Abstract
Background
It has been suggested that the antidepressant effect of laser acupuncture involves modulation of the default mode network (DMN) or resting state network (RSN). In this study, the authors investigated changes in the DMN during laser acupuncture in depressed and nondepressed participants.
Objective
To aim of this study was to determine if the modulation of the DMN effects by laser acupuncture in depressed participants are different from those of nondepressed participants.
Design
Randomized stimulation was performed with laser acupuncture on four putative antidepressant acupoints (LR 14, LR 8, CV 14, and HT 7) in a block on–off design, while the blood oxygenation level–dependent (BOLD) fMRI response was recorded from each subject's whole brain on a 3T scanner. DMN patterns of the participants were identified, using an independent component analysis. The identified DMN components from both the nondepressed group and the depressed group were then analytically compared using SPM5.
Setting
This study took place at a research institute.
Subjects
Ten nondepressed participants and 10 depressed participants (DS) as confirmed by the Hamilton Depression Rating Scale (HAM-D) participated in this study.
Intervention
Low Intensity Laser Acupuncture.
Main outcome measures
Significant DMN patterns in one group were greater than those in the other group.
Results
The nondepressed participants had significant modulation of DMN in the frontal region at the medial frontal gyrus (verum laser>rest, p<0.001) for three acupoints (LR 14, LR 8, and CV 14). For the depressive participants, the DMN modulation occurred at the inferior parietal cortex and the cerebellum (verum laser>rest, p<0.001).
Conclusions
Laser acupuncture on LR 8, LR 14, and CV 14 stimulated both the anterior and posterior DMN in both the nondepressed and depressed participants. However, in the nondepressed participants, there was consistently outstanding modulation of the anterior DMN at the medial frontal gyrus across all three acupoints. In the depressed participants, there was wider posterior DMN modulation at the parieto–temporal–limbic cortices. This is part of the antidepressant effect of laser acupuncture.
Key Words: Low Intensity Laser, Acupuncture, Acupoints, Default Mode Network
Introduction
The default mode network (DMN) is a network of brain regions that are active when the brain is at rest. This network is also referred to as the resting state network (RSN). When the brain is at rest, it is task-negative (for example sitting quietly while waiting to catch a bus and not doing anything in particular, hence, not engaging in a task). The brain is task-positive when it has an actual task to complete (for example, reading this article is a task).1,2 There has been some preliminary evidence to support the hypothesis that the resting brain and DMN are involved in stimulus-independent thought (SIT), which may be important for mental well-being in the individual.3 Mason et al. suggest that SIT allows a “sense of coherence to the individual's past, present and future experiences,” in other words, a sense of self. This resting state and SIT help modulate the individual's ability to manage concurrent tasks mentally when the brain is next in task-positive mode.3 Although the place of the DMN in depression is unclear, there is emerging evidence that there are shifts in functionality of the DMN in depression,4–8 with heightened activity within the DMN and failure to downregulate in the resting brain.
Functional magnetic resonance imaging (fMRI) studies over the last decade have revealed the central effects of needle acupuncture, including the modulation of the default DMN or RSN.9,10 However, it is still unclear what this DMN modulation achieves during needle acupuncture. The regulatory role of acupuncture is modality-independent, as there has been some evidence that laser acupuncture also modulates the DMN.11
The current authors previously reported the differing brain patterns of healthy individuals, compared to depressed individuals during laser acupuncture to depression-specific acupoints.12 This investigation was extended to the current study to identify and compare the modulatory effects of laser acupuncture on the DMN components of nondepressed versus depressed participants.
Methods
Ethics Statement
This study was approved by the research ethics board of the South Eastern Sydney-Illawarra Area Health Service. Subjects provided written informed consent before participation.
Participants
The study was conducted in two stages. Two groups (10 nondepressed participants and 10 participants with major depression, all right-handed) were independently randomly stimulated with laser acupuncture on four acupoints (LR 14, LR 8, CV 14, and HT7), which are used clinically in the treatment of depression according to Traditional Chinese Medicine (TCM).13–14
Inclusion Criteria for Nondepressed Participants
In stage one, the participants (5 women and 5 men) were healthy, nondepressed, volunteers ages 18–50 (mean age=39.8 years), who were recruited from staff and student advertisements by the University of New South Wales and the Prince of Wales Hospital, in Sydney, Australia. In each of these participants, there was no past history of depression or other psychiatric disorders (Beck Depression Inventory15 score of < 10), no history of drug or alcohol abuse, and no psychotropic medication or herbal intake within 3 months of the study.
Inclusion Criteria for Depressed Participants
Depressed participants (5 women and 5 men), ages 18–50 (mean age=43.7 years) were recruited for stage two of the study. The recruitment process was similar to that of the healthy, nondepressed participants, but also included general practice patients with confirmed clinical depression in metropolitan Sydney, Australia. All participants were currently depressed according to the Diagnostic and Statistical Manual of Mental Disorders, 4th ed., (DSM-IV) classification.16 All depressed participants scored ≥12 on both the self-reporting Beck Depression Inventory (BDI: mean score: 22.8) and scored ≥14 (mean score 21.0) on the clinician-assessed Montgomery and Asberg Depression Rating Scale (MADRS).17 Their individual scores on the clinician-assessed Hamilton Psychiatric Reporting Scale (HAM-D)18 were > 10 (mean score: 18.5). Among these participants, there was no history of alcohol abuse or psychotropic medication or herbals taken within 3 months of the scans.
Exclusion Criteria for Both Groups
Presence of systemic or neurological disorder, mania, or any other psychiatric disorders (apart from depression) were grounds for exclusion from the study in depressed participants. Any contraindications to magnetic resonance imaging (MRI; e.g., pacemakers, ferromagnetic implants or foreign bodies, claustrophobia) were also grounds for exclusion in both groups.
Sample Size
The sample size for this study (n=20) was a composite of both the nondepressed participants and the depressed participants.
Choice of Acupoints
The chosen acupoints were those used in a pilot clinical study that found laser acupuncture (LA) to be efficacious for treating of mild-to-moderate depression (p<0.001).19 These acupoints are on the classically named Liver (LR), Heart (HT), and Conception Vessel (CV) meridians and are acupoints useful for treating depression.11 LR 14 is the “alarm point” on the LR meridian and was selected for its rapid onset in action. LR 8 is the “water point” on the same meridian and is useful for calming “Liver Fire” according to TCM, which may present as somatic symptoms of depression, such as headaches, pectoral pains, and “heartburn.” In TCM, reference to the Heart is usually the emotional heart and CV 14 is the “alarm point” of the Heart meridian; this point useful for addressing upper gastrointestinal somatic changes of depression. HT 7 on the Heart meridian is classically used as a calmative or sedation point to help an individual relax.
We used left-sided LR 8, which is in the region of the left medial knee area, between the insertions of the sartorius and semitendinosis muscles. Right-sided LR 14 is in the vicinity of the right sixth intercostal space on the mid clavicular line. Left-sided HT 7 is at the wrist crease, in the vicinity of the radial side of the left flexor carpi ulnaris. CV 14 is in the anterior midline, approximately 5 cm below the xiphisternum.
Laser Stimulation
A Moxla® prototype fiberoptic infrared light laser (Euryphaessa AB, Orgnr: 556746-8987, Stockholm, Sweden; 808 nm) with a 25-mW capacity and a fiberoptic arm was developed for usage in the MRI scanner. The laser parameters were similar for both groups.11,12 The acupoints were clearly circled with a skin-marking pen (circumference slightly more than the laser probe) before scanning commenced. A stably held laser was applied to the skin by the acupuncturist (I. Q-S.) who moved the laser from point to point according to the time signal. The laser “on” and “off” was achieved with a computer signal time-locked to the MRI acquisition. In the first round, each run had 25 mW×20 seconds of laser-energy delivery (0.5J), and each acupoint received 4 active laser runs (0.5J×4=2J). In the second round, the procedure was repeated (another 2J). The total energy delivered was 4J per acupoint per participant.
fMRI Design
A “block on–block off” design (Active–Rest–Active–Rest) was applied while the blood oxygen level–dependent (BOLD) fMRI response was recorded from each subject's whole brain on a 3T scanner. Blocks of 24 seconds' duration during which the subject received either laser (the laser is switched “on”) or placebo (the laser is switched “off”) were applied at each acupoint. As has been previously mentioned, the 24 seconds included a 2-second built-in time delay for laser safety, 2 seconds TR (or rest time) and the actual active or placebo laser blocks of 20 seconds. Eight blocks comprise one session (192 seconds). There was randomization of the two sessions for each of the 4 acupoints (LR 14, CV 14, HT 7, and LR8). In total there were 8 sessions for the whole fMRI design.
fMRI parameters
Acquisition
Imaging was performed on a 3T Philips Intera MRI scanner (Philips Medical Systems, Best, The Netherlands) for both T1-weighted three-dimensional (3D) structural and BOLD contrast fMRI. The 3D structural MRI was acquired in sagittal orientation, using a T1-weighted Turbo Field Echo (TFE) sequence (repetition time/echo time [TR/TE]=6.39/2.9 ms; flip angle=8; matrix size=256×256; field of view [FOV]=256×256 mm; slice thickness 1.0 mm), yielding sagittal slices of 1.0-mm thick and an in-plane spatial resolution of 1.0×1.0 mm, producing an isotropic voxel of 1.0×1.0×1.0 mm. A gradient echo-planar imaging (EPI) technique (TR/TE=2000/40 ms; matrix size=128×128; FOV=250×250 mm; in plane pixel size 1.953×1.953 mm) was used to acquire T2-weighted BOLD contrast fMRI in axial orientation. Each participant's whole brain was scanned, using 21 slices at a 5.0-mm slice thickness (to reduce subcortical dropout) and a 0.5-mm gap for each volume. Each session of 96 volumes was collected with the rate of 2 seconds/volume.
Image preprocessing and statistical analysis
Imaging data were analyzed using statistical parametric mapping (SPM2, Wellcome Department of Cognitive Neurology, London, UK) implemented in Matlab version 6 (The Mathworks Inc., Natick, MA). All volumes were realigned spatially to the first volume and the time-series for voxels within each slice realigned temporally to acquisition of the middle slice. Resulting volumes were normalized to a standard EPI template based on the Montreal Neurological Institute (MNI). The normalized images were smoothed with an isotropic 8 mm full-width half-maximal Gaussian kernel. The time-series in each voxel were high pass-filtered to 1/120 Hz to remove low-frequency noise.
Independent component analysis
All samples underwent independent component analysis (ICA) with the Group ICA of fMRI Toolbox (GIFT),20–23 using the estimated total component numbers. ICA allows for extraction of independent data from fMRI data. In this study, group ICA analysis was applied on fMRI data separately on depressed and nondepressed subjects. The different sessions with the acupoints were input as different scan sessions. First, GIFT estimated the suggested component number for each group. Second, all the components of the intragroup mean were ranked according to a standard DMN template.24 The anterior and posterior components of DMN were decided based on the spatial ranking and temporal frequency attributes. 25,26 Finally the DMN components of each individual and each acupoint were extracted and entered into the full factorial group comparison.
Second-level post processing
Given that the DMN consisted of two parts, a full factorial design was used to perform the group comparison, using SPM. There are two second-level factors in the design matrix: DMN (anterior/posterior) and group (nondepressed subjects/depressed subjects). Group comparisons were conducted four times for each acupoint separately. To avoid a multiple comparisons error, all results were corrected using the FWE [Family Wise type 1 Error] threshold of p<0.05 with cluster threshold (k)>15 contiguous voxels for each cluster.
Results
ICA
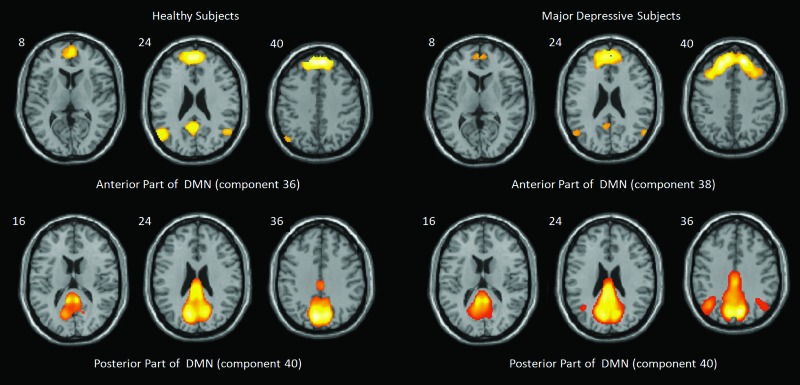
In the nondepressed subjects group, 42 independent components were isolated using GIFT. All 42 components were carefully ranked according to spatial and temporal attributes. Component 36 was selected as the anterior DMN, and component 40 was selected as the posterior part of the DMN for the nondepressed subjects. Similarly, in the depressed subjects, components 38 and 40 were selected as the anterior and the posterior parts of the DMN respectively (Fig. 1).
FIG. 1.
Default mode network (DMN). The independent components of the default mode network (DMN) for the nondepressed, healthy subject group and the major depressive subject group during acupuncture. In the nondepressed healthy group, components 36 (left-top) and 40 (left-bottom) indicated the anterior and posterior parts of the DMN. However, in the depressive group, the anterior and posterior parts of DMN were components 38 (right-top) and 40 (right-bottom).
Full Factorial Analysis
Using full factorial analysis on SPM, both nondepressed and depressed subjects had modulation of both anterior and posterior DMNs. However, each of nondepressed subjects had outstanding significant modulation of DMN in the frontal region at medial frontal gyrus. All clusters were presented with p<0.05 (FWE correction) and k (cluster size) >40 voxels. LR 8, LR 14, and CV 14 produced a significant difference in DMN modulation between nondepressed and depressed subjects. HT7 produced no significant difference between the two groups.
LR 8
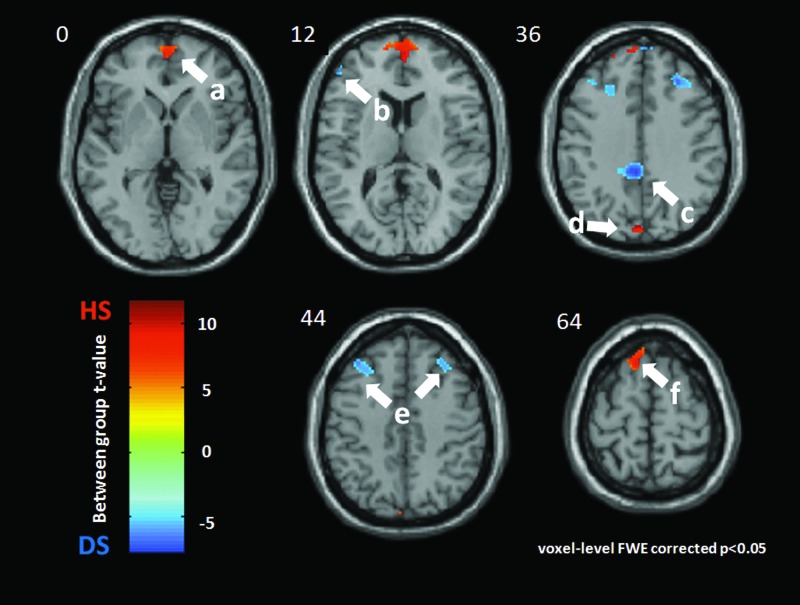
In each of the nondepressed subjects, LR 8 modulated the bilateral medial frontal gyrus (BA10, [4 58 6], t=11.64, k=556), left inferior frontal gyrus (BA46, [−46 42 12], t=5.18, k=43), left cuneus (BA19, [−2 82 34], t=10.62, k=44), and left superior frontal gyrus (BA6, [34 36 42), t=8.96, k=64).
In the depressed subjects, LR 8 produced more DMN activation at the bilateral superior and middle frontal gyrus (left BA9, [−28 32 38], t=5.19, k=192; right BA9, [34 36 42], t=5.18, k=266) and the middle cingulate (BA 31, [0−42 40], t=5.21, k=157). See Figures 2 and 3.
FIG. 2.
Medial frontal gyrus (MFG) in nondepressed healthy subjects (HS). Compared with depressed subjects, nondepressed subjects' DMN showed more activation at the bilateral medial frontal gyrus, consistently among different acupuncture points. Regions of interests were the bilateral medial frontal gyrus cluster of the comparison between depressed subjects' DMN and nondepressed subjects DMN during acupuncture. Different colors were used to label the different acupuncture points. The middle slice at the sagittal direction was chosen as the background.
FIG. 3.
LR 8 acupoint. Group difference between nondepressed healthy subjects (HS) and major depressive subjects (DS) during acupuncture at LR 8. Warm color indicates more DMN activation for HS, while the cold color shows the increased DMN activation for DS. All clusters were presented with p<0.05 (Family Wise type 1 Error correction) and k (cluster size)>15 voxels. The left side of this scan is the left side of a subject. The DMN of HS was significantly more activated during LR 8–point acupuncture at: a: the bilateral medial frontal gyrus (BA10, [4 58 6], t=11.64, k=556); b: the left inferior frontal gyrus (BA46, [−46 42 12], t=−5.18, k=43); d: the left cuneus (BA19, [−2 −82 34], t=10.62, k=44); and f: the left superior frontal gyrus (BA6, [−10 26 64], t=8.96, k=64). DS had more DMN activations at these regions: c: middle cingulate gyrus (BA31, [0 −42 40], t=−5.21, k=157); and e: bilateral superior and middle frontal gyrus (left: BA9, [−28 32 38], t=−5.19, k=192; right: BA9, [34 36 42], t=−5.18, k=266).
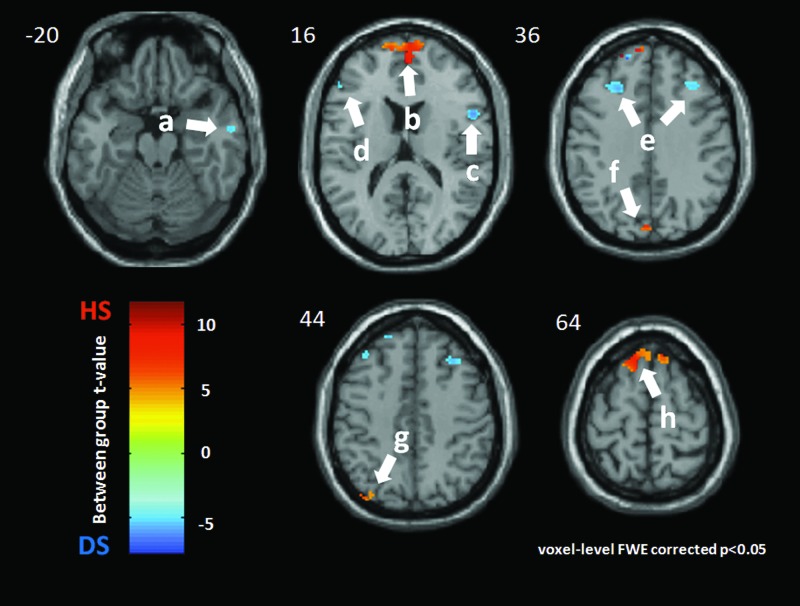
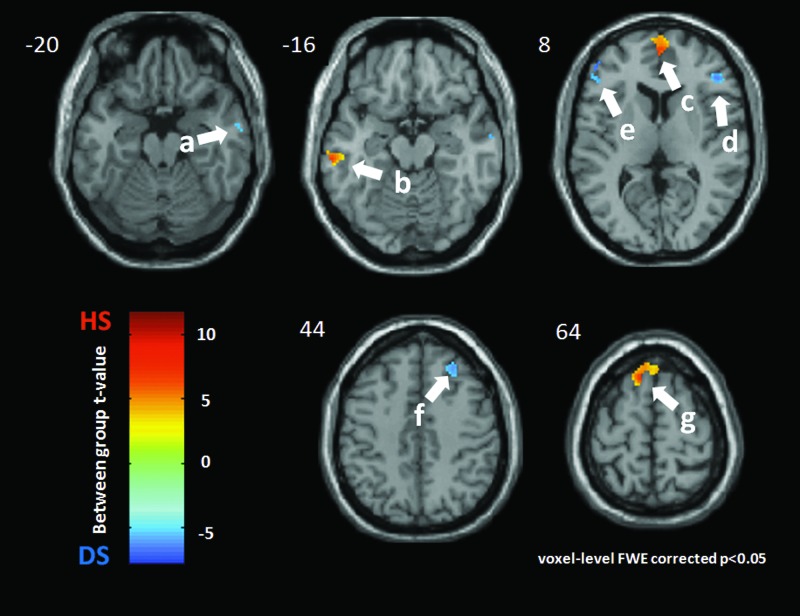
LR 14
The DMN of each nondepressed subject was significantly activated at the bilateral medial frontal gyrus again with a large activation size (BA10, [2 58 10], t=10.35, k=581) in the following regions: left cuneus (BA19, [−2 −82 34], t=8.24, k=20); left precuneus (BA19, [−38 −78 46], t=6.69, k=25); and left superior frontal gyrus (BA6, [−12 34 62], t=9.08, k=85.
In the depressed subjects, there were more DMN activations in the following regions: right inferior temporal gyrus (BA21, [60 −10 −20, t=5.19, k=16); right inferior frontal gyrus (BA 44, [50 10 18], t=5.20, k=79); left inferior frontal gyrus (BA46, [−52 34 12], t=5.21, k=30); bilateral middle frontal gyrus (left BA9, [−40 32 38], t=5.19, k=119; and right BA9, [38 28 44], t=5.20, k=114). See Figures 2 and 4.
FIG. 4.
LR 14 acupoint. Group difference between nondepressed healthy subjects (HS) and major depressive subjects (DS) during acupuncture at LR 14. Warm color indicates more DMN activation for HS, while the cold color shows the increased DMN activation for DS. All clusters were presented with p<0.05 (Family Wise type 1 Error correction) and k (cluster size)>15 voxels. The left side of this scan is the left side of a subject. The DMN of HS was significantly more activated during LR 14–point acupuncture at: b: the bilateral medial frontal gyrus (BA 10, [2 58 10], t=10.35, k=581); f: the left cuneus (BA19, [−2 −82 34], t=8.24, k=20); g: the left precuneus (BA19, [−38 −78 46], t=6.69, k=25); and h: the left superior frontal gyrus (BA6, [−12 34 62], t=9.08, k=85). DS had more DMN activations at following regions: a: the right inferior temporal gyrus (BA21, [60 −10 −20], t=−5.19, k=16); c: the right inferior frontal gyrus (BA44, [50 10 18], t=−5.20, k=79); d: the left inferior frontal gyrus (BA46, [−52 34 12], t=−5.21, k=30); and e: the bilateral middle frontal gyrus (left: BA9, [−40 32 38], t=−5.19, k=119; right: BA9, [38 28 44], t=−5.20, k=114);
CV 14
For CV 14, the DMN of nondepressed subjects, again, was activated more significantly in the bilateral medial frontal gyrus (BA10, [2 52 18], t=15.20, k=535), right middle temporal gyrus (BA21, [−62 −26 −16], t=9.05, k=54), and left superior frontal gyrus (BA6, [−8 26 64], t=9, k=85).
Depressed subjects had more DMN activations in the following regions: right inferior temporal gyrus (BA21, [60 −4 −18], t=5.19, k=21); right inferior frontal gyrus (BA46, [42 36 12], t=5.20, k=65); left inferior frontal gyrus (BA46, [−48 32 16], t=5.20, k=137); and right middle frontal gyrus (BA9, [24 36 46], t=5.19, k=180). See Figures 2 and 5.
FIG. 5.
CV 14 acupoint. Group difference between healthy subjects and major depressive subjects during acupuncture at CV 14. Warm color indicates more DMN activation for nondepressed healthy subjects (HS), while the cold color shows the increased DMN activation for depressive subjects (DS). All clusters were presented with p<0.05 (Family Wise type 1 Error correction) and k (cluster size)>15 voxels. The left side of the scan the left side of a subject. The DMN of HS was more significantly activated during LR 14–point acupuncture at: b: the right middle temporal gyrus (BA21, [−62 −26 −16], t=9.05, k=54); c: the bilateral medial frontal gyrus (BA10, [2 52 18], t=15.20, k=535); and g: the left superior frontal gyrus (BA6, [−8 26 64], t=9.10, k=85). DS had more DMN activations at the following regions: a: the right inferior temporal gyrus (BA21, [60 −4 −18], t=−5.19, k=21); d: the right inferior frontal gyrus (BA46, [42 36 12], t=−5.20, k=65); e: the left inferior frontal gyrus (BA46, [−48 32 16], t=−5.20, k=137); and f: the right middle frontal gyrus (BA9, [24 36 46], t=−5.19, k=180).
HT 7
There were no significant differences between nondepressed and depressed subjects' activations with this acupoint.
Discussion
Emerging evidence describing the normal function of the DMN as being necessary for mental and physical well-being has widened interest in the part the DMN plays in many conditions. Needle acupuncture has been suggested to be effective for reestablishing this regulation.27,28
In the current authors' previously reported work on laser acupuncture on “depression-specific” acupoints in a healthy sample, there was activation of the limbic system (cingulate) and the frontal cortex (middle frontal and superior frontal gyrii) suggestive of anterior DMN modulation.11 This experimental condition was also extended by the current authors to a sample of major depressed subjects and this was noted as having increased brain regions of activation to include the parietal cortex, temporal cortex, and cerebellum.12 These brain regions had an important involvement in the posterior DMN.
Although the ICA findings support the DMN results from these two previously observed groups, the modulation did not include exclusive anterior DMN modulation in the nondepressed subjects or exclusive posterior DMN modulation in the depressed subjects. The anterior and the posterior DMN are most likely to be anticorrelated, meaning that, when the anterior DMN is more active, the posterior DMN is less active and vice versa.29 The magnitude of the BOLD signal at the medial frontal gyrus was constantly high for nondepressed subjects, DMN compared to the depressed subjects' DMN across the three acupoints. This outstanding and consistent modulation of the medial frontal gyrus across all three significant acupoints (LR 8, LR 14, and CV 14) in nondepressed subjects may be one of the key features linked to a sense of well-being. Anterior medial cortical activity is linked to self-reflection and a healthy perspective regarding oneself.30 Activation changes in the posterior DMN is implicated in major depression.31,32 Connectivity between the temporal cortex and regions of the posterior DMN, including the precuneus and posterior cingulate, has been previously reported,30 and there is perhaps a change in this connectivity in depressed individuals. Further, negative reflections or ruminations (during SIT) by a depressed individual changes brain connectivity between the posterior cingulate and the subgenual cingulate,31 and this may be where laser acupuncture modulation also occurs. In other words, stimulation by laser acupuncture of wider areas of the DMN—including the temporal and posterior cingulate regions of the posterior DMN—in depressed participants (brain regions involved in over-reflection or rumination and distortion of the sense of self32) may be part of the regulatory role of laser acupuncture.
There are, of course, limitations in this study. It had small sample size. The depression symptoms that were chosen for the psychological measures in this study may be representative of this depressed group of participants alone. Hence, the comparison of DMN changes being tested by this study may be restricted to this sample of nondepressed and depressed participants. The findings therefore need to be replicated in an independent sample. Furthermore, given that major depression is a diverse disorder, and the subjects in the current study were outpatients (a primary-care scenario) with mild-to-moderate severity of depression, the findings may not be applicable to all types of depressive disorders.
Finally, this was a cross-sectional study. Change in DMN before and after treatment with acupuncture and other antidepressant treatments must be examined. The significance or function of the medial frontal gyrus needs to be evaluated further with respect to the health and well-being of the individual. This brain region, being involved in hopes and aspirations, may be synonymous with a healthy sense of self.30
Conclusions
Laser acupuncture on LR 14, LR 8, and CV 14 significantly modulates both the anterior and the posterior components of the DMN in both nondepressed and depressed participants. The medial frontal gyrus, as part of the anterior DMN, was significantly activated in nondepressed participants across all acupoints. There was a shift away from this consistent anterior modulation in the depressed subjects toward a greater posterior network modulation. The modulation of the posterior DMN may be part of the antidepressant effect of laser acupuncture. Rumination or over-reflection and the distortion of the sense of self are targeted.
Acknowledgments
With grateful thanks to Oliver Rochesson, Director, EDR Electronics and Peter Jenkins, MBA, for their technical expertise. The authors acknowledge Louise and Gary Dobson, James Fairfax, and the Thyne Reid Foundation for their philanthropy. Thanks also to Kate Crosbie and Angie Russell for their help with the preparation of the manuscript, as well as the staff at Neuroscience Research Australia (NeuRa) for their support.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Gusnard DA. Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 2.Greicius MD. Krasnow B. Reiss AL. Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mason MF. Norton MI. Van Horn JD. Wegner DM. Grafton SD. Macrae CN. Wandering minds: The default network and stimulus independent thought. Science. 2007;315(58):393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheline YI. Barch DM. Price JL, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckner RL. Andrews-Hanna JR. Schacter DL. The brain's default network: Anatomy, function and relevance to disease. Ann N Y Acad Sci. 2008 Mar;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 6.Broyd SJ. Demanuelle C. Debener S. Helps SK. James CJ. Sonuga-Barke EJS. Default mode brain dysfunction in mental disorders: A systematic review. Neurosci Biobehav Rev. 2009;33(3):279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54(3):338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- 8.Gotlib IH. Hamilton JP. Neuroimaging and depression: Current status and unresolved issues. Curr Dir Psychol Sci. 2008;17(2):159–163. [Google Scholar]
- 9.Dhond RP. Yeh C. Park KY. Kettner N. Napadow V. Acupuncture modulates resting state connectivity in default and sensorimotor brain networks. Pain. 2008;136(3):407–418. doi: 10.1016/j.pain.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong C. Bai L. Dai R, et al. Modulatory effects of acupuncture on resting-state networks: A functional MRI study combining independent component analysis and multivariate granger causality analysis. J Magn Reson Imaging. 2011;35(3):572–581. doi: 10.1002/jmri.22887. [DOI] [PubMed] [Google Scholar]
- 11.Quah-Smith I. Sachdev P. Wen W. Chen X. Williams MA. The brain effects of laser acupuncture in healthy individuals: An fMRI investigation. PLoS One. 2010;5(9):e12619. doi: 10.1371/journal.pone.0012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quah-Smith I. Wen W. Chen X. Williams MA. Sachdev P. The brain effects of laser acupuncture in depressed individuals: An fMRI investigation. Med Acupunct. 2012;24(3):161–171. [Google Scholar]
- 13.Maciocia G. New York: Churchill-Livingstone; 1994. Mental–emotional problems. In: The Practice of Chinese Medicine: The Treatment of Diseases with Acupuncture and Chinese Herbs; pp. 197–280. [Google Scholar]
- 14.Aung SKH. Chen WPD. New York: Thieme; 2007. Clinical Introduction to Medical Acupuncture. [Google Scholar]
- 15.Beck AT. Ward CH. Mendelson M. Mock J. An inventory for measuring depression. Arch Gen Psych. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association (APA) Washington, DC: APA; 2000. Diagnostic and Statistical Manual of Mental Disorders V4—DSM-IV. [Google Scholar]
- 17.Montgomery SA. Asberg M. A new depression scale designed to be sensitive to change. Brit J Psych. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton M. Development of arating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 19.Quah-Smith JI. Tang WM. Russell J. Laser acupuncture for mild to moderate depression in a primary care setting—a randomised controlled trial. Acupunct Med. 2005;23(3):103–111. doi: 10.1136/aim.23.3.103. [DOI] [PubMed] [Google Scholar]
- 20.Calhoun VD. Adali T. Unmixing fMRI with independent component analysis. IEEE Eng Med Biol Mag. 2006;25(2):79–90. doi: 10.1109/memb.2006.1607672. [DOI] [PubMed] [Google Scholar]
- 21.Calhoun VD. Adali T. Pearlson GD. Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14(3):140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egolf E. Rachakonda S. Calhoun VD Group ICA fMRI Toolbox (GIFT) New Signal Processing Techniques Applied to Brain Imaging. The Institute of Living. icatb.sourceforge.net/gift/SOBP_poster.pdf. [Jan 6;2013 ]. icatb.sourceforge.net/gift/SOBP_poster.pdf
- 23.Calhoun VD. Liu J. Adali T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. NeuroImage. 2009;45(1suppl):S163–S172. doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seely WW. Menon V. Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2149–2156. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esposito F. Aragri A. Pesaresi I, et al. Independent component model of the default-mode brain function: Combining individual-level and population-level analyses in resting-state fMRI. Magn Reson Imaging. 2008;26(7):905–913. doi: 10.1016/j.mri.2008.01.045. [DOI] [PubMed] [Google Scholar]
- 26.Esposito F. Bertolino A. Scarabino T, et al. Independent component model of the default-mode brain function: Assessing the impact of active thinking. Brain Res Bull. 2006;70(4–6):263–269. doi: 10.1016/j.brainresbull.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Liu P. Zhou G. Zhang Y, et al. The hybrid GLM-ICA investigation on the neural mechanism of acupoint ST 36: An fMRI study. Neurosci Lett. 2010;479(3):267–271. doi: 10.1016/j.neulet.2010.05.077. [DOI] [PubMed] [Google Scholar]
- 28.Qin W. Bai L. Dai J, et al. The temporal–spatial encoding of acupuncture effects in the brain. Mol Pain. 2011;23(7):19. doi: 10.1186/1744-8069-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uddin L Q. Kelly AMC. Biswal BB. Castellanos XF. Milham MP. Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30(2):625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson MK. Nolen-Hoeksema S. Mitchell KJ. Levin Y. Medial cortical activity, self-reflection and depression. Soc Cogn Affect Neurosci. 2009;4(4):313–327. doi: 10.1093/scan/nsp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fransson P. Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. NeuroImage. 2008;42(3):1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 32.Berman MG. Peltier S. Nee DE. Kross E. Deldin PJ. Jonides J. Depression, rumination and the default mode network. Soc Cogn Affect Neurosci. 2011;6(5):548–555. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]