Abstract
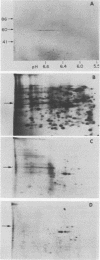
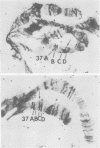
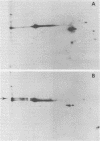
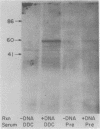
We have isolated chromosomal deoxyribonucleic acid clones containing the Drosophila dopa decarboxylase gene. We describe an isolation procedure which can be applied to other nonabundantly expressed Drosophila genes. The dopa decarboxylase gene lies within or very near polytene chromosome band 37C1-2. The gene is interrupted by at least one intron, and the primary mode of regulation is pretranslational. At least two additional sequences hybridized by in vivo ribonucleic acid-derived probes are found within a 35-kilobase region surrounding the gene. The developmental profile of ribonucleic acid transcribed from one of these regions differs from that of the dopa decarboxylase transcript.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bantle J. A., Maxwell I. H., Hahn W. E. Specificity of oligo (dT)-cellulose chromatography in the isolation of polyadenylated RNA. Anal Biochem. 1976 May 7;72:413–427. doi: 10.1016/0003-2697(76)90549-2. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bonner J. J., Pardue M. L. Ecdysone-stimulated RNA synthesis in imaginal discs of Drosophila melanogaster. Assay by in situ hybridization. Chromosoma. 1976 Oct 12;58(1):87–99. doi: 10.1007/BF00293443. [DOI] [PubMed] [Google Scholar]
- Brandhorst B. P. Two-dimensional gel patterns of protein synthesis before and after fertilization of sea urchin eggs. Dev Biol. 1976 Sep;52(2):310–317. doi: 10.1016/0012-1606(76)90248-7. [DOI] [PubMed] [Google Scholar]
- Brutlag D., Appels R., Dennis E. S., Peacock W. J. Highly repeated DNA in Drosophila melanogaster. J Mol Biol. 1977 May 5;112(1):31–47. doi: 10.1016/s0022-2836(77)80154-x. [DOI] [PubMed] [Google Scholar]
- Chen T. T., Hodgetts R. B. The appearance of dopa decarboxylase activity in imaginal discs of Sarcophaga bullata, undergoing development in vitro. Dev Biol. 1974 Jun;38(2):271–284. doi: 10.1016/0012-1606(74)90006-2. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Maniatis T., Kafatos F. C., Jeffrey A., Vournakis J. N. Full length and discrete partial reverse transcripts of globin and chorion mRNAs. Cell. 1975 Apr;4(4):367–378. doi: 10.1016/0092-8674(75)90157-9. [DOI] [PubMed] [Google Scholar]
- Fragoulis E. G., Sekeris C. E. Translation of mRNA for 3,4-dihydroxyphenylalanine decarboxylase isolated from epidermis tissue of Calliphora vicina R. -D. in a heterologous system. Dependence of mRNA concentration on the insect steroid hormone ecdysone. Eur J Biochem. 1975 Feb 3;51(1):305–316. doi: 10.1111/j.1432-1033.1975.tb03930.x. [DOI] [PubMed] [Google Scholar]
- Hodgetts R. B., Sage B., O'Connor J. D. Ecdysone titers during postembryonic development of Drosophila melanogaster. Dev Biol. 1977 Oct 1;60(1):310–317. doi: 10.1016/0012-1606(77)90128-2. [DOI] [PubMed] [Google Scholar]
- Hodgetts R. B. The response of dopa decarboxylase activity to variations in gene dosage in Drosophila: a possible location of the structural gene. Genetics. 1975 Jan;79(1):45–54. doi: 10.1093/genetics/79.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. The rabbit beta-globin gene contains a large large insert in the coding sequence. Cell. 1977 Dec;12(4):1097–1108. doi: 10.1016/0092-8674(77)90172-6. [DOI] [PubMed] [Google Scholar]
- Kaback D. B., Angerer L. M., Davidson N. Improved methods for the formation and stabilization of R-loops. Nucleic Acids Res. 1979 Jun 11;6(7):2499–2317. doi: 10.1093/nar/6.7.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Kraminsky G. P., Clark W. C., Estelle M. A., Gietz R. D., Sage B. A., O'Connor J. D., Hodgetts R. B. Induction of translatable mRNA for dopa decarboxylase in Drosophila: an early response to ecdysterone. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4175–4179. doi: 10.1073/pnas.77.7.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh J. L., Wright T. R. Developmental relationship between dopa decarboxylase, dopamine acetyltransferase, and ecdysone in Drosophila. Dev Biol. 1980 Dec;80(2):379–387. doi: 10.1016/0012-1606(80)90412-1. [DOI] [PubMed] [Google Scholar]
- McCaman M. W., McCaman R. E., Lees G. J. Liquid cation exchange--a basis for sensitive radiometric assays for aromatic amino acid decarboxylases. Anal Biochem. 1972 Jan;45(1):242–252. doi: 10.1016/0003-2697(72)90024-3. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudkin G. T. Replication in polytene chromosomes. Results Probl Cell Differ. 1972;4:59–85. doi: 10.1007/978-3-540-37164-9_3. [DOI] [PubMed] [Google Scholar]
- Rudkin G. T. The relative mutabilities of DNA in regions of the X chromosome of Drosophila melanogaster. Genetics. 1965 Sep;52(3):665–681. doi: 10.1093/genetics/52.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura A., Sorsa V. Electron microscopic analysis of the banding pattern in the salivary gland chromosomes of Drosophila melanogaster: divisions 37, 38 and 39 of 2L. Hereditas. 1979;91(1):5–18. doi: 10.1111/j.1601-5223.1979.tb01635.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sparrow J. C., Wright T. R. The selection for mutants in Drosophila melanogaster hypersensitive to alpha-methyl dopa, a dopa decarboxylase inhibitor. Mol Gen Genet. 1974 May 21;130(2):127–141. doi: 10.1007/BF00269084. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensink P. C., Finnegan D. J., Donelson J. E., Hogness D. S. A system for mapping DNA sequences in the chromosomes of Drosophila melanogaster. Cell. 1974 Dec;3(4):315–325. doi: 10.1016/0092-8674(74)90045-2. [DOI] [PubMed] [Google Scholar]
- Woolford J. L., Jr, Rosbash M. The use of R-looping for structural gene identification and mRNA purification. Nucleic Acids Res. 1979 Jun 11;6(7):2483–2497. doi: 10.1093/nar/6.7.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright T. R., Bewley G. C., Sherald A. F. The genetics of dopa decarboxylase in Drosophila melanogaster. II. Isolation and characterization of dopa-decarboxylase-deficient mutants and their relationship to the alpha-methyl-dopa-hypersensitive mutants. Genetics. 1976 Oct;84(2):287–310. doi: 10.1093/genetics/84.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright T. R., Hodgetts R. B., Sherald A. F. The genetics of dopa decarboxylase in Drosophila melanogaster. I. Isolation and characterization of deficiencies that delete the dopa-decarboxylase-dosage-sensitive region and the alpha-methyl-dopa-hypersensitive locus. Genetics. 1976 Oct;84(2):267–285. doi: 10.1093/genetics/84.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]