Abstract
Background
Secondary lymphedema is a life-long disease of painful tissue swelling that often follows axillary lymph node dissection to treat breast cancer. It is hypothesized that poor lymphatic regeneration across the obstructive scar tissue during the wound healing process may predispose the tissue to swell at a later date. Treatment for lymphedema remains suboptimal and is in most cases palliative. The purpose of this study was to evaluate the ability of Lymphomyosot to treat tissue swelling and promote lymphangiogenesis in experimental models of murine lymphedema.
Methods
Experimental models of mouse lymphedema were injected with varied amounts of Lymphomyosot and saline as control. Measurements of tail swelling and wound closure were taken and compared amongst the groups. Three separate groups of mice were analyzed for lymphatic capillary migration, lymphatic vessel regeneration, and macrophage recruitment.
Results
Lymphomyosot significantly reduced swelling and increased the rate of surgical wound closure. Lymphomyosot did not increase the migration of lymph capillaries in a mouse tail skin regeneration model or regeneration of lymph vessels following murine axillary lymph node dissection.
Conclusions
Lymphomyosot may act through inflammatory and wound repair pathways to reduce experimental lymphedema. Its ability to regulate inflammation as well as assist in tissue repair and extracellular formation may allow for the production of a scar-free matrix bridge through which migrating cells and accumulated interstitial fluid can freely spread.
Introduction
Secondary lymphedema is a life-long disease of increased fluid retention, tissue swelling, and impaired immune function due to reduced lymphatic drainage. Lymphedema often occurs after axillary lymph node dissection to treat breast cancer.1 It is one of the most frequent long-term side effects of breast cancer and its treatment, and usually develops several years after the original lymphatic-related injury has healed. Various studies have estimated that anywhere from 30%–60% of breast cancer survivors report symptoms of lymphedema.2 Despite recent improvements in surgical techniques, such as the use of sentinel node biopsy for staging, as opposed to full axillary node dissection, the risk of lymphedema has declined but has not been eliminated.1,3 Furthermore, both mastectomy and conservative surgery patients have similar rates of developing lymphedema.4 The mechanisms that regulate its development remain largely unknown and so it remains unclear why some patients develop lymphedema and others who are identically treated do not.5
Lymphedema often causes breast cancer survivors to experience severe limitations in their daily activities, measurably affecting their overall quality of life. Specifically, women with lymphedema are more disabled, report symptoms from the upper extremity (shoulder or arm pain) and difficulties in arm movement, pain and swelling from the operated breast, recurrent infections, and higher psychological distress.5,6 Treatment for lymphedema remains suboptimal and is in most cases palliative, with a goal of preventing disease progression rather than a cure.5 Currently there are no pharmacologic treatments to improve lymphatic recovery following breast cancer surgery. There are widely used noncurative approaches to manage the swelling after it appears, such as compression garments and manual lymphatic drainage therapy.5,7
It is important to recognize that the axillary dissection removes not only the axillary lymphatic vessels and their associated lymph nodes, but also an abundance of surrounding soft tissue, thereby producing a large cavity in the axilla. The surgery is performed in an effort to prevent tumor metastasis through the lymphatic system, remove tumorous cells residing within the lymphatic system, and to determine tumor staging for subsequent treatments. However, the ensuing wound repair process inside the axillary cavity often leads to the development of fibrotic tissue and scarring. Fibrotic tissue has been found to inhibit lymphatic regeneration and interstitial flows, which may predispose upstream tissue to the disfiguring thickening and chronic infection of lymphedema.5,8,9 Therefore, treatments targeted at improving the natural healing of the wound site may indirectly increase lymphangiogenesis and interstitial fluid drainage and prevent or reduce the incidence of secondary lymphedema.8–12 There are presently no prescription drugs successful in the prevention or treatment of fibrotic scarring.13,14
The healing of an adult wound is a complex and dynamic process involving many cell lineages.15,16 The early stage of wound repair is dominated by an inflammatory phase,15,17–19 consisting of an initial influx of neutrophilic granulocytes into the damaged tissue, followed by monocytes that later differentiate into macrophages.20–22 These cells and mediators are thought to drive the excess scar formation.15,21,23 It is becoming increasingly evident that the normal and successful outcome of the acute inflammatory process that leads to good quality matrix deposition is the complete resolution of inflammation.16,17,24 Inflammation resolution is essential for restoration of full functionality and a 'normal' appearance to the injured tissue.16 The persistence of the inflammatory response leads to scarring and fibrosis.16,24–27 The lymphatic system plays a pivotal role in the resolution process by transporting the immune cells and mediators away from the inflammatory milieu. Inflammatory mediators in turn have been shown to compromise lymphatic pumping.28,29 The functionality of the lymphatic system and ongoing inflammatory processes are closely interlinked. Targeting not only one single pathway but the network of underlying mechanisms at different entry points might be a beneficial therapeutic approach to improve the wound healing and the regeneration of the lymphatic system at the same time.
The multicomponent/multitargeting medication Lymphomyosot has been shown in several observational studies to act on inflammatory pathways and reduce tissue edema of thrombotic or inflammatory etiology.30–33 Its components in other extraction forms and dosages have been shown to regulate inflammation,34–40 as well as assist in tissue repair and extracellular matrix formation through the regulation of matrix metalloproteinases, fibroblast activation, and collagen synthesis.41–45 The mechanism of action and the effectiveness of Lymphomyosot for treating lymphedema specifically are unclear. Here, we employed established experimental models of lymphedema and lymphangiogenesis to clarify whether Lymphomyosot acts to reduce tissue swelling by targeting functional lymphatic regeneration, inflammation, or by assisting with wound repair.
Materials and Methods
Experimental drug
Lymphomyosot N (Biologische Heilmittel Heel GmbH, Baden-Baden, Germany) is a multicomponent medication containing highly diluted ingredients. One ampoule (containing fluid for the injection of 1 mL of Lymphomyosot) contains 0.55 mg Gentiana lutea D5, 0.55 mg Pinus sylvestris D4, 0.55 mg Scrophularia nodosa D3, 0.55 mg Equisetum hiemale D4, 1.1 mg Ferrum jodatum D12, 0.55 mg Calcium phosphoricum D12, 0.55 mg Aranea diadematus D6, 0.55 mg Fumaria officinalis D4, 1.1 mg Geranium robertianum D4, 0.55 mg Levothyroxinum D12, 1.1 mg Nasturtium officinale D4, 0.55 mg Natrium sulfuricum D4, 0.55 mg Sarsaparillae radix D6, 0.55 mg Myosotis arvensis D3, 0.55 mg Teucrium scorodonia D3, 0.55 mg Veronica officinalis D3, sodium chloride, and water. All components are prepared according to the European Pharmacopoeia.46 Lymphomyosot is recommended to treat lymphatic diseases30,33 in humans by application of 1 ampoule per day in acute cases and otherwise 1–3 ampoules per week. For the experiments, Lymphomyosot and controls were administered intraperitoneally in dosages of 5 μL, 25 μL, or 50 μL per animal.
Experimental models
Female balb/c mice (Jackson Labs; 6–8 weeks) were used. Mice were anesthetized with 2.5% isoflurane mixed with oxygen gas for surgical procedures and were killed at experimental endpoints by CO2 asphyxiation. All protocols were approved by the Animal Care and Use Committee of Michigan Technological University. All mice used for experiments were randomized, with the surgeon blind to the treatment.
Model 1. Tail lymphedema: normal wound healing
Tail skin edema was created in balb/c mice by excising a 1-mm circular band of dermis (which contained the lymphatic capillary network) 2 cm from the base of the tail, leaving the underlying bone, muscle, tendons, and major blood vessels intact, similar to previous studies.47–49 Mice were divided into four groups (10 mice per group) for tail swelling and wound closure measurements and received 5, 25, or 50 μL i.p. injections of Lymphomyosot or 50 μL saline on the day of surgery and then every other day until euthanization at day 30. A separate group of mice were divided into three groups (10 mice per group) and received 25 or 50 μL i.p. injections of Lymphomyosot or 50 μL of saline on the day of surgery and then every other day until euthanization at day 9 for analysis of macrophage recruitment into the swollen tail skin.
Model 2. Tail lymphedema: covered wound healing
A scar-free/enhanced tissue repair model of tail skin edema was created in balb/c mice by removal of dermis as described above, with the regenerating region placed 2 cm down the tail. Following the injury, the wound region was covered with a close-fitting, gas-permeable silicone sleeve similar to previous studies.14,50–55 The sleeve protected the injury site, allowing the tissue to repair more rapidly and in a relatively scar-free manner. Placement of a covered circular wound 2 cm down the tail did not result in edema of the distal tail skin by day 10. Mice were divided into two groups (10 mice per group) and received 25 μL i.p. injections of Lymphomyosot or saline on the day of surgery and then every other day until euthanization at day 10 for analysis of lymphatic capillary migration.
Model 3. Foreleg lymphedema
A murine foreleg model of lymphedema was produced as previously described.56 Balb/c mice were anesthetized with 2.5% isoflurane mixed with oxygen gas. A 5 mm long surgical incision through the dermis was placed across the axilla on the right side and the axillary lymph nodes were identified and excised along with visible portions of pre and post-nodal collecting vessels under an Olympus SZX7 stereo microscope. The dermis was sutured with 6-0 suture thread (Harvard Apparatus, Holliston, MA) and mice were allowed to recover to day 10 or 15. Mice were divided into two groups (10 mice per group) and received 25 μL i.p. injections of Lymphomyosot or saline on the day of surgery and then every other day until euthanization at days 10 (5 mice per group) and 15 (5 mice per group) for IC-Green imaging and analysis of lymphatic vessel regeneration.
Immunofluorescence and immunohistochemistry
Tail specimens from model 2 were cut into 10 or 100 μm longitudinal cryosections and immunostained. Immunostaining of tail cryosections proceeded with antibodies against lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) in the thick sections in order to detect lymphatic endothelial cells (LECs). A goat polyclonal antibody against the lymphatic-specific hyaluronan receptor LYVE-1 (R&D Systems) was used along with an Alexa Fluor 488 donkey anti-goat secondary antibody (Invitrogen). Macrophages were detected in the 10 μm cryosections of mouse tail skin by labeling the F4/80 macrophage-specific marker.57 A biotinylated anti F4/80 antibody (Serotec) was used along with Alexa Fluor 555 conjugated streptavidin (Invitrogen). Cell nuclei in LYVE-1 and F4/80 labeled sections were counterstained with 4′,6-diamino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA).
Physiological measurements
Mouse tail images from the uncovered regenerating skin lymphedema model were captured every 3 days for each mouse tail with a DP71 color camera mounted to a stereo microscope. Tail swelling was determined with Metamorph software (Molecular Devices) by measuring tail diameters from digital images of the swollen tail distal to the wound site at the most swollen location. Wound closure was measured along the longitudinal axis of the tail as the distance between the distal and proximal regenerating tissue across the wound site. Macrophage percent coverage was determined within the swollen skin distal to the wound in F4/80-labeled tissue sections with a threshold analysis using Metamorph software.
Imaging of functional lymphatic vessels via IC-Green fluorescence lymphography
We used a recently developed imaging system to detect functional lymphatic vessels and lymph nodes in the mouse foreleg.58–60 Five microliters of a 5 mg/mL solution of the fluorescent near-infrared dye Indocyanine Green (ICG; Akorn) was injected into the mouse paw. Detection of ICG was performed with an electron multiplying charge-coupled device (CCD) (Hamamatsu, C9100-13). ICG was illuminated with an array of 760 nm LEDs (Epitex Inc) placed before a 760 nm band pass filter (model 760FS10-50, Andover Corporation) to provide the excitation light for activating ICG. A 785 nm and 763 nm custom-made holographic notch band rejection filter (model HNPF-785.0-2.0 and HNPF-763.0-2.0, Kaiser Optical Systems) and a 830 nm image quality bandpass filter (model 830FS20-25, Andover Corporation) were placed before the camera lens to selectively reject the excitation light and pass the emitted 830 nm wavelength.
Statistical methods
At least five animals were used for each data point. Ten animals were used for most data points. Data are presented as means with standard deviations. P values were calculated using ANOVA or Student's t-test, as indicated.
Results
Reduced tissue swelling by Lymphomyosot administration
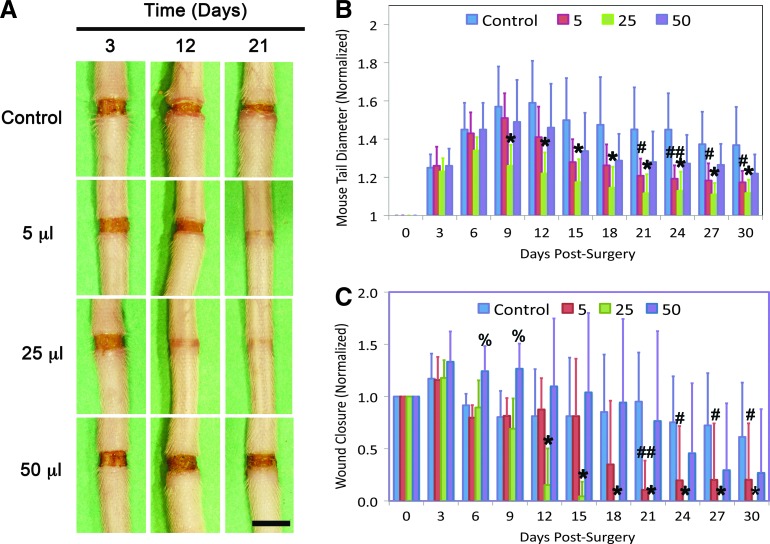
The ability of Lymphomyosot to reduce tissue swelling during lymphedema was assessed by employing an experimental model of lymphedema. Lymphedema was induced in mouse tails as per model 1 and tissue swelling was measured over a 30-day period in mice that received either 5, 25, or 50 μL of Lymphomyosot or 50 μL of saline, administered via i.p. injections (Fig. 1A and B). We found a reduction in tail swelling in mice that had received 25 μL of the drug relative to the saline control group. Differences were highly significant at day 9 and remained highly significant for the duration of the study (p<0.005 by ANOVA). We also found a reduction in tail swelling during days 21–30 in mice that had received 5 μL of the drug. This difference was highly significant at day 24 (p<0.005 by ANOVA) and significant at the other days (p<0.05 by ANOVA). Differences in tail diameter between the lymph edematous mice receiving 50 μL of the drug relative to saline administration were not significant at any time. These results show that Lymphomyosot reduces swelling of the mouse tail and that 25 μL of Lymphomyosot was the most effective dose for reducing tail diameter, followed by the 5 μL dose, with no effect from the 50 μL dose.
FIG. 1.
Lymphomyosot reduces tissue swelling and increases wound closure. Lymphedema in the mouse tail skin was induced over a period of 30 days by a 1 mm wide surgical excision of the skin that was left unprotected. Injury sites at days 3, 12, and 21 for control, 5 μL, 25 μL, and 50 μL drug injection doses are shown from top to bottom, respectively (A). Scale bar in lower right panel=5 mm. The evolution of tail swelling (B) and wound closure (C) over the 30-day period for the different conditions is graphically depicted. For the swelling data, tail diameters are normalized to the average initial (pre-swelling) diameter of the tails within each group. For the wound closure data, wound lengths are normalized to the initial 1 mm long wound; n=10 per group. High statistical significance of the 25 μL drug injection group relative to the control group (p<0.005) is represented by *. Statistical difference between the 5 μL and control group is represented by # (p<0.05) and ## (p<0.005). Statistical difference between the 50 μL and control group is represented by % (p<0.05). A color version of this figure is available in the online article at www.liebertpub.com/lrb.
Reduced tissue swelling coincides with improved wound repair
We have recently shown that tissue swelling in the mouse tail model of lymphedema was worsened when repair of the surgical wound was inhibited by combined neutralization of vascular endothelial growth factor receptor (VEGFR)-2 and VEGFR-3 signaling.50 In contrast, the swelling was improved when wound repair was augmented with a protective silicone cuff.50 Inhibition of lymphatic regeneration by neutralizing either VEGFR-2 or VEGFR-3 signaling did not impair resolution of the tail swelling.50 These results suggested that tissue swelling may be related to wound repair of the lymphatic-related injury rather than, or in addition to, lymphangiogenesis across the wound site. Because we found a rapid reduction in the tissue swelling in Lymphomyosot-treated tails, we examined the surgical obstruction for progression of wound repair to determine whether wound repair was related to the tissue swelling. To this end, we quantified and compared wound closure between the different groups. We found that administration of 25 μL of Lymphomyosot accelerated wound closure relative to treatment with saline (Fig. 1C). Differences between these groups were highly significant at day 12 and remained highly significant for the duration of the study (p<0.005, by ANOVA). Administration of 5 μL of Lymphomyosot was also found to accelerate wound closure relative to control wounds (Fig. 1C). These differences were highly significant at day 21 (p<0.005, by ANOVA) and significant at days 24–30 (p<0.05, by ANOVA). Thus, improved wound repair (Fig. 1C) and reduced tail diameter (Fig. 1B) by Lymphomyosot appeared to be temporally coincident. This finding confirms our earlier report that tissue swelling may be relieved by wound repair of the surgical injury, which provides a matrix bridge for interstitial and lymphatic flows of accumulating interstitial fluid.50 This suggests that Lymphomyosot may reduce tissue swelling by increasing wound repair of the surgical obstruction.
Lymphomyosot does not affect lymphatic capillary or lymphatic vessel regeneration
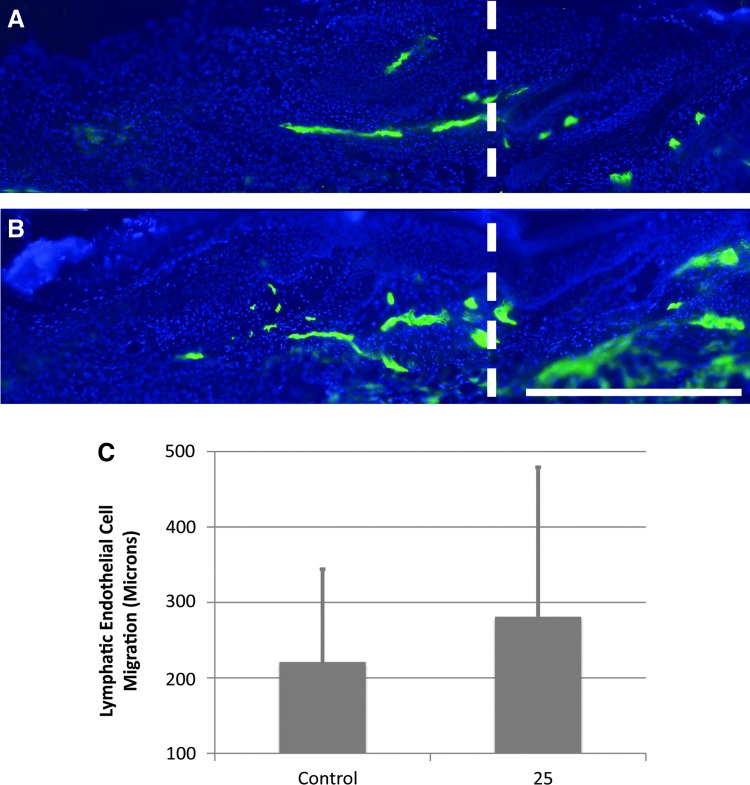
We have found previously that improved repair of the surgical obstruction promotes functional lymphangiogenesis indirectly, as interstitial fluid and lymphatic cells are able to spread freely through a restored, relatively scar-free, matrix bridge.9,14,50 In order to determine if Lymphomyosot has a direct effect on lymphangiogenesis, we produced a clearly defined region of scar-free regenerating skin within which capillary lymphangiogenesis occurs (as per model 2). In this model, both the Lymphomyosot-treated and the saline-treated control skin experience an augmented wound repair due to protection of the wound site with a silicone cuff. Thus, following drug treatment, any differences obtained in lymphatic cell migration across the site of surgical obstruction would be due to a direct action of the drug rather than an indirect action secondary to improved wound repair. We found from a previous study that lymphatic migration into the protected regenerating region is detectable in immunolabeled cross sections by post-surgery day 10.55 In order to determine the ability of Lymphomyosot to increase lymphatic capillary regeneration, we prepared groups of mice with covered regenerating regions as per model 2 and administered either 25 μL of drug or saline via i.p. injections. After animal euthanization at day 10, regenerating regions were cryo-sectioned into thick sections for LYVE-1 immunolabeling of lymphatic endothelial cells and 3-dimensional fluorescence microscopy (Fig. 2). The distance of lymphatic endothelial cell migration into the regenerating region was measured from LYVE-1-labeled sections for all tails (Fig. 2C). Comparisons between groups showed that application of 25 μL Lymphomyosot did not increase the distance of lymphatic endothelial cell migration (p>0.05 by Student's t-Test).
FIG. 2.
Lymphomyosot does not increase lymphangiogenesis of lymphatic capillaries. Lymphedema in the mouse tail skin was induced by a 1 mm wide surgical excision of the skin that was protected with a silicone cuff. Images of LYVE-1 labeled (green color) thick cryo-sections from 10-day post-surgery tail skin are shown for mice treated with 25 μL of saline (A) or drug (B). Scale bar in lower right panel=0.5 mm. The vertical white dashed line in A and B marks the distal border between native and regenerating tissue. The graph shows distance of lymphatic capillary migration into the regenerating region in the saline and drug treated groups (C). There were no statistical differences between these groups; n=10. A color version of this figure is available in the online article at www.liebertpub.com/lrb.
Because lymphedema in humans is caused primarily by disruption of lymphatic vessels rather than by disruption of lymphatic capillaries, we aimed to determine whether Lymphomyosot may act directly on regenerating lymphatic vessels. To clarify the ability of Lymphomyosot to increase lymphatic vessel regeneration, we removed axillary lymph nodes as per model 3. Mice underwent surgery for axillary lymph node dissection and received either 25 μL of drug or saline via i.p. injections until live animal lymphatic imaging at day 10 or 15 with IC-Green dye (5 mice per group). Ten minutes following an injection of IC-Green into the unoperated mouse foreleg, fluorescent lymph tracer can be seen flowing from the injection site at the paw, through lymphatic vessels in the foreleg, and reaching the site of the axillary lymph nodes (Fig. 3A—yellow arrow identifies fluorescence signal at axilla), signifying functional transport of the fluorescent dye from the paw injection site to the axilla. Following excision of the axillary lymph nodes in the mouse foreleg, we found a lack of dye accumulation at the axilla in both drug and saline treated mice at day 10, indicative of poorly functioning lymphatic vessels in both groups of mice at this time (Fig. 3B and 3C). In contrast, IC-Green dye accumulation was recovered near the site of the axilla by day 15 in both groups (Fig. 3D and 3E) indicative of a regenerated lymphatic vessel transport system in the mouse foreleg. The data indicate that Lymphomyosot may not influence lymph drainage by regeneration of lymphatic vessels.
FIG. 3.
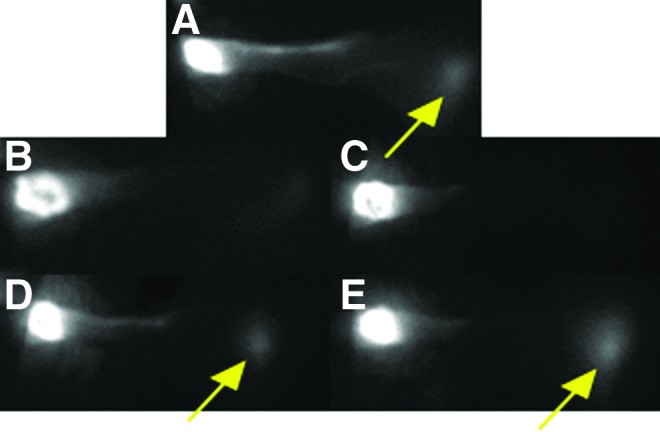
Lymphomyosot does not increase lymphangiogenesis of lymphatic vessels. Axillary lymph nodes were removed from the mouse and lymphatic vessels in the foreleg were visualized via IC-green lymphangiography. Shown are an un-operated control foreleg (A), operated mouse forelegs that received 25 μL of saline at day 10 (B) and day 15 (D), and operated mouse forelegs that received 25 μL of drug at day 10 (C) and day 15 (E). Yellow arrow identifies fluorescence signal at the axilla; n=5 per group. A color version of this figure is available in the online article at www.liebertpub.com/lrb.
Lymphomyosot modulates the macrophage infiltration in the swollen tissue
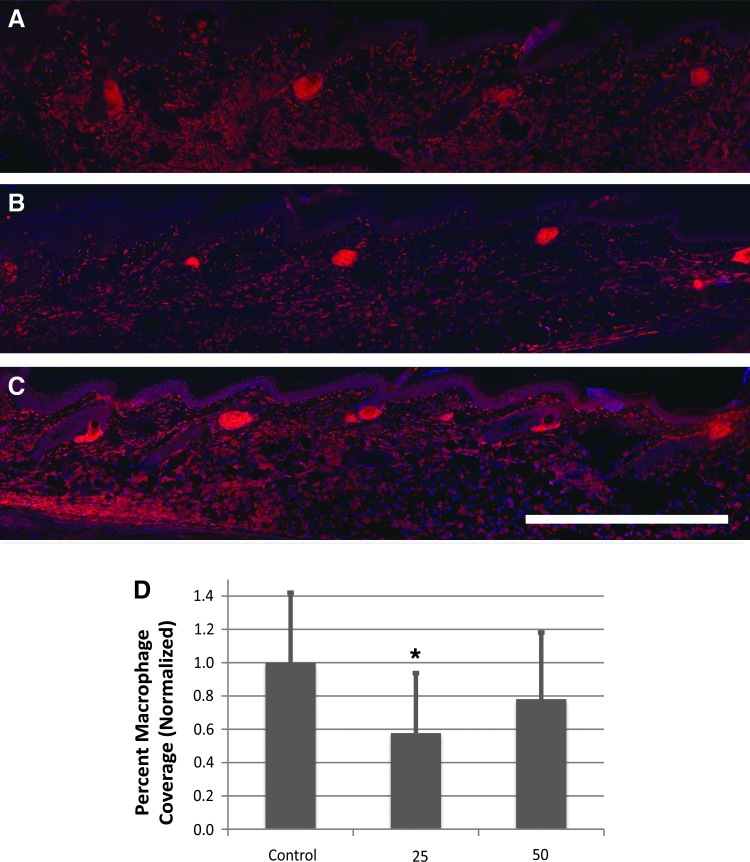
Because Lymphomyosot had no effect on regenerating lymphatic capillaries or vessels, we wondered if the drug may reduce tissue swelling by acting on inflammatory pathways, in addition to improving wound repair. Because we saw a significant reduction in swelling by day 9 in the 25 μL drug-treated group, lymphedema was induced in the mouse tail (as per model 1) and tails were assessed at day 9 for macrophage recruitment. Mice received 25 or 50 μL i.p. injections of Lymphomyosot or 50 μL of saline (10 mice per group). Collected tails were cryo-sectioned and immunolabeled against the F4/80 macrophage marker (Fig. 4). Macrophage coverage was found to be highest in the saline control group and significantly reduced in the 25 μL drug-treated group (p<0.05, by ANOVA; Fig. 4D). There was no reduction in the 50 μL drug-treated group. Lymphomyosot may have accelerated migration of the macrophages out of the affected tissue by the time the tissue was measured and/or modulated their recruitment or inhibited their recruitment. These are a few possible mechanisms of action contributing to reduction of the tissue swelling.
FIG. 4.
Modulated macrophage infiltration during experimental lymphedema by Lymphomyosot administration. Lymphedema in the mouse tail skin was induced by a 1 mm wide surgical excision of the skin that was placed 10 mm from the tail base and left unprotected. Shown are fluorescence images of the F4/80 macrophage marker (A–C) (immune-detected antigen in each image is shown in red). Macrophages were detected in tissue sections of lymph edematous mouse tail skin treated with saline (A), 25 μL of the drug (B), or 50 μL of the drug (C). The skin epidermis is located at the top of each image. Scale bar in lower right panel of C=1 mm. Wound margin is to the left of each image (not shown). Distal direction is to the right for each image. Blue color in each image is DAPI-labeled cell nuclei. Measurement of macrophage percent coverage for the different treatments (D) demonstrated possibly an accelerated migration of the macrophages out of the tested tissue by the time of measurement and/or modulation of their recruitment or inhibition of their recruitment in the swollen tissue by the drug treatment; n=10 per group. A significant difference relative to the control condition is indicated by *p<0.05. A color version of this figure is available in the online article at www.liebertpub.com/lrb.
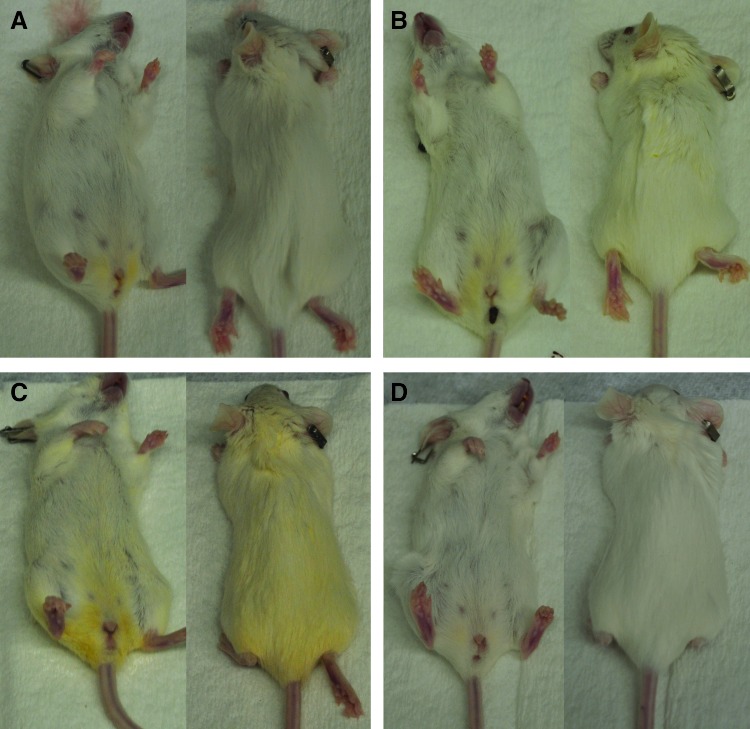
Yellowish fur discoloration found in Lymphomyosot-treated mice
During the course of the experimentation to produce the data for Figure 1, a striking yellowish fur discoloration became apparent on the mice receiving the 25 μL injections of Lymphomyosot (Fig. 5). As the discoloration in this group became darker over time, a more mild yellowish discoloration in the fur of mice receiving the 5 μL injections of Lymphomyosot also became apparent. Mice receiving control saline injections or 50 μL injections of Lymphomyosot did not become discolored to the same extent. The origin of the discoloration was not clear, but the yellowish color initiated at the genitalia and spread outwardly over time to discolor the fur at more distant sites. We are unable to explain these findings but may suggest a possible diuretic effect brought on by the Lymphomyosot treatment causing the yellowish fur discoloration. It is noteworthy that the dose response with respect to the yellowish fur discoloration follows a similar dose response pattern as was observed in the wound healing, tissue swelling, and macrophage coverage measurements.
FIG. 5.
Yellow fur discoloration. Images collected immediately post-euthanization for mice treated with saline (A), 5 μL of the drug (B), 25 μL of the drug (C), or 50 μL of the drug (D) depict an unusual dose-dependent pattern of yellowish discoloration; n=10 per group. A color version of this figure is available in the online article at www.liebertpub.com/lrb.
Discussion
Secondary lymphedema of the upper limb is often a consequence of a surgical procedure that removes lymph nodes, lymphatic collecting vessels, and surrounding tissue from the axilla in an effort to treat breast cancer and reduce the risk of secondary metastasis. It has been hypothesized that lymphedema develops due to poor lymphatic regeneration across the fibrotic scar that forms at the site of surgery.9–12,50 Therefore, therapies that improve wound repair and reduce scarring may be effective for reducing the development of post-surgical lymphedema.
Lymphomyosot has been shown to reduce acute edema.30,33 Components of this medication have also been found to have an effect on the inflammatory process in addition to assisting with tissue repair.35,37,38,42,44 However, the mechanism and the potential for Lymphomyosot to treat lymphedema specifically remained unknown. We found that Lymphomyosot reduced tissue swelling in a mouse tail model of lymphedema through a number of possible mechanisms: acceleration of the inflammatory and wound healing processes and/or modulation or inhibition of inflammation and/or influencing extracellular matrix secretion and remodeling during wound repair. Although the mouse tail swelling is caused by disconnecting the tail skin lymphatic network, the swelling reduction achieved by Lymphomyosot in this study was not mediated by lymphangiogenesis of capillaries or vessels. Thus, Lymphomyosot may act to reduce tissue swelling in the human by regulating inflammation and/or improving wound repair. The results of this study suggest that Lymphomyosot may be more effective in the prevention of lymphedema in humans if drug treatment is applied immediately following surgery to modulate inflammation and aid in wound repair, relative to being applied subsequent to lymphedema appearance, which typically occurs many years after the original lymphatic-related injury with concomitant inflammation has healed.
Wound repair is a complex and well-orchestrated biological process consisting of three sequential phases, inflammation, proliferation/granulation, and remodeling.15,20 It is the inflammatory phase with its vast array of growth factors and mediators produced by the influx of granulocytes and macrophages, that if not resolved and restored to their pre-inflammatory state leads to excess scarring, fibrosis, and chronic inflammatory disease.15,21,23 Events occur early in acute inflammation that engage an active and coordinated resolution programme (e.g., a switch to local production of specialized intercellular messengers, programmed leukocyte death by apoptosis, and subsequent clearance of dying cells by phagocytes), that is responsible for restoring the pre-inflammatory state and homeostasis.17,24,25 The multicomponent medicine Lymphomyosot may have targeted one or more of these pathways. The results of this study suggest that substances within Lymphomyosot may modulate the inflammatory response and/or promote wound healing.34,36,38,42,44
The exact mechanism of action is not clear, but several possibilities are proposed. The first mechanism of action is a general acceleration of the entire inflammatory and wound repair process. This may explain the results yielded in this study of faster wound closure and decreased concentration of macrophages. Indeed, components of Lymphomyosot have been reported to activate the production of nitric oxide (NO), reactive oxygen species (ROS), and tumour necrosis factor alpha (TNF-α), supporting a successful initial activation of inflammation followed by the simultaneous regulation of these and other early phase proinflammatory cytokines such as interleukin-1 beta (IL-1 β) and interleukin-6 (IL-6).35–37,61 The activation of nuclear factor kappa B (NF-kappa B), an important transcription factor involved in the regulation of the transcription of genes encoding these proinflammatory cytokines, is also reported to be inhibited by several components of Lymphomyosot, further assisting with the control of early stages of inflammation.38,62,63 Interferon gamma (IFN-γ),39,64 interleukin-10 (IL-10),65 as well as interleukin-8 (IL-8)66 and monocyte chemoattractant protein 1 (MCP-1),67,68 molecules that are involved in the recruitment of the appropriate immune mediators to the damaged tissues have been found to be influenced by components of Lymphomyosot. Other early phase reactions involving the modulation of important proinflammatory enzymes such as cyclooxygenase 1 and 2,69 together with the synthesis and release of prostaglandin E270 and histamine,71 are also reported to be affected by Lymphomyosot's components, as are immune system molecules that can play critical roles in early stages of an immune system response, the dendritic cells.72 Furthermore, the regulation of leukocyte infiltration by macrophages73 at least partly by influencing the expression of macrophage migration inhibitory factor74 and the activity of important adhesion molecules38,75 such as vascular cell adhesion protein 1 (VCAM-1) and intracellular adhesion molecule 1 (ICAM-1) seem to be regulated by certain components of Lymphomyosot. Finally, Lymphomyosot also helps modulate the synthesis and release of nitric oxide.76–78 an important signaling molecule in a wide variety of physiological and pathological processes including inflammation, vasodilation, immune system response, wound healing, and tissue remodeling.
The decreased number of macrophages could also be explained by the effect that certain Lymphomyosot ingredients have on leukocyte trafficking. With several components displaying a possible inhibitory effect on early proinflammatory activity, and in particular influencing the expression of macrophage migration inhibitory factor74 and the activity of vascular adhesion molecules,38,75 this could result in Lymphomyosot acting as an anti-inflammatory agent and inhibiting key pro-inflammatory mediator recruitment to the inflammatory site. Macrophages are thought to be vital in not only clearing a wound of invading microbes but also as crucial coordinators of the repair process acting as both professional phagocytes to clear the wound debris and as major sources of wound growth factor signals.16,23,79 Indeed, a healing response that lacks macrophages recruitment during the early stage of repair results in the lack of vascular endothelial growth factor (VEGF) and transforming growth factor beta-1 (TGF-b1), vital for wound angiogenesis and myofibroblast differentiation.23,79,80 However, unbalanced inflammation characterized by increased numbers of macrophages is a hallmark of excess scarring and fibrosis,23 as uncontrolled activity of macrophages can be detrimental to tissue repair due to the release of their proinflammatory and cytotoxic mediators.23 It is possible that by inhibiting this key inflammatory mediator, Lymphomyosot may have acted as an anti-inflammatory agent, reducing inflammation and swelling in addition to possibly assisting in modulating scar formation and fibrosis.
Repair of tissue after injury depends upon the synthesis of fibrous extracellular matrix to replace lost or damaged tissue. Newly deposited extracellular matrix is then remodeled over time to emulate normal tissue. The inflammatory phase, re-epithelization and contraction all depend on cell–extracellular matrix interactions to minimize infection and promote rapid wound closure.81 Several components of Lymphomyosot have been found to have stimulating effects on fibroblast proliferation,41,44,45 collagen production,13,45 keratinocyte activity,82 and increasing glycosaminoglycan content, vital components of the extracellular matrix ground substance.13 This, together with the modulatory effects on matrix metalloproteinases activity42,83 in the remodeling of the extracellular matrix, may assist with more rapid and efficient formation of both provisional extracellular matrix directly after surgery and a better overall quality wound at the end of the repair process. Furthermore, components of Lymphomyosot not only have stimulating effects on fibroblast and collagen formation but others seem to also inhibit their activities,84,85 thus displaying a modulating effect on collagen formation and possibly preventing excess scarring and fibrosis in the final stages of wound repair. The ability to modulate fibroblast proliferation, collagen production, and the proteolytic activities of the matrix metalloproteinases may allow for the production of a relatively scar-free matrix bridge directly after surgery through which migrating cells and accumulated interstitial fluid can freely spread through.86 This may suggest another possible mechanism of action in Lymphomyosot for achieving faster wound closure and reducing tissue swelling in this study design.
The effectiveness of most drugs can be demonstrated by a linear dose–response curve. However, we found that Lymphomyosot was increasingly effective from 5 to 25 μL and that the effects were suppressed at 50 μL. Although we are unable at this time to explain the response suppression at the 50 μL dose, we find it intriguing that all the outcome variables measured in the study consistently follow a similar dose–response pattern. A possible explanation could be that Lymphomyosot follows a dose–response pattern that is nonlinear. In the literature, there are several examples of inverted U-shaped dose responses reported for other pharmacological, biological, and toxicological investigations.87–91 Multiple studies have shown that effects of endocrine-disrupting chemicals at high doses cannot predict the effects at low doses.92,93 In the field of environmental toxicology and radiation biology, the concept that low doses have opposite effects than high doses is known as hormesis.88,93–95 In addition, several studies analyzing the effects of pharmacological or nonpharmacological treatments on cognitive function and memory revealed a stimulation with increasing doses up to a maximum, following an inhibition at higher doses.90,91,96,97 In another example related to vaccine development, the authors observed a similar immune suppression effect at above threshold doses.98
In the present study, we have shown that Lymphomyosot improves wound repair, modulates macrophage activity, and reduces acute lymphedema. Several potential mechanisms of action with opposing theories have been put forward in our discussion. Modulation and acceleration of wound healing through the resolution of inflammation is one such theory that may explain the reduced swelling, decreased macrophage numbers, and faster wound closure. Lymphomyosot may also be acting as an anti-inflammatory agent inhibiting important early phase inflammatory reactors, possibly blocking the early onset of inflammation in addition to reducing the influx of granulocytes into the wound site. Effects on the extracellular matrix throughout the healing process is another way that Lymphomyosot may assist in improving repair of a surgical obstruction site, thus reducing tissue swelling and increasing wound closure. The results are unlikely to be due to only one of these mechanisms of action, but rather a combination of them. This is a multicomponent medication, which seems to have a wide range of effects on different pathways. Each major component of the drug, listed separately in the methods section, contains multiple subcomponents. There may be multiple active ingredients within each major component. Furthermore, the cumulative biological responses that were seen in the mice may depend upon a synergistic or additive effect of multiple active ingredients acting in concert.
The existence of a variety of pathways contributing to the development of secondary lymphedema points to the necessity of such multitargeting medications. The variant (synergistic and additive) effects mediated by 16 different major ingredients in Lymphomyosot suggest a use for this medication as supportive treatment after surgeries. Underlying processes like inflammation, drainage, and tissue remodeling are targeted and modulated at the same time in low doses with little side effects. Lymphomyosot may provide a new systemic or local medication for wound therapy or skin repair to improve healing rate and quality and its effect on modulating inflammation could help to prevent chronic inflammatory states and prevent scarring and fibrosis.
Perspective
Secondary lymphedema is hypothesized to develop due to poor lymphatic regeneration across the fibrotic scar that develops at the site of surgery in an effort to treat breast cancer. The complexities of adult wound repair and its multiple cellular and extracellular pathways displayed in secondary lymphedema may have been shown to be directly influenced by the multitargeting medication Lymphomyosot. The underlying processes of inflammation, drainage and tissue remodeling were targeted and modulated at the same time in low doses with little side effects. These results provide the opportunity to explore the potential therapeutic effects that Lymphomyosot may provide as a new systemic or local medication for wound therapy or skin repair to improve rate and quality of healing.
Abbreviations Used
- ANOVA
analysis of variance
- CCD
charge-coupled device
- DAPI
4′,6-diamino-2-phenylindole
- ICAM-1
intracellular adhesion molecule 1
- ICG
Indocyanine Green
- IFN-γ
interferon gamma
- IL-1β
interleukin-1 beta
- IL-6
interleukin-6
- IL-8
interleukin-8
- IL-10
interleukin-10
- i.p.
intraperitoneal
- LEC
lymphatic endothelial cell
- LYVE-1
lymphatic vessel endothelial hyaluronan receptor 1
- MCP-1
monocyte chemoattractant protein 1
- NF-kappa B
nuclear factor kappa B
- NO
nitric oxide
- ROS
reactive oxygen species
- SE
standard errors
- TGF-b1
transforming growth factor beta-1
- TNF-α
tumor necrosis factor alpha
- VCAM-1
vascular cell adhesion protein 1
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
Acknowledgments
This study was performed on behalf of the Biologische Heilmittel Heel GmbH.
We would like to thank Dr. David Lescheid for his valuable comments on the manuscript.
Author Disclosure Statement
This study was performed on behalf of the Biologische Heilmittel Heel GmbH, manufacturer of the Lymphomyosot formulation evaluated in this study.
References
- 1.Lumachi F. Basso SM. Bonamini M, et al. Incidence of arm lymphoedema following sentinel node biopsy, axillary sampling and axillary dissection in patients with breast cancer. In Vivo. 2009;23:1017–1020. [PubMed] [Google Scholar]
- 2.Seifart U. Albert US. Heim ME, et al. Lymphedema in patients with breast cancer—A consensus regarding diagnostics and therapy in patients with postoperative lymphedema after primary breast cancer. Rehabilitation (Stuttg Dec. 2007;46:340–348. doi: 10.1055/s-2007-985170. [DOI] [PubMed] [Google Scholar]
- 3.Leidenius M. Leivonen M. Vironen J. von Smitten K. The consequences of long-time arm morbidity in node-negative breast cancer patients with sentinel node biopsy or axillary clearance. J Surg Oncol. 2005;92:23–31. doi: 10.1002/jso.20373. [DOI] [PubMed] [Google Scholar]
- 4.Paskett ED. Stark N. Lymphedema: Knowledge, treatment, and impact among breast cancer survivors. Breast J. 2000;6:373–378. doi: 10.1046/j.1524-4741.2000.99072.x. [DOI] [PubMed] [Google Scholar]
- 5.Cemal Y. Pusic A. Mehrara BJ. Preventative measures for lymphedema: separating fact from fiction. J Am Coll Surg. 2011;213:543–551. doi: 10.1016/j.jamcollsurg.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chachaj A. Malyszczak K. Pyszel K, et al. Physical and psychological impairments of women with upper limb lymphedema following breast cancer treatment. Psychooncology. 2010;19:299–305. doi: 10.1002/pon.1573. [DOI] [PubMed] [Google Scholar]
- 7.Hamner JB. Fleming MD. Lymphedema therapy reduces the volume of edema and pain in patients with breast cancer. Ann Surg Oncol. 2007;14:1904–1908. doi: 10.1245/s10434-006-9332-1. [DOI] [PubMed] [Google Scholar]
- 8.Suami H. Pan WR. Taylor GI. The lymphatics of the skin filled by a dermal backflow: An observation in a scarred cadaver leg. Lymphology. 2007;40:122–126. [PubMed] [Google Scholar]
- 9.Uzarski J. Drelles MB. Gibbs SE, et al. The resolution of lymphedema by interstitial flow in the mouse tail skin. Am J Physiol Heart Circ Physiol. 2008;294:H1326–1334. doi: 10.1152/ajpheart.00900.2007. [DOI] [PubMed] [Google Scholar]
- 10.Avraham T. Clavin NW. Daluvoy SV, et al. Fibrosis is a key inhibitor of lymphatic regeneration. Plast Reconstr Surg. 2009;124:438–450. doi: 10.1097/PRS.0b013e3181adcf4b. [DOI] [PubMed] [Google Scholar]
- 11.Clavin NW. Avraham T. Fernandez J, et al. TGF-beta1 is a negative regulator of lymphatic regeneration during wound repair. Am J Physiol Heart Circ Physiol. 2008;295:H2113–2127. doi: 10.1152/ajpheart.00879.2008. [DOI] [PubMed] [Google Scholar]
- 12.Avraham T. Yan A. Zampell JC, et al. Radiation therapy causes loss of dermal lymphatic vessels and interferes with lymphatic function by TGF-beta1-mediated tissue fibrosis. Am J Physiol Cell Physiol. 2010;299:C589–605. doi: 10.1152/ajpcell.00535.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlisle EM. In vivo requirement for silicon in articular cartilage and connective tissue formation in the chick. J Nutr. 1976;106:478–484. doi: 10.1093/jn/106.4.478. [DOI] [PubMed] [Google Scholar]
- 14.Goldman J. Conley KA. Raehl A, et al. Regulation of lymphatic capillary regeneration by interstitial flow in skin. Am J Physiol Heart Circ Physiol. 2007;292:H2176–2183. doi: 10.1152/ajpheart.01011.2006. [DOI] [PubMed] [Google Scholar]
- 15.Stramer BM. Mori R. Martin P. The inflammation-fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. J Invest Dermatol. 2007;127:1009–1017. doi: 10.1038/sj.jid.5700811. [DOI] [PubMed] [Google Scholar]
- 16.Shaw TJ. Martin P. Wound repair at a glance. J Cell Sci. 2009;122:3209–3213. doi: 10.1242/jcs.031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nathan C. Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 18.Chen GY. Nunez G. Sterile inflammation: Sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishida Y. Gao JL. Murphy PM. Chemokine receptor CX3CR1 mediates skin wound healing by promoting macrophage and fibroblast accumulation and function. J Immunol. 2008;180:569–579. doi: 10.4049/jimmunol.180.1.569. [DOI] [PubMed] [Google Scholar]
- 20.Mercandetti M. Wound healing and repair. http://emedicine.medscape.com/article/1298129-overview. [May 29;2012 ]. http://emedicine.medscape.com/article/1298129-overview
- 21.Wilgus TA. Immune cells in the healing skin wound: Influential players at each stage of repair. Pharmacol Res. 2008;58:112–116. doi: 10.1016/j.phrs.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Eming SA. Werner S. Bugnon P, et al. Accelerated wound closure in mice deficient for interleukin-10. Am J Pathol. 2007;170:188–202. doi: 10.2353/ajpath.2007.060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucas T. Waisman A. Ranjan R, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184:3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 24.Serhan CN. Savill J. Resolution of inflammation: The beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 25.Serhan CN. Chiang N. Van Dyke TE. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serhan CN. Brain SD. Buckley CD, et al. Resolution of inflammation: State of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perretti M. Dalli J. Exploiting the Annexin A1 pathway for the development of novel anti-inflammatory therapeutics. Br J Pharmacol. 2009;158:936–946. doi: 10.1111/j.1476-5381.2009.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Von Der Weid PY. Rehal S. Lymphatic pump function in the inflamed gut. Ann NY Acad Sci. 2010;1207:E69–74. doi: 10.1111/j.1749-6632.2010.05715.x. [DOI] [PubMed] [Google Scholar]
- 29.von der Weid PY. Rehal S. Dyrda P, et al. Mechanisms of VIP-induced inhibition of the lymphatic vessel pump. J Physiol. 2012;590:2677–2691. doi: 10.1113/jphysiol.2012.230599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moyseyenko V CV. Effectiveness and tolerability of Lymphomyosot: Solution for injection in treating oedemas and swellings of thrombotic or inflammatory aetiology in general clinical practice. Eur J Integ Med. 2009:1. [Google Scholar]
- 31.Eiber A KP. Weiser M. Diabetic peripheral neuropathy. Adjuvant homeopathic treatment enhances the effects of conventional therapy. Der Allgemeinarzt. 2003;25:610–614. [Google Scholar]
- 32.Dietz A-R. Possibilities for lymph therapy with diabetic polyneuropathy. Intl J Biomed Res Ther. 2000;299:4–9. [Google Scholar]
- 33.Zenner SMH. Therapeutic use of Lymphomyosot. Results of a multicentre use observation study on 3,512 patients. Biol Ther. 1990:8. [Google Scholar]
- 34.Gabay O. Sanchez C. Salvat C, et al. Stigmasterol: A phytosterol with potential anti-osteoarthritic properties. Osteoarth Cartil. 2010;18:106–116. doi: 10.1016/j.joca.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 35.Guo YJ. Zhao L. Li XF, et al. Effect of Corilagin on anti-inflammation in HSV-1 encephalitis and HSV-1 infected microglias. Eur J Pharmacol. 2010;635:79–86. doi: 10.1016/j.ejphar.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 36.Ikeda Y. Murakami A. Fujimura Y, et al. Aggregated ursolic acid, a natural triterpenoid, induces IL-1beta release from murine peritoneal macrophages: Role of CD36. J Immunol. 2007;178:4854–4864. doi: 10.4049/jimmunol.178.8.4854. [DOI] [PubMed] [Google Scholar]
- 37.Jeong HJ. Koo HN. Na HJ, et al. Inhibition of TNF-alpha and IL-6 production by Aucubin through blockade of NF-kappaB activation RBL-2H3 mast cells. Cytokine. 2002;18:252–259. doi: 10.1006/cyto.2002.0894. [DOI] [PubMed] [Google Scholar]
- 38.Moon MK. Lee YJ. Kim JS. Kang DG. Lee HS. Effect of caffeic acid on tumor necrosis factor-alpha-induced vascular inflammation in human umbilical vein endothelial cells. Biol Pharm Bull. 2009;32:1371–1377. doi: 10.1248/bpb.32.1371. [DOI] [PubMed] [Google Scholar]
- 39.Ahmad SF. Khan B. Bani S. Suri KA. Satti NK. Qazi GN. Amelioration of adjuvant-induced arthritis by ursolic acid through altered Th1/Th2 cytokine production. Pharmacol Res. 2006;53:233–240. doi: 10.1016/j.phrs.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Schmolz MMH. Immunopharmacological effect of individual antihomotoxic agents in Lymphomyosot N. Biol Med. 2001;30:177–183. [Google Scholar]
- 41.Song HS. Park TW. Sohn UD, et al. The effect of caffeic acid on wound healing in skin-incised mice. Korean J Physiol Pharmacol. 2008;12:343–347. doi: 10.4196/kjpp.2008.12.6.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang ST. Wang CY. Yang RC, et al. Ellagic acid, the active compound of Phyllanthus urinaria, exerts in vivo anti-angiogenic effect and inhibits MMP-2 activity. Evid Based Complement Alternat Med. 2011;2011:215035. doi: 10.1093/ecam/nep207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho JN. Lee YH. Park JS, et al. Protective effects of aucubin isolated from Eucommia ulmoides against UVB-induced oxidative stress in human skin fibroblasts. Biol Pharm Bull. 2005;28:1244–1248. doi: 10.1248/bpb.28.1244. [DOI] [PubMed] [Google Scholar]
- 44.Stevenson PC. Simmonds MS. Sampson J. Houghton PJ. Grice P. Wound healing activity of acylated iridoid glycosides from Scrophularia nodosa. Phytother Res. 2002;16:33–35. doi: 10.1002/ptr.798. [DOI] [PubMed] [Google Scholar]
- 45.Ozturk N. Korkmaz S. Ozturk Y. Baser KH. Effects of gentiopicroside, sweroside and swertiamarine, secoiridoids from gentian (Gentiana lutea ssp. symphyandra), on cultured chicken embryonic fibroblasts. Planta Med. 2006;72:289–294. doi: 10.1055/s-2005-916198. [DOI] [PubMed] [Google Scholar]
- 46.Europe Co. European Pharmacopoeia. Strasbourg: Council of Europe. 2010.
- 47.Rutkowski JM. Moya M. Johannes J. Goldman J. Swartz MA. Secondary lymphedema in the mouse tail: Lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc Res. 2006;72:161–171. doi: 10.1016/j.mvr.2006.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swartz MA. Kaipainen A. Netti PA, et al. Mechanics of interstitial-lymphatic fluid transport: Theoretical foundation and experimental validation. J Biomech. 1999;32:1297–1307. doi: 10.1016/s0021-9290(99)00125-6. [DOI] [PubMed] [Google Scholar]
- 49.Roberts MA. Mendez U. Gilbert RJ. Keim AP. Goldman J. Increased hyaluronan expression at distinct time points in acute lymphedema. Lymphat Res Biol. 2012;10:122–128. doi: 10.1089/lrb.2012.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ongstad EL. Bouta EM. Roberts JE, et al. Lymphangiogenesis-independent resolution of experimental edema. Am J Physiol Heart Circ Physiol. 2010;299:H46–54. doi: 10.1152/ajpheart.00008.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boardman KC. Swartz MA. Interstitial flow as a guide for lymphangiogenesis. Circ Res. 2003;92:801–808. doi: 10.1161/01.RES.0000065621.69843.49. [DOI] [PubMed] [Google Scholar]
- 52.Goldman J. Le TX. Skobe M. Swartz MA. Overexpression of VEGF-C causes transient lymphatic hyperplasia but not increased lymphangiogenesis in regenerating skin. Circ Res. 2005;96:1193–1199. doi: 10.1161/01.RES.0000168918.27576.78. [DOI] [PubMed] [Google Scholar]
- 53.Pytowski B. Goldman J. Persaud K, et al. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst. 2005;97:14–21. doi: 10.1093/jnci/dji003. [DOI] [PubMed] [Google Scholar]
- 54.Rutkowski JM. Boardman KC. Swartz MA. Characterization of lymphangiogenesis in a model of adult skin regeneration. Am J Physiol Heart Circ Physiol. 2006;291:H1402–1410. doi: 10.1152/ajpheart.00038.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goldman J. Rutkowski JM. Shields JD, et al. Cooperative and redundant roles of VEGFR-2 and VEGFR-3 signaling in adult lymphangiogenesis. FASEB J. 2007;21:1003–1012. doi: 10.1096/fj.06-6656com. [DOI] [PubMed] [Google Scholar]
- 56.Tammela T. Saaristo A. Holopainen T, et al. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med. 2007;13:1458–1466. doi: 10.1038/nm1689. [DOI] [PubMed] [Google Scholar]
- 57.Austyn JM. Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11:805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- 58.Kwon S. Sevick-Muraca EM. Noninvasive quantitative imaging of lymph function in mice. Lymphat Res Biol. 2007;5:219–231. doi: 10.1089/lrb.2007.1013. [DOI] [PubMed] [Google Scholar]
- 59.Sevick-Muraca EM. Sharma R. Rasmussen JC, et al. Imaging of lymph flow in breast cancer patients after microdose administration of a near-infrared fluorophore: feasibility study. Radiology. 2008;246:734–741. doi: 10.1148/radiol.2463070962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma R. Wang W. Rasmussen JC, et al. Quantitative imaging of lymph function. Am J Physiol Heart Circ Physiol. 2007;292:H3109–3118. doi: 10.1152/ajpheart.01223.2006. [DOI] [PubMed] [Google Scholar]
- 61.Liu R. Zhang L. Lan X, et al. Protection by borneol on cortical neurons against oxygen-glucose deprivation/reperfusion: Involvement of anti-oxidation and anti-inflammation through nuclear transcription factor kappaappaB signaling pathway. Neuroscience. 2011;176:408–419. doi: 10.1016/j.neuroscience.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 62.Rose P. Won YK. Ong CN. Whiteman M. Beta-phenylethyl and 8-methylsulphinyloctyl isothiocyanates, constituents of watercress, suppress LPS induced production of nitric oxide and prostaglandin E2 in RAW 264.7 macrophages. Nitric Oxide. 2005;12:237–243. doi: 10.1016/j.niox.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 63.Umesalma S. Sudhandiran G. Differential inhibitory effects of the polyphenol ellagic acid on inflammatory mediators NF-kappaB, iNOS, COX-2, TNF-alpha, and IL-6 in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Basic Clin Pharmacol Toxicol. 2010;107:650–655. doi: 10.1111/j.1742-7843.2010.00565.x. [DOI] [PubMed] [Google Scholar]
- 64.Okubo T. Washida K. Murakami A. Phenethyl isothiocyanate suppresses nitric oxide production via inhibition of phosphoinositide 3-kinase/Akt-induced IFN-gamma secretion in LPS-activated peritoneal macrophages. Mol Nutr Food Res. 2010;54:1351–1360. doi: 10.1002/mnfr.200900318. [DOI] [PubMed] [Google Scholar]
- 65.Zhao L. Zhang SL. Tao JY, et al. Preliminary exploration on anti-inflammatory mechanism of Corilagin (beta-1-O-galloyl-3,6-(R)-hexahydroxydiphenoyl-D-glucose) in vitro. Int Immunopharmacol. 2008;8:1059–1064. doi: 10.1016/j.intimp.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 66.Zhao Z. Shin HS. Satsu H. Totsuka M. Shimizu M. 5-Caffeoylquinic acid and caffeic acid down-regulate the oxidative stress- and TNF-alpha-induced secretion of interleukin-8 from Caco-2 cells. J Agric Food Chem. 2008;56:3863–3868. doi: 10.1021/jf073168d. [DOI] [PubMed] [Google Scholar]
- 67.Chao CY. Mong MC. Chan KC. Yin MC. Anti-glycative and anti-inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol Nutr Food Res. 2010;54:388–395. doi: 10.1002/mnfr.200900087. [DOI] [PubMed] [Google Scholar]
- 68.Chao PC. Hsu CC. Yin MC. Anti-inflammatory and anti-coagulatory activities of caffeic acid and ellagic acid in cardiac tissue of diabetic mice. Nutr Metab (Lond) 2009;6:33. doi: 10.1186/1743-7075-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anauate MC. Torres LM. de Mello SB. Effect of isolated fractions of Harpagophytum procumbens D.C. (devil's claw) on COX-1, COX-2 activity and nitric oxide production on whole-blood assay. Phytother Res. 2010;24:1365–1369. doi: 10.1002/ptr.3124. [DOI] [PubMed] [Google Scholar]
- 70.Karonen M. Hamalainen M. Nieminen R, et al. Phenolic extractives from the bark of Pinus sylvestris L. and their effects on inflammatory mediators nitric oxide and prostaglandin E2. J Agric Food Chem. 2004;52:7532–7540. doi: 10.1021/jf048948q. [DOI] [PubMed] [Google Scholar]
- 71.Choi YH. Yan GH. Ellagic Acid attenuates immunoglobulin E-mediated allergic response in mast cells. Biol Pharm Bull. 2009;32:1118–1121. doi: 10.1248/bpb.32.1118. [DOI] [PubMed] [Google Scholar]
- 72.Litjens NH. Rademaker M. Ravensbergen B, et al. Monomethylfumarate affects polarization of monocyte-derived dendritic cells resulting in down-regulated Th1 lymphocyte responses. Eur J Immunol. 2004;34:565–575. doi: 10.1002/eji.200324174. [DOI] [PubMed] [Google Scholar]
- 73.Schilling S. Goelz S. Linker R. Luehder F. Gold R. Fumaric acid esters are effective in chronic experimental autoimmune encephalomyelitis and suppress macrophage infiltration. Clin Exp Immunol. 2006;145:101–107. doi: 10.1111/j.1365-2249.2006.03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ikeda Y. Murakami A. Ohigashi H. Ursolic acid promotes the release of macrophage migration inhibitory factor via ERK2 activation in resting mouse macrophages. Biochem Pharmacol. 2005;70:1497–1505. doi: 10.1016/j.bcp.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 75.Spelman K. Aldag R. Hamman A, et al. Traditional herbal remedies that influence cell adhesion molecule activity. Phytother Res. 2011;25:473–483. doi: 10.1002/ptr.3350. [DOI] [PubMed] [Google Scholar]
- 76.Picerno P. Autore G. Marzocco S. Meloni M. Sanogo R. Aquino RP. Anti-inflammatory activity of verminoside from Kigelia africana and evaluation of cutaneous irritation in cell cultures and reconstituted human epidermis. J Nat Prod. 2005;68:1610–1614. doi: 10.1021/np058046z. [DOI] [PubMed] [Google Scholar]
- 77.Kim SR. Koo KA. Sung SH. Ma CJ. Yoon JS. Kim YC. Iridoids from Scrophularia buergeriana attenuate glutamate-induced neurotoxicity in rat cortical cultures. J Neurosci Res. 2003;74:948–955. doi: 10.1002/jnr.10828. [DOI] [PubMed] [Google Scholar]
- 78.Lee S. Park HS. Notsu Y, et al. Effects of hyperin, isoquercitrin and quercetin on lipopolysaccharide-induced nitrite production in rat peritoneal macrophages. Phytother Res. 2008;22:1552–1556. doi: 10.1002/ptr.2529. [DOI] [PubMed] [Google Scholar]
- 79.Mosser DM. Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peters T. Sindrilaru A. Hinz B, et al. Wound-healing defect of CD18(-/-) mice due to a decrease in TGF-beta1 and myofibroblast differentiation. EMBO J. 2005;24:3400–3410. doi: 10.1038/sj.emboj.7600809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.von der Weid PY. Muthuchamy M. Regulatory mechanisms in lymphatic vessel contraction under normal and inflammatory conditions. Pathophysiology. 2010;17:263–276. doi: 10.1016/j.pathophys.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 82.Agyare C. Lechtenberg M. Deters A. Petereit F. Hensel A. Ellagitannins from Phyllanthus muellerianus (Kuntze) Exell: Geraniin and furosin stimulate cellular activity, differentiation and collagen synthesis of human skin keratinocytes and dermal fibroblasts. Phytomedicine. 2011;18:617–624. doi: 10.1016/j.phymed.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 83.Cai Y. Chen T. Xu Q. Astilbin suppresses collagen-induced arthritis via the dysfunction of lymphocytes. Inflamm Res. 2003;52:334–340. doi: 10.1007/s00011-003-1179-3. [DOI] [PubMed] [Google Scholar]
- 84.Dai JP. Chen J. Bei YF. Han BX. Wang S. Influence of borneol on primary mice oral fibroblasts: A penetration enhancer may be used in oral submucous fibrosis. J Oral Pathol Med. 2009;38:276–281. doi: 10.1111/j.1600-0714.2008.00738.x. [DOI] [PubMed] [Google Scholar]
- 85.Mai LM. Lin CY. Chen CY. Tsai YC. Synergistic effect of bismuth subgallate and borneol, the major components of Sulbogin, on the healing of skin wound. Biomaterials. 2003;24:3005–3012. doi: 10.1016/s0142-9612(03)00126-1. [DOI] [PubMed] [Google Scholar]
- 86.Midwood KS. Williams LV. Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol. 2004;36:1031–1037. doi: 10.1016/j.biocel.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 87.Murado MA. Vazquez JA. The notion of hormesis and the dose-response theory: A unified approach. J Theor Biol. 2007;244:489–499. doi: 10.1016/j.jtbi.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 88.Calabrese EJ. Baldwin LA. Hormesis: U-shaped dose responses and their centrality in toxicology. Trends Pharmacol Sci. 2001;22:285–291. doi: 10.1016/s0165-6147(00)01719-3. [DOI] [PubMed] [Google Scholar]
- 89.Calabrese EJ. Baldwin LA. The frequency of U-shaped dose responses in the toxicological literature. Toxicol Sci. 2001;62:330–338. doi: 10.1093/toxsci/62.2.330. [DOI] [PubMed] [Google Scholar]
- 90.Zhang X. Kan Q. Fu Y. Liu S. Dai Z. Dong Y. Noradrenergic activity regulated dexamethasone-induced increase of 5-HT(3) receptor-mediated glutamate release in the rat's prelimbic cortex. Biochim Biophys Acta. 2012;1823:2157–2167. doi: 10.1016/j.bbamcr.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 91.Baldi E. Bucherelli C. The inverted “u-shaped” dose-effect relationships in learning and memory: Modulation of arousal and consolidation. Nonlinearity Biol Toxicol Med. 2005;3:9–21. doi: 10.2201/nonlin.003.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vandenberg LN. Colborn T. Hayes TB, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kendig EL. Le HH. Belcher SM. Defining hormesis: Evaluation of a complex concentration response phenomenon. Int J Toxicol. 2010;29:235–246. doi: 10.1177/1091581810363012. [DOI] [PubMed] [Google Scholar]
- 94.Calabrese E. Iavicoli I. Calabrese V. Hormesis: Its impact on medicine and health. Hum Exp Toxicol. 2013;32:120–152. doi: 10.1177/0960327112455069. [DOI] [PubMed] [Google Scholar]
- 95.Mushak P. How prevalent is chemical hormesis in the natural and experimental worlds? Sci Total Environ. 2013;443:573–581. doi: 10.1016/j.scitotenv.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 96.Thirugnanasambandam N. Grundey J. Paulus W. Nitsche MA. Dose-dependent nonlinear effect of L-DOPA on paired associative stimulation-induced neuroplasticity in humans. J Neurosci. 2011;31:5294–5299. doi: 10.1523/JNEUROSCI.6258-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Horiguchi M. Hannaway KE. Adelekun AE. Huang M. Jayathilake K. Meltzer HY. D(1) receptor agonists reverse the subchronic phencyclidine (PCP)-induced novel object recognition (NOR) deficit in female rats. Behav Brain Res. 2013;238:36–43. doi: 10.1016/j.bbr.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 98.Serafini P. Carbley R. Noonan KA. Tan G. Bronte V. Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]