Abstract
Mice deficient in the transcription factor methyl-CpG-binding protein 2 (Mecp2), a mouse model of Rett syndrome, display reduced CO2 chemosensitivity, which may contribute to their breathing abnormalities. In addition, patients with Rett syndrome and male mice that are null for Mecp2 show reduced levels of brain serotonin (5-HT). Serotonin is known to play a role in central chemosensitivity, and we hypothesized that increasing the availability of 5-HT in this mouse model would improve their respiratory response to CO2. Here we determined the apnoeic threshold in heterozygous Mecp2-deficient female mice and examined the effects of blocking 5-HT reuptake on the CO2 response in Mecp2-null male mice. Studies were performed in B6.129P2(C)-Mecp2τm1.1Bird null males and heterozygous females. In an in situ preparation, seven of eight Mecp2-deficient heterozygous females showed arrest of phrenic nerve activity when arterial CO2 was lowered to 3%, whereas the wild-types maintained phrenic nerve amplitude at 53 ± 3% of maximal. In vivo plethysmography studies were used to determine CO2 chemosensitivity in null males. These mice were exposed sequentially to 1, 3 and 5% CO2. The percentage increase in minute ventilation in response to increased inspired CO2 was less in Mecp2−/y than in Mecp2+/y mice. Pretreatment with citalopram, a selective 5-HT reuptake inhibitor (2.5 mg kg−1 I.P.), 40 min prior to CO2 exposure, in Mecp2−/y mice resulted in an improvement in CO2 chemosensitivity to wild-type levels. These results suggest that decreased 5-HT in Mecp2-deficient mice reduces CO2 chemosensitivity, and restoring 5-HT levels can reverse this effect.
A recent study showed that CO2 chemosensitivity is depressed in male mice with deletion of the transcription factor methyl-CpG-binding protein 2 (Mecp2; Zhang et al. 2011). MECP2 is mutated in the autism spectrum disorder Rett syndrome (RTT), and the phenotype includes abnormal respiration characterized by hyperventilation followed by apnoea (Southall et al. 1988; Smeets et al. 2006). An elevated CO2 threshold in RTT would contribute to apnoea, and significant hypocapnia has been reported in affected individuals (Southall et al. 1988). Zhang and co-authors reported a blunted CO2 response, relative to wild-type (WT) mice, at 1, 2 and 3% CO2, but no difference at 6 and 9%. Earlier reports had not shown a difference in the respiratory response to CO2 between Mecp2-deficient and WT mice (Bissonnette & Knopp, 2006; Voituron et al. 2009). Bissonnette & Knopp (2006) used CO2 concentrations of 3, 5 and 7%, with the balance being oxygen. The hyperoxia would blunt contributions from peripheral chemoreceptors that are important to the integrated CO2 response (Forster et al. 2000; Blain et al. 2010; Ramanantsoa et al. 2011). Voituron and co-authors (2009) used a single CO2 level of 4% in air. In view of the findings of Zhang et al. (2011), which showed no difference in response to CO2 at higher levels (i.e. 6 and 9%), this single 4% exposure may have failed to show the depressed respiratory response in Mecp2-null mice that is present at 1 and 3% CO2.
Subjects with RTT and mouse models have deficits in serotonin (5-HT). 5-Hydroxyindoleacetic acid levels are low in the cerebrospinal fluid of individuals with RTT (Samaco et al. 2009). Brain 5-HT levels in Mecp2-null males, while initially normal, are depressed at postnatal day 42 and thereafter (Ide et al. 2005; Viemari et al. 2005; Panayotis et al. 2011). In addition, a recent study has shown that a serotonin 1a receptor agonist reduces apnoea, corrects the irregular breathing pattern and prolongs survival in Mecp2-null males (Abdala et al. 2010). The importance of 5-HT in CO2 chemosensitivity is well documented (reviewed by Hilaire et al. 2010; Hodges & Richerson, 2010a,b). The goal of the present study was to determine whether increasing brain 5-HT will correct the blunted CO2 response in Mecp2-deficient mice.
Methods
Ethical approval
The experiments were performed according to the UK Home Office Animals (Scientific Procedures) Act (1986) and were in agreement with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996).
Animals
Studies were performed with B6.129P2(C)-Mecp2τm1.1Bird mice (Jackson Laboratory, Bar Harbor, ME, USA; stock no. 003890) and their WT littermates. Heterozygous females were between 4.0 and 18.7 months of age and null males between postnatal day 40 and 72 at the time of study. Heterozygous female mice were used because they are thought to better recapitulate the disease in female human patients. Mecp2-null males were chosen for the citalopram protocol because they have been shown to have depressed brain 5-HT concentrations (Ide et al. 2005; Viemari et al. 2005; Panayotis et al. 2011).
In situ studies
We used an in situ decerebrated ‘working heart–brainstem preparation’ of the mouse (Paton, 1996), which is a modified version of cardiopulmonary bypass and allows direct recordings to be made from the phrenic nerve (PN), as well as the ability to manipulate the arterial CO2 concentration precisely. This preparation has previously been used to define an apnoeic threshold in juvenile rats (St-John & Paton, 2000) and is not confounded by depressant action of anaesthetic agents or changes in sleep/arousal states. Briefly, female heterozygous Mecp2−/+ mice were heparinized (1500 IU I.P.) and subsequently anaesthetized deeply with halothane until loss of their paw withdrawal reflex. Mice were decerebrated, then bisected subdiaphragmatically and the head and thorax immersed in ice-cold carbogenated artificial cerebrospinal fluid. The thoracic phrenic nerve was cut distally. Preparations were moved to a recording chamber. A double-lumen catheter (Braintree Scientific, Braintree, MA, USA) was inserted into the descending aorta for retrograde perfusion by a peristaltic roller pump (505Du; Watson Marlow, Falmouth, UK). The perfusion solution consisted of carbogenated modified Ringer solution at 32°C with an oncotic agent replacing serum albumin (Ficoll 1.25%). The second lumen of the catheter was used to monitor aortic perfusion pressure. The baseline perfusate flow was preset between 18 and 20 ml min−1.
The level of CO2 within the perfusate was monitored using an in-line gas analyser (FM2 CO2 analyser; P.K. Morgan, Sequim, WA, USA), and the mix of CO2 to oxygen was altered manually to achieve different levels of CO2 ranging from 3 to 9% in 3% incremental steps (balance oxygen). Maximal phrenic amplitude was obtained with 7 or 9% CO2 in the perfusate.
Arterial pressure, ECG and phrenic nerve bursts (via a suction electrode) were recorded in Spike2 software (Cambridge Electronic Design, Cambridge, UK) on a PC. Data were saved to disk and analysed offline.
Plethysmography studies
Respiratory frequency, tidal volume (VT) and their product, minute ventilation (V̇E), were determined in a body plethysmograph (Mortola & Noworaj, 1983; Bissonnette & Knopp, 2006). Briefly, individual unanesthetized animals were placed in a 65 ml chamber with their head exposed through a close-fitting hole in Parafilm.® A pneumotachograph (Mortola & Noworaj, 1983) was connected to the chamber and a differential pressure transducer (Model PT5A; Grass Instrument Co., West Warwick, RI, USA). The pressure signal was integrated to give tidal volume. Volume changes were calibrated by injecting known amounts of air into the chamber. The analog signal from the transducer was amplified, converted to digital, displayed on a monitor, and stored to computer disk for later analysis. The studies were begun after the animal was given time to become adjusted to the chamber. A cone was fitted over the animal’s head that allowed inspired gases to be delivered. The gas was rapidly (5 s) changed in succession from air to 1, 3 and then 5% CO2, with the balance being air. The last 2 min of each 5 min exposure to air and to CO2 was used for analysis (as used previously by Davis et al. 2006). Segments of the record that contained apnoea were not used to calculate frequency. The minute ventilation in response to CO2 is reported as the percentage increase relative to the control. Recorded data were analysed with custom functions in Chart v5.5.6 (AD Instruments Inc., Colorado Springs, CO, USA). In order to determine the effect of raising brain 5-HT in Mecp2-null males, citalopram (2.5 mg kg−1, I.P.) was administered 40 min prior to a CO2 study. This dose and time interval were based on a microdialysis study that showed effective increases in 5-HT in the prefrontal cortex of mice (Calcagno et al. 2007).
Analysis
Results are reported as means ± SEM. Comparisons between Mecp2−/+ and WT phrenic amplitude in the in situ studies were made with Student’s unpaired t test. Comparisons between Mecp2−/y mice and WT and between control and citalopram treatment were made with one-way repeated-measures ANOVA and the Bonferroni method used for post hoc analysis. A P value of <0.05 was used for significance.
Results
Effect of lowering CO2 in the in situ preparation
Previous studies of CO2 chemosensitivity in Mecp2-deficient mice have used additions to inspired gases (Bissonnette & Knopp, 2006; Voituron et al. 2009; Zhang et al. 2011) that preclude an estimate of apnoeic threshold. Arterial perfusion in the in situ preparation allowed us to lower the CO2 concentration to a level that resulted in phrenic apnoea (see representative trace in Fig. 1A). Six of seven Mecp2-deficient heterozygous females (Mecp2−/+) showed arrest of phrenic nerve activity when CO2 was lowered to 3% (Fig. 1A and B). The seventh animal had a phrenic amplitude that was 38.6% of its maximal value. In two Mecp2−/+ mice, CO2 was lowered to only 4%. One became apnoeic, and in the other the phrenic amplitude fell to 55.9% of maximal. In the Mecp2−/+ mice that developed apnoea, phrenic activity returned in 126 ± 42 s after the perfusate was set back to 6% CO2. In contrast to Mecp2−/+ mice, phrenic amplitude was sustained at 53 ± 3% of maximal in WT females perfused with 3% CO2 (n = 7). Intermediate CO2 levels resulted in non-significant decreases in phrenic amplitude in Mecp2−/+ mice compared with WT; 72.2 ± 7.5 versus 81.2 ± 2.4% of maximal at 6% CO2 and 62.5 ± 7.6 versus 71.5 ± 4.7% at 5% CO2.
Figure 1. The apnoeic CO2 threshold is higher in Mecp2+/− mice in situ.
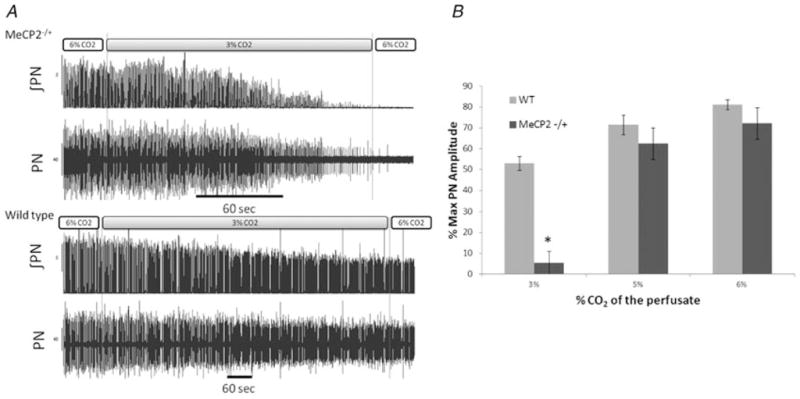
A, representative phrenic nerve (PN) traces for wild-type and Mecp2−/+ female mice. Recordings were made in situ at baseline (6% arterial CO2) and 3% CO2 and show both raw and integrated data. The Mecp2+/− mice become apnoeic at 3% CO2, whereas the WT do not. Note the different time scales. B, summary of PN response to changes in arterial CO2 for wild-type (n = 5–7) and Mecp2−/+ mice (n = 5–7). Six of seven Mecp2−/+ mice were apnoeic at 3% CO2. In two Mecp2−/+ mice, CO2 was only lowered to 4%. One became apnoeic, and phrenic amplitude decreased to 55.9% of maximal in the other animal. Values are means ± SEM. * P = <0.001, Student’s unpaired t test.
Carbon dioxide chemosensitivity in Mecp2-null males in vivo
Baseline respiratory parameters were similar in Mecp2-null males (Mecp2−/y; n = 9) and WT (n = 10; frequency, 250 ± 11 versus 242 ± 11 breaths min−1; VT, 5.0 ± 0.6 versus 5.5 ± 0.6 μl g−1; and VE, 1.23 ± 0.17 versus 1.29 ± 0.09 ml g−1) as was age at time of study (48.8 ± 2.2 versus 47.9 ± 2.1 days; ANOVA). The response to CO2 in Mecp2−/y mice was significantly depressed at all three levels of inspired gas (P = 0.013 at 1%, P = 0.02 at 3% and P = 0.049 at 5% CO2; ANOVA; Fig. 2A).
Figure 2. Summary of CO2 chemosensitivity.
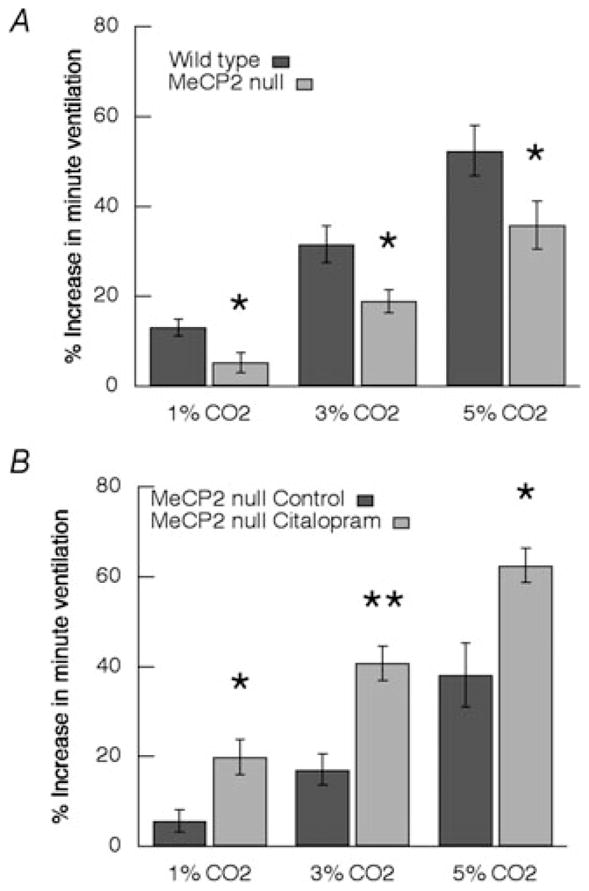
A, increase in minute ventilation in wild-type (n = 10) compared with Mecp2-null mice (n = 9). B, effect of citalopram (2.5 mg kg−1) on CO2 response in Mecp2-null mice (n = 6); * P ≤ 0.05, * * P ≤ 0.01 (ANOVA).
Effect of citalopram on CO2 chemosensitivity in male mice
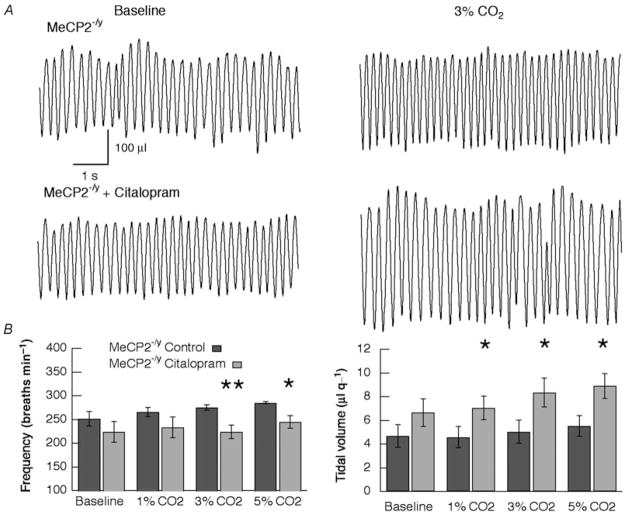
Forty minutes after administering the 5-HT reuptake blocker citalopram (2.5 mg kg−1. I P.), respiratory frequency was increased from 242 ± 11 to 284 ± 9 breaths min−1 (P = 0.007, Student’s paired t test) in WT mice (Table 1). There was a tendency for V̇E also to increase (1.58 ± 0.08 versus 1.29 ± 0.09 ml g−1; P = 0.054) in WT. The reuptake antagonist did not significantly affect baseline respiratory parameters in Mecp2−/y mice (frequency, 250 ± 11 versus 224 ± 22 breaths min−1; V̇E, 5.0 ± 0.6 versus 6.6 ± 1.2 μl g−1; and VT, 1.23 ± 0.17 versus 1.39 ± 0.17 ml g−1; n = 6). This dose of the 5-HT reuptake blocker significantly increased the CO2 response at all levels of inspired gas in Mecp2−/y mice (P = 0.04 at 1%, P = 0.003 at 3% and P = 0.002 at 3%; ANOVA; Fig. 2B). The increases in minute ventilation that resulted after pretreatment with citalopram restored CO2 chemosensitivity in Mecp2−/y mice to that of WT, but baseline ventilation was unaffected. In the baseline study prior to administration of citalopram, increases in both respiratory frequency and tidal volume contributed to the V̇E response to CO2 (Fig. 3B). After inhibition of 5-HT reuptake, VT alone accounted for the increase in V̇E (Fig. 3). Indeed, frequency decreased at 3 and 5% CO2 compared with the corresponding CO2 response during baseline conditions (Fig. 3B). In contrast to the effects of citralopram in Mecp2-deficient males, the drug did not significantly change CO2 responses in WT mice (n = 8; data not shown).
Table 1.
Baseline respiratory parameters
| Strain | Age (days) | Weight (g) | Treatment | Number | Frequency (breaths min−1) | Tidal volume (μl g−1) | Minute ventilation (ml g−1) |
|---|---|---|---|---|---|---|---|
| Mecp2+/y | 47.9 ± 2.1 | 21.5 ± 0.4 | Control | 10 | 242 ± 11 | 5.5 ± 0.6 | 1.29 ± 0.09 |
| Citalopram | 8 | 284 ± 9* | 5.6 ± 0.4 | 1.58 ± 0.08 | |||
| Mecp2−y | 48.4 ± 2.2 | 20.1 ± 1.4 | Control | 9 | 251 ± 11 | 5.0 ± 0.6 | 1.23 ± 0.17 |
| Citalopram | 6 | 224 ± 22 | 6.6 ± 1.2 | 1.39 ± 0.17 |
Values are means ± SEM.
P = 0.007 compared with control (Student’s paired t test).
Figure 3. Respiratory pattern in Mecp2-null male mice before and after citalopram.
A, representative traces from a single mouse showing respiratory pattern during baseline and during 3% CO2 inspired gas. The top two traces were obtained before and the bottom two traces after administration of citalopram (2.5 mg kg−1 I.P.) to block 5-HT reuptake. Calibration bars are the same for all panels. B, summary of effects of citalopram on respiratory frequency (left panel) and tidal volume (right panel) in Mecp2−/y mice (n = 6). * P ≤ 0.05, * * P ≤ 0.01 (ANOVA).
Discussion
This report shows that arrest of activity in the phrenic nerve of heterozygous Mecp2-deficient mice occurs at a CO2 concentration that sustains discharge in wild-type animals at half their maximal amplitude. In addition, the results reveal that a depressed 5-HT concentration in Mecp2-null male mice underlies their blunted CO2 chemosensitivity. The results add to those of Zhang et al. (2011), who studied Mecp2-null males, by demonstrating an altered CO2 apnoeic threshold in heterozygous females. Their previous work showed that 5–7 days of treatment with the noradrenaline reuptake blocker desipramine partly (50–70%) restored sensitivity to WT levels. Here we add serotonin to the neuromodulators whose deficiency contributes to the blunted CO2 response in Mecp2-deficient mice.
The Mecp2-null males exposed to 1, 3 and 5% inspired CO2 in air showed depressed ventilatory response at all levels. The heterozygous female mice with arterial perfused artificial cerebrospinal fluid at 5 and 6% CO2 in balance oxygen displayed a reduced phrenic activity compared with WT, but the result was not significant. The difference most probably lies in the oxygen concentrations used. With hyperoxia, the peripheral carotid body ventilator drive (oxygen sensor) will be depressed, but CO2 will still have a peripheral stimulatory effect. This peripheral CO2 drive is important for setting the apnoeic threshold, as seen when the perfusate is lowered to 3% CO2. The results suggest that peripheral CO2 contributes less to elevated carbon dioxide responses.
Most previous studies examining the role of 5-HT have concentrated on the effects of lowering its level on chemosensitivity. The hypercapnic ventilatory response was decreased by 50% in mice with near-complete absence of central 5-HT neurons (Hodges et al. 2008). This marked lack of 5-HT neurons was achieved by using a conditional knockout mouse in which Lmx1b is deleted in Pet-1-expressing cells. A lesser effect was observed in Pet-1-null animals (Hodges et al. 2011). These animals had 73% fewer tryptophan hydroxylase-immunoreactive neurons in mid-line raphe and 50% fewer in ventrolateral medulla compared with WT mice. At 7% inspired CO2, minute ventilation was 40% depressed in male mice. There was no significant effect on CO2 sensitivity at 3 or 4%. In addition, female mice lacking Pet-1 did not show a depressed CO2 response. The effect of loss of 5-HT transporter has been examined (Li & Nattie, 2008). These mice develop with an elevated brain 5-HT environment and, as adults, have decreased brain 5-HT content. Minute ventilation in 5% CO2 was 50% less in males that lacked the 5-HT transporter, but there was no effect in females (Li & Nattie, 2008). Recently, the confounding effects of germline removal of 5-HT genes has been eliminated by a genetic strategy that allowed ligand-induced acute hyperpolarization of 5-HT neurons (Ray et al. 2011). Baseline ventilation was not affected by the ligand. The V̇E, however, was depressed by 47% compared with control mice on exposure to 5% CO2. Depletion of 5-HT may also affect the interaction between peripheral and central CO2 chemosensitivity. Depletion of 5-HT neurons that ranged from 37 to 68% at various caudal–rostral levels in the nucleus raphe obscurus of rats when combined with carotid body removal depressed the response to 7% CO2 by 31.2%. This depression was greater than the sum of 5-HT depletion in raphe obscurus alone and carotid body removal alone (da Silva et al. 2011). Conversely, Depuy et al. (2011) recently showed that selective optogenetic stimulation of serotoninergic raphe obscurus neurons increases ventilation and potentiates ventilatory responses to high CO2 in mice.
Our observation that an increase in central 5-HT in mice whose serotonin levels are low restores CO2 chemosensitivity is not singular. In the animals with near-complete absence of 5-HT neurons discussed above (Hodges et al. 2008), the authors used an intracerebroventricular infusion of 5-HT for 2 days. On the second day of infusing 1 and 5 mM 5-HT, the CO2 response was restored to WT levels. It was estimated that these infusions effected a cerebrospinal fluid 5-HT concentration of 55–275 μM. This estimate is considerably higher than the 0.25 nM found with microdialysis studies of extracellular 5-HT in medulla and prefrontal cortex (Taylor & Basbaum, 1995; Calcagno et al. 2007). Following I.P. injection of citalopram (1.25 mg kg−1) in C57BL/6J mice, prefrontal cortex 5-HT increased by 50%, and after 5.0 mg kg−1 it increased twofold (Calcagno et al. 2007). The intermediate dose used (2.5 mg kg−1) in the present study in Mecp2-null males corrected the CO2 sensitivity to WT levels.
In normal rats, microdialysis of the 5-HT reuptake inhibitor fluoxetine into raphe magnus augmented the response to 7% CO2 (Taylor et al. 2004). There was no effect at 1 day of treatment, and changes were present at 7 and 15 days. Additionally, systemic fluoxetine, administered via osmotic minipump, did not alter the response to CO2. These results are consistent with our findings in WT mice, where acute systemic 5-HT reuptake inhibition did not affect CO2 chemosensitivity.
In Mecp2-null male mice pretreated with citalopram to increase brain 5-HT, the increase in V̇E with added inspiratory CO2 was entirely due to increases in VT (Fig. 3). In man, this pattern, an increase in tidal volume and no change in frequency, was observed during pressure-support ventilation (Georgopoulos et al. 1997). The hyperventilation significantly lowered the end-tidal CO2. Assuming that the pattern had the same effect in mice, this would result in lower arterial CO2 in the postcitalopram study compared with the baseline study, where the response to CO2 showed increases in both frequency and in VT.
Recent studies have shown that both astrocytes (Lioy et al. 2011) and microglia (Derecki et al. 2012) play important roles in generating apnoea and breath cycle irregularity in mouse models of RTT. These cell types express 5-HT receptors (Whitaker-Azmitia & Azmitia, 1994; Krabbe et al. 2012); therefore, glia may contribute to the depressed CO2 chemosensitivity seen in Mecp2-deficient mice.
Reports in Mecp2-deficient mice that established depressed brain 5-HT have not completely elucidated the underlying mechanisms (Ide et al. 2005; Viemari et al. 2005; Panayotis et al. 2011). The number of 5-HT neurons is not reduced (Viemari et al. 2005), indicating that synthesis, secretion or turnover must be affected. The reported increase in 5-hydroxyindoleacetic acid:5-HT ratio (Ide et al. 2005; Viemari et al. 2005; Panayotis et al. 2011) is consistent with an elevated turnover. Recently, Nguyen et al. (2012) measured a number of synaptic proteins in male mice whose Mecp2 protein was markedly depleted at 5 weeks postnatal. Two of the synaptic proteins that were depressed may contribute to the low level of 5-HT in brain of Mecp2-deficient mice. Synapsin 1 has been shown to regulate 5-HT release (Browning et al. 1985) and calcium, calmodulin-dependent protein kinase II phosphorylation of tryptophan hydroxylase 2 increases the activity of this enzyme that is rate limiting for 5-HT production (Kuhn et al. 2007).
The present findings that there is an elevated apnoeic threshold in heterozygous Mecp2-deficient female mice and that an agent which blocks 5-HT reuptake corrects defective CO2 chemosensitivity have clinical implications. Hypocapnia secondary to hyperventilation has been documented in RTT subjects (Southall et al. 1988; Smeets et al. 2006) and, in one case, supplemental CO2 was reported to be beneficial (Smeets et al. 2006). In addition, one of two patients with low cerebrospinal fluid 5-HT metabolite was clinically improved when treated with the reuptake blocker fluoxetine, although breathing was not mentioned (Temudo et al. 2009). A separate single case report found marked reduction in hyperventilation/apnoeic episodes following treatment with fluoxetine (Gökben et al. 2012). Although the underlying mechanisms generating rhythm irregularity, apnoeas and low CO2 sensitivity are likely to be separate, there is strong evidence that low CO2 sensitivity could exacerbate periods of hyperventilation followed by apnoeas (Khoo, 2000).
Our findings also have special significance for the critical care of Rett syndrome patients suffering from acute respiratory distress. Patients on the more severe end of the spectrum often experience periods of severe recurrent apnoeas (or breath-holds) that result in low oxygen saturation (<60%), with some requiring hospital admission and ventilatory support. Currently, there are no standardized critical care guidelines for treating low oxygen saturation due to severe episodes of apnoeas in Rett patients. Our data indicate that interventions to increase oxygen saturation that may also result in even mild lowering of partial pressures of CO2 may further exacerbate the episodes of breath holding.
In summary, we have shown that heterozygous Mecp2-deficient female mice have a hypocapnic threshold that exceeds that of wild-type mice. In addition, we have confirmed that Mecp2-null males have a depressed response to CO2 and have extended this finding to show that elevation of brain 5-HT corrects their CO2 chemosensitivity.
New Findings.
-
What is the central question of this study?
Mice deficient in methyl-CpG-binding protein 2 (Mecp2; a model of Rett syndrome) display reduced CO2 chemosensitivity, which may contribute to their breathing abnormalities. Patients and mice show reduced levels of brain 5-HT, which is important in central chemosensitivity. It is not known whether increasing 5-HT in this mouse model would improve their respiratory response to CO2.
-
What is the main finding and its importance?
We show, for the first time, that females heterozygous for the Mecp2 mutation have a higher hypocapnic threshold for apnoeas versus wild-type mice and that increasing 5-HT in Mecp2-null males, with citalopram, can correct CO2 chemosensitivity, a finding that may be important clinically.
Acknowledgments
This work was supported by New Life Foundation for Disabled Children, UK and the International Rett Syndrome Foundation.
References
- Abdala AP, Dutschmann M, Bissonnette JM, Paton JF. Correction of respiratory disorders in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2010;107:18208–18213. doi: 10.1073/pnas.1012104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette JM, Knopp SJ. Separate respiratory phenotypes in methyl-CpG-binding protein 2 (Mecp2) deficient mice. Pediatr Res. 2006;59:513–518. doi: 10.1203/01.pdr.0000203157.31924.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blain GM, Smith CA, Henderson KS, Dempsey JA. Peripheral chemoreceptors determine the respiratory sensitivity of central chemoreceptors to CO2. J Physiol. 2010;588:2455–2471. doi: 10.1113/jphysiol.2010.187211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning MD, Huganir R, Greengard P. Protein phosphorylation and neuronal function. J Neurochem. 1985;45:11–23. doi: 10.1111/j.1471-4159.1985.tb05468.x. [DOI] [PubMed] [Google Scholar]
- Calcagno E, Canetta A, Guzzetti S, Cervo L, Invernizzi RW. Strain differences in basal and post-citalopram extracellular 5-HT in the mouse medial prefrontal cortex and dorsal hippocampus: relation with tryptophan hydroxylase-2 activity. J Neurochem. 2007;103:1111–1120. doi: 10.1111/j.1471-4159.2007.04806.x. [DOI] [PubMed] [Google Scholar]
- da Silva GS, Giusti H, Benedetti M, Dias MB, Gargaglioni LH, Branco LG, Glass ML. Serotonergic neurons in the nucleus raphe obscurus contribute to interaction between central and peripheral ventilatory responses to hypercapnia. Pflugers Arch. 2011;462:407–418. doi: 10.1007/s00424-011-0990-x. [DOI] [PubMed] [Google Scholar]
- Davis SE, Solhied G, Castillo M, Dwinell M, Brozoski D, Forster HV. Postnatal developmental changes in CO2 sensitivity in rats. J Appl Physiol. 2006;101:1097–1103. doi: 10.1152/japplphysiol.00378.2006. [DOI] [PubMed] [Google Scholar]
- Depuy SD, Kanbar R, Coates MB, Stornetta RL, Guyenet PG. Control of breathing by raphe obscurus serotonergic neurons in mice. J Neurosci. 2011;31:1981–1990. doi: 10.1523/JNEUROSCI.4639-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Cronk JC, Lu Z, Xu E, Abbott SBG, Guyenet PG, Kipnis J. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature. 2012;484:105–109. doi: 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster HV, Pan LG, Lowry TF, Serra A, Wenninger J, Martino P. Important role of carotid chemoreceptor afferents in control of breathing of adult and neonatal mammals. Respir Physiol. 2000;119:199–208. doi: 10.1016/s0034-5687(99)00115-2. [DOI] [PubMed] [Google Scholar]
- Georgopoulos D, Mitrouska I, Bshouty Z, Webster K, Patakas D, Younes M. Respiratory response to CO2 during pressure-support ventilation in conscious normal humans. Am J Respir Crit Care Med. 1997;156:146–154. doi: 10.1164/ajrccm.156.1.9606055. [DOI] [PubMed] [Google Scholar]
- Gökben S, Ardiç UA, Serdaroğlu G. Use of buspirone and fluoxetine for breathing problems in Rett syndrome. Pediatr Neurol. 2012;46:192–194. doi: 10.1016/j.pediatrneurol.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Voituron N, Menuet C, Ichiyama RM, Subramanian HH, Dutschmann M. The role of serotonin in respiratory function and dysfunction. Respir Physiol Neurobiol. 2010;174:76–88. doi: 10.1016/j.resp.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Best S, Richerson GB. Altered ventilatory and thermoregulatory control in male and female adult Pet-1 null mice. Respir Physiol Neurobiol. 2011;177:133–140. doi: 10.1016/j.resp.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Medullary serotonin neurons and their roles in central respiratory chemoreception. Respir Physiol Neurobiol. 2010a;173:256–263. doi: 10.1016/j.resp.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. The role of medullary serotonin (5-HT) neurons in respiratory control: contributions to eupneic ventilation, CO2 chemoreception, and thermoregulation. J Appl Physiol. 2010b;108:1425–1432. doi: 10.1152/japplphysiol.01270.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide S, Itoh M, Goto Y. Defect in normal developmental increase of the brain biogenic amine concentrations in the mecp2-null mouse. Neurosci Lett. 2005;386:14–17. doi: 10.1016/j.neulet.2005.05.056. [DOI] [PubMed] [Google Scholar]
- Khoo MCK. Determinents of ventilatory instability and variability. Respir Physiol. 2000;122:167–182. doi: 10.1016/s0034-5687(00)00157-2. [DOI] [PubMed] [Google Scholar]
- Krabbe G, Matyash V, Pannasch U, Mamer L, Boddeke HW, Kettenmann H. Activation of serotonin receptors promotes microglial injury-induced motility but attenuates phagocytic activity. Brain Behav Immun. 2012;26:419–428. doi: 10.1016/j.bbi.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Sakowski SA, Geddes TJ, Wilkerson C, Haycock JW. Phosphorylation and activation of tryptophan hydroxylase 2: identification of serine-19 as the substrate site for calcium, calmodulin-dependent protein kinase II. J Neurochem. 2007;103:1567–1573. doi: 10.1111/j.1471-4159.2007.04855.x. [DOI] [PubMed] [Google Scholar]
- Li A, Nattie E. Serotonin transporter knockout mice have a reduced ventilatory response to hypercapnia (predominantly in males) but not to hypoxia. J Physiol. 2008;586:2321–2329. doi: 10.1113/jphysiol.2008.152231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, Kasper BK, Hirrlinger PG, Kirchoff F, Bissonnette JM, Ballas N, Mandel G. A role for glia in the progression of Rett’s syndrome. Nature. 2011;475:497–500. doi: 10.1038/nature10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortola JP, Noworaj A. Two-sidearm tracheal cannula for respiratory airflow measurements in small animals. J Appl Physiol. 1983;55:250–253. doi: 10.1152/jappl.1983.55.1.250. [DOI] [PubMed] [Google Scholar]
- Nguyen MV, Du F, Felice CA, Shan X, Nigam A, Mandel G, Robinson JK, Ballas N. MeCP2 is critical for maintaining mature neuronal networks and global brain anatomy during late stages of postnatal brain development and in the mature adult brain. J Neurosci. 2012;32:10021–10034. doi: 10.1523/JNEUROSCI.1316-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayotis N, Ghata A, Villard L, Roux JC. Biogenic amines and their metabolites are differentially affected in the Mecp2-deficient mouse brain. BMC Neurosci. 2011;12:47. doi: 10.1186/1471-2202-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JFR. A working heart-brainstem preparation of the mouse. J Neurosci Methods. 1996;65:63–68. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- Ramanantsoa N, Hirsch MR, Thoby-Brisson M, Dubreuil V, Bouvier J, Ruffault PL, Matrot B, Fortin G, Brunet JF, Gallego J, Goridis C. Breathing without CO2 chemosensitivity in conditional Phox2b mutants. J Neurosci. 2011;31:12880–12888. doi: 10.1523/JNEUROSCI.1721-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637–642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-John WM, Paton JFR. Characterizations of eupnea, apneusis and gasping in a perfused rat preparation. Respir Physiol. 2000;123:201–213. doi: 10.1016/s0034-5687(00)00177-8. [DOI] [PubMed] [Google Scholar]
- Samaco RC, Mandel-Brehm C, Chao HT, Ward CS, Fyffe-Maricich SL, Ren J, Hyland K, Thaller C, Maricich SM, Humphreys P, Greer JJ, Percy A, Glaze DG, Zoghbi HY, Neul JL. Loss of MeCP2 in aminergic neurons causes cell-autonomous defects in neurotransmitter synthesis and specific behavioral abnormalities. Proc Natl Acad Sci U S A. 2009;106:21966–21971. doi: 10.1073/pnas.0912257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets EE, Julu PO, van Waardenburg D, Engerström IW, Hansen S, Apartopoulos F, Curfs LM, Schrander-Stumpel CT. Management of a severe forceful breather with Rett syndrome using carbogen. Brain Dev. 2006;28:625–632. doi: 10.1016/j.braindev.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Southall DP, Kerr AM, Tirosh E, Amos P, Lang MH, Stephenson JB. Hyperventilation in the awake state: potentially treatable component of Rett syndrome. Arch Dis Child. 1988;63:1039–1048. doi: 10.1136/adc.63.9.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BK, Basbaum AI. Neurochemical characterization of extracellular serotonin in the rostral ventromedial medulla and its modulation by noxious stimuli. J Neurochem. 1995;65:578–589. doi: 10.1046/j.1471-4159.1995.65020578.x. [DOI] [PubMed] [Google Scholar]
- Taylor NC, Li A, Green A, Kinney HC, Nattee EE. Chronic fluoxetine microdialysis into the medullary raphe of the rat, but not systemic administration, increases the ventilatory response to CO2. J Appl Physiol. 2004;97:1763–1773. doi: 10.1152/japplphysiol.00496.2004. [DOI] [PubMed] [Google Scholar]
- Temudo T, Rios M, Prior C, Carrilho I, Santos M, Maciel P, Sequeiros J, Fonseca M, Monteiro J, Cabral P, Vieira JP, Ormazabal A, Artuch R. Evaluation of CSF neurotransmitters and folate in 25 patients with Rett disorder and effects of treatment. Brain Dev. 2009;31:46–51. doi: 10.1016/j.braindev.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Roux JC, Tryba AK, Saywell V, Burnet H, Peña F, Zanella S, Bévengut M, Barthelemy-Requin M, Herzing LB, Moncla A, Mancini J, Ramirez JM, Villard L, Hilaire G. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J Neurosci. 2005;25:11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voituron N, Zanella S, Menuet C, Dutschmann M, Hilaire G. Early breathing defects after moderate hypoxia or hypercapnia in a mouse model of Rett syndrome. Respir Physiol Neurobiol. 2009;168:109–118. doi: 10.1016/j.resp.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM, Azmitia EC. Astroglial 5-HT1a receptors and S-100 beta in development and plasticity. Perspect Dev Neurobiol. 1994;2:233–238. [PubMed] [Google Scholar]
- Zhang X, Su J, Cui N, Gai H, Wu Z, Jiang C. The disruption of central CO2 chemosensitivity in a mouse model of Rett syndrome. Am J Physiol Cell Physiol. 2011;301:C729–C738. doi: 10.1152/ajpcell.00334.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]