Abstract
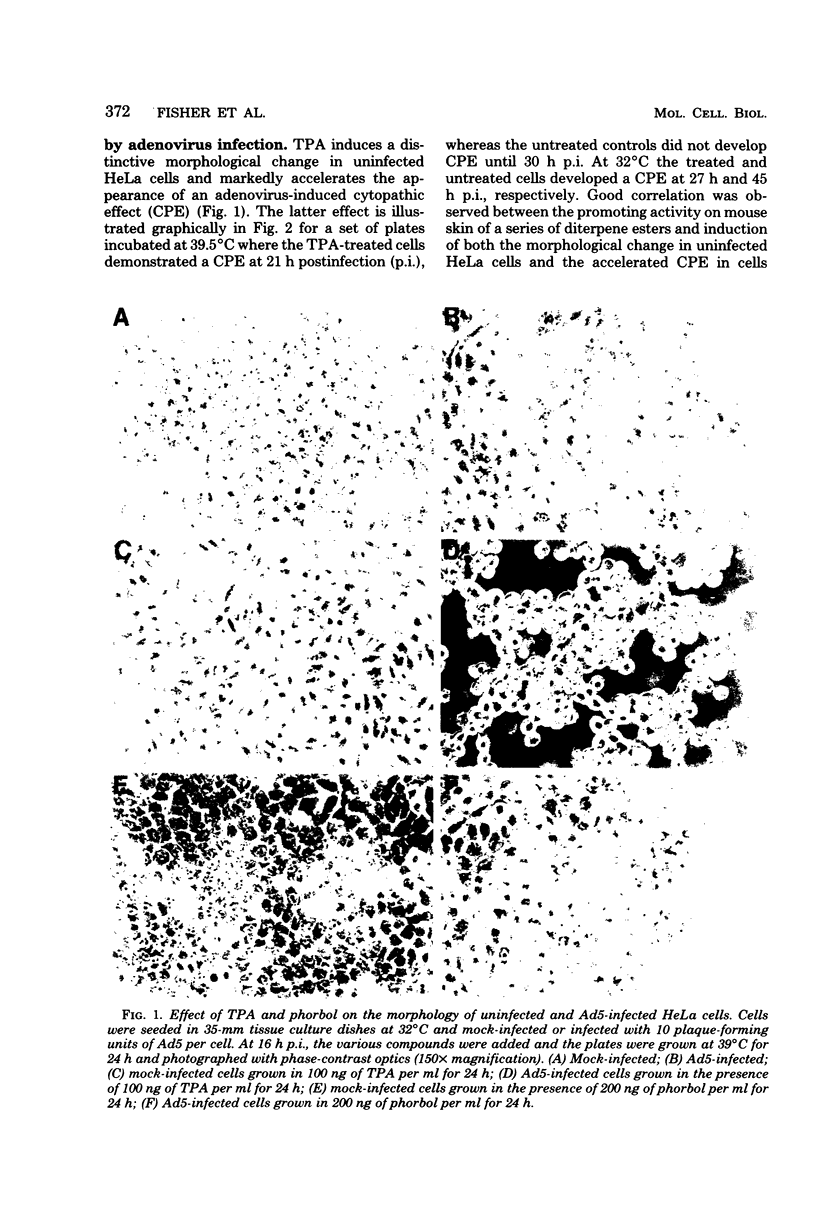
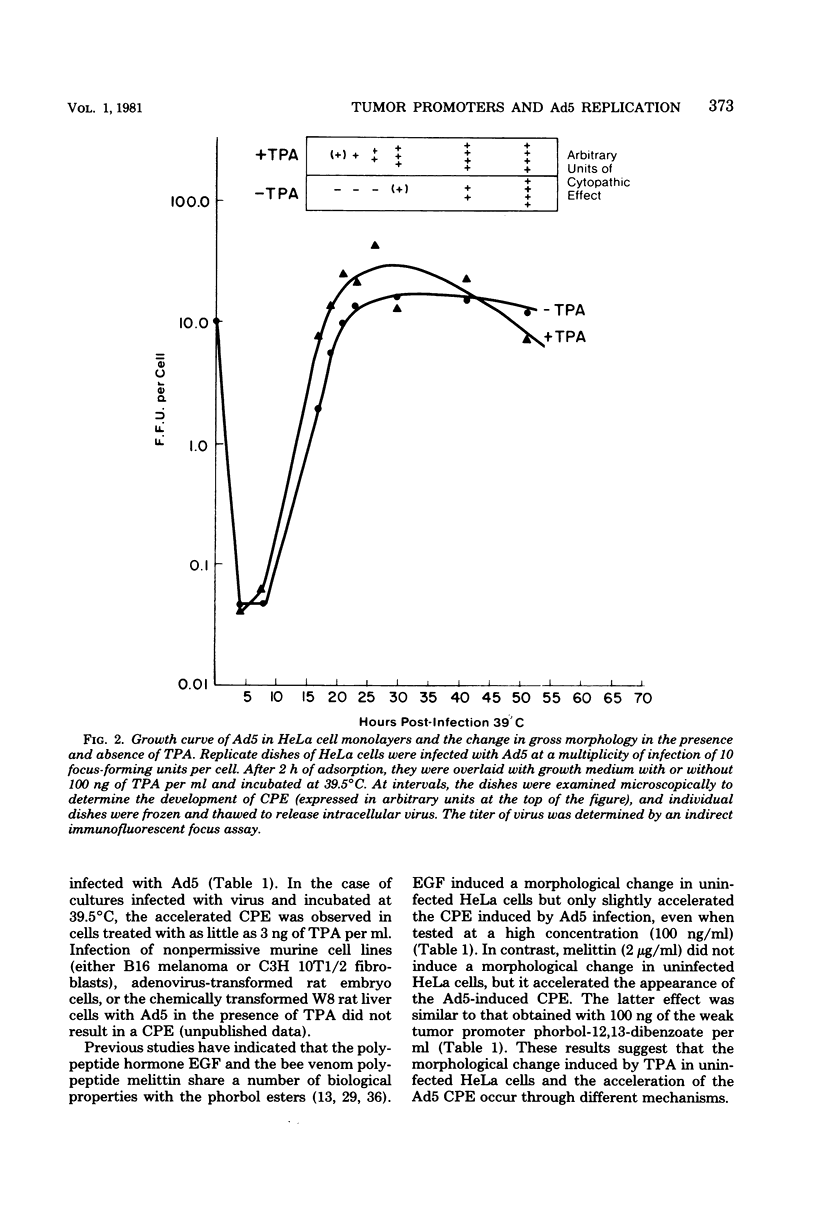
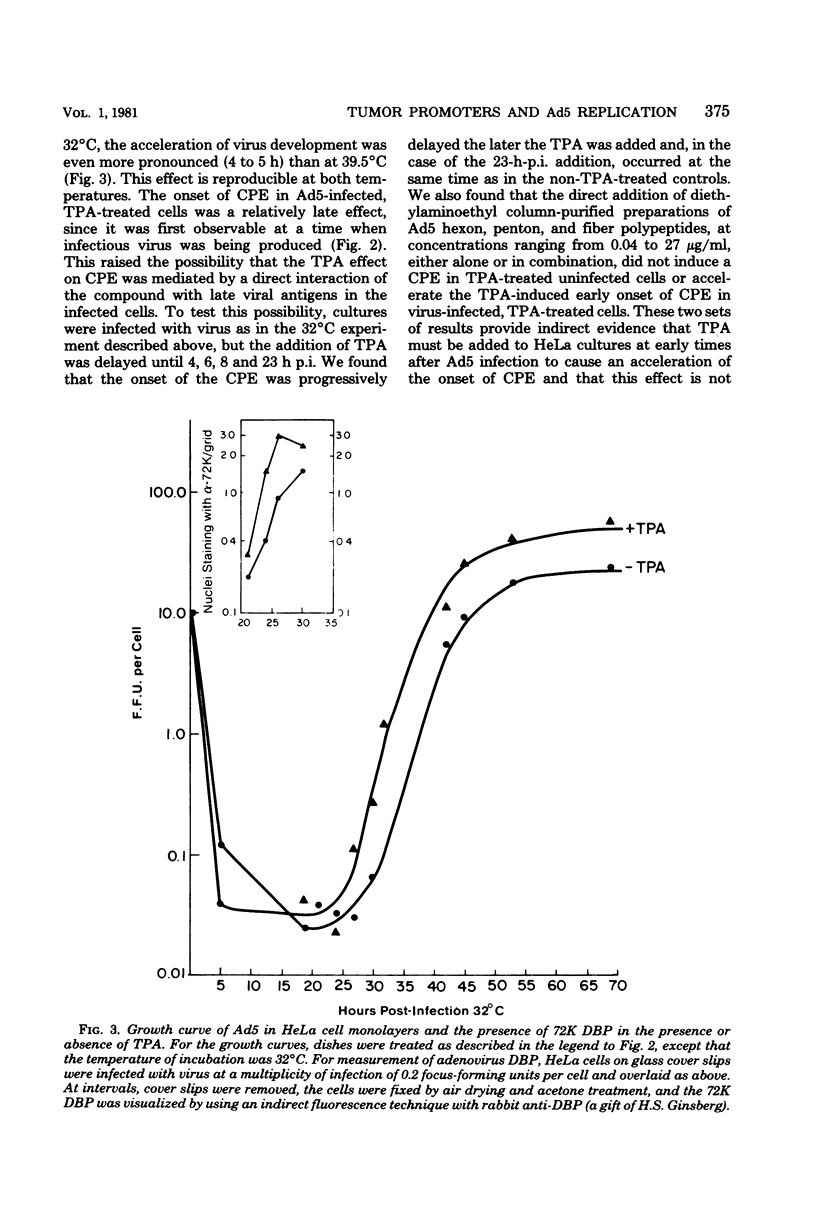
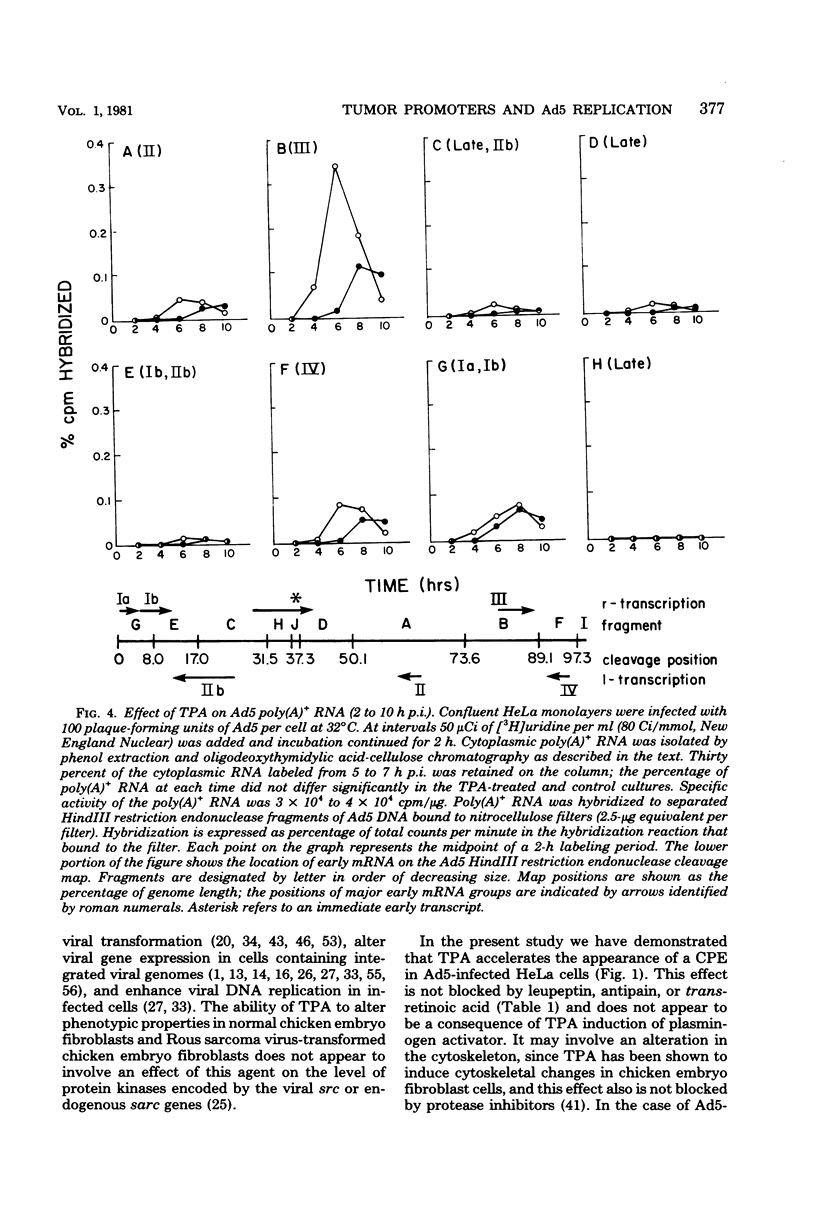
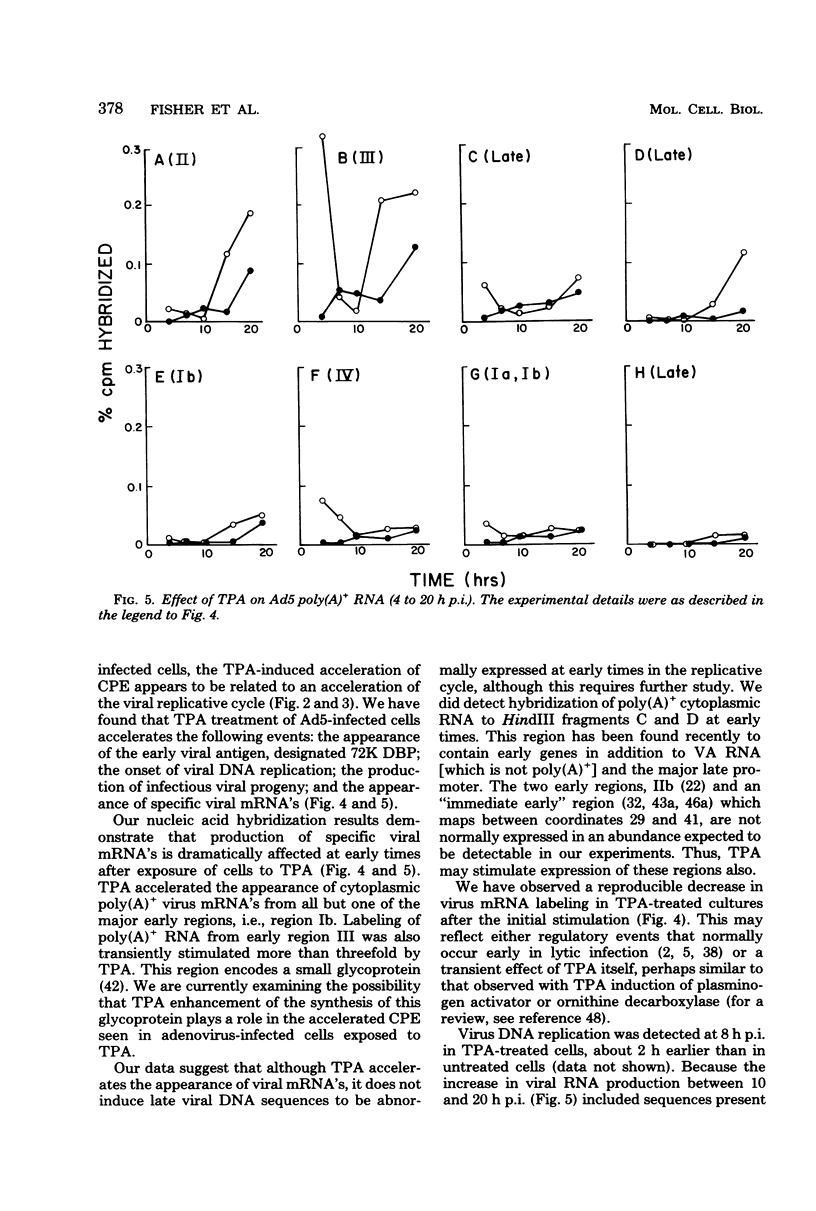
In this study we evaluated the effect of phorbol ester tumor promoters on the kinetics of adenovirus type 5 (Ad5) replication in human cells. When added at the time of infection, 12-O-tetradecanoyl-phorbol-13-acetate (TPA) accelerated the appearance of an early virus antigen (72,000-molecular-weight [72K] deoxyribonucleic acid-binding protein), the onset of viral deoxyribonucleic acid synthesis, and the production of infectious virus. The appearance of an Ad5-specific cytopathic effect (CPE) was also accelerated in infected cultures exposed to TPA, whereas phorbol, 4 alpha-phorbol-12,13-didecanoate and 4-OmeTPA, which are inactive as tumor promoters, were ineffective in inducing this morphological change. The acceleration of the CPE seen in TPA-treated Ad5-infected cells was not caused by TPA induction of the protease plasminogen activator, since the protease inhibitors leupeptin and antipain do not inhibit the earlier onset of this CPE and, in contrast, epidermal growth factor, which induces plasminogen activator in HeLa cells, does not induce an earlier CPE. Evidence for a direct effect of TPA on viral gene expression was obtained by analyzing viral messenger ribonucleic acid (mRNA) synthesis. TPA accelerated the appearance of mRNA from all major early regions of Ad5, transiently stimulated the accumulation of region III mRNA, and accelerated the appearance of late Ad5 mRNA. Thus, TPA altered the temporal program of Ad5 mRNA production and accelerated the appearance of at least some Ad5-specific polypeptides during lytic infection of human cells. These effects presumably explain the earlier onset of the Ad5-specific CPE in TPA-treated cells and may have relevance to the effects of TPA on viral gene expression in nonpermissive cells carrying integrated viral deoxyribonucleic acid sequences.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arya S. K. Phorbol ester-mediated stimulation of the synthesis of mouse mammary tumour virus. Nature. 1980 Mar 6;284(5751):71–72. doi: 10.1038/284071a0. [DOI] [PubMed] [Google Scholar]
- Blanton R. A., Carter T. H. Autoregulation of adenovirus type 5 early gene expression. III. Transcription studies in isolated nuclei. J Virol. 1979 Feb;29(2):458–465. doi: 10.1128/jvi.29.2.458-465.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Driedger P. E., Rossow P. W. Effect of a phorbol ester on a transformation-sensitive surface protein of chick fibroblasts. Nature. 1976 Dec 2;264(5585):446–447. doi: 10.1038/264446a0. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Dicker P., Rozengurt E. Inhibition of epidermal growth factor binding to surface receptors by tumor promotors. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1037–1043. doi: 10.1016/0006-291x(79)90221-3. [DOI] [PubMed] [Google Scholar]
- Carter T. H., Blanton R. A. Possible role of the 72,000 dalton DNA-binding protein in regulation of adenovirus type 5 early gene expression. J Virol. 1978 Feb;25(2):664–674. doi: 10.1128/jvi.25.2.664-674.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T. H., Ginsberg H. S. Viral transcription in KB cells infected by temperature-sensitive "early" mutants of adenovirus type 5. J Virol. 1976 Apr;18(1):156–166. doi: 10.1128/jvi.18.1.156-166.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna M., Rochette-Egly C., Rosenfeld C., Mishal Z. Altered lipid microviscosity in lymphoblastoid cells treated with 12-O-tetradecanoyl phorbol 13-acetate, a tumor promoter. FEBS Lett. 1979 Apr 1;100(1):62–66. doi: 10.1016/0014-5793(79)81131-x. [DOI] [PubMed] [Google Scholar]
- Colburn N. H., Bruegge W. F., Bates J. R., Gray R. H., Rossen J. D., Kelsey W. H., Shimada T. Correlation of anchorage-independent growth with tumorigenicity of chemically transformed mouse epidermal cells. Cancer Res. 1978 Mar;38(3):624–634. [PubMed] [Google Scholar]
- Colburn N. H., Former B. F., Nelson K. A., Yuspa S. H. Tumour promoter induces anchorage independence irreversibly. Nature. 1979 Oct 18;281(5732):589–591. doi: 10.1038/281589a0. [DOI] [PubMed] [Google Scholar]
- Craig E. A., Raskas H. J. Effect of cycloheximide on RNA metabolism early in productive infection with adenovirus 2. J Virol. 1974 Jul;14(1):26–32. doi: 10.1128/jvi.14.1.26-32.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond L., O'Brien S., Donaldson C., Shimizu Y. Growth stimulation of human diploid fibro-blasts by the tumor promoter, 12-0-tetradecanoly-phorbol-13-acetate. Int J Cancer. 1974 May 15;13(5):721–730. doi: 10.1002/ijc.2910130516. [DOI] [PubMed] [Google Scholar]
- Driedger P. E., Blumberg P. M. The effect of phorbol diesters on chicken embryo fibroblasts. Cancer Res. 1977 Sep;37(9):3257–3265. [PubMed] [Google Scholar]
- Fisher P. B., Bozzone J. H., Weinstein I. B. Tumor promoters and epidermal growth factor stimulate anchorage-independent growth of adenovirus-tranformed rat embryo cells. Cell. 1979 Nov;18(3):695–705. doi: 10.1016/0092-8674(79)90124-7. [DOI] [PubMed] [Google Scholar]
- Fisher P. B., Dorsch-Häsler K., Weinstein I. B., Ginsberg H. S. Tumour promoters enhance anchorage-independent growth of adenovirus-transformed cells without altering the integration pattern of viral sequences. Nature. 1979 Oct 18;281(5732):591–594. doi: 10.1038/281591a0. [DOI] [PubMed] [Google Scholar]
- Fisher P. B., Flamm M., Schachter D., Weinstein I. B. Tumor promoters induce membrane changes detected by fluorescence polarization. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1063–1068. doi: 10.1016/0006-291x(79)90225-0. [DOI] [PubMed] [Google Scholar]
- Fisher P. B., Goldstein N. I., Weinstein I. B. Phenotypic properties and tumor promotor-induced alterations in rat embryo cells transformed by adenovirus. Cancer Res. 1979 Aug;39(8):3051–3057. [PubMed] [Google Scholar]
- Fisher P. B., Lee L. S., Weinstein I. B. Changes in epidermal growth factor receptors associated with adenovirus transformation, chemical carcinogen transformation and exposure to a phorbol ester tumor promoter. Biochem Biophys Res Commun. 1980 Apr 29;93(4):1160–1166. doi: 10.1016/0006-291x(80)90611-7. [DOI] [PubMed] [Google Scholar]
- Fisher P. B., Weinstein I. B. Chemical-viral interactions and multistep aspects of cell transformation. IARC Sci Publ. 1980;(27):113–131. [PubMed] [Google Scholar]
- Fisher P. B., Weinstein I. B. Effects of tumor promoters and extracellular calcium on the growth of normal, transformed and temperature sensitive rat liver epithelial cells. Cancer Lett. 1980 Jul;10(1):7–17. doi: 10.1016/0304-3835(80)90059-2. [DOI] [PubMed] [Google Scholar]
- Fisher P. B., Weinstein I. B., Eisenberg D., Ginsberg H. S. Interactions between adenovirus, a tumor promoter, and chemical carcinogens in transformation of rat embryo cell cultures. Proc Natl Acad Sci U S A. 1978 May;75(5):2311–2314. doi: 10.1073/pnas.75.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galos R. S., Williams J., Binger M. H., Flint S. J. Location of additional early gene sequences in the adenoviral chromosome. Cell. 1979 Aug;17(4):945–956. doi: 10.1016/0092-8674(79)90334-9. [DOI] [PubMed] [Google Scholar]
- Gielen J., Aviv H., Leder P. Characteristics of rabbit globin mRNA purification by oligo(dT) cellulose chromatography. Arch Biochem Biophys. 1974 Jul;163(1):146–154. doi: 10.1016/0003-9861(74)90464-0. [DOI] [PubMed] [Google Scholar]
- Ginsberg H. S., Lundholm U., Linné T. Adenovirus DNA-binding protein in cells infected with wild-type 5 adenovirus and two DNA-minus, temperature-sensitive mutants, H5ts125 and H5ts149. J Virol. 1977 Jul;23(1):142–151. doi: 10.1128/jvi.23.1.142-151.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. R., Delclos K. B., Blumberg P. M. Phorbol ester action is independent of viral and cellular src kinase levels. Science. 1980 Apr 11;208(4440):191–193. doi: 10.1126/science.6244621. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Sobel M. E. Tumor promoters and Kirsten sarcoma virus increase synthesis of a secreted glycoprotein by regulating levels of translatable mRNA. Cell. 1980 Feb;19(2):449–455. doi: 10.1016/0092-8674(80)90519-x. [DOI] [PubMed] [Google Scholar]
- Hudewentz J., Bornkamm G. W., Zur Hausen H. Effect of the diterpene ester TPA on Epstein-Barr virus antigen- and DNA synthesis in producer and nonproducer cell lines. Virology. 1980 Jan 15;100(1):175–178. doi: 10.1016/0042-6822(80)90563-2. [DOI] [PubMed] [Google Scholar]
- Lawrence W. C., Ginsberg H. S. Intracellular uncoating of type 5 adenovirus deoxyribonucleic acid. J Virol. 1967 Oct;1(5):851–867. doi: 10.1128/jvi.1.5.851-867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Epidermal growth factor, like phorbol esters, induces plasminogen activator in HeLa cells. Nature. 1978 Aug 17;274(5672):696–697. doi: 10.1038/274696a0. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Membrane effects of tumor promoters: stimulation of sugar uptake in mammalian cell cultures. J Cell Physiol. 1979 Jun;99(3):451–460. doi: 10.1002/jcp.1040990319. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Tumor-promoting phorbol esters inhibit binding of epidermal growth factor to cellular receptors. Science. 1978 Oct 20;202(4365):313–315. doi: 10.1126/science.308698. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Mathews M. B. Control of adenovirus early gene expression: a class of immediate early products. Cell. 1980 Aug;21(1):303–313. doi: 10.1016/0092-8674(80)90138-5. [DOI] [PubMed] [Google Scholar]
- Lin J. C., Shaw J. E., Smith M. C., Pagano J. S. Effect of 12-O-tetradecanoyl-phorbol-13-acetate on the replication of Epstein-Barr virus. I. Characterization of viral DNA. Virology. 1979 Nov;99(1):183–187. doi: 10.1016/0042-6822(79)90052-7. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P., Edwards C. A., Vembu D. The roles of the simian virus 40 tumor antigens in transformation of Chinese hamster lung cells. Cell. 1979 Jul;17(3):635–643. doi: 10.1016/0092-8674(79)90271-x. [DOI] [PubMed] [Google Scholar]
- Mufson R. A., DeFeo D., Weinstein I. B. Effects of phorbol ester tumor promoters on arachidonic acid metabolism in chick embryo fibroblasts. Mol Pharmacol. 1979 Sep;16(2):569–578. [PubMed] [Google Scholar]
- Mufson R. A., Laskin J. D., Fisher P. B., Weinstein I. B. Melittin shares certain cellular effects with phorbol ester tumour promoters. Nature. 1979 Jul 5;280(5717):72–74. doi: 10.1038/280072a0. [DOI] [PubMed] [Google Scholar]
- Nagle D. S., Blumberg P. M. Activity of phorbol ester tumor promoters on enucleated Swiss 3T3 cells. Cancer Res. 1980 Apr;40(4):1066–1072. [PubMed] [Google Scholar]
- Nevins J. R., Winkler J. J. Regulation of early adenovirus transcription: a protein product of early region 2 specifically represses region 4 transcription. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1893–1897. doi: 10.1073/pnas.77.4.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi K., Levine L. Stimulation of prostaglandin synthesis by tumor-promoting phorbol-12, 13-diesters in canine kidney (MDCK) cells. Cycloheximide inhibits the stimulated prostaglandin synthesis, deacylation of lipids, and morphological changes. J Biol Chem. 1978 Jul 10;253(13):4783–4790. [PubMed] [Google Scholar]
- Quigley J. P. Phorbol ester-induced morphological changes in transformed chick fibroblasts: evidence for direct catalytic involvement of plasminogen activator. Cell. 1979 May;17(1):131–141. doi: 10.1016/0092-8674(79)90301-5. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B., Crowe R. M., Pollack R. Tumor promoters induce changes in the chick embryo fibroblast cytoskeleton. Cell. 1979 Oct;18(2):361–368. doi: 10.1016/0092-8674(79)90055-2. [DOI] [PubMed] [Google Scholar]
- Ross S., Levine A. J. The genomic map position of the adenovirus type 2 glycoprotein. Virology. 1979 Dec;99(2):427–430. doi: 10.1016/0042-6822(79)90023-0. [DOI] [PubMed] [Google Scholar]
- Seif R. Factors which disorganize microtubules or microfilaments increase the frequency of cell transformation by polyoma virus. J Virol. 1980 Nov;36(2):421–428. doi: 10.1128/jvi.36.2.421-428.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., De Larco J. E., Todaro G. J. Biologically active phorbol esters specifically alter affinity of epidermal growth factor membrane receptors. Nature. 1979 May 31;279(5712):387–391. doi: 10.1038/279387a0. [DOI] [PubMed] [Google Scholar]
- Sundar S. K., Ablashi D. V., Bengali Z. H., Levine P. H., Nonoyama M. Mitogenic effect of 12-0 tetradecanoyl phorbol 13-acetate on non-human primate mononuclear cells and in vitro interaction with Epstein-Barr virus transformation. Arch Virol. 1980;64(2):141–153. doi: 10.1007/BF01318018. [DOI] [PubMed] [Google Scholar]
- Weinstein I. B., Lee L. S., Fisher P. B., Mufson A., Yamasaki H. Action of phorbol esters in cell culture: mimicry of transformation, altered differentiation, and effects on cell membranes. J Supramol Struct. 1979;12(2):195–208. doi: 10.1002/jss.400120206. [DOI] [PubMed] [Google Scholar]
- Wigler M., DeFeo D., Weinstein I. B. Induction of plasminogen activator in cultured cells by macrocyclic plant diterpene esters and other agents related to tumor promotion. Cancer Res. 1978 May;38(5):1434–1437. [PubMed] [Google Scholar]
- Wigler M., Weinstein I. B. Tumour promotor induces plasminogen activator. Nature. 1976 Jan 22;259(5540):232–233. doi: 10.1038/259232a0. [DOI] [PubMed] [Google Scholar]
- Williams J. F. Enhancement of adenovirus plaque formation on HeLa cells by magnesium chloride. J Gen Virol. 1970 Dec;9(3):251–255. doi: 10.1099/0022-1317-9-3-251. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., zur Hausen H. Tumour promoter TPA enhances transformation of human leukocytes by Epstein-Barr virus. Nature. 1979 Jul 19;280(5719):244–245. doi: 10.1038/280244a0. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Lichti U., Ben T., Patterson E., Hennings H., Slaga T. J., Colburn N., Kelsey W. Phorbol esters stimulate DNA synthesis and ornithine decarboxylase activity in mouse epidermal cell cultures. Nature. 1976 Jul 29;262(5567):402–404. doi: 10.1038/262402a0. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., Bornkamm G. W., Schmidt R., Hecker E. Tumor initiators and promoters in the induction of Epstein-Barr virus. Proc Natl Acad Sci U S A. 1979 Feb;76(2):782–785. doi: 10.1073/pnas.76.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., O'Neill F. J., Freese U. K., Hecker E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature. 1978 Mar 23;272(5651):373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]




