beta-lactamase-producing bacteria in intensive care units of Sanandaj general hospitals (Kurdistan, Iran) Chemotherapy. 2009;55:287–292. doi: 10.1159/000224656. [DOI] [PubMed] [Google Scholar]
- 32.Mansouri M, Ramazanzadeh R. Spread of extended-spectrum beta-lactamase producing Escherichia coli clinical isolates in Sanandaj hospitals. Journal of Biological Sciences. 2009;9:362–366. [Google Scholar]
- 33.Ramazanzadeh R. Prevalence and characterization of extended-spectrum beta-lactamase production in clinical isolates of Klebsiella spp. African Journal of Microbiology Research. 2010;4:1359–1362. [Google Scholar]
- 34.Ramazanzadeh R. Etiologic agents and extended-spectrum beta-lactamase production in urinary tract infections in Sanandaj, Iran. Eastern Journal of Medicine. 2010;15:57–62. [Google Scholar]
- 35.Ramazanzadeh R, Farhadifar F, Mansouri M. Etiology and Antibiotic Resistance Patterns of Community-Acquired Extended-Spectrum Beta-Lactamase-Producing Gram Negative Isolates in Sanandaj. Research Journal of Medical Sciences. 2010;4:243–247. [Google Scholar]
- 36.Hoseini D. The Prevalence of TEM and SHV Genes among Extended-Spectrum Beta-Lactamases Producing Escherichia Coli and Klebsiella Pneumoniae. Iranian Journal of Basic Medical Sciences. 2012;15:654–660. [PMC free article] [PubMed] [Google Scholar]
- 37.Herzer PJ, Inouye S, Inouye M, Whittam TS. Phylogenetic distribution of branched RNA-linked multicopy single-stranded DNA among natural isolates of Escherichia coli. J Bacteriol. 1990;172:6175–6181. doi: 10.1128/jb.172.11.6175-6181.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson JR, Delavari P, Kuskowski M, Stell AL. Phylogenetic distribution of extraintestinal virulence-associated traits in Escherichia coli. J Infect Dis. 2001;183:78–88. doi: 10.1086/317656. [DOI] [PubMed] [Google Scholar]


