Abstract
A new class of treatment processes called advanced reduction processes (ARPs) is proposed. ARPs combine activation methods and reducing agents to form highly reactive reducing radicals that degrade oxidized contaminants. Batch screening experiments were conducted to identify effective ARPs by applying several combinations of activation methods (ultraviolet light, ultrasound, electron beam, and microwaves) and reducing agents (dithionite, sulfite, ferrous iron, and sulfide) to degradation of four target contaminants (perchlorate, nitrate, perfluorooctanoic acid, and 2,4 dichlorophenol) at three pH-levels (2.4, 7.0, and 11.2). These experiments identified the combination of sulfite activated by ultraviolet light produced by a low-pressure mercury vapor lamp (UV-L) as an effective ARP. More detailed kinetic experiments were conducted with nitrate and perchlorate as target compounds, and nitrate was found to degrade more rapidly than perchlorate. Effectiveness of the UV-L/sulfite treatment process improved with increasing pH for both perchlorate and nitrate. We present the theory behind ARPs, identify potential ARPs, demonstrate their effectiveness against a wide range of contaminants, and provide basic experimental evidence in support of the fundamental hypothesis for ARP, namely, that activation methods can be applied to reductants to form reducing radicals that degrade oxidized contaminants. This article provides an introduction to ARPs along with sufficient data to identify potentially effective ARPs and the target compounds these ARPs will be most effective in destroying. Further research will provide a detailed analysis of degradation kinetics and the mechanisms of contaminant destruction in an ARP.
Key words: nitrate; oxidized contaminant; perchlorate; reducing radicals, reduction; sulfite; ultraviolet light
Introduction
Anew class of water and wastewater treatment processes called the advanced reduction processes (ARPs) is proposed, and results of preliminary experiments are reported. An ARP degrades oxidized contaminants by producing highly reactive reducing radicals by combining reagents and activation methods. This mode of operation is similar to that employed by advanced oxidation processes (AOPs), but differs in that reducing radicals are produced rather than oxidizing radicals such as the hydroxyl radical.
A free radical can be defined as any species having an odd number of electrons and thus having an unpaired electron. A free radical normally has a strong tendency to either give up the unpaired electron or accept another electron to form a pair. Therefore, they act as effective reductants (donating electrons) or oxidants (accepting electrons).
In general, the kinetics of the redox reactions involved in the degradation of a target compound are the crucial factor in deciding the feasibility of a treatment process. The formation of the highly reactive reducing radicals will make the kinetics of the desired reactions feasible, when they might be too slow with typical reductants. Many radicals are not selective and are thus well-suited for use as effective reductants in water/wastewater treatment.
Many of the current water treatment techniques for contaminated water, such as ion exchange, reverse osmosis, and nanofiltration/ultrafiltration, only concentrate the contaminant without degrading or eliminating it. Employing ARPs, which make use of such highly reactive and minimally selective radicals, will lead to transformation of target contaminants into more innocuous or simpler products. Also, the partial decomposition of nonbiodegradable organic pollutants can lead to biodegradable intermediates.
Prospective reducing agents and activation methods were chosen for initial experiments testing the concept of ARPs based on their ability to either produce or promote formation of reducing radicals. The target contaminants in these experiments included organics, inorganics, and emerging contaminants.
Background
Much research has been conducted on reactions involving reducing free radicals (Neta et al., 1987; Buxton et al., 1988; Smith et al., 2004), but very little research exists on applications of reducing radicals to water treatment/contaminant degradation. The present research is one of the first of its kind in considering the application of activation methods to produce reducing radicals from reductants to reductively degrade oxidized contaminants.
Activation methods
Ultraviolet (UV) light of a variety of wavelengths could be used in an ARP, and the desired wavelength would depend on the absorption spectra of the reagent to be activated. One type of lamp that is currently used in water and wastewater treatment is the low-pressure mercury vapor lamp (UV-L), and this lamp produces light that is almost entirely at 254 nm. Another type of lamp is a narrowband ultraviolet (UV-N) lamp that primarily emits light at 313 nm, which is more effectively absorbed by reagents such as dithionite.
When a liquid is irradiated with ultrasound, the ultrasound waves pass through the medium in a series of alternate compression and expansion cycles leading to the creation of microbubbles. The extreme conditions generated during cavitation cause thermal decomposition of water to create both oxidizing (OH•) and reducing (H•) radical species (Skov et al., 1997; Kang and Hoffmann, 1998).
In electron beam (E-beam) treatment, ionizing radiation from an electron beam source is used to pass electrons through water, producing free radicals that can degrade aqueous contaminants. (Siddiqui et al., 1996). The oxidizing free radical (OH•) and the reducing species (H• and e−aq) are the most reactive products of this reaction and generally control the rate of degradation observed during E-beam treatment.
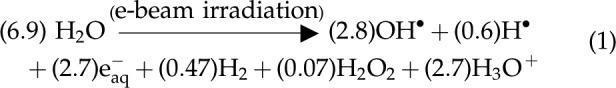
The values in parenthesis are called G values and they represent the efficiency of the ionizing radiation in producing reactive species. A G value is defined as the moles of radicals, excited species or other products, formed (or lost) due to absorption of 107 J of energy. (Nickelsen et al., 1992).
Microwave energy is a nonionizing electromagnetic radiation with frequencies in the range of 300 MHz to 300 GHz. (Haque, 1999). Degradation or enhanced degradation of target compounds by these treatment processes is brought about by the rapid heating caused by microwave irradiation, by direct microwave action, or by both (Haque, 1999; Lo et al., 2010).
Reducing agents
Different reductants can be chosen for ARPs based upon their ability to be activated by one or more activation methods and produce reducing radicals or effective reducing agents. Dithionite is known to have a long, weak S-S bond that can be broken to produce two sulfur dioxide radical anions (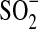 ) (Makarov, 2001).
) (Makarov, 2001).
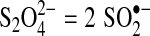 |
(2) |
The sulfur dioxide radical anion exists in aqueous dithionite solutions at very low concentrations, as evidenced by the low equilibrium constant for this reaction of 1.4×10−9 M (Mayhew, 1978; Neta et al., 1987). This free radical anion is a strong reductant with a reported standard reduction potential of −0.66 V (Mayhew, 1978). Dithionite has an absorption peak in the ultraviolet near 315 nm (Ohlsson et al., 1986; McKenna et al., 1991; Pukhovskaya et al., 2005), so irradiation near this wavelength can provide energy to break the weak S-S bond.
Although sulfite (SO32−) is a particular anion, it will be used as a general term to describe the group that includes sulfurous acid (H2SO3), bisulfite (HSO3−), and sulfite (SO32−). The UV absorption peak of sulfite solutions depends on the pH and concentration of the solutions (Getman, 1926). Both the hydrated electron (Devonshire and Weiss, 1968) and the sulfite radical anion (SO3•−) (Dogliotti and Hayon, 1968; Chawla et al., 1973; Buxton et al., 1988; Jeevarajan and Fessenden, 1989) are formed in irradiated sulfite solutions.
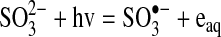 |
(3) |
The hydrated electron would be a strong reductant and the sulfite radical anion could act as an oxidant or reductant, because it can accept an electron to return to sulfite or it can donate an electron and react with water to form sulfate.
Sulfide solutions absorb UV light with a maximum at 230 nm (Kotronarou et al., 1992; Dzhabiev and Tarasov, 1993; Melsheimer and Schlogl, 1997) and irradiation with UV has promoted formation of hydrogen (Dzhabiev and Tarasov, 1993; Hara et al., 1999).
Solutions of ferrous iron absorb UV light with a maximum at 220 nm and UV irradiation promotes formation of hydrogen (Potterill et al., 1936). This could occur through a reaction of hydrated electrons with hydrogen ions, because hydrated electrons have been shown to be formed in ferrous iron solutions irradiated with UV light (Airey and Dainton, 1966).
Target contaminants
Four target compounds were investigated in this study—perchlorate, nitrate, perfluorooctanoic acid (PFOA), and 2,4-dichlorophenol (2,4-DCP). Perchlorate is a highly oxidized form of chlorine that isdifficult to reduce. This is of concern as it can disturb the functioning of the thyroid gland by interfering with its iodide uptake. (ITRC Perchlorate Team, 2007). Most chemical processes that degrade perchlorate are slow and require high temperatures or high pressures, or both. Physical treatment processes like ion exchange, reverse osmosis, nanofiltration/ultrafiltration, electrodialysis, and capacitive deionization can remove perchlorate from impacted media, but they do not degrade it. Biological processes also have been employed to treat contaminated ground and surface water, soil and wetlands (Urbansky, 2002; ITRC Perchlorate Team, 2007). Biological processes can be limited by their poor performance with toxic compounds present in the water and by poor performance at extreme temperatures or ionic strength. Another potential problem is that it can be difficult to monitor the addition of electron donors (organics) to match incoming electron acceptors (perchlorate, nitrate). Furthermore, in some cases, biological treatment processes have the potential to allow growth of undesirable microorganisms such as pathogens.
Nitrate is one of the most widespread contaminants of ground water in the United States, due to its use as a fertilizer and its formation from other nitrogen forms in human and animal wastes. Nitrate adversely affects human health by causing methemoglobinemia in infants as well as inhibiting iodine uptake by the thyroid gland, leading to thyroid dysfunction (ITRC EISBD Work Team, 2000). Active metals, ammonia, borohydride, formate, hydrazine, hydroxylamine, hydrogen, and ferrous iron are some of the chemical-reducing agents that have been used to chemically reduce nitrate in the presence of catalysts, or high temperatures, and pressures. Electrochemical and photochemical techniques are some of the nitrate reduction mechanisms that employ energy sources (Fanning, 2000).
PFOA is a synthetic, completely fluorinated organic acid that does not occur naturally in the environment. The physiochemical stability of PFOA makes it difficult to treat using most conventional treatment methods (Hoffmann et al., 2009).
2,4-DCP is a chlorinated derivative of phenol, which is highly toxic to aquatic organisms. 2,4-DCP is used primarily as intermediate in the preparation of the herbicide 2,4 dichlorophenoxyacetic acid (2,4-D). It is a high-volume chemical, which is highly toxic to aquatic organisms (Exon and Koller, 1985).
Materials and Methods
Materials
Chemical reagents and samples were prepared in an anaerobic chamber (Coy Laboratory Products, Inc.) containing an atmosphere of 95% N2 and 5% H2. Deaerated deionized water was used to make all solutions and was prepared by deoxygenating ultrapure water (18 MΩ·cm) with 99.99% nitrogen for 2 h, and then with the atmosphere in the anaerobic chamber for 12 h. Oxygen levels in the anaerobic chamber were minimized by a palladium-catalyzed reaction with hydrogen and were monitored using an Oxygen and Hydrogen Analyzer produced by Coy Labs and a resazurin-based visual indicator. Target compounds and reductants for this research were American Chemical Society grade or higher.
Analytical procedures
Concentrations of perchlorate and nitrate were measured to monitor degradation of these anions. PFOA and 2,4-DCP degradation were measured indirectly by monitoring the increased concentrations of fluoride and chloride, respectively. Percent removals for each compound were calculated based on the relative amounts of the anions released (8 mole F/mole PFOA, 2 mole Cl/mole 2,4-DCP). All of the analytes (ClO4−, NO3−, Cl−, F−) were analyzed by ion chromatography on a Dionex 500 ion chromatograph equipped with a 4-mm Dionex AS–16 analytical column. Analysis of perchlorate was conducted with a 40 mM sodium hydroxide eluent at a 1 mL/min flow rate with a 250-μL sample loop. Analysis of fluoride, chloride, and nitrate was conducted with a 10 mM sodium hydroxide eluent at a 1.25 mL/min flow rate with a 250-μL sample. The accuracy, precision, and detection limits for the analytical procedures are presented in Table 1. No replicate experiments were conducted. The solutions for e-beam experiments contained a 0.005 M carbonate buffer and all other solutions contained a 0.005 M phosphate buffer.
Table 1.
Analytical Procedure Data
| MDL (μg/L) | Accuracy (% recovery) | Precision (RSD%) | |
|---|---|---|---|
| Nitrate | 7.3 | 94.7 | 1.56 |
| Perchlorate | 6.1 | 95.8 | 1.46 |
| Chloride | 11.7 | 97.1 | 1.80 |
| Fluoride | 300 | 94.6 | 4.37 |
MDL, method detection limit; RSD, relative standard deviation.
Reactor systems
Two sources of UV radiation were used. UV light from low-pressure bulbs (UV-L) was provided by a Phillips TUV PL-L36W/4P lamp positioned 11 cm above the target solution within an enclosure. UV light from a narrow band source (UV-N) was provided by a Phillips PL-L36W/01/4P lamp in the same enclosure. The UV-L source produced UV light with a wavelength of 253.7 nm and the UV-N source produced UV light with a wavelength of 311 nm. The ultrasound reactor system consisted of the target solutions in plastic centrifuge vials, sonicated using a Hielscher ultrasonic processor, UP50H (50 W, 30 kHz). A commercial microwave was used with plastic bottles as the microwave reactor system. The electron beam at the National Center for Electron Beam Research at the Texas A&M University was used as the e-beam irradiation source. It consists of two vertically mounted opposing 10 MeV (million electron volts), 18 kW electron beam linear accelerators. Samples irradiated by the electron beam were placed in 7″×7″ (∼17.78 cm×17.78 cm) plastic bags wrapped in Saran wrap and the combination was placed within a 10″×10″ (∼25.4 cm×25.4 cm) plastic bag. The Saran wrap was used to reduce the oxygen transport into the samples.
Kinetic experiments on degradation of perchlorate and nitrate were conducted using cylindrical quartz reactors. The quartz reactors were obtained from Starna cells (Atascadero). The reactors have an interior diameter of 47 mm and a depth of 10 mm. The UV512C Digital UV C Meter obtained from General Tools was used to measure the light intensity at the top of the reactor.
Results and Discussion
A series of screening experiments were conducted to determine what ARPs (combinations of reagent and activating method) were most effective. Batch experiments were conducted with all combinations of four reagents and five activating methods (20 potential ARPs) with four target compounds at three pH levels (2.4, 7.0, and 11.2). The experiments cover time periods or energy doses that were found appropriate for the activating agent/reagent pair. Samples were taken from the reactors over a wide range of times to allow estimation of first-order rate constants that might vary over several orders of magnitude. The limited data collected in these screening experiments did not allow comparison of alternative rate models. The experimental conditions (time periods or energy doses) are summarized in Table 2.
Table 2.
Screening Experiment Time Periods or Energy Doses
| Activation method | Experiment time or energy dose |
|---|---|
| UV-L and UV-N | 20 h |
| Ultrasonics | 5 h |
| Microwaves | 1 min |
| Electron beam dose | 10 kGy* |
1 Gy=1 J/kg.
UV, ultraviolet; UV-L, UV light from low-pressure bulbs; UV-N, UV light from a narrow band source.
Results of the screening experiments and relevant control experiments are summarized semiquantitatively in Tables 3 and 4. Specific levels of treatment are provided in Supplementary Tables S1–S4. These results indicate that the E-beam and UV-L generally were successful in activating different reagents to degrade the target contaminants. 2,4-DCP and nitrate were more readily degraded compared to perchlorate and PFOA, which are particularly difficult to destroy and any degradation is indicative of potentially effective degradation processes. Both reductive and oxidative processes could be involved in the degradation of the organic compounds PFOA and 2,4-DCP. However, reduction processes are believed to be the sole mechanisms for degradation of perchlorate and nitrate, because they can only be reduced. Furthermore, the literature suggests that reducing radicals will be produced by the combinations of reagents and activation methods that were used in these experiments. These results demonstrate that a wide range of ARPs can degrade a wide variety of contaminants. Degradation of the target contaminant was observed in some of the control experiments that were conducted with an activation method in the absence of a reductant. In all such cases, more extensive degradation or faster kinetics was observed when a reagent was present during application of the activation method.
Table 3.
Summary of Screening Experiments
| |
Activation method |
||||
|---|---|---|---|---|---|
| Reagent | UV-L | UV-N | Ebeam | US | Microwave |
| Nitrate removal | |||||
| Dithionite | +++ | 0 | +++ | 0 | 0 |
| Sulfite | +++ | 0 | +++ | 0 | 0 |
| Sulfide | +++ | 0 | n.a. | 0 | 0 |
| Ferrous | +++ | + | ++ | 0 | 0 |
| Perchlorate removal | |||||
| Dithionite | 0 | 0 | 0 | 0 | 0 |
| Sulfite | + | 0 | 0 | 0 | 0 |
| Sulfide | 0 | 0 | n.a. | 0 | 0 |
| Ferrous | 0 | 0 | 0 | 0 | 0 |
| DCP removal | |||||
| Dithionite | ++ | n.a. | + | 0 | 0 |
| Sulfite | +++ | ++ | ++ | 0 | 0 |
| Sulfide | +++ | +++ | n.a. | 0 | 0 |
| Ferrous | +++ | ++ | ++ | 0 | 0 |
| PFOA removal | |||||
| Dithionite | + | + | n.a. | 0 | 0 |
| Sulfite | + | 0 | n.a. | 0 | 0 |
| Sulfide | 0 | 0 | n.a. | 0 | 0 |
| Ferrous | 0 | 0 | n.a. | 0 | 0 |
DCP, dichlorophenol; PFOA, perfluorooctanoic acid; Ebeam, electron beam; US, ultrasound; n.a., not available (experiment has not yet been conducted); 0, negligible removal (0%–10%); +, low removal (10%–40%); ++, moderate removal (40%–70%); +++, good removal (>70%).
Table 4.
Summary of Screening Control Experiments
| |
Removal |
|||
|---|---|---|---|---|
| Nitrate | Perchlorate | DCP | PFOA | |
| Only reagent | ||||
| Dithionite | 0 | 0 | 0 | n.a. |
| Sulfite | 0 | 0 | 0 | 0 |
| Sulfide | 0 | 0 | 0 | 0 |
| Ferrous | 0 | 0 | + | 0 |
| Only activation method | ||||
| UV-L | + | 0 | +++ | 0 |
| UV-N | 0 | 0 | +++ | 0 |
| Ebeam | ++ | 0 | +++ | n.a. |
| US | 0 | 0 | 0 | 0 |
| Microwave | 0 | 0 | 0 | 0 |
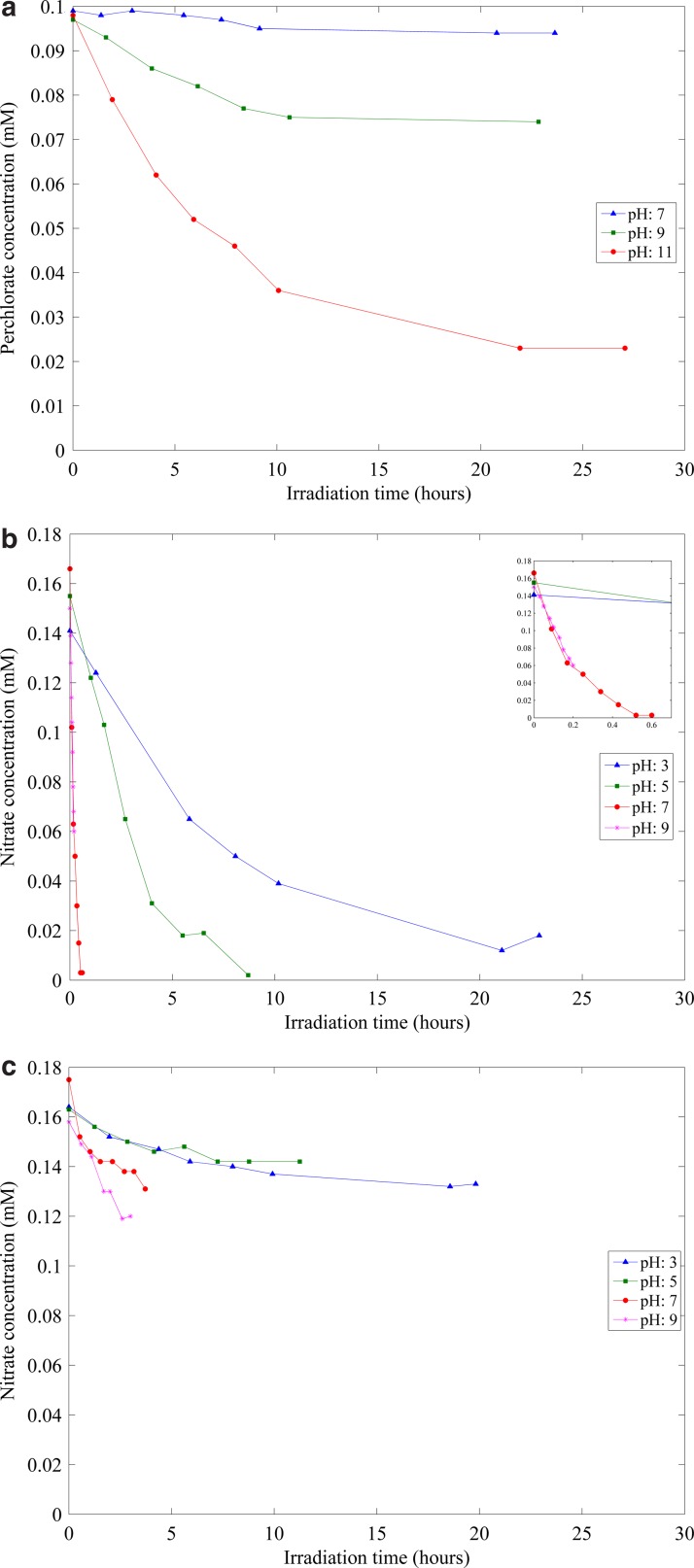
The ARP that combines sulfite with UV-L provided the most consistently high levels of removal across all contaminants. In particular, it is the only combination tested that was able to achieve destruction of perchlorate. Therefore, this ARP was the first one chosen for further tests. Perchlorate kinetic experiments were conducted with initial concentrations of 0.1 mM perchlorate and 11 mM sulfite and a light intensity of 8 mW/cm2 measured at the top of the reactor. Nitrate kinetic experiments were conducted with initial concentrations of 0.16 mM nitrate and 2.8 mM sulfite and a light intensity of 4 mW/cm2 measured at the top of the reactor. The results of the perchlorate and nitrate kinetic experiments are presented in Fig. 1a and b, respectively. Better perchlorate and nitrate removal was observed in the kinetic experiments than in the screening experiments. This is due to a more efficient UV reactor being used in the kinetic experiments. It is thus believed that better removal can be achieved for PFOA and 2,4-DCP if more efficient reactors than those used in the screening experiments were employed. No removal of perchlorate was observed in the control experiments that were conducted with either sulfite or UV-L, but not both. However, some removal of nitrate was observed in the control experiment with only UV-L (Fig. 1c).
FIG. 1.
Summary of (a) perchlorate and (b) nitrate kinetic experiments. (c) Summary of nitrate control experiments.
Figure 1a shows that perchlorate removal is incomplete, even at the highest pH. The concentration–time plots show some tendency to be linear early in the experiment, but become nonlinear later. The nonlinearity is believed to be caused by consumption of sulfite by a photochemical reaction (Dogliotti and Hayon, 1968; Chawla et al., 1973; Fischer and Warneck, 1996). The actual degradation reaction is believed to be the result of the reaction between radicals produced by photolysis of sulfite, so as the concentration of sulfite decreases, the rate of degradation of perchlorate decreases.
Figure 1b shows that nitrate was removed more rapidly than perchlorate, but that the same behavior was observed (linear early, nonlinear later), although a nearly complete nitrate removal was observed in all experiments.
The effectiveness of UV-L in stimulating contaminant degradation was measured for each experiment by calculating the quantum yield for removal of perchlorate or nitrate using Equation (4):
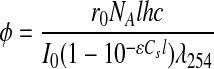 |
(4) |
where I0=irradiance entering reactor (J/[m2·s]), ɛ=molar absorptivity of sulfite (m2/mole), Cs=concentration of sulfite (mole/m3), l=depth of reactor (m), λ254=wavelength of UV light (m), r0=initial rate of removal of target compound (mole/[m3·s]), NA=Avogadro's number (mole−1), h=Planck's constant (J·s), and c=speed of light (m/s). This quantum yield is the ratio of molecules of the target compound degraded per photon absorbed by sulfite. The molar absorptivity of sulfite is needed for these calculations and the values used are given in Table 5. UV-L was more strongly absorbed at low pH, where sulfurous acid [pKa1=1.8, pKa2=7.2 (Smith et al., 2004)] dominates and at high pH, where sulfite predominates.
Table 5.
Sulfite Molar Absorptivity at 254 nm
| pH | Molar absorptivity (M−1·cm−1) |
|---|---|
| 2.5 | 25.5 |
| 5.2 | 7.6 |
| 7.5 | 15.2 |
| 9.0 | 17.4 |
| 10.9 | 18.2 |
The results of the calculations for the quantum yield are tabulated in Table 6 for perchlorate and in Table 7 for nitrate. The reported quantum yields compare favorably with the average quantum yields for decolorization of reactive azo dyes by AOPs like UV/TiO2 (0.100), UV/H2O2 (0.018), and UV/H2O2/Fe2+ (0.051) (Muruganandham et al., 2007).
Table 6.
Initial Degradation Rates and Quantum Yields for Perchlorate Degradation
| pH | Initial ClO4− degradation rate (mM/hour) | Quantum yield for ClO4− degradation |
|---|---|---|
| 7 | 0.0003 | 0.13×10−4 |
| 9 | 0.0031 | 1.2×10−4 |
| 11 | 0.0088 | 3.6×10−4 |
Table 7.
Initial Degradation Rates and Quantum Yields for Nitrate Degradation
| pH | Initial NO3− degradation rate (mM/hour) | Quantum yield for NO3− degradation |
|---|---|---|
| 3 | 0.013 | 0.0027 |
| 5 | 0.033 | 0.02 |
| 7 | 0.59 | 0.20 |
| 9 | 0.48 | 0.14 |
The effectiveness of the UV-L/sulfite treatment process improved with increasing pH for both perchlorate and nitrate. This is believed to be due to the higher concentration of SO32−, which absorbs more ultraviolet light, and therefore produces more reactive species (aqueous electrons and the sulfite radical anion). However, SO32− will be the dominant species at both pH 9 and pH 11, but perchlorate reduction is much more rapid at the higher pH. Also, the concentration of SO32− would be higher at pH 9 than at pH 7, but the initial rate of nitrate removal was observed to be higher at pH 7. Therefore, there are other effects of pH on degradation of target compounds beyond speciation of sulfite/bisulfite/sulfurous acid. Nitrate is much more rapidly degraded than perchlorate and has more efficient use of photons at the higher pH.
Nitrate reduction using chemical-reducing agents, such as active metals, ammonia, borohydride, formate, hydrogen, and ferrous iron, and energy sources, such as electrochemical, photochemical, and thermal processes, needed 1–20 h to degrade 90% nitrate (Fanning, 2000).
Slow degradation of perchlorate is expected, because it has been reported to be a very difficult compound to chemically reduce at room temperature (Table 8). One hypothesis to explain the mechanism by which the sulfite/UV-L ARP is able to degrade perchlorate is that the sulfite radical ion reacts directly with perchlorate to extract an oxygen atom. This hypothesis is based on literature reports that sulfite acts through an oxygen abstraction mechanism to reduce chlorite/chlorate (Halperin and Taube, 1950, 1952) and perbromate, the bromine analog of perchlorate. Sulfite radical anions also react directly with oxygen as the first step in the formation of sulfuric acid (Haber and Wansbrough-Jones, 1932; Ermakov et al., 1997; Zuo et al., 2005). If perchlorate was reduced by oxygen abstraction, chlorate would be produced, which is easily reduced by sulfite to chloride (Taube, 1982). The sulfate radical anion that is produced could be reduced by the aqueous electron produced by photochemical decay of sulfite. The following equations summarize the oxygen abstraction mechanism, with Equation (5) being the chain initiation reaction, Equations (6) and (7) being chain propagation reactions, and Equation (8) being the chain termination reaction.
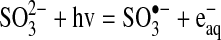 |
(5) |
 |
(6) |
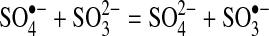 |
(7) |
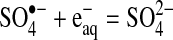 |
(8) |
Table 8.
Summary of Chemical Reduction of Perchlorate and Calculated Half Lives
| Reductant | Typical half-lifea | Conditions | References |
|---|---|---|---|
| CH3ReO2 | 0.26 h | 25°C, pH 0 | Abu-Omar et al.,1996; Abu-Omar and Espenson, 1995 |
| Fe(0) | 252 days | 25°C, [HClO4]=1 M, [Fe(0)]=0.037 M | Abu-Omar et al.,1996; Lang et al.,2002 |
| Ru(II) | 12.5 days | 25°C, [H+]=0.09–0.30 M | Kallen and Earley, 1970, 1971 |
| Sn(II)/Mo | 890 days | 25°C, [H2SO4]=2.5 M, [Mo] (catalyst)=10−5 M | Haight and Sager, 1952 |
| Ti(III) | 53 days | 50°C, [H+]=0.2–1.0 M | Abu-Omar and Espenson, 1995; Duke and Quinney, 1954 |
| Ti(III) | 668 days | 25°C, [H+]=0.1 M | Liu et al.,1984 |
| Ti(III)-Hedta | 2000 days | 25°C, [H+]=0.1 M | Liu et al.,1984 |
| V(II) | 4400 days | 49.95°C, [H+]=0.11 M | King and Garner, 1954 |
| V(III) | 14,000 days | 49.95°C, [H+]=0.11 M | King and Garner, 1954 |
Initial concentrations of reductant and perchlorate assumed to be 10−4 M and 10−5 M.
A similar oxygen abstraction mechanism is possible for nitrate reduction. Additionally, the aqueous electron could also play a direct role in the degradation of nitrate. Another hypothesis to explain the mechanism by which the sulfite/UV-L ARP is able to degrade perchlorate is that perchlorate reduction is due to the catalytic effects of trace metals that were present in the experimental solution. Taube (1982) has proposed a mechanism for reduction of perchlorate that is based on the transfer of an oxygen atom to metals such as V, Mo, Ti, Ru, Os, and U.
The effectiveness of an ARP is expected to decrease under aerobic conditions due to scavenging of reducing radicals by oxygen.
Summary
The results of the screening experiments validate the theory motivating ARPs, that is, that combining activation methods and reducing agents to produce reducing radicals can degrade oxidized contaminants. The UV-L/sulfite ARP is an effective ARP and shows the ability to destroy perchlorate and PFOA, which are both compounds that are very difficult to degrade chemically at room temperature. The effectiveness of the UV-L/sulfite treatment process improved with increasing pH for both perchlorate and nitrate.
Much research remains to be done to develop the potential of ARPs. This includes investigating the effects of oxygen, ionic strength, and natural organic matter on degradation by ARPs; evaluating different ARPs against a range of target contaminants; and identifying the degradation mechanisms for those that well degraded.
Supplementary Material
Acknowledgments
This project was funded, in part, with funds from the State of Texas as part of the program of the Texas Hazardous Waste Research Center (THWRC). The contents do not necessarily reflect the views and policies of the sponsor nor does the mention of trade names or commercial products constitute endorsement of recommendation for use. THWRC did not play a role in conducting the research nor in preparing this manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- Abu-Omar M.M. Appelman E.H. Espenson J.H. Oxygen-transfer reactions of methylrhenium oxides. Inorg. Chem. 1996;35:7751. [Google Scholar]
- Abu-Omar M.M. Espenson J.H. Facie abstraction of successive oxygen atoms from perchlorate ions by methylrhenium dioxide. Inorg. Chem. 1995;34:6239. [Google Scholar]
- Airey P.L. Dainton F.S. The photochemistry of aqueous solutions of Fe(II). I. Photoelectron detachment from ferrous and ferrocyanide ions. Proc. R. Soc. Lond. A Mat. 1966;291:340. [Google Scholar]
- Buxton G.V. Greenstock C.L. Helman W.P. Ross A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O−) in aqueous solution. J. Phys. Chem. Ref. Data. 1988;17:513. [Google Scholar]
- Chawla O.P. Arthur N.L. Fessenden R.W. Electron spin resonance study of the photolysis of aqueous sulfite solutions. J. Phys. Chem. 1973;77:772. doi: 10.1021/j100625a009. [DOI] [PubMed] [Google Scholar]
- Devonshire R. Weiss J.J. Nature of the transient species in the photochemistry of negative ions in aqueous solution. J. Phys. Chem. 1968;72:3815. [Google Scholar]
- Dogliotti L. Hayon E. Flash photolysis study of sulfite thiocyanate and thiosulfate ions in solution. J. Phys. Chem. 1968;72:1800. [Google Scholar]
- Duke F.R. Quinney P.R. The kinetics of the reduction of perchlorate ion by Ti(III) in dilute solution. J. Am. Chem. Soc. 1954;76:3800. [Google Scholar]
- Dzhabiev T.S. Tarasov B.B. Photochemical decomposition of an aqueous solution of sodium sulfide. J. Photochem. Photobiol. A. 1993;72:23. [Google Scholar]
- Ermakov A.N. Poskrebyshev G.A. Purmal A.P. Sulfite oxidation: The state-of-the-art of the problem. Kinet. Catal. 1997;38:295. [Google Scholar]
- Exon J.H. Koller L.D. Toxicity of 2-chlorophenol, 2,4-dichlorophenol, and 2,4,6-trichlorophenol. In: Jolley R.L., editor; Davis W.P., editor; Katz S., editor; Roberts M.H., editor; Bull R.J., editor. Water Chlorination: Chemistry, Environmental Impact, and Health Effects; Proceedings of the Fifth Conference on Water Chlorination: Environmental Impact and Health Effects; Williamsburg, VA. Chelsea, MI: Lewis; 1985. Jun 3–8, 1984. p. 307. [Google Scholar]
- Fanning J.C. The chemical reduction of nitrate in aqueous solution. Coord. Chem. Rev. 2000;199:159. [Google Scholar]
- Fischer M. Warneck P. Photodecomposition and photooxidation of hydrogen sulfite in aqueous solution. J. Phys. Chem. 1996;100:15111. [Google Scholar]
- Getman F.H. The ultra-violet absorption spectra of aqueous solutions of sulfur dioxide and some of its derivatives. J. Phys. Chem. 1926;30:266. [Google Scholar]
- Haber F. Wansbrough-Jones O.H. The impact of light on oxygen free and oxygen rich sulphite solutions. (VI. Announcement on auto oxydation.) Z. Phys. Chem. B-Chem. E. 1932;18:103. [Google Scholar]
- Haight G.P. Sager W.F. Evidence for preferential one-step divalent changes in the molybdate-catalyzed reduction of perchlorate by stannous ion in sulfuric acid solution. J. Am. Chem. Soc. 1952;74:6056. [Google Scholar]
- Halperin J. Taube H. Oxygen atom transfer in the reaction of chlorate with sulfite in aqueous solution. J. Am. Chem. Soc. 1950;72:3319. [Google Scholar]
- Halperin J. Taube H. The transfer of oxygen atoms in oxidation-reduction reactions—4. The reaction of hydrogen peroxide with sulfite and thiosulfate, and of oxygen, manganese dioxide and of permanganate with sulfite. J. Am. Chem. Soc. 1952;74:380. [Google Scholar]
- Haque K.E. Microwave energy for mineral treatment processes—A brief review. Int. J. Miner. Process. 1999;57:1. [Google Scholar]
- Hara K. Sayama K. Arakawa H. UV photoinduced reduction of water to hydrogen in Na2S, Na2SO3, and Na2S2O4 aqueous solutions. J. Photochem. Photobiol. A. 1999;128:27. [Google Scholar]
- Hoffmann M.R. Vecitis C.D. Park H. Cheng J. Mader B.T. Treatment technologies for aqueous perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) Front. Environ. Sci. Eng. China. 2009;3:129. [Google Scholar]
- Emerging Technologies for Enhanced In Situ Biodenitrification (EISBD) of Nitrate-Contaminated Ground Water. Washington, DC: Interstate Technology & Regulatory Council; 2000. Interstate Technology & Regulatory Council (ITRC) Enhanced In Situ Biodenitrification (EISBD) Work Team. [Google Scholar]
- ITRC Perchlorate Team. Washington, DC: Interstate Technology & Regulatory Council; 2007. Remediation Technologies for Perchlorate Contamination in Water and Soil. PERC-2. [Google Scholar]
- Jeevarajan A.S. Fessenden R.W. ESR studies of solvated electron in liquid solution using photolytic production. J. Phys. Chem. 1989;93:3511. [Google Scholar]
- Kallen T.W. Earley J.E. Substitution as rate-determining step in reduction of perchlorate ion by ruthenium(II) J. Chem. Soc. D. 1970;1970:851. [Google Scholar]
- Kallen T.W. Earley J.E. Reduction of perchlorate ion by aquoruthenium(II) Inorg. Chem. 1971;10:1152. [Google Scholar]
- Kang J.-W. Hoffmann M.R. Kinetics and mechanism of the sonolytic destruction of methyl tert-butyl ether by ultrasonic irradiation in the presence of ozone. Environ. Sci. Technol. 1998;32:3194. [Google Scholar]
- King W.R. Garner C.S. Kinetics of the oxidation of vandium(II) and vanadium(III) ions by perchlorate ion. J. Phys. Chem. 1954;58:29. [Google Scholar]
- Kotronarou A. Mills G. Hoffmann M.R. Oxidation of hydrogen sulfide in aqueous solution by ultrasonic irradiation. Environ. Sci. Technol. 1992;26:2420. [Google Scholar]
- Lang G. Ujvari M. Horanyi G. On the reduction of ClO4− ions in the course of metal dissolution in HClO4 solutions. Corros. Sci. 2002;45:1. [Google Scholar]
- Liu B.Y. Wagner P.A. Earley J.E. Reduction of perchlorate ion by (N-(hydroxyethyl)ethylenediaminetracetato)aquotitanium(III) Inorg. Chem. 1984;23:3418. [Google Scholar]
- Lo K.V. Chan W.I. Lo I.W. Liao P.H. The effects of irradiation intensity on the microwave-enhanced advanced oxidation process. J. Environ. Sci. Heal. A. 2010;45:257. doi: 10.1080/10934520903430087. [DOI] [PubMed] [Google Scholar]
- Makarov S.V. Novel trends in chemistry of sulfur-containing reductants. Uspekhi Khimii. 2001;70:1005. [Google Scholar]
- Mayhew S.G. The redox potential of dithionite and sulfur dioxide(1−) from equilibrium reactions with flavodoxins, methyl viologen and hydrogen plus hydrogenase. Eur. J. Biochem. 1978;85:535. doi: 10.1111/j.1432-1033.1978.tb12269.x. [DOI] [PubMed] [Google Scholar]
- McKenna C.E. Gutheil W.G. Song W. A method for preparing analytically pure sodium dithionite. Dithionite quality and observed nitrogenase-specific activities. Biochim. Biophys. Acta. 1991;1075:109. doi: 10.1016/0304-4165(91)90082-r. [DOI] [PubMed] [Google Scholar]
- Melsheimer J. Schlogl R. Identification of reaction products of mild oxidation of H2S in solution and in solid state by UV-VIS spectroscopy. Fresen. J. Anal. Chem. 1997;357:397. [Google Scholar]
- Muruganandham M. Selvam K. Swaminathan M. A comparative study of quantum yield and electrical energy per order (E(Eo)) for advanced oxidative decolourisation of reactive azo dyes by UV light. J. Hazard. Mater. 2007;144:316. doi: 10.1016/j.jhazmat.2006.10.035. [DOI] [PubMed] [Google Scholar]
- Neta P. Huie R.E. Harriman A. One-electron-transfer reactions of the couple sulfur dioxide/sulfur dioxide radical anion in aqueous solutions. Pulse radiolytic and cyclic voltammetric studies. J. Phys. Chem. 1987;91:1606. [Google Scholar]
- Nickelsen M.G. Cooper W.J. Kurucz C.N. Waite T.D. Removal of benzene and selected alkyl-substituted benzenes from aqueous solution utilizing continuous high-energy electron irradiation. Environ. Sci. Technol. 1992;26:144. [Google Scholar]
- Ohlsson P.I. Blanck J. Ruckpaul K. Reduction of lactoperoxidase by the dithionite anion monomer. Eur. J. Biochem. 1986;158:451. doi: 10.1111/j.1432-1033.1986.tb09774.x. [DOI] [PubMed] [Google Scholar]
- Potterill R.H. Walker O.J. Weiss J. Electron affinity spectrum of ferrous ion in aqueous solution. Proc. R. Soc. Lond. A. 1936;156:561. [Google Scholar]
- Pukhovskaya S.G. Guseva L.Z. Makarov S.V. Naidenko E.V. A new procedure for the spectrophotometric determination of nitrogen(II) oxide in solutions. J. Anal. Chem. 2005;60:21. [Google Scholar]
- Siddiqui M.S. Amy G.L. Cooper W.J. Kurucz C.N. Waite T.D. Nickelsen M.G. Bromate ion removal by HEEB irradiation. J. Am. Water Works Ass. 1996;88:90. [Google Scholar]
- Skov E.R. Pisani J.A. Beale S.E. Industrial wastewater treatment using hydroxyl radical oxidation by hydraulically induced cavitation. In AIChE Spring National Meeting Proceedings, Session 86a; Houston, TX. 1997. [Google Scholar]
- Smith R.M. Martell A.E. Motekaitis R.J. In NIST Standard Reference Database 46. Standard Reference Data Program, National Institute of Standards and Technology; Gaithersburg, MD: 2004. NIST critically selected stability constants of metal complexes database. [Google Scholar]
- Taube H. Observations on atom-transfer reactions. ACS Symp. Ser. 1982;198:151. [Google Scholar]
- Urbansky E.T. Perchlorate as an environmental contaminant. Environ. Sci. Pollut. Res. 2002;9:187. doi: 10.1007/BF02987487. [DOI] [PubMed] [Google Scholar]
- Zuo Y. Zhan J. Wu T. Effects of monochromatic UV-visible light and sunlight on Fe(III)-catalyzed oxidation of dissolved sulfur dioxide. J. Atmos. Chem. 2005;50:195. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.