Abstract
CD28 is one of the main costimulatory receptors responsible for the proper activation of T lymphocytes. We have isolated two aptamers that bind to the CD28 receptor. As a monomer, one of them interfered with the binding of CD28 to its ligand (B7), precluding the costimulatory signal, whereas the other one was inactive. However, dimerization of any of the anti-CD28 aptamers was sufficient to provide an artificial costimulatory signal. No antibody has featured a dual function (i.e., the ability to work as agonist and antagonist) to date. Two different agonistic structures were engineered for each anti-CD28 aptamer. One showed remarkably improved costimulatory properties, surpassing the agonistic effect of an anti-CD28 antibody. Moreover, we showed in vivo that the CD28 agonistic aptamer is capable of enhancing the cellular immune response against a lymphoma idiotype and of prolonging survival of mice which receive the aptamer together with an idiotype vaccine. The CD28 aptamers described in this work could be used to modulate the immune response either blocking the interaction with B7 or enhancing vaccine-induced immune responses in cancer immunotherapy.
Keywords: aptamer, costimulation, immunotherapy
Introduction
All naive lymphocytes need to receive at least two signals to be properly activated: the first one is triggered by T-cell receptor, whereas the second one (also known as costimulatory signal) comes mainly from CD28.1 This latter molecule is expressed on the surface of naive lymphocytes, whereas its ligands, B7.1 and B7.2, are expressed on the surface of activated antigen-presenting cells (APCs).1,2 Lack of costimulation turns the lymphocyte into a stage of anergy, being thus unable to respond to its antigen.3 Substantial efforts have been made in the field of immunotherapy to develop simple, translational, clinical tools that could promote or reduce T-cell tolerance depending on the immunological context, e.g., potentiation of either anergy in autoimmunity or immune responses in cancer immunotherapy. Antibodies have been used to achieve both the goals. Yet, so far, there is no evidence that an agonistic antibody could be converted into an antagonistic one, or vice versa.
Tumor antigens usually trigger very weak, or even undetectable, natural immune responses due to low antigenicity and absence of proper activation of the immune cells in the tumor environment. APCs within the tumor are constantly receiving immunosuppressive signals that turn them into a state of tolerance.4 Besides lacking costimulatory ligands, APCs also express immunosuppressive receptors and release immunosuppressive cytokines. As a plausible immunotherapy strategy to bypass the tolerogenic APC, we can provide an artificial costimulatory ligand to the tumor antigen-specific lymphocytes. This is commonly achieved with antibodies that bind to costimulatory receptors. Antibodies are bivalent ligands, bringing together two costimulatory receptors on the cell membrane, that triggers the costimulatory signal. Some humanized antibodies with costimulatory capacity, such as CD40, OX40, and 4-1BB, are currently in clinical trials.5 They are cell-derived products that pose substantial regulatory challenges when used in an academic setting. Furthermore, access to this kind of reagents, especially for clinical development, is usually very limited.
Aptamers are single-stranded DNA or RNA oligonucleotides which fold into complex secondary structures, acquiring an excellent affinity and specificity for their ligands.6 SELEX (Systematic Evolution of Ligands by Exponential Enrichment) is the method of aptamer selection used to screen millions of short RNA sequences with high affinity for a given ligand.7 Once selected, aptamers can easily be chemically synthesized, which would greatly simplify the manufacturing process aimed at translational, clinical development.8
Over the last few years, new aptamers with immunomodulatory ability have been described. It should be emphasized that the agonistic aptamer targeting 4-1BB and OX40 shows similar or even superior costimulatory activity to the corresponding agonistic antibodies, with similar antitumor effects.9,10
This is a groundbreaking study, showing the immunomodulatory capacity of two anti-CD28 aptamers. Also, it shows that these aptamers can easily be engineered, depending on the needs of the immunological approach, to function as antagonistic (blocking) or agonistic (activating) by means of a simple dimerization. Finally, it shows that agonistic anti-CD28 aptamers boost the antitumor immune response elicited by idiotypic vaccination in a murine lymphoma model, increasing the overall survival to a considerable extent.
Results
Isolation of aptamers that bind to murine CD28
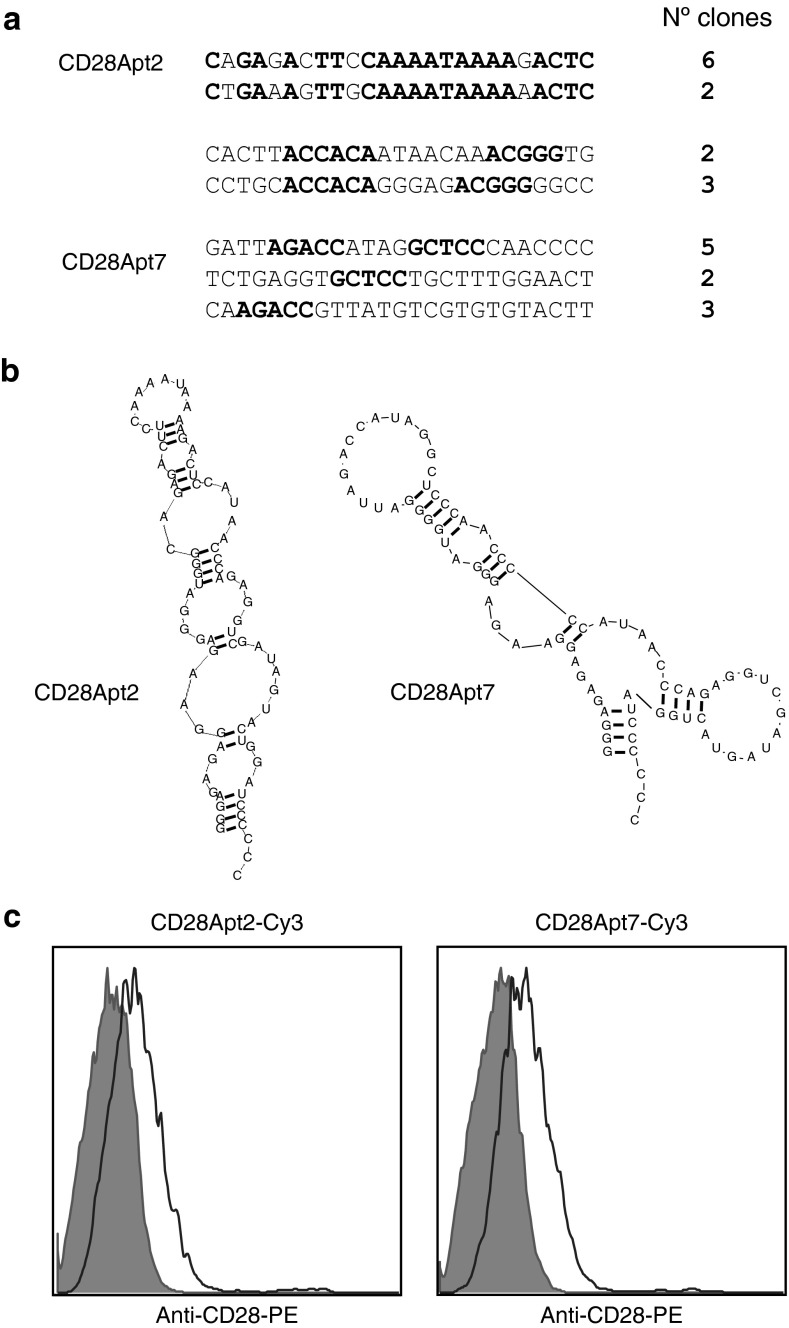
SELEX was used for the selection of aptamers that bind to CD28. We started with a 2′-fluoropyrimidine–modified RNA library of 25 N, which would have about four11 different starting sequences. After nine rounds of selection against CD28-Fc murine recombinant protein (Supplementary Table S1), the library was cloned into pGEMT plasmid, and the 25 random sequences from each clone were analyzed. An enrichment of certain sequences was observed (Figure 1a), especially two of them (20 and 16.6% of all the clones), indicating that the selection was reaching an endpoint. The Kd (dissociation constant) of the two main 2′-fluoropyrimidine–modified RNA aptamers (resistant to RNAses) was 40 nmol/l (CD28Apt2) and 60 nmol/l (CD28Apt7), as determined by filter-binding assay.9 No binding against human IgG1, or CTLA4-Fc, or the human CD28-Fc proteins was detected, nor competition with the anti-CD28 antibody 37.51. Secondary structures predicted by the computer program RNAstructure are shown in Figure 1b. The fact of having started with a shorter library of 25 nt (randomized sequence), versus one of 40 nt (the usual length), obviates the tedious labor of truncation needed for the chemical synthesis (current technology only allows for the generation of short sequence RNA aptamers).
Figure 1.
Selection and characterization of CD28 aptamers. (a) Summary of the aptamer sequences binding to soluble extracellular CD28 murine recombinant protein isolated from round 9 of selection. The fixed sequences have been removed to simplify the figure from the 5′ end (5′-GGGGGAATTCTAATACGA CTCACTATAGGGAGAGAGGAAGAGGGATGGG-3′) and from the 3′ end (5′-CATAACCCAGAGGTCGATAGTACTGGATCCCCCC-3′). Sequence similarities among aptamers of the same family are shown in bold. (b) Sequence and computer secondary structure prediction of the two aptamers against CD28. (c) Binding of CD28Apt7 and CD28Apt2 to CD28 expressed on the cell surface. CD28-transfected 293 and the parental cell line (gray filled) were stained with the Cy3-labeled aptamers and analyzed by flow cytometry.
Despite specific CD28 binding, i.e., aptamer binding to just CD28-transfected but not parental HEK293 cells (Figure 1c), none of the aptamers in the monomeric form presented any costimulatory capacity (data not shown). However, this outcome was expected, because the CD28 receptor needs cross-linking to initiate the downstream activation cascade.
Monomeric CD28Apt2 blocks B7.2 interaction and reduces the costimulatory signal
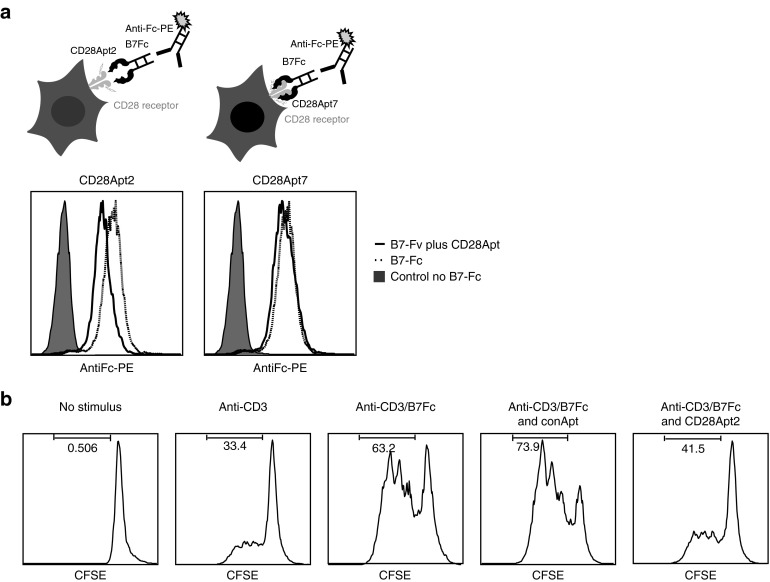
Aptamers have been previously used to block ligand–receptor interactions.11,12 To test whether our aptamers were able to prevent the interaction of CD28 with its main ligand B7.2, a blocking assay was used (described in the upper panel of Figure 2a). As shown in Figure 2a, the addition of CD28Apt2 in its monomeric form, as opposed to that of CD28Apt7, reduces the binding of B7.2-Fc to CD28. In particular, 2.5 ng/μl of CD28Apt2 can reduce the binding of 5 ng/μl of B7.2 by 1 log to CD28 on the surface of HEK-293-CD28. So as to prove whether this could have an effect on the grade of T-cell activation, we performed a proliferation assay by carboxyfluorescein succinimidyl ester (CFSE) dilution on CD4 T lymphocytes, with suboptimal amount of CD3 activation signal and in the presence of bivalent B7.2-Fc recombinant protein (Figure 2b). Cross-linking CD28 by dimeric B7.2-Fc recombinant protein launches a potent costimulatory signal, thereby enhancing the proliferation of T cells, as shown by CD4 polyclonal activation with anti-CD3. The blocking effect of the CD28Apt2 aptamer in its monomeric form at 1:5 ratio (5 ng/μl of B7.2-Fc versus 25 ng/μl of CD28Apt2 monomeric aptamer) has a very strong inhibitory impact on the proliferation of purified CD4 lymphocytes, which is measured by CFSE dilution. No inhibitory effect was observed in the proliferation of CD4 lymphocytes with the addition of a control RNA aptamer at the same concentration, proving that the effect is directly mediated by the steric interaction impediment between CD28 and B7.2.
Figure 2.
CD28 costimulatory signal blockade using CD28Apt2. (a) Schematic representation of B7-CD28 interaction blockade by CD28Apt2. HEK293 transfected with CD28 cDNA were incubated with the chimera B7-Fc recombinant protein in the presence of equal amounts of CD28Ap2 or CD28Apt7. The binding of B7-Fc to CD28 was detected with an anti-human–Fc PE-conjugated antibody by flow cytometry. (b) Demonstration of an inhibitory effect of CD28Apt2 on the proliferation of CD4+ lymphocytes. CD4+ lymphocytes were labeled with CSFE and suboptimally activated with an anti-CD3 antibody and the recombinant protein B7-Fc. CFSE dilution was evaluated by flow cytometry. The experiments were repeated twice with similar results. CFSE, carboxyfluorescein succinimidyl ester.
Dimeric CD28-aptamer costimulates CD8 and CD4 in vitro
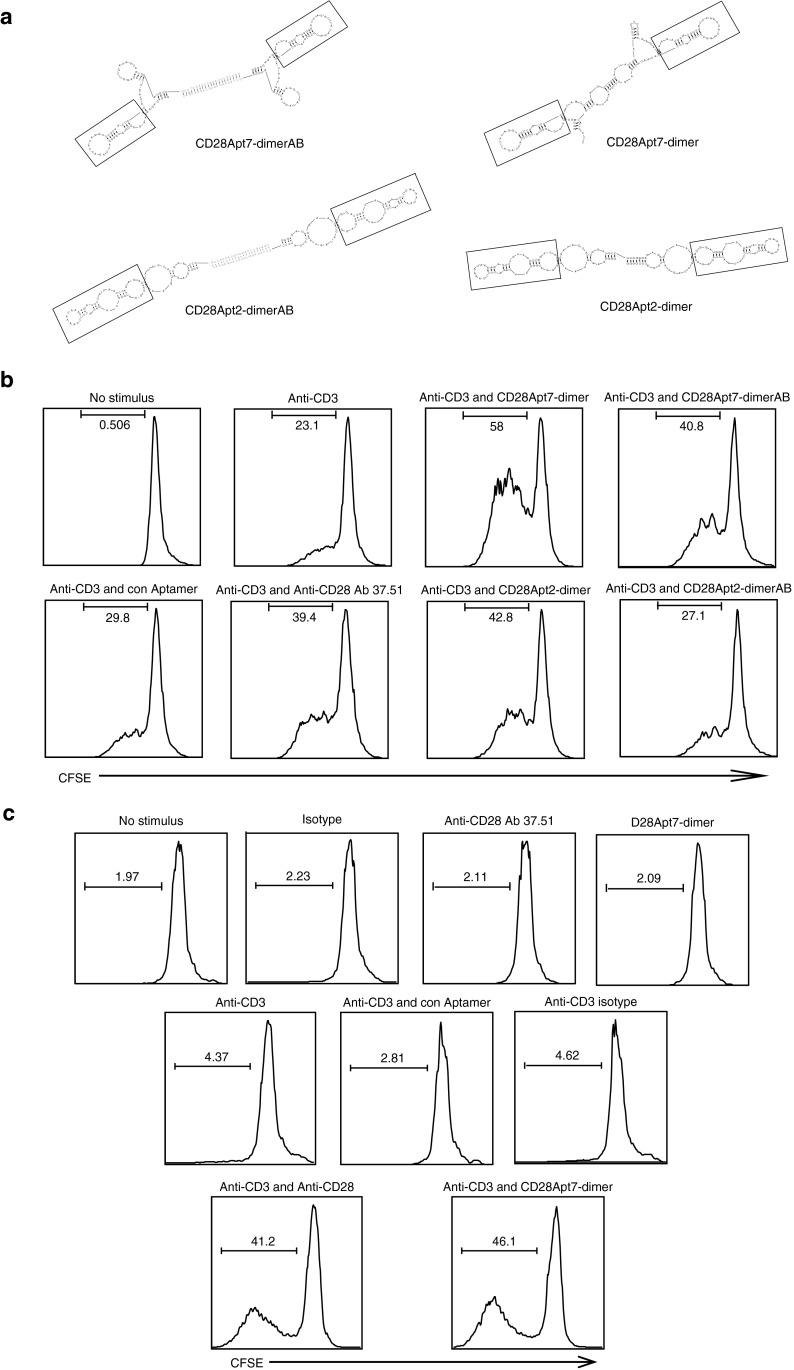
The selected aptamers were tested for their agonistic capacity. To cross-link the CD28 receptors so that the aptamers may trigger the costimulation signal, they need to be dimerized. The binding region of an antibody to the CD28 receptors has an indirect effect on the potency of the costimulatory signal, affecting both distribution and proximity of the two cognate receptors that are brought together by the agonistic antibody itself.13,14 This is the reason why two different dimeric aptamer constructs were tested, with and without a linker. The dimeric constructs with the linker were similar in size to those previously described for 4-1BB agonistic aptamers9 and were generated by hybridization of two monomers with a 21-nucleotide complementary sequence which was added to each monomer by PCR extension in the 3′-region. This linker provides a 21-nucleotide double-stranded stem, which reflects the average distance between the two Fv of an Ig (65 Å) and guarantees a more rigid structure. The other kind of dimeric aptamer was transcribed as a single strand of two consecutive monomers without any extra linker between the two units, reducing the distance between each aptamer to the minimum. The predicted secondary structure of each dimer is shown in Figure 3a. In both dimeric constructs, the core of the aptamer corresponding to the variable region of the RNA library from which they were selected is preserved (shown within the chart). No remarkable changes in affinity or specificity between the two different dimeric constructs were detected (Supplementary Figure S1).
Figure 3.
Dimerization of CD28Apt2 and CD28Apt7 turns the aptamers into agonist agents. (a) Secondary structure predicted by the computer program RNAstructure of two different dimeric constructs of each aptamer: dimers generated without a linker, which have a shorter distance between each functional aptamer (CD28Apt2-dimer, CD28Apt7-dimer); dimers generated with a double-stranded linker of two nucleotides by hybridization of two complementary sequences engineered in 3′ end of each monomer (CD28Apt2-AB, CD28Apt7-AB). The core of each aptamer is shown within a chart. (b) Each dimeric aptamer construct was tested for its costimulation capacity on CD4 lymphocytes labeled with CFSE after suboptimal activation with anti-CD3 antibody. CFSE dilution was assessed by flow cytometry. (c) Costimulation of CD8 lymphocytes by CFSE dilution with the best aptamer construct selected from the CD4 assay after suboptimal activation with anti-CD3 antibody. All experiments were repeated at least three times. CFSE, carboxyfluorescein succinimidyl ester.
As shown in Figure 3, the construct without linker CD28Apt7-dimer has the strongest costimulatory capacity, outranging the agonistic monoclonal antibody 37.51 (Figure 3b). The CD28Apt7-dimer has a 60 nmol/l Kd and, despite not featuring the highest affinity for CD28, shows a better costimulation rate than CD28Apt2-dimer. The proliferative effect exerted on the CD4 population after CD3 stimulus reached 18.5–23%. However, after the addition of a CD28 agonistic antibody, there was a 35–39.4% increase (approximately a twofold increase), whereas proliferation with CD28Apt7-dimer reached 52.1–58% (approximately a 2.5-fold increase), and the one with CD28Apt7-AB reached 37–40.8% (similar to the antibody 37.51). CD28Apt2-dimer had a similar proliferation ratio to that of the anti-CD28 monoclonal antibody (42.8%). No proliferative effect in human CD4 lymphocytes stimulated with anti-CD3 (OKT3) plus the CD28Apt7-dimer was observed (data not shown), proving that the agonistic effect on murine lymphocytes is certainly CD28-mediated, since the aptamer does not bind to human CD28.
CD8 T cells suboptimally activated with anti-CD3 were treated with the CD28Apt7-dimer agonistic aptamer, reaching a 40.2–46.1% proliferation rate (approximately a tenfold proliferation increase). In parallel, the proliferation obtained with the agonistic antibody was 38.5–41.2 % (approximately a ninefold increase). No proliferation was observed with the CD28Apt7-dimer alone and without the anti-CD3 stimulus, discarding the possibility of having developed a superagonistic anti-CD28 aptamer.
CD28Apt7-dimer aptamer promotes a strong cellular response
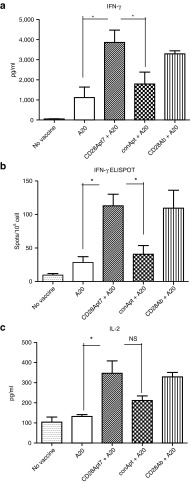
As shown in Figure 4, mice immunized with irradiated A20 plus the CD28 agonistic antibody or the agonistic aptamer CD28Apt7-dimer generated a stronger cellular immune response than those receiving control vaccinations, which is proven by a higher production of interleukin-2 and interferon-γ. Interferon-γ production by lymphocytes was confirmed by ELISpot. Indeed, the interaction between lymphocytes and irradiated A20 from these two groups caused a nearly twofold increase in cytokine release compared with the negative control groups.
Figure 4.
Boosting cellular immune response through CD28Apt7-dimer. (a) Detection of IFN-γ by ELISA from supernatant obtained after a 48-hour co-culture of irradiated A20 cells and lymphocytes obtained from mice previously immunized with irradiated A20 cells plus 400 pmol of the CD28Apt7-dimer, or CD28 agonistic antibody 37.51, or a scramble aptamer. The results are expressed as mean and SEM of triplicates. (b) Detection of IFN-γ producing lymphocytes through ELISpot after 24 hours co-culture of irradiated A20 cells and lymphocytes obtained from mice previously immunized with irradiated A20 cells plus 400 pmol of the CD28Apt7-dimer, or CD28 agonistic antibody 37.51, or a scramble aptamer. The results are expressed as mean and SEM of triplicates. (c) Detection of IL-2 by ELISA from supernatant obtained after a 48-hour co-culture of irradiated A20 cells and lymphocytes obtained from mice previously immunized with irradiated A20 cells plus 400 pmol of the CD28Apt7-dimer, or CD28 agonistic antibody 37.51, or a scramble aptamer. The results are expressed as mean and SEM of triplicates. *P < 0.05. IFN, interferon; IL, interleukin; NS, not significant.
CD28Apt7-dimer aptamer promotes a strong humoral response
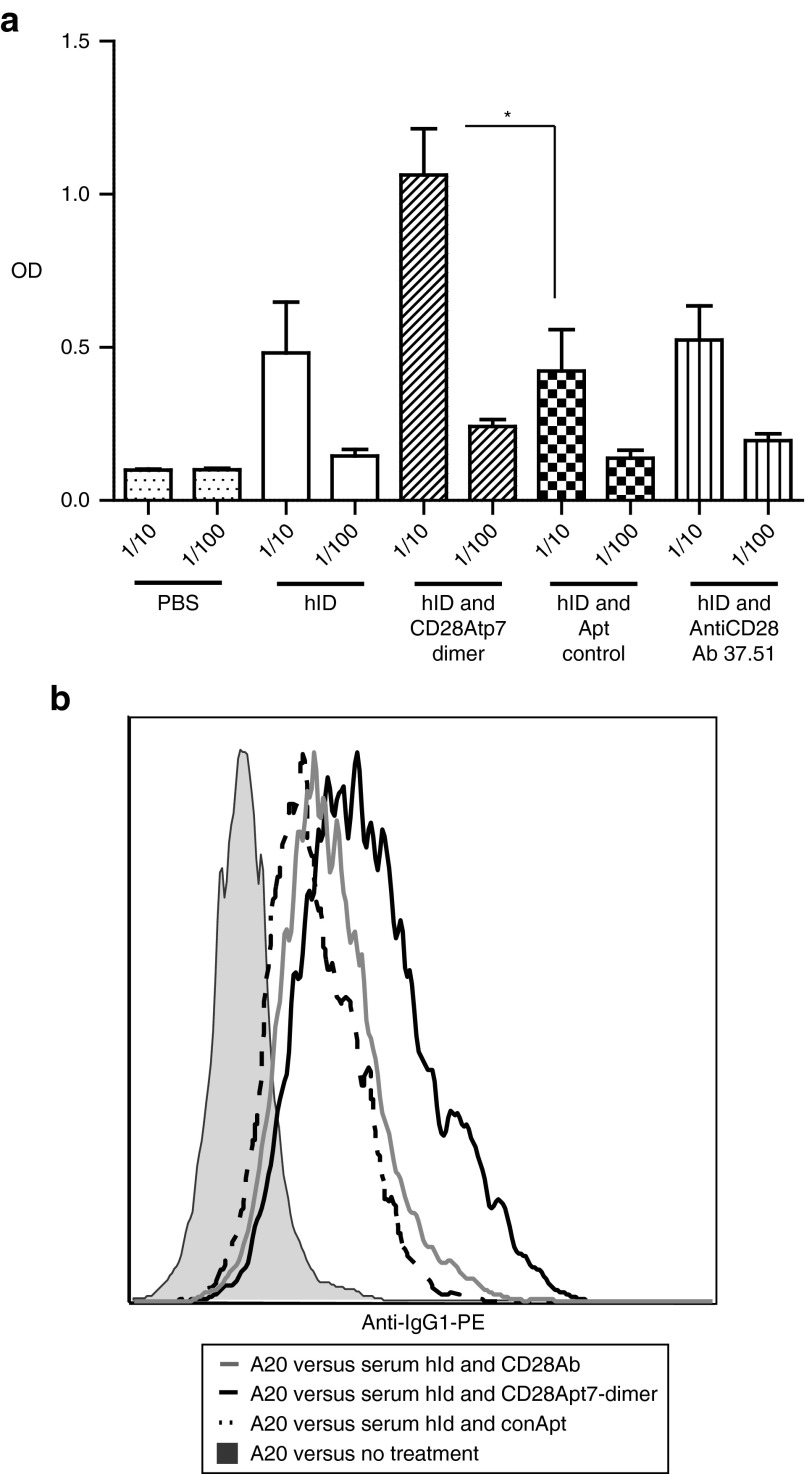
To evaluate the capacity of the dimeric agonistic aptamer CD28Apt7-dimer to enhance the humoral response, an idiotypic vaccination protocol was chosen. In this immunotherapy strategy,15 the humoral immune response is considered very important to control the follicular lymphoma progression. As shown in Figure 5a, a significant increase in the titer of anti-Id antibodies was achieved through the Id vaccine in combination with CD28 agonistic aptamer, but not with the agonistic CD28 antibody. To confirm that the anti-Id antibodies detected by ELISA were able to bind the native Id on the surface of the tumor cells, a flow cytometry assay was performed (Figure 5b). The average median fluorescence intensity was increased in the group of mice vaccinated with hId and the agonistic CD28Apt7-dimer (40.9), versus the group vaccinated with the hId and the agonistic anti-CD28 antibody 37.51 (29.6), the group vaccinated with hId and Apt control (20.6), and the untreated group (6.2).
Figure 5.
Boosting humoral immune response through CD28Apt7-dimer. (a) Anti-idiotype (A20) antibodies were identified in the serum collected 2 weeks after completion of the two injection vaccination schedule with hId (three mice), hId-control aptamer (five mice), hId-AntiCD28 Ab 37.51 (five mice), and hId-CD28Apt7-dimer (five mice). ELISA was performed, coating the plate with the mouse A20 idiotype IgG2-κ and using an anti-IgG1 as a secondary antibody. The experiment was repeated twice with similar results. *P < 0.05. (b) A20 tumor cells were incubated with the serum of immunized mice obtained 2 weeks after vaccination. Anti-idiotypic antibodies were detected through an anti-IgG1 PE antibody using flow cytometry. A representative histogram of one mouse per group is shown. OD, optical density; PBS, phosphate-buffered saline.
Potentiation of antitumor vaccine efficacy by CD28Apt7-dimer
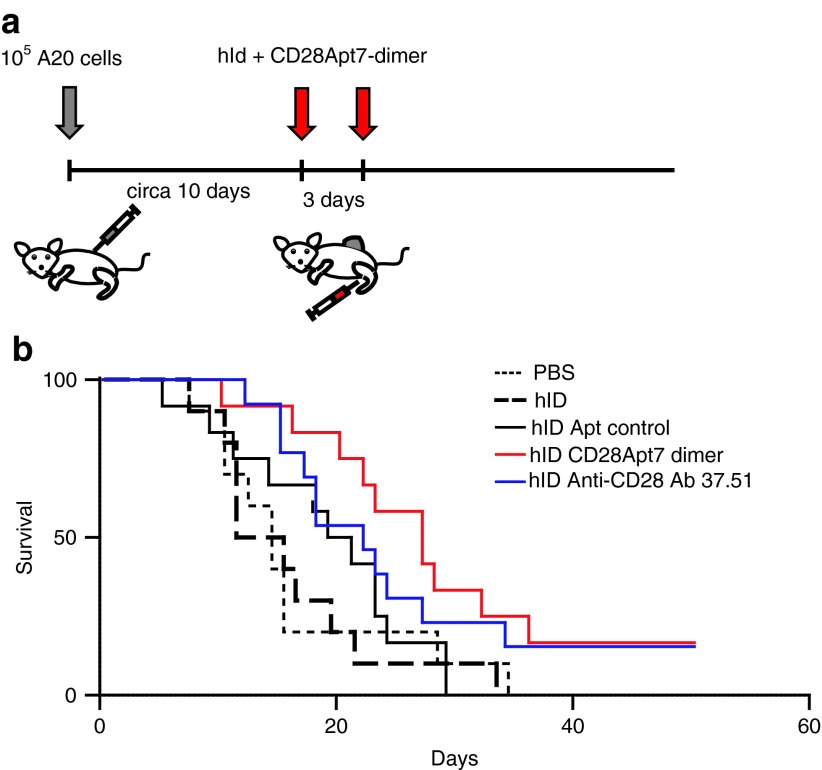
We next determined whether the agonistic CD28-aptamer was able to enhance the antitumor effect of the Id vaccine in A20 lymphoma-bearing mice, following the vaccine protocol described in Materials and Methods and shown in Figure 6a. The CD28 agonist antibody has been documented to exert a slight antitumor effect in certain tumor models.16 However, no effect on its own was detected in this tumor model (data not shown). On the contrary, treatment with hId together with the aptamer caused a slow tumor growth (Supplementary Figure S2) and a considerable survival rate increase (Figure 6b), which reached a statistical significance of P = 0.0356 compared with the association of hId and scramble aptamer. The scramble aptamer in combination with hId also had a small effect on tumor progression, but it did not reach statistical significance. It is noteworthy that similar effects were observed in some other tumor models with different control aptamers.17,18 This could be assumed to be part of the immunostimulatory capacity of the RNA on the innate immune system. Finally, the treatment with hId vaccine in combination with the agonistic antibody 37.51 exerted a small effect on tumor progression but did not reach statistical significance either.
Figure 6.
Antitumor effect of the idiotypic vaccine in combination with CD28Apt7-dimer. (a) Mice (10–13 mice per group) were subcutaneously injected in the flank with 105 A20 cells. When tumors reached a 5-mm diameter, mice were vaccinated twice, 3 days apart, subcutaneously with hId alone, or with 37.51 antibody, or with CD28Apt7-dimer, or with a control aptamer. (b) Results of the tumor vaccination experiment. Reported here are the days to tumor onset after idiotypic vaccination. The difference between control aptamer and CD28Apt7-dimer was statistically significant by log-rank test (P < 0.05). The experiment was repeated twice with similar results. PBS, phosphate-buffered saline.
Discussion
It is known that aptamers can act as inhibitors of certain receptors. Nevertheless, it has never been shown that an inhibitory aptamer can be engineered to also function as an agonist. We were able to do so through dimerization of two functional aptamer units. The same aptamer that works as a blocker of the B7-CD28 interaction (CD28Apt2), after dimerization, switches toward an agonistic effect similar to that exerted by antibodies commonly used for this purpose. This is actually a new relevant feature of aptamers, since antibodies are either antagonists (i.e., CTLA-4 antibody) or agonists (i.e., 4-1BB antibody) and, while it might be desirable to convert them into antibodies with the opposite function, this has been technically infeasible so far. On the other hand, we have generated an improved dimeric form of the CD28 agonistic aptamer by reducing the size of the linker between the two functional aptamer units. Furthermore, we have shown that the closer the interaction between two CD28 receptors, the stronger the costimulatory signal. Thus, the simple manipulation of the linker can be used to optimize the agonistic effect of an aptamer.
Genetically modifying antibodies implies a tedious process, featuring several steps of cloning and purification. Besides, antibodies are proteins and, as such, potential antigens which can induce a neutralizing humoral immune response. In order to be cleared for use in patients, they need to be humanized through a long process that removes the xenogenic epitopes from the constant regions of the IgG. Moreover, the humanizing process can sometimes affect their binding affinity.19 Even with a humanized antibody, there is a theoretical risk of inducing an anti-Id response that would hamper its function. Instead, aptamers are modified nucleic acids with very low antigenicity. Therefore, a neutralizing humoral immune response against them is very unlikely to occur.
Another major obstacle towards the clinical development of agonistic anti-CD28 antibodies for immune costimulatory purposes is the high toxicity that is induced upon their inoculation.20,21 Aptamers have a shorter half-life in blood (12–24 hours) than antibodies. Thus, any artificial costimulation would be more time-controlled. We have recently developed the first bispecific aptamers to target costimulation to distal tumor metastatic lesions, which enhance overall efficacy, thereby reducing therapeutic doses and toxicity.18
The aptamers described in this study could also be used to target T lymphocytes with small interfering RNA, which would knockdown immunosuppressive factors hampering the development of antitumor immune responses. Among the costimulatory receptors present on T cells, CD28 is the one that operates at the earliest timepoint and therefore, from our point of view, the ideal candidate as small interfering RNA target receptor to shut down the immune suppression at the early stages of vaccination, when the balance between tolerance and immune response is not yet established.
Blockade of B7-CD28 interaction has already been proven to induce tolerance.22,23 When combined with the blockade of the CD40/CD40L pathway, the survival of allogeneic grafts is dramatically improved.24 Clinical use of CD28 blockade aptamer together with the antagonistic CD40 aptamer that abrogates these two costimulatory signals could be very effective when treating autoimmune diseases as well as inducing tolerance towards allogeneic transplants.
We also developed an anti-CD28 agonistic aptamer that functions as a potent adjuvant and is capable of inducing strong immune responses (surpassing those of the CD28 agonistic antibody, especially in the humoral immune response) in the context of a tumor vaccination strategy. Although, we speculated that this is due to the higher agonistic capacity of the aptamer versus the antibody, further studies need to be performed to discard the possible interaction of Apt7-dimer aptamer with other immune stimulatory receptors, which could have an additive effect in triggering the immune response.25 Remarkably, the aptamer-vaccine combination succeeded in reducing the tumor progression in mice that had started receiving treatment with tumors of 5-mm diameter and two shots of vaccine formulation (Idh-CD28Apt7dimer).
Materials and methods
SELEX CD28-aptamer. A 25 N randomized RNA library (with two constant flanking sequences of 22 nt at the 5′ end and 49 nt at the 3′ end) was created by transcription of partially randomized DNA oligonucleotides: template 5′-TCGGGCGAGTCGTCTG-25N-CCGCATCGTCCTCCC-3′ forward 5′-GGGGGAATTCTAATACGACTCACTAT AGGGAGAGAGGAAGAGGGATGGG-3′ reverse 5′-GGGGGGATCCAGTACTATCGACCTCTGGGTTATG-3′. It is known that a library of 25 N has about four11 different starting sequences. The binding reactions were carried out in 150 mmol/l NaCl, 2 mmol/l CaCl2, 20 mmol/l HEPES (pH 7.4), 0.01% BSA buffer. The RNA library was subjected to counter-selection before each round of selection to remove RNAs specific to human IgG-Fc, as well as protein G. To this end, the randomized RNA library was incubated with 800 pmol of human IgG1 (Sigma, St Louis, MO) at 37 °C for 30 minutes. The counter-selection was subsequently followed by incubation with sepharose protein G-coated beads (GE Healthcare Bio-science, Uppsala, Sweden). After bead pelleting by centrifugation, the supernatant was used for the selection.
Murine CD28 human IgG-Fc fusion protein (R&D systems, Minneapolis, MN) was immobilized by coupling to protein G-coated sepharose beads (GE Healthcare Bio-science). The bead-coupled CD28 fusion protein was incubated with the pre-cleared RNA pool and washed three times with a wash buffer (150 mmol/l NaCl, 2 mmol/l CaCl2, 20 mmol/l HEPES (pH 7.4)) to remove non-interacting RNA. RNA bound to CD28 was extracted through a 30-minute incubation in phenol:chloroform:isoamyl alcohol (25:24:1) and amplified by reverse transcription followed by PCR.
Nine rounds of selection were performed with increasing stringency throughout the selection process, by increasing the RNA-protein ratio in the selection reaction. Aptamers from round 9 were retrotranscribed and cloned into pGMET-easy (Promega, Madison, WI). Single colonies were sequenced and amplified by PCR amplification, followed by in vitro transcription with Durascribe T7 Transcription kit (Epicenter, Madison, WI).
CD28-aptamer dimerization. The production of the dimeric aptamer with a double-stranded linker was carried out by engineering the 3′ end of each aptamer to create two complementary regions so that they could be attached together by hybridization, as previously described.9 Dimeric aptamers were purified by polyacrylamide gel electrophoresis and concentrated on a 30 kDa Amicon Ultra-4 column (Millipore, Billerica, MA). Dimers generated without a double-stranded linker were transcribed contiguously from a template created by first overlapping two oligos for each aptamer-oligo, followed by extension with Taq (Invitrogen, Carlsbad, CA). CD28Apt2: forward GAATTCTAATACGACTCACTATAGGGAGAGAGGAAGAGGGATGGGCAGAGACTTCCAA AATAAAAGACTCCATAACCCAGAGGTCGATAGTACTGGATCC, reverse, CCCGGGATCCAGTACTATCGAC CTCTGGGTTATGGAGTCTTTTATTTTGGAAGTCTCTGCCCATCCCTCTTCCTCTC TCCCGGGGGGATCCAGTACTATC; CD28Apt7: forward GAATTCTAATACGACTCACTATAGGGAGAGAGGAAG AGGGATGGGGATTAGACCATAGGCTCCCAACCCCCATAACCCAGAGGTCGATAG TACTGGATCC, reverse GGGGGGATCCAGTACTATCGACCTCTGGGT TATGGGGGTTGGGAGCCTATGGTCTAATCCCCATCCCTC TTCCTCTCTCCCGGGGGGATCCAGTACTATC.
Binding to CD28. Binding of the aptamer to the nitrocellulose filter was performed as previously described.9 However, since the inhibitory or costimulatory effect of the aptamers would take place on the cell surface, we tested their binding to HEK293 stable cells transfected with a CD28-expressing plasmid. To do so, we first generated a stable cell line that expressed CD28. CD28 cDNA was obtained from the Open Biosystems (Huntsville, AL) clone (3418931); cDNA was amplified by PCR using the forward (ACCATGACACTCAGGCTGCTGTTC) and reverse (GTCAGGGGCGGTACGCTGCAAAG) primers and recloned into pCDNA3.1 TOPOTA system (Invitrogen). Then, the plasmid was used to generate a stable HEK293 cell line expressing CD28. CD28 aptamers were transcribed in the presence of N6-(6-aminohexyl)-ATP (Gibco, Bethesda Research Laboratories, Bethesda, MD) and, in order for them to contain Cy3, RNA pellets were suspended in 0.2 mol/l NaHCO3 (pH 8.2) and mixed with an equal volume of Cy3 monofunctional dye (Amersham-Pharmacia Biotech, Piscataway, NJ) for 1 hour at room temperature. Both the transfected and the parental HEK293 cell lines were pre-blocked with random fluorinated RNA (2.5 μg/ml) and incubated with the Cy3-labeled aptamer for flow cytometry analysis.
CD28–B7 interaction blocking assay. To test whether or not our aptamers were able to block the interaction of CD28 with its main ligand B7-2, HEK293-CD28 cells (2 × 105) were incubated with 0.5 μg of chimera recombinant bivalent protein B7-Fc (R&D systems) and 1 μg of CD28Apt2 or CD28Apt7 at 37 °C for 30 minutes. After the incubation, the binding of B7-Fc to CD28 was confirmed by flow cytometry with an anti-Fc antibody (Anti-human-Fc-PE, HP6017; BioLegend, San Diego, CA).
Proliferation assays. To evaluate the costimulatory capacity of each aptamer construct, a CD4 polyclonal proliferation assay by CFSE dilution was performed. CD4 T lymphocytes purified from the spleen of BALB/c mice were suboptimally activated with an anti-CD3 antibody and the different CD28 agonistic aptamer constructs. Given the importance of CD8 T lymphocytes in tumor control, particularly through their cytotoxic action, we also tested the costimulatory capacity of CD28Apt7-dimer on polyclonal CD8 lymphocytes isolated from the spleen by diluting CFSE with suboptimal anti-CD3 stimulus. CD8+ and CD4+ lymphocytes were isolated from the spleen using the Miltenyi negative selection kit (Miltenyi Biotec, Auburn, CA). Purified CD8+ and CD4+ lymphocytes were stained with 2 μmol/l of CFSE. CFSE-labeled cells were incubated with 1 mg/ml of anti-CD3 antibody (BD Biosciences, San Jose, CA) and 5 mg/ml of anti-murine CD28 (37.51) purified from hybridoma supernatant, or 5 mg/ml isotype control Ab, or 100 nmol/l of CD28-aptamer dimers from each clone, and/or analyzed by flow cytometry.
Tumor immunotherapy studies. To evaluate the capacity of the dimeric agonistic aptamer CD28Apt7-dimer to enhance the humoral response, an idiotypic vaccination protocol was chosen. In this immunotherapy strategy,15 the humoral immune response is considered very important to control the follicular lymphoma progression. A20 tumor cells (2 × 105) were implanted subcutaneously in one flank of 8-week-old BALB/c female mice. Mice were monitored for tumor development. The vaccine was started when tumors reached a diameter of 5 mm, i.e., about 10 days after tumor inoculation. Mice were vaccinated intrainguinally twice, 3 days apart, with an idiotype vaccine which consisted every time of a recombinant Ig26 featuring both the A20 lymphoma idiotype and the human-Fc region (hId), alone or admixed together with 400 pmol of the dimeric agonistic aptamer CD28Apt7 as an adjuvant, or a scramble aptamer, or 400 pmol of the agonistic CD28 antibody 37.51. On day 7 after the first vaccine, mice were bled to assay the amount of anti-idiotype (Id) antibodies. To ensure that the humoral response was specific to the A20 idiotype rather than to the humanized portion of the recombinant idiotype, i.e., the human-Fc fraction (xenotopos), we used a hybridoma-derived A20 Ig whose amino acid sequence is identical to the native A20 tumor Ig to perform an ELISA.
In vivo cellular immune response. To determine whether the agonistic aptamer CD28Apt7-dimer was able to enhance the cellular immune response in the context of tumor vaccination, 8-week-old female BALB/c mice were immunized intrainguinally with plain 5 × 105 A20 irradiated cells (5,000 rad) per leg. Two days later, they were injected subcutaneously in the vaccination area (a spot corresponding to the remnant where irradiated cells can be observed) with 400 pmol of CD28 agonistic antibody 37.51, or CD28Apt7-dimer, or controlApt, or saline buffer. Two weeks later, mice were killed, and leukocytes from spleen and inguinal lymph nodes were cultured with A20 irradiated cells at a 1:10 ratio (104 A20 irradiated cell per 105 leukocytes). After 72 hours, the supernatant was collected, and interleukin-2 and interferon-γ were measured by ELISA (BD Biosciences, San Diego, CA). For the ELISpot assay, leukocytes extracted from the inguinal lymph nodes of immunized mice were cultured for 24 hours with A20 irradiated cells at a 1:10 ratio (104 A20 irradiated cell per 105 leukocytes). Animal studies have been approved by the authors' Institutional Review Board.
Anti-idiotypic antibody detection by ELISA. Also to ensure that humoral responses were specific to the A20 idiotype rather than to the humanized portion of the recombinant idiotype, the IgG2/κ A20 idiotype was purified from A20 hybridoma and used to coat the ELISA plate at 10 μg/ml. Serum from mice immunized with hId (collected on day 14 postimmunization) was diluted at 1/10 and 1/100 in phosphate-buffered saline, and 100 μl were added to each well of the ELISA plate. After 1 hour of incubation, the anti-idiotype antibodies were detected with an anti-mouse IgG1 secondary antibody conjugated with peroxidase.
Anti-idiotypic antibody detection by flow cytometry. A20 cells (106) were incubated with the serum of immunized mice at ½ dilution in phosphate-buffered saline for 30 minutes at room temperature. After incubation, cells were washed twice and stained with an anti-IgG1 PE antibody. Next, cells were analyzed by flow cytometry.
Statistical analysis. For survival studies, P values were determined using the log-rank (Mantel–Cox) test. As for the other assays, the Student's t-test was used.
SUPPLEMENTARY MATERIAL Figure S1. CD28-aptamer dimers interact specifically with the cognate receptor. Figure S2. Antitumor effect of the idiotypic vaccine in combination with CD28Apt7-dimer. Table S1. SELEX conditions.
Acknowledgments
The study was partially supported by Union Temporal de Empresas and Fundacion para la Investigacion Medica Aplicada. No external grants were used to support this research. The authors declared no conflict of interest.
Supplementary Material
CD28-aptamer dimers interact specifically with the cognate receptor.
Antitumor effect of the idiotypic vaccine in combination with CD28Apt7-dimer.
SELEX conditions.
References
- Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- Boussiotis VA, Gribben JG, Freeman GJ, Nadler LM. Blockade of the CD28 co-stimulatory pathway: a means to induce tolerance. Curr Opin Immunol. 1994;6:797–807. doi: 10.1016/0952-7915(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007;7:95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- Bunka DH, Stockley PG. Aptamers come of age - at last. Nat Rev Microbiol. 2006;4:588–596. doi: 10.1038/nrmicro1458. [DOI] [PubMed] [Google Scholar]
- Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- Sullenger BA, Gilboa E. Emerging clinical applications of RNA. Nature. 2002;418:252–258. doi: 10.1038/418252a. [DOI] [PubMed] [Google Scholar]
- McNamara JO, Kolonias D, Pastor F, Mittler RS, Chen L, Giangrande PH, et al. Multivalent 4-1BB binding aptamers costimulate CD8+ T cells and inhibit tumor growth in mice. J Clin Invest. 2008;118:376–386. doi: 10.1172/JCI33365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollins CM, Nair S, Boczkowski D, Lee J, Layzer JM, Gilboa E, et al. Assembling OX40 aptamers on a molecular scaffold to create a receptor-activating aptamer. Chem Biol. 2008;15:675–682. doi: 10.1016/j.chembiol.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezhnoy A, Stewart CA, Mcnamara JO, 2nd, Thiel W, Giangrande P, Trinchieri G, et al. Isolation and optimization of murine IL-10 receptor blocking oligonucleotide aptamers using high-throughput sequencing. Mol Ther. 2012;20:1242–1250. doi: 10.1038/mt.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santulli-Marotto S, Nair SK, Rusconi C, Sullenger B, Gilboa E. Multivalent RNA aptamers that inhibit CTLA-4 and enhance tumor immunity. Cancer Res. 2003;63:7483–7489. [PubMed] [Google Scholar]
- Hünig T, Dennehy K. CD28 superagonists: mode of action and therapeutic potential. Immunol Lett. 2005;100:21–28. doi: 10.1016/j.imlet.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Lühder F, Huang Y, Dennehy KM, Guntermann C, Müller I, Winkler E, et al. Topological requirements and signaling properties of T cell-activating, anti-CD28 antibody superagonists. J Exp Med. 2003;197:955–966. doi: 10.1084/jem.20021024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendandi M. Idiotype vaccines for lymphoma: proof-of-principles and clinical trial failures. Nat Rev Cancer. 2009;9:675–681. doi: 10.1038/nrc2717. [DOI] [PubMed] [Google Scholar]
- Townsend SE, Allison JP. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993;259:368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- Pastor F, Kolonias D, Giangrande PH, Gilboa E. Induction of tumour immunity by targeted inhibition of nonsense-mediated mRNA decay. Nature. 2010;465:227–230. doi: 10.1038/nature08999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor F, Kolonias D, McNamara JO, 2nd, Gilboa E. Targeting 4-1BB costimulation to disseminated tumor lesions with bi-specific oligonucleotide aptamers. Mol Ther. 2011;19:1878–1886. doi: 10.1038/mt.2011.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getts DR, Getts MT, McCarthy DP, Chastain EM, Miller SD. Have we overestimated the benefit of human(ized) antibodies. MAbs. 2010;2:682–694. doi: 10.4161/mabs.2.6.13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- Römer PS, Berr S, Avota E, Na SY, Battaglia M, ten Berge I, et al. Preculture of PBMCs at high cell density increases sensitivity of T-cell responses, revealing cytokine release by CD28 superagonist TGN1412. Blood. 2011;118:6772–6782. doi: 10.1182/blood-2010-12-319780. [DOI] [PubMed] [Google Scholar]
- Guillot C, Ménoret S, Guillonneau C, Braudeau C, Castro MG, Lowenstein P, et al. Active suppression of allogeneic proliferative responses by dendritic cells after induction of long-term allograft survival by CTLA4Ig. Blood. 2003;101:3325–3333. doi: 10.1182/blood-2002-07-2076. [DOI] [PubMed] [Google Scholar]
- Tzachanis D, Berezovskaya A, Nadler LM, Boussiotis VA. Blockade of B7/CD28 in mixed lymphocyte reaction cultures results in the generation of alternatively activated macrophages, which suppress T-cell responses. Blood. 2002;99:1465–1473. doi: 10.1182/blood.v99.4.1465. [DOI] [PubMed] [Google Scholar]
- Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Bendandi M, Marillonnet S, Kandzia R, Thieme F, Nickstadt A, Herz S, et al. Rapid, high-yield production in plants of individualized idiotype vaccines for non-Hodgkin's lymphoma. Ann Oncol. 2010;21:2420–2427. doi: 10.1093/annonc/mdq256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CD28-aptamer dimers interact specifically with the cognate receptor.
Antitumor effect of the idiotypic vaccine in combination with CD28Apt7-dimer.
SELEX conditions.