Abstract
Background
The adherence to the Mediterranean Diet (Med Diet) seems to reduce the incidence of metabolic syndrome. The present study aimed to explore whether the adherence to the overall Med Diet pattern and to specific Med Diet items is associated with the presence of metabolic syndrome, impaired fasting glucose (IFG), insulin resistance (IR), and microinflammation in subjects free of diabetes and cardiovascular diseases.
Measurements
Each patient underwent clinical assessment. Adherence to the Med Diet was measured by a previously validated 14-item questionnaire. Metabolic syndrome was defined by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria; IR was defined by homeostasis model assessment of insulin resistance (HOMA-IR); inflammation was assessed through a high-sensitivity C-reactive protein (hsCRP) assay.
Results
A total of 120 subjects (64.2% women, mean age 59.8±10.2 years) were enrolled at this study. Subjects with lower Med Diet pattern adherence exhibited higher occurrence of metabolic syndrome and all its components and higher HOMA-IR and hsCRP values (P for all <0.0001). Subjects with metabolic syndrome were less likely to consume olive oil (P=0.002) and vegetables (P=0.023). By multivariable analyses, the overall Med Diet score was found to be strongly and inversely associated with the presence of metabolic syndrome [B=−0.066; 95% confidence interval (CI) −0.105 to −0.028; P=0.001], IFG (B=−0.076; 95% CI −0.114 to −0.038; p<0.0001), high HOMA-IR (B=−0.071; 95% CI −0.108 to −0.034; P<0.0001) and high hsCRP (B=−0.082; 95% CI −0.125 to −0.045; P<0.0001). None of specific Med Diet items independently predicted metabolic syndrome, IFG, and high HOMA-IR. Instead, the consumption of white meat over red meat (B=−0.324; 95% CI −0.467 to −0.178; P<0.0001) was found to be inversely associated with increased hsCRP.
Conclusions
The inverse associations between adherence to Med Diet and the prevalence of metabolic syndrome and prediabetes may be due more to the effects of the entire dietary pattern rather than to individual food components. Metabolic syndrome–related microinflammation may further be linked to specific Med Diet components.
Introduction
Western countries are facing a pandemic of metabolic syndrome. According to the Third Adult Treatment Panel (ATP III) criteria, the prevalence of metabolic syndrome among adults is 25%, increasing with aging up to 40%.1,2 Metabolic syndrome is a multifactorial condition regulated by a complex interaction between genetic and environmental factors such as the quality of dietary pattern. The Mediterranean Diet (Med Diet), first described from observations on dietary habits of people living in different regions of the Mediterranean basin,3 is based on the consumption of minimally processed foods, including most of the dietary protective factors, such as vegetables, fruits, unrefined grains, fish, vegetable proteins from pulses, vegetable fats mainly from olive oil, moderate consumption of red wine, and more rarely poultry. Conversely, fast food, red meat, and processed meat products are far from the principles of the traditional Med Diet.
The adherence to an overall food pattern in line with the Med Diet has been demonstrated to significantly reduce the prevalence of metabolic syndrome.4–8 However, it remains unclear whether the Med Diet selectively and independently impacts on specific metabolic syndrome features such as dysglycemia.
Epidemiological longitudinal evidence for an association between the Med Diet and insulin resistance (IR) in subjects free of diabetes is limited. In a large prospective study, adherence to the Med Diet pattern was associated with a small reduction in the incidence of type 2 diabetes during follow-up, but IR was not assessed.9 A recent investigation showed cross-sectional associations between Med Diet adherence with fasting blood glucose (FBG) and IR in nondiabetic subjects, regardless of obesity, but not with incident diabetes.10 Contrariwise, in a large sample of individuals at high cardiovascular risk, the Med Diet was strongly associated with reduction in the incidence of diabetes independently of weight loss and physical activity.11 Data on IR alone are sparse. Some authors revealed a modest, not-significant association between IR and adherence to the Med Diet12,13 in overweight/obese subjects. Other authors revealed a cross-sectional association with IR and higher FBG only in normoglycemic subjects, while they failed to achieve the same result in diabetics and in those with impaired fasting glucose.14
Chronic microinflammation plays a key role in metabolic syndrome, being closely related to IR and abdominal fat amount. C-reactive protein (CRP) is a suitable marker to assess metabolic syndrome-related chronic microinflammation, and it is considered as an independent cardiovascular risk factor. The adoption of the Med Diet has been demonstrated to reduce CRP by 20%15; moreover, this effect seems to be independent of weight loss.16,17
The aims of the present study were to investigate the degree of adherence to Med Diet pattern in a sample of outpatients referred for evaluation of cardiovascular risk factors, free of diabetes and cardiovascular disease, and whether the adherence to the overall Med Diet pattern more than to the specific Med Diet items was associated with the prevalence of metabolic syndrome, impaired fasting glucose (IFG), IR, and microinflammation.
Materials and Methods
Study population
Patients who visited the Ambulatory of our Department in the period between September, 2012, and November, 2012, for evaluation of cardiovascular risk factors were considered for this study. Subjects who did not meet any of the following criteria were enrolled consecutively: Relevant changes in dietary habits within the last year; diabetes or current antidiabetic treatment; history of coronary heart disease, stroke/transient ischemic attack, other symptomatic cardiovascular events; and severe chronic diseases except blood hypertension, obesity, dyslipidemia, and IFG. We also excluded those who were taking anti-inflammatory agents or statins or other lipid-lowering agents; current or former smokers; those who referred a current or former daily consumption of more than two glasses (1 glass=100 mL) of alcoholic beverages for women, and more than three for men, for a consecutive period of at least 6 months.
Measurements
During the same morning of the visit, each patient completed all elements of the study assessment. Venous blood was withdrawn after an overnight fast. FBG, triglycerides, and high-density lipoprotein cholesterol (HDL-C) were assessed through enzymatic assays; fasting insulin and high sensitivity C-reactive protein (hsCRP) were determined through enzyme-linked immunosorbent assay (ELISA) kits. Insulin concentrations were expressed in U/mL, hsCRP concentrations in μg/mL.
A face-to-face interview investigated medical history, general habits, and home therapy of the patients. The presence of family history of type 2 diabetes mellitus was carefully investigated. Waist circumference (WC) was measured at the upper iliac crest with the patients standing; body weight was measured with light clothing; body mass index (BMI) was calculated by dividing the weight (kg) by the square of height (meters).
Blood pressure was measured by a physician, after a rest period of 10 min, in sitting position, using a mercury sphygmomanometer. Three measurements were taken, and the mean value was calculated. Patients were classified as hypertensive if they were on antihypertensive treatment or if they had systolic blood pressure (SBP) ≥130 mmHg and/or diastolic blood pressure (DBP) ≥85 mmHg. Metabolic syndrome was diagnosed, according to National Cholesterol Education Program (NCEP) ATP III 2001 criteria,18 by the presence of three or more of the following features: WC ≥88 cm in women and ≥102 cm in men; HDL-C <50 mg/dL in women and <40 mg/dL in men; FBG ≥100 mg/dL and/or antidiabetic treatment; fasting triglycerides ≥150 mg/dL; SBP ≥130 mmHg and/or DBP ≥90 mmHg and/or antihypertensive treatment. IFG was defined by FBG concentrations within the range 100–125 mg/dL.19
IR was estimated by homeostasis model assessment index of IR (HOMA-IR), calculated as follows: HOMA-IR=[fasting insulin (U/mL)×fasting glucose (mmol/L)]/22.5. By convention, patients with HOMA-IR >2.5 were considered to have IR.
Microinflammatory status was defined by hsCRP concentrations >1000 μg/mL. Med Diet adherence was measured by administration of a previously validated 14-item questionnaire.20 The total score ranges from 0 to 14: The higher the score, the higher the degree of adherence to the Med Diet pattern. Each item was scored 0 or 1. One point was given for: (1) Using olive oil as the main source of culinary fat; (2) consumption of 4 or more tablespoons (1 tablespoon=13.5 grams) of olive oil/day; (3) consumption of 2 or more servings (1 serving=200 grams) of vegetables/day; (4) consumption of 3 or more pieces of fruit/day; (5) consumption of less than 1 serving (1 serving=100 grams) of red meat or hamburger or sausages/day; (6) consumption of less than 1 serving (=12 grams) of animal fat such as butter, margarine, or cream/day; (7) consumption of less than 1 glass (=100 mL) of sugar-sweetened beverages/day; (8) consumption of 7 or more glasses (=100 mL) of red wine/week; (9) consumption of 3 or more servings (1 serving=150 grams) of pulses/week; (10) consumption of 3 or more servings (1 serving=150 grams) of fish/week; (11) consumption of less than 2 commercial pastries/week; (12) consumption of 3 or more servings (1 serving=30 grams) of tree nuts/week; (13) preferring white meat over red meat; (14) consumption of “soffritto” (a sauce made with tomato, garlic, onion or leeks sautéed in olive oil) 2 or more times/week.
Physical activity habits were self-reported by patients. Housework was considered to be “low-intensity physical activity”; steady-pace walking was considered “moderate-intensity physical activity”; cycling or playing aerobic activities such as foot race or gym exercises were considered “high-intensity physical activities”. The total amount of physical activity was quantified for each subject through an arbitrary scoring system, by using the following formula: Physical activity score=[hours/week of low-intensity activity×1] + [hours/week of moderate-intensity activity×2] + [hours/week of high-intensity physical activity×3].
Statistical analysis
All analyses were performed via Statistical Package for Social Sciences (SPSS) software, version 17.0 for Windows. The differences in average 14-item Med Diet questionnaire score were compared across age tertiles and by gender. According to the 14-item Med Diet questionnaire score, subjects were grouped as follows: Score 0–5, lowest adherence to the Med Diet; score 6–9; score ≥10, highest adherence to the Med Diet. Continuous variables are presented as mean±standard deviation (SD), categorical variables as frequencies. One-way analysis of variance (ANOVA) was adopted to compare continuous variables across groups, chi-squared test to compare categorical variables. The percentage of fulfillment of each specific item of the 14-item Med Diet questionnaire was compared according to the presence of metabolic syndrome, by using a chi-squared test. Multivariable regression models were constructed to identify whether Med Diet score and specific Med Diet items independently predicted higher occurrence of metabolic syndrome, IFG, and higher values of hsCRP and HOMA-IR.
Results
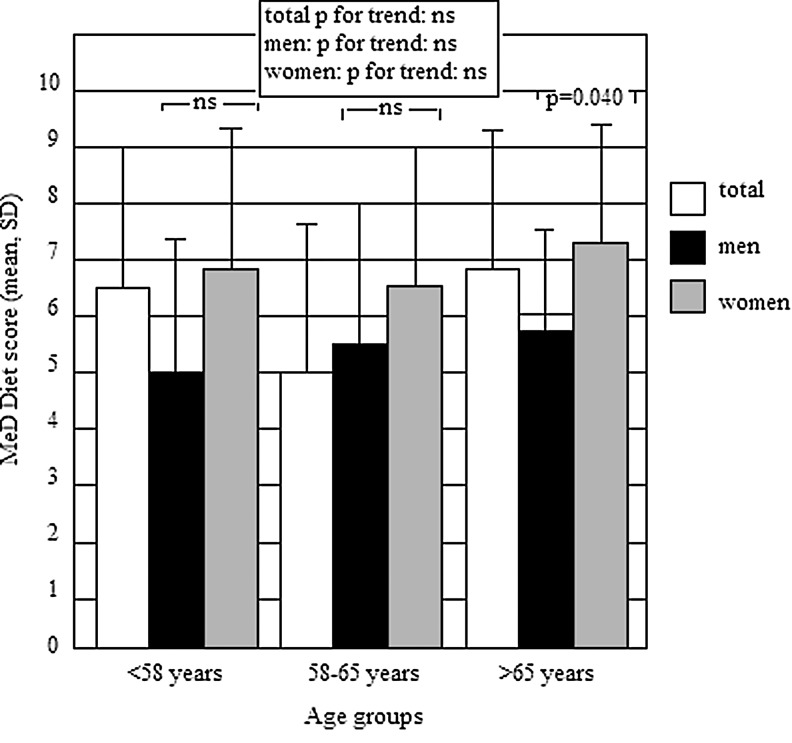
A total of 120 subjects (64.2% women), mean age 59.8±10.2 years, were enrolled at this study; 44 subjects were diagnosed to have metabolic syndrome. None of the subjects enrolled received dietary counseling prior to the study. In all, mean value (±SD) for the 14-item score questionnaire was 6.6±2.4. When subjects were grouped according to age tertiles, as shown by Fig. 1, no differences in average score were observed across age groups. No differences between men and women were observed in those aged <58 years and 58–65 years, whereas women aged >65 years achieved a significantly higher score (P=0.040) than men in the same age group.
FIG. 1.
Average Mediterranean diet (MeD) score by gender and age tertiles.
As shown in Table 1, 37 subjects achieved a Med Diet score within 0–5, 65 a score within 6–9, 18 a score ≥10. No differences in mean age, education, physical activity, and consumption of antiplatelet and β-blocker agents were found across categories of adherence. The prevalence of metabolic syndrome and all its components strongly decreased across groups, as did the proportion of subjects with IFG and the average values of hsCRP and HOMA-IR. Moreover subjects with higher adherence were more likely to be women. Mean values of BMI decreased across categories, without achieving statistical significance. Moreover, women were more likely to adhere to the Med Diet than men.
Table 1.
Characteristics of the Sample According to Med Diet Score
| Score | 0–5 (n=37) | 6–9 (n=65) | ≥10 (n=18) | P |
|---|---|---|---|---|
| Females (%) | 54.0 | 67.7 | 72.2 | 0.020 |
| Age (years) | 59.6±10.2 | 60.0±10.5 | 60.0±9.4 | NS |
| Education (years) | 9.3±3.8 | 8.4±4.2 | 9.4±4.0 | NS |
| Current workers (%) | 54.0 | 41.5 | 50.0 | NS |
| BMI (kg/m2) | 30.0±5.0 | 29.0±4.8 | 27.6±3.8 | NS |
| Physical activity score (n) | 26.7±12.5 | 28.1±13.1 | 34.5±13.8 | NS |
| Metabolic syndrome (%) | 64.8 | 27.7 | 11.1 | <0.0001 |
| Hypertension (%) | 64.8 | 67.7 | 50.0 | 0.027 |
| Central obesity (%) | 73.0 | 71.0 | 44.4 | <0.0001 |
| Glucose (mg/dL) | 111.0±20.2 | 97.4±17.6 | 93.0±9.3 | <0.0001 |
| IFG (%) | 73.0 | 34.0 | 16.7 | <0.0001 |
| Triglycerides (mg/dL) | 168.2±89.3 | 125.8±47.0 | 95.5±50.7 | <0.0001 |
| Triglycerides criteria (%) | 46.0 | 24.6 | 5.5 | <0.0001 |
| HDL-C (mg/dL) | 48.0±13.3 | 55.7±12.8 | 60.0±14.6 | <0.0001 |
| HDL-C criteria (%) | 48.6 | 23.1 | 11.0 | <0.0001 |
| CRP (log10) | 3.0±0.45 | 2.93±0.34 | 2.48±0.40 | <0.0001 |
| HOMA-IR (n) | 3.67±1.80 | 2.56±1.65 | 1.51±0.80 | <0.0001 |
| Medications | ||||
| Beta-blockers (%) | 8.1 | 1.5 | 5.5 | NS |
| Other antihypertensives (%) | 51.3 | 61.5 | 39.0 | 0.007 |
| Antiplatelet (%) | 13.5 | 15.4 | 16.5 | NS |
Med Diet, Mediterranean diet; NS, not significant; BMI, body mass index; IFG, impaired fasting glucose; HDL-C, high-density lipoprotein cholesterol; CRP, C-reactive protein; HOMA-IR, homeostasis model assessment of insulin resistance.
When the specific Med Diet items were analyzed, as shown by Table 2, the percentage of achievement of the specific items was compared according to the presence of metabolic syndrome. We found that subjects with metabolic syndrome (n=44) were less likely to consume olive oil as the main culinary fat (47.7% vs. 69.7%; P=0.002), four tablespoons of olive oil/day (47.7% vs. 69.7%; P=0.002), and vegetables (39.3% vs. 56.1%; P=0.023) than those without (n=76). No further differences were found. All subjects who declared they used olive oil as the main culinary fat were consuming more than 4 tablespoons/day. These two items were considered as a single variable in the multivariable regression analysis.
Table 2.
Fulfillment of the Specific Med Diet Items According to the Presence of Metabolic Syndrome
| Items | With MetS (n=44) | Without MetS (n=76) | P |
|---|---|---|---|
| Using olive oil as the main culinary fat (%) | 47.7 | 69.7 | 0.002 |
| ≥4 Tablespoons olive oil/day (%) | 47.7 | 69.7 | 0.002 |
| ≥2 Servings of vegetables/day (%) | 39.3 | 56.1 | 0.023 |
| ≥3 Pieces of fruit/day (%) | 36.0 | 39.2 | NS |
| <1 Serving of red/processed meat (%) | 43.3 | 40.0 | NS |
| <1 Serving of animal fat (%) | 29.0 | 28.7 | NS |
| <1 Glass of sugar-sweetened beverages/day (%) | 40.1 | 47.5 | NS |
| Moderate wine consumption (%) | 10.7 | 11.5 | NS |
| ≥3 Servings of pulses/week (%) | 7.4 | 11.0 | NS |
| ≥3 Servings of fish/week (%) | 7.4 | 11.0 | NS |
| <2 Commercial pastries/week (%) | 21.1 | 22.3 | NS |
| ≥3 Servings of tree nuts/week (%) | 4.8 | 6.0 | NS |
| Preferring white meat over red meat (%) | 24.0 | 31.3 | NS |
| ≥2 “Soffritto”/week (%) | 12.0 | 12.9 | NS |
Med Diet, Mediterranean diet; MetS, metabolic syndrome; NS, not significant.
As shown in Table 3, multivariable regression models were constructed. All models were controlled for education, marital status, smoking, family history of diabetes, WC, physical activity score, and medications. All the specific Med Diet items were added to the models. The presence of metabolic syndrome, IFG, hsCRP >1000 μg/mL, and HOMA-IR >2.5 were considered as the dependent variables one by one. In the models in which IFG, hsCRP >1000 μg/mL and HOMA-IR >2.5 were considered as dependent variables, the presence of metabolic syndrome was added as covariate.
Table 3.
Multivariable Regression Analysis
| |
Presence of MetS |
IFG |
||||||
|---|---|---|---|---|---|---|---|---|
| Covariates | B | 95% CI | P | B | 95% CI | P | ||
| Sex | −0.128 | −0.302 | 0.046 | NS | −0.151 | −0.324 | 0.021 | NS |
| Age | 0.002 | −0.009 | 0.012 | NS | 0.022 | 0.011 | 0.032 | <0.0001 |
| BMI | 0.038 | 0.020 | 0.056 | <0.0001 | 0.021 | 0.003 | 0.038 | 0.023 |
| Med Diet score | −0.066 | −0.105 | −0.028 | 0.001 | −0.076 | −0.114 | −0.038 | <0.0001 |
| Olive oil | −0.108 | −0.276 | 0.061 | NS | −0.059 | −0.226 | 0.108 | NS |
| Vegetables | 0.053 | −0.094 | 0.206 | NS | 0.013 | −0.129 | 0.150 | NS |
| White meat | 0.079 | −0.098 | 0.255 | NS | 0.096 | −0.068 | 0.244 | NS |
| Wine | −0.004 | −0.188 | 0.180 | NS | 0.162 | −0.021 | 0.345 | NS |
| Fish | 0.094 | −0.111 | 0.300 | NS | −0.084 | −0.288 | 0.120 | NS |
| |
hsCRP>1000 μg/mL |
HOMA-IR>2.5 |
||||||
|---|---|---|---|---|---|---|---|---|
| Covariates | B | 95% CI | P | B | 95% CI | P | ||
| Sex | −0.048 | −0.233 | 0.136 | NS | 0.007 | −0.160 | 0.175 | NS |
| Age | −0.005 | −0.015 | 0.006 | NS | 0.006 | −0.003 | 0.016 | NS |
| BMI | −0.005 | −0.026 | 0.015 | NS | 0.041 | 0.024 | 0.058 | <0.0001 |
| Med Diet score | −0.082 | −0.125 | −0.045 | <0.0001 | −0.071 | −0.108 | −0.034 | <0.0001 |
| Olive oil | 0.060 | −0.082 | 0.202 | NS | −0.124 | −0.286 | 0.038 | NS |
| Vegetables | −0.046 | −0.190 | 0.112 | NS | −0.065 | −0.216 | 0.079 | NS |
| White meat | −0.324 | −0.467 | −0.178 | <0.0001 | −0.084 | −0.252 | 0.084 | NS |
| Wine | −0.164 | −0.346 | 0.021 | NS | 0.075 | −0.102 | 0.253 | NS |
| Fish | −0.194 | −0.410 | −0.023 | NS (P=0.079) | 0.004 | −0.192 | 0.201 | NS |
The presence of metabolic syndrome, IFG, HOMA-IR >2.5, and hsCRP >1000 μg/mL were singly considered as dependent variables.
All models were controlled for education, marital status, working status, current smoking, family history of diabetes, waist circumference, physical activity score, medications. All Med Diet items were added. The presence of metabolic syndrome was added in IFG, hsCRP, and HOMA-IR models.
MetS, metabolic syndrome; IFG, impaired fasting glucose; B, regression coefficient; 95% CI, 95% confidence intervals; NS, not significant; BMI, body mass index; Med Diet, Mediterranean diet; HOMA-IR, homeostasis model assessment of insulin resistance; hsCRP, high-sensitivity C-reactive protein.
We found that BMI [B=0.038; 95% confidence interval (CI) 0.020–0.056; P<0.0001] and overall Med Diet score (B=−0.066; 95% CI −0.105 to −0.028; P=0.001) were strongly associated with the presence of metabolic syndrome. None of the specific Med Diet items nor the other covariates predicted the presence of metabolic syndrome.
IFG was found to be independently predicted by age (B=0.022; 95% CI 0.011–0.032; P<0.0001), BMI (B=0.021; 95% CI 0.003–0.038; P=0.023) and overall Med Diet score (B=−0.076; 95% CI −0.114 to −0.038; P<0.0001), whereas none of the specific items showed an association.
Overall Med Diet score (B=−0.082; 95% CI −0.125 to −0.045; P<0.0001) and the consumption of white meat over red meat (B=−0.324; 95% CI −0.476 to −0.178; P<0.0001) were significantly and inversely associated with high hsCRP concentrations, but not sex, age, BMI, and the remaining items.
BMI (B=0.041; 95% CI 0.024–0.058; P<0.0001) and overall Med Diet score (B=−0.071; 95% CI −0.108 to −0.034; P<0.0001) were independently associated with high HOMA-IR, but not sex, age, and Med Diet items.
Discussion
In the present study, we found an overall low level of adherence to the Med Diet in a population of subjects referred for evaluation of cardiovascular risk factors. This finding may suggest that dietary pattern changes toward modern Western diet are occurring, even in Mediterranean countries, indicating a dietary globalization in Western countries. Today, a diet rich in meat, processed foods, and sweets has become more common at a population level, even in adults and older subjects. Elderly women may be more likely to preserve the dietary habits in line with the Med Diet.
In this cross-sectional investigation, we chose to exclude diabetic subjects in order to evaluate whether the adherence to the Med Diet has an impact on prediabetes, metabolic syndrome, IR, and metabolic syndrome–related microinflammation by avoiding the potential confounding role of diabetes and antidiabetic medications. Moreover, BMI could be less specific in assessing whole-body adiposity in the patients with diabetes, because diabetes reduces lean mass. In this sense, the presence of diabetes would have played a confounding role.
When the presence of metabolic syndrome was considered as a dependent variable, we found that the overall Med Diet score was independently associated with it. This is consistent with previous reports suggesting that adherence to the Med Diet may protect against metabolic syndrome4–8; moreover, this effect seems to be independent of whole and abdominal obesity markers. The interesting finding is that none of the specific items of Med Diet predicted the presence of metabolic syndrome. This could indicate that the overall Med Diet pattern, more than the consumption of specific food categories, favorably impacts on metabolic syndrome, probably through a potential beneficial interplaying of food components, beyond weight gain.
In the present study we found strong inverse associations of Med Diet score with IFG and HOMA-IR >2.5. The strength of these associations was not affected by abdominal obesity, BMI, social factors, family history of diabetes, and presence of metabolic syndrome; none of Med Diet items was found to be associated. These findings were partly supported by previous reports. The effects of Med Diet adherence on IR in nondiabetic subjects has been poorly investigated, and evidence of associations with specific Med Diet items is lacking. A longitudinal survey that failed to find an association between Med Diet adherence and incident diabetes showed a cross-sectional association with IR and FBG, but only in nondiabetics. Moreover, the association with FBG disappeared after adjustment for obesity.10 A longitudinal study12 on subjects with abdominal obesity (ATP III criteria) free of diabetes revealed a trend for a reduction of HOMA-IR in the subjects who received the Med Diet, without achieving statistical significance. Probably the follow-up period was too short to obtain a significant reduction of HOMA-IR; moreover, the prevalence of metabolic syndrome was not declared, making it impossible to estimate the potential confounding role of clustered risk factors. A significant 2-year reduction in IR was reported, independent of weight loss and inflammation.17 A cross-sectional investigation13 failed to find a statistically significant association between Med Diet adherence and HOMA-IR and FBG. IFG was not investigated, and the subjects were matched for overweight/obesity, suggesting that the beneficial effects of the Med Diet on insulin sensitivity may be limited in subjects with body weight excess, probably because of the higher inflammatory levels; moreover, this study population did not exclude diabetic subjects. HOMA-IR may be not a reliable index in diabetics.
Our results suggest that the overall Med Diet pattern may exert a beneficial role on glucose metabolism, regardless of the frequency of consumption of specific food categories. The interaction of various components, such as olive oil, whole grain products, large intake of fibers by fruits and vegetables, vitamins, and polyphenolic compounds by fruits or red wine, may exert a pivotal role in modulating glucose homeostasis pathways beyond the amount of adipose tissue. A main limitation of our investigation is that we did not assess the caloric and carbohydrates daily intake of the subjects, so that we cannot exclude their possible confounding role.
Further longitudinal investigations are needed to better understand whether prediabetic subjects may turn back to normoglycemia by closely adhering to the Med Diet pattern, and whether it may substantially reduce the progression rate toward diabetes. The presence of microinflammation, defined as hsCRP concentrations >1000 μg/mL, was found to be strongly and inversely associated not only with Med Diet score, but also with the consumption of white meat over red meat. hsCRP has been widely recognized to be a suitable marker to detect metabolic syndrome–linked inflammation, and previous reports showed a longitudinal reduction of hsCRP levels in subjects who adopted MedDiet pattern, independently of weight loss.15–17
In metabolic syndrome patients, chronic inflammation mainly originates from visceral adipose tissue. We found that the associations between overall Med Diet adherence and hsCRP was not affected by either high WC and metabolic syndrome. This is an interesting finding because it can be hypothesized that several properties of the Med Diet may exert direct or indirect anti-inflammatory effects, such as the high total plasma antioxidant capacity conferred by large amounts of vitamins (such as A, C, and E), the lower lipidic oxidation linked to high dietary availability of monounsaturated or polyunsaturated fatty acids, and the lower omega-6 to omega-3 fatty ratio.
We would emphasize that despite the lack of association found between the regular consumption of fish and inflammation, this Med Diet item was, however, close to statistical significance (B=−0.194; 95%CI=−0.410 to −0.023; P=0.079). Probably a significant result would have been achieved if the sample had been wider.
Specific food categories, such as fish, may particularly prevent inflammation, and it has been suggested formerly that the consumption of fish rich in long-chain omega-3 polyunsaturated fatty acids, such as salmon, is inversely associated with serum hsCRP concentrations. Moreover, several kinds of fish offer a suitable omega-6 to omega-3 fatty ratio. We found that the consumption of white meat over red/processed meat was inversely associated with high hsCRP. The frequent consumption of red or processed meat may be linked to higher inflammatory levels because of the higher unsaturated fat amount of these foods, which may predispose to abdominal obesity and lipidic oxidation.
Our results represent a picture of the typical subjects who came to a medical preventive setting for evaluation of cardiovascular risk factors and for whom prevention should be guaranteed. These results support the hypotheses that dietary habits in line with the traditional Med Diet pattern may beneficially impact metabolic syndrome, prediabetes, and microinflammation in subjects free of diabetes and clinical cardiovascular diseases. Further longitudinal investigations are needed to assess whether these associations may be reversible, and whether the administration of the Med Diet may reduce and reverse the prevalence metabolic derangements at a population level. Moreover, it should be investigated whether specific nutrients of the Med Diet may exert nutrigenomic effects by modulating metabolic and inflammatory pathways at a gene level that may explain individual differences in the dietary modulation of metabolism.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Alberti KG. Zimmet P. Shaw J. The metabolic syndrome—a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 2.Ford E. Giles W. Dietz W. Prevalence of the metabolic syndrome among U.S. adults: Findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 3.Keys A. Mediterranean diet and public health: Personal reflections. Am J Clin Nutr. 1995;61:1321S–1323S. doi: 10.1093/ajcn/61.6.1321S. [DOI] [PubMed] [Google Scholar]
- 4.Kastorini CM. Milionis HJ. Esposito K. Giugliano D. Goudevenos JA. Panagiotakos DB. The effect of Mediterranean diet on metabolic syndrome and its components: A meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol. 2011;57:1299–1313. doi: 10.1016/j.jacc.2010.09.073. [DOI] [PubMed] [Google Scholar]
- 5.Sánchez-Taínta A. Estruch R. Bulló M. Corella D. Gómez-Gracia E. Fiol M. Algorta J. Covas MI. Lapetra J. Zazpe I. Ruiz-Gutiérrez V. Ros E. Martínez-González MA. PREDIMED group. Adherence to a Mediterranean-type diet and reduced prevalence of clustered cardiovascular risk factors in a cohort of 3,204 high-risk patients. Eur J Cardiovasc Prev Rehabil. 2008;15:589–593. doi: 10.1097/HJR.0b013e328308ba61. [DOI] [PubMed] [Google Scholar]
- 6.Paletas K. Athanasiadou E. Sarigianni M. Paschos P. Kalogirou A. Hassapidou M. Tsapas A. The protective role of the Mediterranean diet on the prevalence of metabolic syndrome in a population of Greek obese subjects. J Am Coll Nutr. 2010;29:41–45. doi: 10.1080/07315724.2010.10719815. [DOI] [PubMed] [Google Scholar]
- 7.Rumawas ME. Meigs JB. Dwyer JT. McKeown NM. Jaques PF. Mediterranean-style dietary pattern, reduced risk of metabolic syndrome traits, and incidence in the Framingham Offspring Cohort. Am J Clin Nutr. 2009;90:1608–1614. doi: 10.3945/ajcn.2009.27908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panagiotakos DB. The role of Mediterranean diet in the epidemiology of metabolic syndrome; converting epidemiology to clinical practice. Lipids Health Dis. 2005;4:7. doi: 10.1186/1476-511X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.InterAct Consortium. Mediterranean diet and type 2 diabetes risk in the European Prospective Investigation Into Cancer and Nutrition (EPIC) study. Diabetes Care. 2011;34:1913–1918. doi: 10.2337/dc11-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abiemo EE. Alonso A. Nettleton JA. Steffen LM. Bertoni AG. Jain A. Lutsey PL. Relationships of the Mediterranean dietary pattern with insulin resistance and diabetes incidence in the Multi-Ethnic Study of Atherosclerosis (MESA) Br J Nutr. 2012;30:1–8. doi: 10.1017/S0007114512003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salas-Salvadó J. Bulló M. Babio N. Martínez-González MÁ. Ibarrola-Jurado N. Basora J. Estruch R. Covas MI. Corella D. Arós F. Ruiz-Gutiérrez V. Ros E. PREDIMED Study Investigators. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care. 2011;34:14–19. doi: 10.2337/dc10-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rallidis LS. Lekakis J. Kolomvotsou A. Zampelas A. Vamvakou G. Efstathiou S. Dimitriadis G. Raptis SA. Kremastinos DT. Close adherence to a Mediterranean diet improves endothelial function in subjects with abdominal obesity. Am J Clin Nutr. 2009;90:263–268. doi: 10.3945/ajcn.2008.27290. [DOI] [PubMed] [Google Scholar]
- 13.Tzima N. Pitsavos C. Panagiotakos DB. Skoumas J. Zampelas A. Chrysohoou C. Stefanadis C. Mediterranean diet and insulin sensitivity, lipid profile and blood pressure levels, in overweight and obese people; the Attica study. Lipids Health Dis. 2007;6:22. doi: 10.1186/1476-511X-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panagiotakos DB. Tzima N. Pitsavos C. Chrysohoou C. Zampelas A. Toussoulis D. Stefanadis C. The association between adherence to the Mediterranean diet and fasting indices of glucose homoeostasis: The ATTICA Study. J Am Coll Nutr. 2007;26:32–38. doi: 10.1080/07315724.2007.10719583. [DOI] [PubMed] [Google Scholar]
- 15.Chrysohoou C. Panagiotakos DB. Pitsavos C. Das UN. Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process, in healthy adults: The ATTICA study. J Am Col Cardiol. 2004;44:152–158. doi: 10.1016/j.jacc.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 16.Richard C. Couture P. Desroches S. Lamarche B. Effect of the the mediterranean diet with and without weight loss on markers of inflammation in men with metabolic syndrome. Obesity. 2012 Jun 15; doi: 10.1038/oby.2012.148. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 17.Esposito K. Marfella R. Ciotola M. Di Paolo C. Giugliano F. Giugliano G. D'Armiento M. D'Andrea F. Giugliano D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA. 2004;292:1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 18.The Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Associations. Diabetes Care. 2011;((Suppl 34)):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martínez-González MA. García-Arellano A. Toledo E. Salas-Salvadó J. Buil-Cosiales P. Corella D. Covas MI. Schröder H. Arós F. Gómez-Gracia E. Fiol M. Ruiz-Gutiérrez V. Lapetra J. Lamuela-Raventos RM. Serra-Majem L. Pintó X. Muñoz MA. Wärnberg J. Ros E. Estruch R. PREDIMED Study Investigators. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS One. 2012;7:e43134. doi: 10.1371/journal.pone.0043134. [DOI] [PMC free article] [PubMed] [Google Scholar]