Abstract
Objective
The purpose of this article was to systematically review the literature on stimulant and atomoxetine combination therapy, in particular: 1) Characteristics of patients with attention-deficit/hyperactivity disorder (ADHD) given combination therapy, 2) treatment strategies used, 3) efficacy and effectiveness, and 4) safety and tolerability.
Methods
Literature databases (MEDLINE®, EMBASE, Cochrane Central Register of Controlled Trials, Science Citation Index Expanded, and SciVerse Scopus) were systematically searched using prespecified criteria. Publications describing stimulant and atomoxetine combination therapy in patients with ADHD or healthy volunteers were selected for review. Exclusion criteria were comorbid psychosis, bipolar disorder, epilepsy, or other psychiatric/neurologic diseases that could confound ADHD symptom assessment, or other concomitant medication(s) to treat ADHD symptoms.
Results
Of the 16 publications included for review, 14 reported findings from 3 prospective studies (4 publications), 7 retrospective studies, and 3 narrative reviews/medication algorithms of patients with ADHD. The other two publications reported findings from two prospective studies of healthy volunteers. The main reason for prescribing combination therapy was inadequate response to previous treatment. In the studies of patients with ADHD, if reported, 1) most patients were children/adolescents and male, and had a combined ADHD subtype; 2) methylphenidate was most often used in combination with atomoxetine for treatment augmentation or switch; 3) ADHD symptom control was improved in some, but not all, patients; and 4) there were no serious adverse events.
Conclusions
Published evidence of the off-label use of stimulant and atomoxetine combination therapy is limited because of the small number of publications, heterogeneous study designs (there was only one prospective, randomized controlled trial), small sample sizes, and geographic bias. Existing evidence suggests, but does not confirm, that this drug combination may benefit some, but not all, patients who have tried several ADHD medications without success.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a chronic and common childhood neurobehavioral disorder that can persist into adolescence and adulthood (Kessler et al. 2005; Wolraich et al. 2005). In a recent meta-regression analysis, the worldwide-pooled prevalence of ADHD for children and adolescents was estimated to be 5.29%, with significant variability (Polanczyk et al. 2007). Characterized by the core symptoms of hyperactivity, impulsivity, and inattention (National Institute for Health and Clinical Excellence 2008), ADHD has a diverse clinical presentation because of its heterogeneous origins, the effect of confounding psychiatric comorbidities on core symptoms, and the changes in ADHD symptoms that occur with age (Steinhausen 2009; Geissler and Lesch 2011). Given the diverse clinical presentation of ADHD, optimizing treatment for patients with ADHD is a key clinical concern for physicians.
Psychopharmacological treatment for ADHD includes stimulant or, if warranted, nonstimulant (e.g., atomoxetine, bupropion, clonidine, guanfacine) medications (Pliszka et al. 2006; National Institute for Health and Clinical Excellence 2008; Seixas et al. 2012). In addition to psychopharmacological treatment, nonpsychopharmacological interventions (e.g., behavior modification) are also required in most cases because of the complexity of ADHD symptoms and their diverse presentation (Hodgson et al. 2012). Both stimulants and atomoxetine have been shown to be effective as monotherapy for the treatment of ADHD (Faraone et al. 2006; Cheng et al. 2007; Mészáros et al. 2009; Faraone and Buitelaar 2010; Hanwella et al. 2011). Although proven to be effective treatments, stimulant or atomoxetine monotherapy does not provide adequate coverage of symptoms in a small subset of patients (Spencer et al. 1996; Newcorn et al. 2008; Hazell et al. 2011). Because their presumed mechanisms of action differ (Wilens 2006), stimulants and atomoxetine are sometimes given in combination in clinical practice to help improve patient outcomes (Waxmonsky 2005; Pliszka et al. 2006; Prasad and Steer 2008). Although not approved for use in combination in the United States (Strattera Prescribing Information 2012), stimulant and atomoxetine combination therapy in clinical practice includes add-on or adjunct therapy to previous monotherapy, or a short-term combination phase during the switch from one medication to another. However, despite the use of stimulant and atomoxetine combination therapy in clinical practice, collation and analysis of the evidence for this drug combination is lacking.
Because of the potential clinical relevance of combination therapy, the objective of this systematic review is to examine the current literature on stimulant and atomoxetine combination therapy. Publications describing both patients with ADHD and healthy volunteers were considered in our review, because of our interest in reporting safety and tolerability findings for this drug combination. Specifically, this review will focus on 1) characteristics of patients with ADHD given stimulant and atomoxetine combination therapy, 2) stimulant and atomoxetine combination therapy strategies used, 3) efficacy in controlled studies and effectiveness in open-label studies of stimulant and atomoxetine combination therapy, and 4) safety and tolerability of stimulant and atomoxetine combination therapy.
Materials and Methods
Database search strategy
The following databases were searched on March 15, 2012: MEDLINE® via PubMed, EMBASE, Cochrane Central Register of Controlled Trials, Science Citation Index Expanded via Web of Science, and SciVerse Scopus. Search terms from 2 categories were used: 1) Stimulants (including stimulant, stimulants, dexmethylphenidate [EMTREE], dextromethylphenidate, Focalin, “d amphetamine”, d-amphetamine, dexamfetamine, “dex amphetamine”, dex-amphetamine, dexamphetamine [EMTREE], Dexedrine, “dextro amphetamine”, dextro-amphetamine, dextroamphetamine [MeSH], DextroStat, lisdexamfetamine [MeSH, EMTREE], “lis dexamfetamine”, lis-dexamfetamine, NRP104, NRP-104, Vyvanse, Concerta, Daytrana, Metadate, Methylin, methylphenidate [MeSH, EMTREE], phenidylate, Ritalin, amphetamine [MeSH, EMTREE], Adderall) and 2) atomoxetine (including atomoxetine [MeSH, EMTREE], LY139603, Strattera, tomoxetine). Search terms within each category were separated by the Boolean operator OR, and categories were separated by the operator AND. All database searches were restricted to publications published from 1990 onwards. There were no restrictions on publication language.
Study selection
Publications retrieved by the database searches were collated and duplicate publications were discarded. One author (Dr. Monk) screened the title and abstracts for possible full text review, using prespecified inclusion and exclusion criteria. These criteria were also applied during the review of the full text in selected publications. Publications describing stimulant and atomoxetine combination therapy in patients with ADHD or healthy volunteers and the following outcome measures – patient characteristics; treatment strategies; efficacy, effectiveness, safety, and tolerability measures; medical resource use; and pharmacodynamic or pharmacokinetic data – were included for review. Stimulant and atomoxetine combination therapy included stimulant add-on or adjunct therapy to atomoxetine monotherapy, and atomoxetine add-on or adjunct therapy to stimulant monotherapy or during a treatment switch or crossover. Publications were excluded if patients with ADHD had comorbid psychosis, bipolar disorder, epilepsy, or other psychiatric/neurologic diseases that could confound the assessment of their ADHD symptoms, or if they were receiving concomitant medication(s) other than stimulants and atomoxetine to treat ADHD symptoms. The bibliographies of relevant publications were searched for any additional publications that should be assessed.
Data extraction and analysis
We developed a spreadsheet for data collection, which was refined as data were extracted. Data were extracted by one author (Dr. Monk), reviewed by all authors, and validated by an independent reviewer (non-author). The data that were collected included publication and study information (type, design, treatment, duration, location), patient/healthy volunteer information (number, age, sex, ethnicity, ADHD/healthy volunteer, ADHD subtype, other ADHD characteristics, comorbidities), treatment (previous treatment history, combination therapy strategy, ADHD medication dose, duration), efficacy and effectiveness findings (symptom control measures, other measures, other findings), and safety and tolerability findings (study discontinuations, adverse events, vital signs, electrocardiogram [ECG] parameters, other findings). To compare the ADHD medication doses, we calculated the dose equivalents (eq) using the defined daily doses (DDD) for each ADHD medication dose and the following formulas: DDDeq=dose mg/DDD mg or DDDeq=(dose mg/kg×70 kg) / DDD mg (World Health Organization 2009). The DDDs were 30 mg for methylphenidate, 80 mg for atomoxetine, 15 mg for amphetamine, and 15 mg for dextroamphetamine (www.whocc.no/atcddd). To succinctly summarize all publications included in this review, not all data that were collected in the spreadsheet are reported.
Results
Literature search results
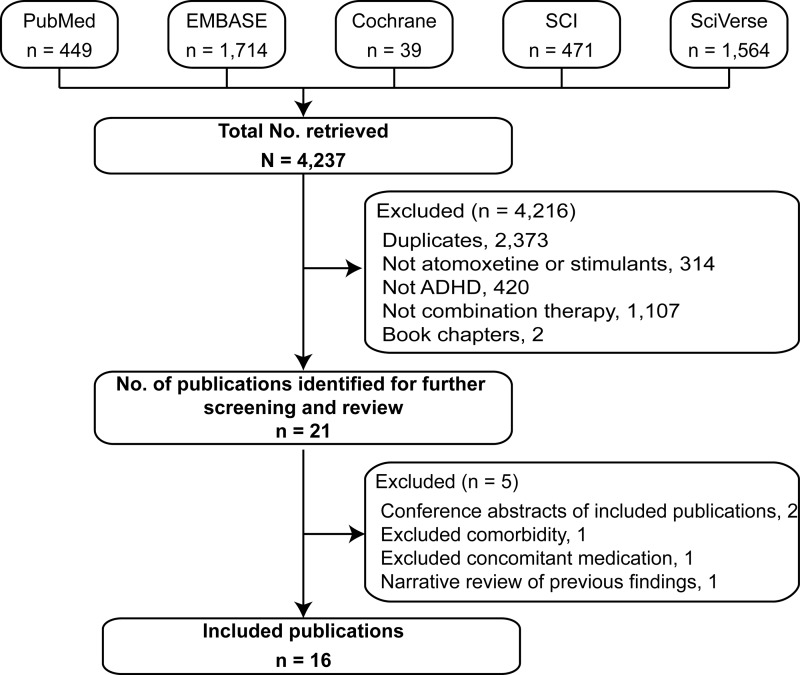
A total of 4237 abstracts were retrieved by the combined database searches (Fig. 1). After the duplicate publications were discarded, 1864 abstracts were screened and 21 publications were selected for full text review. Of the 21 publications reviewed, 5 were excluded because they were conference abstracts of included publications (n=2) (Carlson et al. 2006; Wilens et al. 2008), case reports of patients with an excluded comorbidity (n=1) (Jaworowski et al. 2006) and an excluded concomitant medication (n=1) (Bond et al. 2007), and a narrative review that summarized findings for stimulant and atomoxetine combination therapy reported in other studies (n=1) (Prasad and Steer 2008). The remaining 16 publications were included for review.
FIG1.
Flow diagram of literature search results. Databases were MEDLINE® via PubMed, EMBASE, Cochrane Central Register of Controlled Trials, Science Citation Index (SCI) Expanded via the Web of Science, and SciVerse Scopus. Searches were limited to articles published from 1990 onwards and were not limited to English language articles. ADHD, attention-deficit/hyperactivity disorder.
Publication and study characteristics
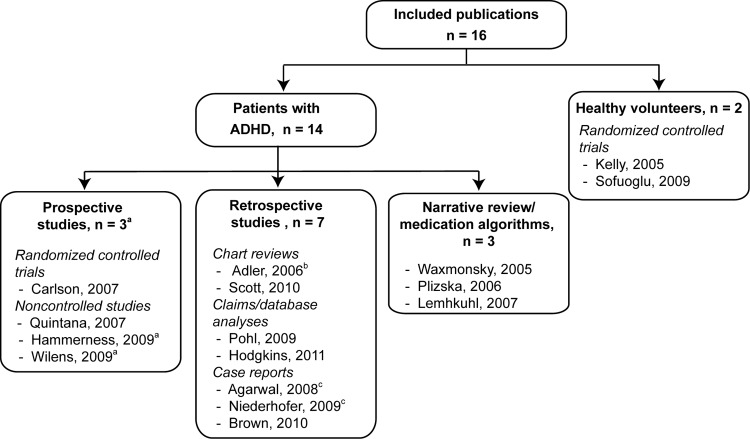
Of the 16 publications, 14 were of patients with ADHD and 2 were of healthy volunteers (Fig. 2). One publication (Lehmkuhl et al. 2007) was written in German. The 14 publications of patients with ADHD reported findings from 3 prospective studies (4 publications), 7 retrospective studies, and 3 narrative reviews/medication algorithms. The three prospective studies were one double-blind, randomized, placebo-controlled, two phase (acute and combination) trial (Carlson et al. 2007), and two non-controlled studies: One open, two phase trial reported in two publications (Hammerness et al. 2009; Wilens et al. 2009); and one switch trial (Quintana et al. 2007). The seven retrospective studies were four observational studies: One ongoing (Adler et al. 2006) and one complete (Scott et al. 2010) chart review, and two claims/medication database analyses (Pohl et al. 2009; Hodgkins et al. 2011); and three case reports (Brown 2004; Agarwal and Sitholey 2008; Niederhofer 2009). Findings from the ongoing chart review were published in a conference abstract (Adler et al. 2006) and findings from two of the three case reports were published in letters to the editors (Agarwal and Sitholey 2008; Niederhofer 2009). Patient numbers in these studies ranged from 1 to 18,609; however, only a small proportion of patients in the complete chart review (Scott et al. 2010) and the two claims/medication database analyses (Pohl et al. 2009; Hodgkins et al. 2011) received combination therapy (Table 1).
FIG. 2.
Overview of articles retrieved from the systematic literature search. ADHD, attention-deficit/hyperactivity disorder. aWilens reports the efficacy outcomes and Hammerness reports the safety outcomes from the same study. bConference abstract. cLetter to the Editor.
Table 1.
Study and Patient Characteristics
|
Publication n, Duration/Period |
Age, years | Male | ADHD subtype | Comorbidities | Treatment history |
|---|---|---|---|---|---|
| Patients with ADHD – prospective studies | |||||
| Carlson, 2007 25, 10 weeks |
Children, mean±SD: 9.6±1.8, range: 6–12 | 83% | Combined: 79% | ODD: 50% | Insufficient response to stimulant triala in previous 12 months |
| Quintana, 2007 62, 8 weeks |
Children & adolescents, mean±SD: 11.5±2.8, range: 6.1–17.5 | 76% | Combined: 63%, Inattentive: 36%, Hyp./Imp.: 2% | CD: 3%, Dysthymia: 2%, GAD: 2%, ODD: 26%, MDD: 2% | Inadequate response (53.2%) or intolerance of AEs (46.8%) with stimulants (MPH: 51.6%, amphetamine: 48.4%) |
| Wilens, 2009 & Hammerness, 2009 50b, 7 weeks |
Children & adolescents, mean±SD: 9.3±2.5 | 76% | Combined: 54%, Inattentive: 38%, Hyp./Imp.: 8% | CD: 4%, ODD: 40% | 44% had previous treatment with medication (type not specified); partial responders to ATMX or MPH included |
| Patients with ADHD – retrospective studies | |||||
| Adler, 2006 29, NA |
Children, adolescents, & adults, mean±SD: 38.3±12.1, range: 6–60 | 69% | NR | NR | ATMX monotherapy for previous 7 months |
| Scott, 2010 285c, 26 Nov 2002 to 20 Apr 2005 |
Children & adolescents, range: 5–17 | 73% | Combined: 61%, Inattentive: 14%, Hyp./Imp.: 4%, NS: 21% | Anxiety dis.: 22%, Bipolar dis.: 11%, CD: 5%, Depression: 28%, Enuresis: 5%, Learning dis.: 24%, ODD: 33%, Substance-related dis.: 3% | ATMX prescribed during study period |
| Pohl, 2009 18,609d, Jul 2003 to Jun 2004 |
Adults, 18–24: 38%, 25–44: 35%, ≥45: 27% | 54% | Hyp.: 31% | Anxiety states: 20%, Bipolar/Mania: 6%, Depression: 39%, Psychotic dis.: 1%, Substance abuse/ dependence: 11% | NR |
| Hodgkins, 2011 4,909e, Jan 2003 to Dec 2006 |
Children & adolescents, 6–9: 46%, 10–12: 27%, 13–15: 19%, 16–17: 8% | 82% | NR | NR | Treatment naïvef |
| Brown, 2004 4, NA |
Children & adolescents, range: 6–17 | 75% | Combined: 75%, Inattentive: 25% | ODD: 25% | Case 1: inadequate coverage of symptoms with OROS MPH monotherapy and adjunct clonidine, intolerable side effects with adjunct IR MPH; Case 2: inadequate coverage of symptoms with amphetamine-XR monotherapy, intolerable side effects with adjunct amphetamine-IR; Case 3: intolerable side effects with MPH, mixed salts of amphetamine, OROS MPH, and nortriptyline monotherapies, inadequate coverage of symptoms with ATMX monotherapy; Case 4: inadequate coverage of symptoms with ATMX |
| Agarwal, 2008 1, 6 weeks |
Child, 8 | Yes | Combined | NR | Tolerance to immediate release MPH and decreased appetite and delayed onset of sleep |
| Niederhofer, 2009 1, NA |
Child, 11 | Yes | NR | NR | Intolerable side effects (depressed mood and appetite loss) with MPH treatment; switch to ATMX but inadequate treatment of inattention |
| Patients with ADHD – medication algorithms/narrative reviews | |||||
| Waxmonsky, 2005 NA, NA |
NA | NA | NA | NA | Insufficient coverage of symptoms with stimulant monotherapy or intolerable side effects |
| Pliszka, 2006 NA, NA |
NA | NA | NA | NA | Inadequate response with ATMX monotherapy and insufficient coverage of symptoms with stimulant monotherapy (even LA forms); combination therapy only considered after full monotherapy trials of 2 stimulants and ATMX |
| Lehmkuhl, 2007 NA, NA |
NA | NA | NA | NA | Inadequate response or intolerable side effects with stimulant monotherapy; combination therapy necessary during switch to help with symptom control |
| Healthy volunteers | |||||
| Kelly, 2005 12, Min. 29 daysg |
Adults, mean: 23, range: 22–27 | 100% | NA | NA | NA |
| Sofuoglu, 2009 10, Min. 12 daysh |
Adults, mean±SD: 29.1±6.4 | 30% | NA | NA | NA |
Adequate stimulant trial was defined as a gradual titration of stimulant medication for at least 2 weeks at specified doses for each medication.
50 of the 82 patients enrolled in this study took atomoxetine and OROS methylphenidate combination therapy. The article reports data for the 50 patients who took atomoxetine and OROS methylphenidate combination therapy.
54 of the 285 patients (atomoxetine treatment success=88 and failure=197) enrolled in this study took stimulant and atomoxetine combination therapy. Overall, 432 patients had sufficient data for study inclusion; however, 147 were categorized in the undetermined group and excluded from analyses.
Patient months, not n values, for individual ADHD medications were reported. Stimulants in combination with atomoxetine represented 1.0–6.7% of patient months.
9 of the 4,909 patients enrolled in this study took stimulant and atomoxetine combination therapy.
Newly prescribed ADHD medication between Jan 2003 and Dec 2006 and no ADHD medication dispensings in the previous 12 months.
Min. 7 day break between each treatment period.
Min. 4 day washout period between each treatment period.
ADHD, attention-deficient/hyperactivity disorder; AE, adverse event; ATMX, atomoxetine; CD, conduct disorder; dis., disorder; GAD, generalized anxiety disorder; Hyp., hyperactive; IR, immediate release; Imp., impulsive; LA, long acting; MDD, major depressive disorder; min., minimum; MPH, methylphenidate; NA, not applicable; NR, not reported; NS, not specified; ODD, oppositional defiant disorder; OROS, Osmotic-controlled Release Oral delivery System; SD, standard deviation; XR, extended release.
The three narrative reviews/medication algorithms briefly discussed possible scenarios and treatment strategies for stimulant and atomoxetine combination therapy (Waxmonsky 2005; Pliszka et al. 2006; Lehmkuhl et al. 2007). The two publications including healthy volunteers reported findings from two randomized, placebo-controlled, crossover trials (Kelly et al. 2005; Sofuoglu et al. 2009). These publications reported safety and tolerability outcomes only.
Patient characteristics and reasons for trying stimulant and atomoxetine combination therapy
The characteristics of patients with ADHD were reported in the three prospective studies and the seven retrospective studies (Table 1). In the prospective studies (Carlson et al. 2007; Quintana et al. 2007; Hammerness et al. 2009; Wilens et al. 2009), patients with ADHD were children or adolescents, predominantly male (76–83%), had the combined ADHD subtype (54–79%), and had oppositional defiant disorder as the predominant comorbidity (26–50%). In two of the prospective studies, the severity of patients' ADHD symptoms were moderate or greater as measured by the Clinical Global Impressions Scale-Severity survey (Carlson et al. 2007) or the ADHD Rating Scale-IV-Parent-Reported Investigator-Rated survey (ADHD-RS-IV-Parent:Inv) (Quintana et al. 2007). In addition, the patients' ADHD-RS-IV-Parent:Inv rating were 1.5 standard deviations (SD) (Carlson et al. 2007) or 2.4 SD (Quintana et al. 2007) above age and gender norms.
The characteristics of patients with ADHD in the three retrospective case reports (Brown 2004; Agarwal and Sitholey 2008; Niederhofer 2009) were similar to those in the prospective studies (e.g., children/adolescents, predominantly male, and predominantly combined ADHD subtype; Table 1). Patients with ADHD in the four remaining retrospective studies (Adler et al. 2006; Pohl et al. 2009; Scott et al. 2010; Hodgkins et al. 2011) were children, adolescents, or adults, and were predominantly male (54–82%).
Reasons for trying stimulant and atomoxetine combination therapy were reported in the three prospective studies, three of the seven retrospective studies, and the three narrative reviews/medication algorithms (Table 1). These reasons included partial or inadequate response or coverage of symptoms with stimulant monotherapy (Brown 2004; Waxmonsky 2005; Pliszka et al. 2006; Carlson et al. 2007; Quintana et al. 2007), inadequate response or coverage of symptoms with atomoxetine monotherapy (Brown 2004; Pliszka et al. 2006; Lehmkuhl et al. 2007; Niederhofer 2009), intolerable side effects with stimulant monotherapy (Brown 2004; Waxmonsky 2005; Lehmkuhl et al. 2007; Niederhofer 2009), or tolerance to stimulant monotherapy (Agarwal and Sitholey 2008). In one study (Hammerness et al. 2009; Wilens et al. 2009), patients received atomoxetine and stimulant combination therapy if they were considered to be partial responders to atomoxetine monotherapy after a 4 week acute atomoxetine treatment phase.
Stimulant and atomoxetine combination therapy strategies
Stimulant and atomoxetine combination therapy strategies for treating patients with ADHD were reported in the three prospective studies, five of the seven retrospective studies, and the three narrative reviews/medication algorithms (Table 2). This drug combination was recommended or implemented to augment stimulant monotherapy (Brown 2004; Waxmonsky 2005; Pliszka et al. 2006; Hodgkins et al. 2011), to augment atomoxetine monotherapy (Brown 2004; Adler et al. 2006; Pliszka et al. 2006; Carlson et al. 2007; Hammerness et al. 2009; Niederhofer 2009; Wilens et al. 2009; Hodgkins et al. 2011), or during a treatment switch from stimulant to atomoxetine monotherapy (Lehmkuhl et al. 2007; Quintana et al. 2007; Agarwal and Sitholey 2008). When specified, methylphenidate (particularly OROS methylphenidate) was most often used in combination with atomoxetine (Brown 2004; Carlson et al. 2007; Quintana et al. 2007; Agarwal and Sitholey 2008; Hammerness et al. 2009; Niederhofer 2009; Wilens et al. 2009; Hodgkins et al. 2011;). The other stimulant used was amphetamine (Brown 2004; Quintana et al. 2007).
Table 2.
Reported Atomoxetine and Stimulant Combination Therapy Strategy, Dosage, and Duration
| |
|
|
Regimen |
|
|---|---|---|---|---|
| Article | Strategy | Duration | Dosage | DDDeqa |
| Patients with ADHD – prospective studies | ||||
| Carlson, 2007 | Acute phase: ATMX+PB | 4 weeks | ATMX: Titrated to 1.2 mg/kg daily (max. 1.4 mg/kg) | 1.05 (1.23) |
| Augmentation: ATMX+OROS MPH (n=9), ATMX+PB (n=8) | 6 weeks | ATMX: Titrated to 1.2 mg/kg daily (max. 1.4 mg/kg) OROS MPH: Titrated to 1.08 mg/kg daily (max. 1.2 mg/kg) |
1.05 (1.23) 2.52 (2.80) |
|
| Quintana, 2007 | Switch: stimulant (MPH or amphetamine) to ATMX | 2 weeks | Week 1 Stimulant: Full doseb Week 1 ATMX: 0.5 mg/kg daily Week 2 Stimulant: Half doseb Week 2 ATMX: 1.2 mg/kg daily |
NC 0.44 NC 1.05 |
| Wilens, 2009 & Hammerness, 2009 | Acute phase: ATMX | 4 weeks | Weeks 1–2 ATMX: 0.5 mg/kg daily Weeks 3–4 ATMX: 1.4 mg/kg daily (max. 100 mg)c |
0.44 1.23 (1.25) |
| Augmentation phase: ATMX+OROS MPH | 3 weeks | ATMX: 1.4 mg/kg daily (max. 100 mg)c OROS MPH: Titrated openly in 18 mg increments weekly to 54 mg |
1.23 (1.25) 0.60–1.80 |
|
| Patients with ADHD – retrospective studies | ||||
| Adler, 2006 | Augmentation: ATMX+stimulant | NR | ATMX mean±SD starting dose: 17.2±4.3 mg daily Stimulant (MPH equivalents): mean±SD final dose 41.4±33.6 mg daily |
0.22 1.38 |
| Pohl, 2009 | NSd: ATMX+LA stimulants, ATMX+SA stimulants, ATMX+IA stimulants | 6.7%, 4.7%, 1.0% months | NR | NC |
| Hodgkins, 2011 | Augmentation: IR MPH+ATMX (n=4), LA MPH+ATMX (n=1), ATMX+IR MPH (n=3), ATMX+LA MPH (n=1) | NR | NR | NC |
| Brown, 2004 | Case 1, Augmentation: OROS MPH+ATMX | 4 months | OROS MPH: 27 mg q. 7:00 am ATMX: 18 mg q. am increased to 36 mg q. am end of week 1 |
0.90 0.23–0.45 |
| Case 2, Augmentation: Amphetamine-XR+ATMX | 5 months | Amphetamine-XR: 20 mg q. 6:30 am ATMX: 18 mg q. am increased to 40 mg q. am |
1.33 0.23–0.50 |
|
| Case 3, Augmentation: ATMX+OROS MPH | 4 months | ATMX: 40 mg bid OROS MPH: 18 mg q. am increased to 27 mg q. am |
0.1 0.60–0.90 |
|
| Case 4, Augmentation: ATMX+amphetamine-XR | 3 months | ATMX: 36 mg q. am (changed to 18 mg bid) Amphetamine-XR: 5 mg q. am |
0.45 0.33 |
|
| Agarwal, 2008 | Switch: IR MPH to ATMX | 3 weeks | IR MPH: 50 mg daily (3–4 divided doses) tapered off ATMX: 0.5 mg/kg daily increased to 1.2 mg/kg daily |
1.67 0.44–1.05 |
| Niederhofer, 2009 | Augmentation: ATMX+MPH | 3 months | ATMX: 40 mg daily MPH: 10 mg daily |
0.50 0.33 |
| Patients with ADHD – medication algorithms/narrative reviews | ||||
| Pliszka, 2006 | Augmentation: stimulant+ATMX | NR | ATMX: 0.5–1.0 mg/kg q. afternoon Stimulant: NR |
0.44–0.88 NC |
| Waxmonsky, 2005 | Augmentation: stimulant+ATMX | NR | AMTX: NR Stimulant: Reduction in dose requirement possible |
NC NC |
| Lehmkuhl, 2007 | Switch: Stimulant to ATMX | 4 weeks | 14 kg child : Weeks 1–2, MPH: 25 mg Weeks 1–2, ATMX:18 mg Weeks 3–4, MPH: 12.5 mg Weeks 3–4, ATMX: 40 mg |
0.83 0.23 0.42 0.50 |
| Healthy volunteers | ||||
| Kelly, 2005 | Monotherapy: ATMX, MPH, or PB Combination: ATMX+MPH, ATMX+PB, MPH+ATMX, MPH+PB, PB+ATMX, PB+MPH, PB+PB |
2 days 3 days |
ATMX: 60 mg bid MPH: 60 mg daily ATMX: 60 mg bid MPH: 60 mg daily |
1.50 2.00 1.50 2.00 |
| Sofuoglu, 2009 | Monotherapy: ATMX or PB | 3 days | ATMX: 40 mg daily | 0.50 |
| Combination: ATMX+dextroamphetamine, PB+dextroamphetamine | 1 day | ATMX: 40 mg daily Dextroamphetamine: 20 mg/70 kg |
0.50 1.33 |
|
DDDeqs were calculated using the DDD for each ADHD medication dose and the following formulas: DDDeq=dose mg/DDD mg or DDDeq=(dose mg/kg×70 kg)/DDD mg (World Health Organization 2009). The DDDs were 30 mg for methylphenidate, 80 mg for atomoxetine, 15 mg for amphetamine, and 15 mg for dextroamphetamine (see www.whocc.no/atcddd).
Dose as prescribed by the patient's physician, who was not associated with the study.
1.4 mg/kg daily taken as either one dose q. am or bid q. am & pm.
Data analyzed as patient months of treatment on any given class of medication.
ADHD, attention-deficit/hyperactivity disorder; ATMX, atomoxetine; bid, twice a day; DDDeq, defined daily dose equivalents; IA, immediate acting; IR, immediate release; LA, long acting; max., maximum; MPH, methylphenidate; NC, not calculated; NR, not reported; NS, not stated; OROS, Osmotic-controlled Release Oral delivery System; PB, placebo; q., every; SA, short acting; SD, standard deviation; XR, extended release.
The DDDeqs of stimulants and atomoxetine when given in combination differed among the studies (Table 2). In the augmentation studies, the DDDeqs for stimulants and atomoxetine were mostly >1 in the prospective studies (Carlson et al. 2007; Wilens et al. 2009) but <1 in the retrospective studies (Brown 2004; Adler et al. 2006; Niederhofer 2009). However, in the switch studies, the DDDeqs for atomoxetine (0.44 increased to 1.05) were similar for the prospective study (Quintana et al. 2007) and retrospective study (Agarwal and Sitholey 2008). Only three studies specified the timing of the atomoxetine dose (e.g., morning [Brown 2004], morning and afternoon [Hammerness et al. 2009; Wilens et al. 2009], or afternoon [Pliska et al. 2006]) or the timing of the stimulant dose (e.g., morning [Brown 2004]).
In the two studies of healthy volunteers, atomoxetine was given in combination with methylphenidate (2.00 DDDeq) (Kelly et al. 2005) or dextroamphetamine (1.34 DDDeq) (Sofuoglu et al. 2009). The DDDeqs for atomoxetine in these studies were 1.50 (Kelly et al. 2005) and 0.50 (Sofuoglu et al. 2009).
Efficacy and effectiveness of stimulant and atomoxetine combination therapy
Efficacy and effectiveness findings of stimulant and atomoxetine combination therapy were reported in the three prospective studies and four of the seven retrospective studies of patients with ADHD (Table 3). Improvements in ADHD symptom control with this drug combination were reported in most of these studies except the prospective double-blind, randomized controlled trial. In this study, adding OROS methylphenidate to the treatment regimen after 4 weeks of atomoxetine monotherapy did not enhance the efficacy of atomoxetine at the end of 6 weeks of combination therapy (Carlson et al. 2007). In the prospective non-controlled study, however, adding OROS methylphenidate to the treatment regimen after 4 weeks of atomoxetine monotherapy did result in statistically significant improvements in ADHD symptom control and severity, and behavior control at the end of 3 weeks of combination therapy (Wilens et al. 2009) (Table 3). In the retrospective chart review, of patients treated with atomoxetine (Scott et al. 2010), a higher proportion of patients classified as treatment success than of those classified as treatment failure had received add-on stimulant therapy. In the retrospective augmentation case reports, adding atomoxetine therapy to stimulant monotherapy extended medication coverage, particularly in the late afternoon and early evening when the effects of stimulant monotherapy had dissipated (Brown 2004), whereas adding methylphenidate therapy to atomoxetine monotherapy improved ADHD symptomatology and the ability to focus and remember (Brown 2004; Niederhofer 2009). From the beginning to the end of the cross-taper phase during a treatment switch from stimulant to atomoxetine monotherapy, improvement in ADHD symptom control (ADHD-RS scores) was statistically significant in one prospective study (Quintana et al. 2007) and substantial in one retrospective study (Agarwal and Sitholey 2008) (Table 3).
Table 3.
Reported Efficacy and Effectiveness Outcomes for Atomoxetine and Stimulant Combination Therapy
| |
|
Quantitative measures |
|
|
|
|
|
|
|
|
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Article statistic | Treatment/comparator groups | ADHDRS- | CGI- | Other quantitative measures or qualitative descriptions | |||||||
| Patients with ADHD – prospective studies | |||||||||||
| Carlson, 2007 | IV-Parent:Inv | Severity | WREMB-AM | WREMB-PM | CPRS | ||||||
| Effect sizea 10 weeks | ATMX+OROS MPH (n=9) | 1.3*** | 1.3* | 0.60 | 0.56 | 1.2* | |||||
| ATMX treatment | ATMX+PB (n=8) | 1.2** | 1.3** | 0.82 | 0.70 | 0.80** | |||||
| Effect sizea 6 weeks | ATMX+OROS MPH (n=9) | 0.05 | 0.34 | 0.43 | 0.33 | 0.18 | |||||
| MPH augmentation | ATMX+PB (n=8) | 0.18 | 0.38 | 0.07 | 0.01 | 0.09 | |||||
| Quintana, 2007 | IV-Parent:Inv | ADHD-I | Inattention ADHDRS subscore | Hyp./Imp. ADHDRS subscore | |||||||
| Mean±SD scores | Baseline (n=61) | 32.1±0.9 vs | NR vs | 18.9±0.5 vs | 13.2±0.5 vs | ||||||
| End (n=60) | 20.8±1.4*** | 2.8±0.1*** | 12.5±0.8*** | 8.3±0.7*** | |||||||
| Wilens, 2009 & Hammerness, 2009 | BRIEF subscales (n=38) | ||||||||||
| Severity | EC | Inhib. | Initiat. | Monit. | O of M | Plan | Shift. | WM | |||
| Mean±SD scores | Baseline (n=40) | 34.3±8.4 | 4.5±0.5 | 55 | 63 | 66 | 65 | 63 | 67 | 57 | 73 |
| Start (n=50) | 21.1±9.9 vs | 3.5±0.6 vs | 53 vs | 60 vs | 59 vs | 62 vs | 59 vs | 63 vs | 54 vs | 66 vs | |
| End (n=50) | 12.8±9.7*** | 2.7±1.1*** | 52 | 55 | 53*** | 56*** | 55** | 56*** | 51* | 59*** | |
| Patients with ADHD – retrospective studies | |||||||||||
| Scott, 2010 | NA | NA | NA | 27% classified as ATMX treatment successb received adjunct stimulant therapy; 15% classified as ATMX treatment failurec received adjunct stimulant therapy | |||||||
| Brown, 2004 | OROS MPH+ATMX | NR | NR | Improved oppositional behavior during periods when MPH had not taken effect or effects had dissipated | |||||||
| Amphetamine XR+ATMX | NR | NR | Calmer, more focused, more alert; able to complete homework in pm and resume afterschool job | ||||||||
| ATMX+OROS MPH | NR | NR | Improved ability to remember and focus; improved school grades | ||||||||
| ATMX+amphetamine XR | NR | NR | Improved cooperative behavior; increased ability to sustain attention at school | ||||||||
| Agarwal, 2008 | Baseline Combination therapy |
50 10 |
NR | NR | |||||||
| Niederhofer, 2009 | ATMX+MPH | NR | NR | Improved ADHD symptomatology | |||||||
p<0.05, **p<0.01, ***p<0.001.
Effect size, mean improvement in standard deviation above the baseline score.
Patients who discontinued ATMX for reasons other than adverse events or treatment ineffectiveness were classified as treatment success.
Patients who discontinued ATMX because of adverse events or treatment ineffectiveness were classified as treatment failure.
ADHD, attention-deficit/hyperactivity disorder; ADHDRS, ADHD Rating Scale; ADHDRS-IV-Parent:Inv, ADHDRS-IV-Parent-Reported Investigator-Rated; ATMX, atomoxetine; BRIEF, Behavioral Rating Inventory of Executive Functioning; CGI, Clinical Global Impressions scale; CGI-ADHD-I, CGI-Improvement of Attention-Deficit/Hyperactivity Disorder scale; CPRS, Conners' Parent Rating Scale; EC, emotional control; Hyp., hyperactive; Imp., impulsive; Inhib., inhibition; Initiat., initiation; Mont., monitor; MPH, methylphenidate; NA, not applicable; NR, not reported; O of M, organization of material; OROS, Osmotic-controlled Release Oral delivery System; PB, placebo; plan, plan/organize; SD, standard deviation; shift., shifting; vs, versus; WM, working memory; WREMB, Weekly Parent Ratings of Evening and Morning Behavior; XR, extended release.
Safety and tolerability of stimulant and atomoxetine combination therapy
Safety and tolerability outcomes for stimulant and atomoxetine combination therapy were reported in the three prospective studies and three of the seven retrospective studies of patients with ADHD (Table 4). Safety and tolerability outcomes reported in the prospective studies were adverse events, changes in blood pressure and pulse and heart rates (Carlson et al. 2007; Quintana et al. 2007; Hammerness et al. 2009). Findings on ECG parameters (Quintana et al. 2007; Hammerness et al. 2009) and blood chemistry (Hammerness et al. 2009) were also reported in some of these studies. No serious adverse events were reported in these studies; however, 10 patients discontinued because of treatment-related adverse events (Carlson et al. 2007; Quintana et al. 2007; Hammerness et al. 2009) (Table 4). Other findings of note in these studies were a mean decrease in weight with combination therapy (0.89 kg [Carlson et al. 2007], 0.82 kg [Hammerness et al. 2009]) and higher rates of insomnia, appetite loss, and irritability, but a lower rate of fatigue, (Hammerness et al. 2009) (Table 4) with combination therapy than with atomoxetine monotherapy. In addition, mean diastolic blood pressure was significantly increased after 3 weeks of stimulant therapy added to atomoxetine (Hammerness et al. 2009) and mean diastolic blood pressure and heart rate were significantly increased after 2 weeks of combination therapy during a switch from stimulant monotherapy to atomoxetine monotherapy (Quintana et al. 2007) (Table 4). There were also two clinically significant changes in ECG parameters (RR interval [Qunitana et al. 2007] and PR [Hammerness et al. 2009]) (Table 4). In the retrospective studies, the safety and tolerability outcomes reported were adverse events (Brown 2004) and descriptive statements (Adler et al. 2006; Agarwal and Sitholey 2008) (Table 4). In the ongoing chart review, 75.9% of patients tolerated and elected to continue with stimulant and atomoxetine combination therapy (Adler et al. 2006).
Table 4.
Reported Safety and Tolerability of Stimulant and Atomoxetine Combination Therapy
| |
AEs, n |
|
||
|---|---|---|---|---|
| Article n | AEs | Discontinuations | Serious | Other safety and tolerability findings |
| Patients with ADHD – prospective studies | ||||
| Carlson, 2007 9 |
6 (cardiac SE, GI discomfort, initial insomnia, rash, toothache, vomiting) | 1 (cardiac SE) | 0 |
Categoric increases BP: n=2 (diastolic; diastolic and systolic); HR: n=1 Change from start to end of treatment, ATMX+OROS MPH vs ATMX+PB, mean±SD Systolic BP: 2.1±11.2 vs 0.25±10.0 mm Hg; Diastolic BP: 3.0±8.5 vs 1.83±7.5 mm Hg; HR: 5.0±12.6 vs −2.0±12.3 bpm |
| Quintana, 2007 61 |
32 (≥2%: nausea, 5; fatigue, 3; headache, 3) | 1 (fatigue) | 0 |
Change from start to end of combination therapy, mean±SD Systolic BP: n.s.; Diastolic BP: +2.8±8.0 mm Hg*; HR: +6.2±10.4 bpm***; ECG RR interval: −58.3±114.1 ms***; no other clinically significant changes for ECG parameters reported |
| Wilens,2009 & Hammerness, 2009 50 |
NR (insomnia, 26; loss of appetite, 22; GI, 20; irritability, 16; headache,11; rhinitis, 11; fatigue, 5; othera, 15) | 8 (insomnia, GI upset, appetite loss, changes in mood after 1 week of treatment, 6) | 0 |
AE OR (95% CI) ATMX vs ATMX+OROS MPHb Fatigue: 0 (0–0.36)***; insomnia: 7.33 (2.20–38.27)***; irritability: 5.0 (1.10–46.93)*; loss of appetite: 6.0 (1.75–31.80)*** Start vs end of combination therapy, mean±SD Systolic BP: 104.5±9.4 vs 104.8±10.6 mm Hg; Diastolic BP: 64.5±9.2 vs 67.3±7.8 mm Hg*, HR: 93.3±12.7 vs 95.0±14.2 bpm; ECG PR: 132.7±19.7 vs 129.3±18.0 ms*; no significant changes for all other ECG parameters tested (QRS, QT, and QTc) or liver function & hematology parameters tested (SGOT, WBC, HCT, HGB) |
| Patients with ADHD – retrospective studies | ||||
| Adler, 2006 29 |
NR | NR | NR | n=22 reported acceptable tolerability |
| Brown, 2004 4 |
2 (initial somnolence, 2; minor GI complaint, 1; difficulty falling asleep, 1) | NA | NR | NR |
| Agarwal, 2008 1 |
NR | NA | NR | Improvement in decreased appetite and delayed onset of sleep associated with MPH use with ATMX+MPH combination therapy; no additional side effects were reported |
| Healthy volunteers | ||||
| Kelly, 2005 12 |
NR (most common: tachycardia, dry mouth, thirst) Frequency was no greater for MPH+ATMX than MPH monotherapy |
1 (ATMX+MPH: palpitations, postural drop in BP, and postural tachycardia) | NR |
Change from baseline Mean systolic BP 1 to 4 hours after dosing, MPH+ATMX:+13 mm Hg vs PB: NR*; max. mean HR, MPH+ATMX: 26 bpm vs PB: 10* at 1.5 to 6 hours; no significant effects for SVR were reported Baseline vs 4 hours after dosing, MPH+ATMX Epinephrine: 147 vs 344 pmol/L; no significant effects for norepinephrine were reported |
| Sofuoglu, 2009 10 |
NR | 0 | NR |
Change from max. post to pre dextroamphetamine dose score, PB or ATMX, treatment effect Systolic BP (F [1, 18]): 8.8**; diastolic BP (F [1, 18]): 610.6**; cortisol (F [1, 62]): 4.4*; POMS: n.s.; DEQ “stimulated” (F [1, 9]): 5.9*; DEQ “high” (F [1, 9]): 5.4*; DEQ “good drug effects” (F [1, 9]): 5.3* |
p<0.05, **p<0.01, ***p<0.001.
Includes enuresis (n=3), talking fast /on edge (n=2), nosebleed (n=1), itchy eyes/dilated pupils (n=2), anxiety (n=1), mouth pain (n=1), irregular mood/decreased personality (n=2), dry mouth (n=2), arm pain (n=1), and urinary (n=1).
Only those ORs that were statistically significant are reported in this table.
ADHD, attention-deficit/hyperactivity disorder; AE, adverse event; ATMX, atomoxetine; BP, blood pressure; bpm, beats per minute; CI, confidence interval; DEQ, Drug Effects Questionnaire; ECG, electrocardiogram; GI, gastrointestinal; HCT, hematocrit; HGB, hemoglobulin; HR, heart rate; max., maximum; MPH, methylphenidate; ms, millisecond; NA, not applicable; NR, not reported; n.s. not significant; OR, odds ratio; OROS, Osmotic-controlled Release Oral delivery System; PB, placebo; POMS, Profile of Mood States; SD, standard deviation; SE, supraventricular extrasystoles; SGOT, serum glutamic oxaloacetic transaminase; SVR, systemic vascular resistance; WBC, white blood cells; vs, versus.
Safety and tolerability outcomes for stimulant and atomoxetine combination therapy were reported in two studies of healthy volunteers (Table 4). Adverse events were reported in one study (Kelly et al. 2005). In this study, the frequency of adverse events was no greater for combination therapy than for methylphenidate monotherapy, and one patient discontinued because of treatment-related adverse events. Significant changes in blood pressure with this drug combination were reported in both studies. Blood pressure was significantly higher 1–4 hours after dosing in patients co-administered atomoxetine and methylphenidate than in those given placebo (Kelly et al. 2005). In addition, co-administration of atomoxetine and dextroamphetamine was found to attenuate increases in blood pressure resulting from dextroamphetamine monotherapy (Sofuoglu et al. 2009). The effect of combination therapy on heart rate was similar to that of methylphenidate monotherapy. Heart rate increased from 1.5 to 6 hours after dosing, with combination therapy having a significant (p<0.05) mean maximum heart rate increase of 26 beats per minute compared with placebo (Kelly et al. 2005). Another treatment effect of combination therapy was increased cortisol concentrations compared with dextroamphetamine monotherapy (Sofuoglu et al. 2009).
Discussion
This is the first systematic review of literature describing the use of stimulant and atomoxetine combination therapy. Although stimulants and atomoxetine are not approved for use in combination in the United States (Strattera Prescribing Information 2012), this drug combination is prescribed (Pohl et al. 2009; Hodgkins et al. 2011) and widely used in clinical practice (Brown 2004; Adler et al. 2006; Pliszka et al. 2006; Scott et al. 2010; Fernàndez and Rojas 2012). Findings from our review showed that there are few studies that describe factors that typify the use of stimulant and atomoxetine combination therapy or that analyze its efficacy, effectiveness, safety, or tolerability. In particular, the strength of evidence for the included studies was limited because of the heterogeneous study designs, small sample sizes, and geographic bias; there was only one prospective, randomized controlled trial of this drug combination. When reported, stimulant and atomoxetine combination therapy was used to maximize treatment effectiveness in patients classified as partial responders to stimulant or atomoxetine monotherapy or to minimize intolerable side effects in patients requiring a reduction in stimulant dose because of intolerable side effects. The findings suggested, but did not confirm, that combination therapy, if used appropriately, may benefit some, but not all, patients who have tried several ADHD medications without success. However, special care and close monitoring of stimulant and atomoxetine combination therapy are required because this drug combination has not been assessed in randomized, controlled, long-term clinical trials.
Current evidence suggests that some patients may respond differently to the various ADHD medications. In a double-blind study of patients with ADHD treated with methylphenidate monotherapy then atomoxetine monotherapy, 43% responded to atomoxetine but not methylphenidate, whereas 42% responded to methylphenidate but not atomoxetine (Newcorn et al. 2008). In the publications retrieved from our systematic review, most patients were given stimulant and atomoxetine combination therapy because of an inadequate response to previous stimulant or atomoxetine monotherapy. Stimulant combination therapy with other nonstimulant medications (e.g., extended-release clonidine [Kollins et al. 2011] and extended-release guanfacine [Sallee et al. 2009; Spencer et al. 2009; Wilens et al. 2012]) has been shown to be effective in patients with an inadequate response to stimulant therapy. A patient's ADHD medication regimen is often developed on a case-by-case basis at the discretion of the patient's physician. Some patients may try several ADHD medications before they experience adequate and tolerable symptom relief (Pliszka et al. 2006; Prasad and Steer 2008), as was the case for some patients who were given stimulant and atomoxetine combination therapy (Brown 2004). This is because current guidelines recommend full monotherapy trials of stimulants and atomoxetine for ADHD treatment (Pliszka et al. 2006; National Institute for Health and Clinical Excellence 2008; Seixas et al. 2012). In addition, identifying patient characteristics or factors that may help physicians tailor treatment regimens has, to date, been mostly unsuccessful (Quintana et al. 2007). In the publications retrieved from our systematic review, the predominant demographic (e.g., male children) and disease characteristics (e.g., combined ADHD subtype), and comorbidities (e.g., oppositional defiant disorder) identified may simply reflect those of the broader ADHD population (Polanczyk et al. 2007; Lee et al. 2008; Weiss et al. 2011). Although we did not exclude studies of adult patients with ADHD from our systematic review, only two studies included adult patients (Adler et al. 2006; Pohl et al. 2009), despite evidence supporting pharmacotherapy for adults with ADHD (Weisler and Childress 2011).
The combination strategies used in the publications included in this review represent a polypharmacy approach that was rationalized by the authors of the publications but not endorsed by the drugs' labels. Stimulant and atomoxetine combination therapy was used to augment previous stimulant or atomoxetine monotherapy to improve ADHD symptom control (e.g., atomoxetine was given to extend ADHD symptom control when the effects of the stimulant medication had dissipated [Brown 2004]) or during a switch from stimulant to atomoxetine monotherapy to maintain adequate symptom control while atomoxetine took effect (Lehmkuhl et al. 2007; Qunitana et al. 2007). In addition to these reasons, physicians may have justified the use of this drug combination because the presumed mechanisms of action for stimulants and atomoxetine differ (Wilens 2006), because it was seen as a “last resort” for treatment success by physicians discouraged by their patient's treatment resistance, or because it improved a wider range of ADHD symptoms than either medication given as monotherapy (Brown 2004). Further understanding of why physicians administer stimulant and atomoxetine combination therapy is required. In most of the reviewed studies, atomoxetine was co-administered with methylphenidate, the stimulant most often recommended as first-line treatment for ADHD (Seixas et al. 2012). The differences in the DDDeqs calculated for stimulant and atomoxetine doses used in the augmentation studies may reflect the different study designs in the included publications. DDDeqs >1 are more likely in the prospective studies because these studies are clinical trials designed to identify the most effective and tolerable medication doses. In contrast, DDDeqs <1 are more likely in retrospective studies because these studies were reports from clinical practice, where a conservative approach is often used to determine medication doses.
The efficacy and effectiveness findings for ADHD symptom control and severity in this systematic review suggested that stimulant and atomoxetine combination therapy was of benefit for some, but not all, patients (Brown 2004; Carlson et al. 2007; Quintana et al. 2007; Agarwal and Sitholey 2008; Wilens et al. 2009). Both stimulants and atomoxetine as monotherapy are effective for the treatment of ADHD (Faraone et al. 2006; Cheng et al. 2007; Mészáros et al. 2009; Faraone and Buitelaar 2010; Hanwella et al. 2011), and combination therapy may be more effective than stimulants as shown in studies of stimulants co-administered with clonidine (Kollins et al. 2011) and guanfacine (Wilens et al. 2012). In this systematic review, OROS methylphenidate and atomoxetine combination therapy for ADHD symptom control and severity did not enhance the efficacy of atomoxetine monotherapy in one prospective randomized controlled study (Carlson et al. 2007), but did enhance the effectiveness of atomoxetine monotherapy in one prospective non-controlled study (Wilens et al. 2009). In the prospective non-controlled study, improvements in executive function for combination therapy exceeded those for atomoxetine monotherapy and were within 0.5 SD of normalization (Wilens et al. 2009). During a switch from stimulant to atomoxetine monotherapy, lower scores for the ADHD-RS were recorded for combination therapy than for either medication alone (Quintana et al. 2007; Agarwal and Sitholey 2008). Although quality of life and functioning were not assessed in most of the reviewed studies, anecdotal improvements (e.g., resumed part-time employment after school) were reported for some patients (Brown 2004). These efficacy and effectiveness findings require validation and further exploration in subsequent studies of stimulant and atomoxetine combination therapy.
The safety and tolerability findings in the publications included in this review suggested that there were no additional safety concerns when stimulants and atomoxetine were co-administered for up to 6 weeks. However, the long-term safety and tolerability of this drug combination has not been tested in controlled clinical trials. Because patient safety is as important as, if not more important than, an improved symptom profile, rigorous monitoring is required when combining stimulants and atomoxetine, even though anecdotal evidence from case reports suggests it is safe and tolerable for up to 5 months of use (Brown 2004). In the prospective augmentation studies, treatment-emergent adverse event rates for methylphenidate and atomoxetine combination therapy compared with atomoxetine were greater in Hammerness et al. (2009) but lower in Carlson et al. (2007). These findings are difficult to interpret, however, because of differing study designs and the small number of patients included in these two studies. In Hammerness et al. (2009), the treatment-emergent adverse events (e.g., weight loss, insomnia, appetite loss, irritability) that were observed more frequently with stimulant and atomoxetine combination therapy than with atomoxetine monotherapy are known stimulant side effects (Vaughan et al. 2012). However, whether combination therapy using stimulant doses lower than those tested in Hammerness et al. (2009) reduces the frequency of these side effects needs to be tested. Both atomoxetine (Wernicke et al. 2003) and stimulants (Wilens et al. 2004) have known long-term cardiovascular effects. In general, the included studies showed that there were no clinically significant differences in cardiovascular parameters for stimulants and atomoxetine coadministered for 1–3 days to healthy volunteers (Kelly et al. 2005; Sofuoglu et al. 2009) and for 2–6 weeks to patients with ADHD (Carlson et al. 2007; Quintana et al. 2007; Hammerness et al. 2009) compared with stimulant or atomoxetine monotherapy. Nevertheless, these findings were limited by the small number of patients in these studies. Although the included studies in our review showed that there were no clinically significant differences in cardiovascular parameters for stimulants and atomoxetine when co-administered, more controlled research data are needed over a longer time frame to monitor any potential cardiovascular effects and, therefore, clinicians should consider monitoring blood pressure and pulse when using combination therapy.
Limitations
There were several limitations with our study. First, we may have inadvertently excluded relevant publications, even though the literature search was comprehensive and included publications written in languages other than English. Second, although we considered all levels of evidence (e.g., from randomized controlled trials to case reports) in our review, the strength of evidence for the included studies was limited because 1) of their heterogeneous methods, trial design, and outcome measures; 2) there was only one randomized controlled trial of atomoxetine and stimulant combination therapy in patients with ADHD; 3) patient numbers in the prospective studies were low (n<100); and 4) of the potential for geographic bias, as most studies were conducted in, or included data from, the United States and Europe. Third, we excluded studies of patients receiving concomitant medication(s) other than stimulants and atomoxetine to treat ADHD symptoms, which may have omitted some findings for difficult-to-treat patients. Fourth, although combination therapy in clinical practice has been administered for up to 5–6 months at a time (Brown 2004; Pohl et al. 2009), most studies assessed combination therapy for only 2–6 weeks (Carlson et al. 2007; Quintana et al. 2007; Agarwal and Sitholey 2008; Hammerness et al. 2009; Wilens et al. 2009) and did not assess adherence or compliance. Last, findings from this systematic review need to be applied with caution and clinical criteria to the broader ADHD population, because most patients given combination therapy were partial responders to or did not tolerate various ADHD medications.
Conclusions
Findings from this systematic review indicate that the published evidence for the off-label combination of atomoxetine and stimulants is limited by the number of publications and the strength of evidence. The existing evidence suggests, but does not confirm, that this drug combination may be of benefit for some, but not all, patients with ADHD classified as partial responders to stimulant or atomoxetine monotherapy, or who experience intolerable side effects with stimulant monotherapy. Further analysis of stimulant and atomoxetine combination therapy will better inform and clarify the validity and benefit of using this drug combination for patients with ADHD.
Clinical Significance
In the clinic, physicians should consider the patient's treatment history, including previous response to ADHD medications and preferences, when choosing treatment strategies for ADHD. The data analyzed in this systematic review suggest that stimulant and atomoxetine combination therapy may be of benefit for those patients with ADHD who have not attained adequate symptom control with either stimulant or atomoxetine monotherapy or who are unable to tolerate late afternoon or evening doses of stimulant medications for 24 hour coverage of symptoms. However, because stimulant and atomoxetine combination therapy has not been assessed in randomized, controlled, long-term clinical trials, special care and close monitoring are required with this drug combination.
Disclosures
Drs. Treuer, Atlin, Dueñas, Lin, Méndez, Montgomery, and Wu are employees of Eli Lilly and Company. Drs. Atlin, Dueñas, and Montgomery own shares in Eli Lilly and Company. Dr. Gau has conducted clinical trials and been on speakers' bureaus for Janssen-Cilag and Eli Lilly and Company, Taiwan. Dr. Monk is an employee of ProScribe Medical Communications contracted by Eli Lilly and Company to provide independent medical writing assistance.
All authors participated in the study design, interpretation of study results, and in the drafting, critical revision, and approval of the final version of the manuscript. As a paid professional medical writer, Dr. Monk drafted the manuscript with input at all stages from all authors.
Acknowledgments
In compliance with the Uniform Requirements for Manuscripts, established by the International Committee of Medical Journal Editors, the sponsor of this study did not impose any impediment, directly or indirectly, on the publication of the study's results. The authors acknowledge the independent medical writing assistance provided by Serina Stretton of ProScribe Medical Communications (www.proscribe.com.au), funded by an unrestricted financial grant from Eli Lilly and Company. ProScribe's services complied with international guidelines for Good Publication Practice (GPP2). Eli Lilly and Company was involved in the study design, data collection, data analysis, and preparation of the manuscript.
References
- Adler L. Morrill M. Shaw D. Raphael F. Chart review of ADHD patients treated with combination atomoxetine and stimulant therapy. Int J Neuropsychopharmacol. 2006;9:S254. [Google Scholar]
- Agarwal V. Sitholey P. Combination of atomoxetine, methylphenidate in attention deficit/hyperactivity disorder: A case report. J Can Acad Child Adolesc Psychiatry. 2008;17:160. [PMC free article] [PubMed] [Google Scholar]
- Bond GR. Garro AC. Gilbert DL. Dyskinesias associated with atomoxetine in combination with other psychoactive drugs. Clin Toxicol (Phila) 2007;45:182–185. doi: 10.1080/15563650600981178. [DOI] [PubMed] [Google Scholar]
- Brown TE. Atomoxetine and stimulants in combination for treatment of attention deficit hyperactivity disorder: Four case reports. J Child Adolesc Psychopharmacol. 2004;14:129–136. doi: 10.1089/104454604773840571. [DOI] [PubMed] [Google Scholar]
- Carlson G. Dunn D. Kelsey DK. Ruff D. Ball S. Ahrbecker L. Allen AJ. A pilot study for augmenting atomoxetine with methylphenidate: Safety of concomitant therapy in children with stimulant-resistant attention-deficit/hyperactivity disorder (ADHD) J Child Adolesc Psychopharmacol. 2006;16:659. doi: 10.1186/1753-2000-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson GA. Dunn D. Kelsey D. Ruff D. Ball S. Ahrbecker L. Allen AJ. A pilot study for augmenting atomoxetine with methylphenidate: Safety of concomitant therapy in children with attention-deficit/hyperactivity disorder. Child Adolesc Psychiatry Ment Health. 2007;1:10. doi: 10.1186/1753-2000-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JY. Chen RY. Ko JS. Ng EM. Efficacy and safety of atomoxetine for attention-deficit/hyperactivity disorder in children and adolescents-meta-analysis and meta-regression analysis. Psychopharmacology. 2007;194:197–209. doi: 10.1007/s00213-007-0840-x. [DOI] [PubMed] [Google Scholar]
- Faraone SV. Biederman J. Spencer TJ. Aleardi M. Comparing the efficacy of medications for ADHD using meta-analysis. MedGenMed. 2006;8:4. [PMC free article] [PubMed] [Google Scholar]
- Faraone SV. Buitelaar J. Comparing the efficacy of stimulants for ADHD in children and adolescents using meta-analysis. Eur Child Adolesc Psychiatry. 2010;19:353–364. doi: 10.1007/s00787-009-0054-3. [DOI] [PubMed] [Google Scholar]
- Fernàndez M. Rojas M. Stimulant and nonstimulant combined treatment for ADHD. (Abstract) Eunethydis 2nd International ADHD Conference; Barcelona, Spain. May 23–25;2012 . [Google Scholar]
- Geissler J. Lesch KP. A lifetime of attention-deficit/hyperactivity disorder: Diagnostic challenges, treatment and neurobiological mechanisms. Expert Rev Neurother. 2011;11:1467–1484. doi: 10.1586/ern.11.136. [DOI] [PubMed] [Google Scholar]
- Hammerness P.Georgiopoulos A.Doyle RL.Utzinger L.Schillinger M.Martelon M.Brodziak K.Biederman J.Wilens TE.An open study of adjunct OROS-methylphenidate in children who are atomoxetine partial responders: II. Tolerability and pharmacokinetics. J Child Adolesc Psychopharmacol 19493–499.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanwella R. Senanayake M. de Silva V. Comparative efficacy, acceptability of methylphenidate, atomoxetine in treatment of attention deficit hyperactivity disorder in children, adolescents: A meta-analysis. BMC Psychiatry. 2011;11:176. doi: 10.1186/1471-244X-11-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell PL. Kohn MR. Dickson R. Walton RJ. Granger RE. Wyk GW. Core ADHD symptom improvement with atomoxetine versus methylphenidate: A direct comparison meta-analysis. J Atten Disord. 2011;15:674–683. doi: 10.1177/1087054710379737. [DOI] [PubMed] [Google Scholar]
- Hodgkins P. Sasané R. Meijer WM. Pharmacologic treatment of attention-deficit/hyperactivity disorder in children: incidence, prevalence, and treatment patterns in The Netherlands. Clin Ther. 2011;33:188–203. doi: 10.1016/j.clinthera.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Hodgson K. Hutchinson AD. Denson L Nonpharmacological treatments for ADHD: A meta-analytic review. J Atten Disord. 2012 May 29; doi: 10.1177/1087054712444732. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Jaworowski S. Benarroch F. Gross-Tsur V. Concomitant use of atomoxetine and OROS®-methylphenidate in a 10-year-old child suffering from attention-deficit/hperactivity disorder with comorbid bipolar disorder and Tourette syndrome. J Child Adolesc Psychopharmacol. 2006;16:365–370. doi: 10.1089/cap.2006.16.365. [DOI] [PubMed] [Google Scholar]
- Kelly RP. Yeo KP. Teng CH. Smith BP. Lowe S. Soon D. Read HA. Wise SD. Hemodynamic effects of acute administration of atomoxetine and methylphenidate. J Clin Pharmacol. 2005;45:851–855. doi: 10.1177/0091270005276737. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Adler LA. Barkley R. Biederman J. Conners CK. Faraone SV. Greenhill LL. Jaeger S. Secnik K. Spencer T. Ustun TB. Zaslavsky AM. Patterns and predictors of attention-deficit/hyperactivity disorder persistence into adulthood: Results from the national comorbidity survey replication. Biol Psychiatry. 2005;57:1442–1451. doi: 10.1016/j.biopsych.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH. Jain R. Brams M. Segal S. Findling RL. Wigal SB. Khayrallah M. Clonidine extended-release tablets as add-on therapy to psychostimulants in children and adolescents with ADHD. Pediatrics. 2011;127:e1406–1413. doi: 10.1542/peds.2010-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SI. Schachar RJ. Chen SX. Ornstein TJ. Charach A. Barr C. Ickowicz A. Predictive validity of DSM-IV and ICD-10 criteria for ADHD and hyperkinetic disorder. J Child Psychol Psychiatry. 2008;49:70–78. doi: 10.1111/j.1469-7610.2007.01784.x. [DOI] [PubMed] [Google Scholar]
- Lehmkuhl G. Poustka F. Schmidt MH. Treatment of attention deficit-hyperactivity disorder with atomoxetine: Recommendations for changing the medication from stimulants to atomoxetine. Monatsschrift fur Kinderheild. 2007;155:645–648. [Google Scholar]
- Mészáros Á. Czobor P. Bálint S. Komlósi S. Simon V. Bitter I. Pharmacotherapy of adult attention deficit hyperactivity disorder (ADHD): A meta-analysis. Int J Neuropsychopharmacol. 2009;12:1137–1147. doi: 10.1017/S1461145709990198. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Clinical Excellence: Attention deficit hyperactivity disorder Diagnosis and management of ADHD in children, young people and adults NICE clinical guideline 722008http://guidance.nice.org.uk/CG72/NICEGuidance/pdf/English Last accessed 24 April 2012.
- Newcorn JH. Kratochvil CJ. Allen AJ. Casat CD. Ruff DD. Moore RJ. Michelson D. Atomoxetine/Methylphenidate Comparative Study Group: Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: Acute comparison and differential response. Am J Psychiatry. 2008;165:721–730. doi: 10.1176/appi.ajp.2007.05091676. [DOI] [PubMed] [Google Scholar]
- Niederhofer H. Atomoxetine may improve methylphenidates' efficacy in treatment of ADHD? Psychiatr Danub. 2009;21:330. [PubMed] [Google Scholar]
- Pliszka SR. Crismon ML. Hughes CW. Conners CK. Emslie GJ. Jensen PS. McCracken JT. Swanson JM. Lopez M. Texas Consensus Conference Project: The Texas children's medication algorithm project: Revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45:642–657. doi: 10.1097/01.chi.0000215326.51175.eb. [DOI] [PubMed] [Google Scholar]
- Pohl GM. Van Brunt DL. Ye W. Stoops WW. Johnston JA. A retrospective claims analysis of combination therapy in the treatment of adult attention-deficit/hyperactivity disorder (ADHD) BMC Health Serv Res. 2009;9:95. doi: 10.1186/1472-6963-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G. de Lima MS. Horta BL. Biederman J. Rohde LA. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Prasad S. Steer C. Switching from neurostimulant therapy to atomoxetine in children and adolescents with attention-deficit hyperactivity disorder: Clinical approaches and review of current available evidence. Paediatr Drugs. 2008;10:39–47. doi: 10.2165/00148581-200810010-00005. [DOI] [PubMed] [Google Scholar]
- Quintana H. Cherlin EA. Duesenberg DA. Bangs ME. Ramsey JL. Feldman PD. Allen AJ. Kelsey DK. Transition from methylphenidate or amphetamine to atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder – a preliminary tolerability and efficacy study. Clin Ther. 2007;29:1168–1177. doi: 10.1016/j.clinthera.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Sallee FR. Lyne A. Wigal T. McGough JJ. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19:215–226. doi: 10.1089/cap.2008.0080. [DOI] [PubMed] [Google Scholar]
- Scott NG. Ripperger-Suhler J. Rajab MH. Kjar D. Factors associated with atomoxetine efficacy for treatment of attention-deficit/hyperactivity disorder in children and adolescents. J Child Adolesc Psychopharmacol. 2010;20:197–203. doi: 10.1089/cap.2009.0104. [DOI] [PubMed] [Google Scholar]
- Seixas M. Weiss M. Muller U. Systematic review of national and international guidelines on attention-deficit hyperactivity disorder. J Psychopharmacol. 2012;26:753–765. doi: 10.1177/0269881111412095. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M. Poling J. Hill K. Kosten T. Atomoxetine attenuates dextroamphetamine effects in humans. Am J Drug Alcohol Abuse. 2009;35:412–416. doi: 10.3109/00952990903383961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer T. Biederman J. Wilens T. Harding M. O'Donnell D. Griffin S. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35:409–432. doi: 10.1097/00004583-199604000-00008. [DOI] [PubMed] [Google Scholar]
- Spencer TJ. Greenbaum M. Ginsberg LD. Murphy WR. Safety and effectiveness of coadministration of guanfacine extended release and psychostimulants in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19:501–510. doi: 10.1089/cap.2008.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhausen HC. The heterogeneity of causes and courses of attention-deficit/hyperactivity disorder. Acta Psychiatr Scand. 2009;120:392–399. doi: 10.1111/j.1600-0447.2009.01446.x. [DOI] [PubMed] [Google Scholar]
- Strattera Prescribing Information. Indianapolis: Eli Lilly and Company; 2012. [Google Scholar]
- Vaughan BS. March JS. Kratochvil CJ. The evidence-based pharmacological treatment of paediatric ADHD. Int J Neuropsychopharmacol. 2012;15:27–39. doi: 10.1017/S1461145711000095. [DOI] [PubMed] [Google Scholar]
- Waxmonsky JG. Nonstimulant therapies for attention-deficit hyperactivity disorder (ADHD) in children and adults. Essent Psychopharmacol. 2005;6:262–276. [PubMed] [Google Scholar]
- Weisler RH. Childress AC. Treating attention-deficit/hyperactivity disorder in adults: focus on once-daily medications. Prim Care Companion CNS Disord. 2011;13 doi: 10.4088/PCC.11r01168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss MD. Wasdell M. Gadow KD. Greenfield B. Hechtman L. Gibbins C. Clinical correlates of oppositional defiant disorder and attention-deficit/hyperactivity disorder in adults. Postgrad Med. 2011;123:177–184. doi: 10.3810/pgm.2011.03.2276. [DOI] [PubMed] [Google Scholar]
- Wernicke JF. Faries D. Girod D. Brown J. Gao H. Kelsey D. Quintana H. Lipetz R. Michelson D. Heiligenstein J. Cardiovascular effects of atomoxetine in children, adolescents, and adults. Drug Saf. 2003;26:729–740. doi: 10.2165/00002018-200326100-00006. [DOI] [PubMed] [Google Scholar]
- Wilens TE. Mechanism of action of agents used in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(Suppl 8):32–38. [PubMed] [Google Scholar]
- Wilens TE. Biederman J. Lerner M. Concerta Study G. Effects of once-daily osmotic-release methylphenidate on blood pressure and heart rate in children with attention-deficit/hyperactivity disorder: Results from a one-year follow-up study. J Clin Psychopharmacol. 2004;24:36–41. doi: 10.1097/01.jcp.0000106223.36344.df. [DOI] [PubMed] [Google Scholar]
- Wilens TE. Bukstein O. Brams M. Cutler AJ. Childress A. Rugino T. Lyne A. Grannis K. Youcha S. A controlled trial of extended-release guanfacine and psychostimulants for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:74–85. doi: 10.1016/j.jaac.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Wilens TE. Hammerness P. Utzinger L. Schillinger M. Georgiopoulous A. Doyle RL. Martelon M. Brodziak K. An open study of adjunct OROS-methylphenidate in children and adolescents who are atomoxetine partial responders: I. Effectiveness. J Child Adolesc Psychopharmacol. 2009;19:485–492. doi: 10.1089/cap.2008.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willens T. Hammerness P. Doyle RL. Utzinger L. Schillinger M. Sawtelle R. Biederman J. OROS (R) Methylphenidate adjunct use in atomoxetine, partial responders. J Child Adolesc Psychopharmacol. 2008;18:633–633. [Google Scholar]
- Wolraich ML. Wibbelsman CJ. Brown TE. Evans SW. Gotlieb EM. Knight JR. Ross EC. Shubiner HH. Wender EH. Wilens T. Attention-deficit/hyperactivity disorder among adolescents: A review of the diagnosis, treatment, and clinical implications. Pediatrics. 2005;115:1734–1746. doi: 10.1542/peds.2004-1959. [DOI] [PubMed] [Google Scholar]
- World Health Organization: DDD: Definition and general considerations. 2009. www.whocc.no/ddd/definition_and_general_considera/ [Jun 3;2012 ]. www.whocc.no/ddd/definition_and_general_considera/