Abstract
The gastroenterologist, whether in academic or clinical practice, must face the reality that an increasingly large percentage of adult patients are morbidly obese. Morbid obesity is associated with significant morbidity and mortality including enhanced morbidity from cardiovascular, cerebrovascular, hepatobiliary and colonic diseases. Most of these associated diseases are actually preventable. Based on the 1991 NIH consensus conference criteria, for most patients with a body mass index (BMI=weight in kilograms divided by the height in meters squared) of 40 or more, or for patients with a BMI of 35 or more and significant health complications, surgery may be the only reliable option. Currently in the United States, over 250,000 bariatric surgical procedures are being performed annually. The practicing gastroenterologist in every community, large and small, must be familiar with the various surgical procedures together with their associated anatomic changes. These changes may dramatically increase the prevalence of nutritional deficiencies and profoundly alter the clinical and endoscopic approaches to diagnosis and management.
INTRODUCTION
Over 100 million adult Americans are currently overweight or obese. It has been widely recognized by physicians, insurance adjusters, nutritionists and the public at large that these patients are at increased risks for hypertension, type II diabetes, coronary artery disease, gallbladder disease, certain cancers (colon, ovarian, breast), dyslipidemia, cerebral vascular disease, osteoarthritis and sleep apnea. The pandemic in the United States and indeed around the globe has spread dramatically.1, 2, 3, 4, 5 In 1988, for example, there were only 19 states with an adult obesity prevalence of 10–14%. In 1994, however, there were 16 states with a prevalence of adult obesity of 15–19% and 34 states with 10–14% prevalence of obesity. In the year 2000, there were 22 states in which the prevalence of obesity was≥20%, 27 states with 15–19% obesity and only 1 state with a prevalence of 10–14% obesity. In the second decade of the new millennium, the reality for the gastroenterologist is that a large and increasing percentage of patients are obese, a disease that is associated with significant morbidity and mortality, and most of these associated deaths are actually preventable. The problem for all of us is the extraordinary healthcare costs. There are estimated between 250 and 300 billion dollars annually spent on conditions directly related to obesity.
Obesity is by definition an excess storage of fat. The medical definition of morbid obesity is a patient whose weight is ≥twice ideal body weight and/or 100 pounds or more overweight. Body mass index (BMI) is commonly used to describe the status of weight. BMI is calculated as weight in kilograms divided by the height in meters squared (m2). An overweight individual has a BMI between 25 and 29.9 kg/m2. Obesity is defined as a BMI≥30 kg/m2. The prevalence of hypertension is virtually linearly related to the BMI as is diabetes, lumbosacral spine disease, chronic cholecystitis and an excess use of five or more healthcare specialists. Coronary artery disease is especially prevalent among obese men, with a prevalence of 44% in men over the age of 50 years with a BMI of 30 kg/m2 or more.
MEDICAL OPTIONS
The average overall efficacy of an unsupervised rigorous weight-loss program is about 20 kg/year. Other options typically used by patients outside of medical supervision include support groups, behavior modification, specialized structured diets, and non-medically supervised very-low calorie diets. Commercial options cost consumers billions of dollars annually and do produce definite though transiently positive results. Several unorthodox methods including bulimia, purging, starvation diets or even jaw wiring are considered hazardous to a patient's health. Additionally, prescription pharmacological agents are available; however some of these, including thyroid derivatives and amphetamines, are not licensed for bariatric use and should not be used.
In general, drugs that can treat obesity work through three different mechanisms: appetite suppression, increased metabolism of dietary nutrients, and interference with the routine absorption of ingested nutrients.6, 7, 8, 9, 10, 11, 12 Multiple single-agent drugs are in later stages of development, including Contrave, Empatic, Qnexa, and an injectable combination of leptin and pranlintide. Qnexa has recently been approved by the FDA as an adjunct to dietary control in patients with a BMI 30 kg/m2. This is a combination of two drugs including phentermine and topiramate.
An application was recently rejected by the FDA for a novel drug used outside the United States called Rimonabant.12 It is a selective cannabinoid receptor-1 blocker, and its major side effect is vacillating mood disorders. Unfortunately, it has only a limited efficacy with a 4–5 kg additional weight loss over that achieved with a 1,500 calorie/day diet alone.
Orlistat, currently the only approved medication for obesity, is a pancreatic lipase inhibitor that costs about $200 per month. Given its pharmacological mechanism of action, untoward side effects of steatorrhea, diarrhea, cramping and flatulence are common. The overall efficacy is marginal however. Patients only lose an additional 3–4 kg/year with Orlistat over that achieved by a 1,500 calorie/day diet alone.
Sibutramine, recently discontinued by order of the FDA is a monoamine reuptake inhibitor. It cost about $120 a month and had the side effects of tachycardia, hypertension, seizures, cholelithiasis, depression and even suicide ideation. Its efficacy was demonstrated to be limited also. It's estimated that 4–5 kg of extra weight loss could occur over a year with sibutramine in addition to a 1,500 calorie/day diet.
NOVEL INTERVENTIONAL THERAPIES
There are many novel therapies that are being investigated around the world.13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 The most promising, currently licensed for use in much of Europe and Latin America, is the BioEnteric Intragastric Balloon, also called the BIB system. It is licensed overseas for short-term use (6 months) and it consists of an intragastric balloon that is placed and removed endoscopically, similar to the Garren balloon used decades ago. Many investigations are being conducted in the use of this and other endoscopically-placed gastric restriction and volume-reduction devices, including those that create gastric partitions and restrictions.
SURGICAL OPTIONS—WHY SURGERY?
It has been well documented by extensive studies that despite impressive weight loss with medical or pharmacological programs, diet or exercise, many if not most patients with clinically severe or morbid obesity are refractory in the long run to these conservative treatments. On the basis of the 1991 NIH consensus conference criteria, for most patients with a BMI of 40 or more, or for patients with a BMI of 35 or more and significant health complications, surgery may be an option if they have failed to lose weight with more conservative approaches. The practicing gastroenterologist in every community, large and small, must know the current surgical options and their different benefits and potential problems. Since most non-surgical programs fail in the long term, patients are becoming increasingly interested in surgery as more effective and durable. Patients know these options often by information accessible on the internet, and clinicians must be vigilant in keeping up to date with these options. Bariatric surgery introduces several unique issues to the practicing internist and gastroenterologist, and knowledge of these must be gained to adequately address the increasing proportion of the patient population who have had bariatric surgery.
SURGICAL OPTIONS FOR MORBID OBESITY—MULTIDISCIPLINARY APPROACH NECESSARY
It is quite clear from past experience that patients should be carefully screened in a multidisciplinary manner before being considered acceptable candidates for surgery for morbid obesity. Unfortunately, limited screening may be undertaken by the patient who seeks surgery at sites remote from the community physicians who will be ultimately caring for the patient. The disciplines that routinely should be involved in providing these patients with a preoperative evaluation include gastroenterology, anesthesia, cardiology, pulmonology, psychiatry, endocrinology and clinical nutrition. In many instances, however, few if any of these specialties have interacted with the individual patient before being considered for bariatric surgery.
The surgical options that are currently available include those that are a) predominantly malabsorptive, those that are b) primarily restrictive, and c) those that have a combination of malabsorptive and restrictive components.
The surgical options (Figures 1, 2, 3) are as follows:
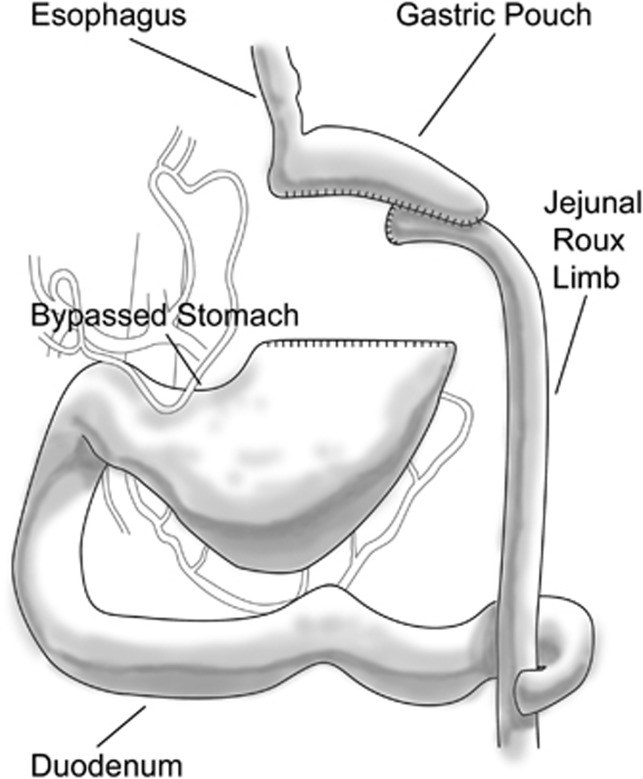
Figure 1.
The Roux-en-Y gastric bypass. A small gastric pouch is created from the proximal stomach with the remnant stomach remaining in continuity with the duodenum. A ‘Roux' limb of jejunum beyond the ligament of Treitz is connected to the gastric pouch and a jejunojeunostomy formed 100–150 cm distal to the gastrojejunostomy.
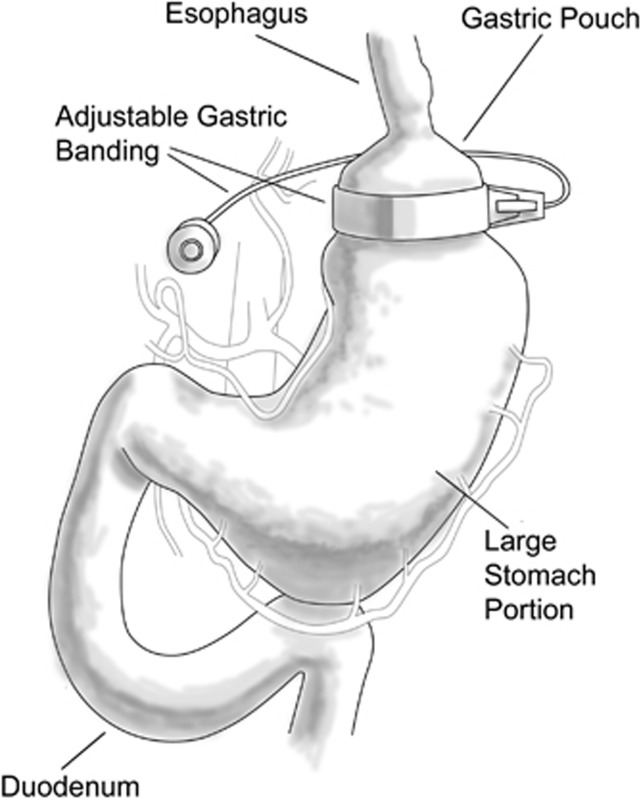
Figure 2.
Gastric band. An inflatable band is placed around the proximal stomach with a port for fluid instillation in the abdominal wall. The band may be tightened or loosened by either adding of withdrawing liquid from the port. The device constricts the proximal stomach ‘restricting' the amount of food that can be consumed at any one time.
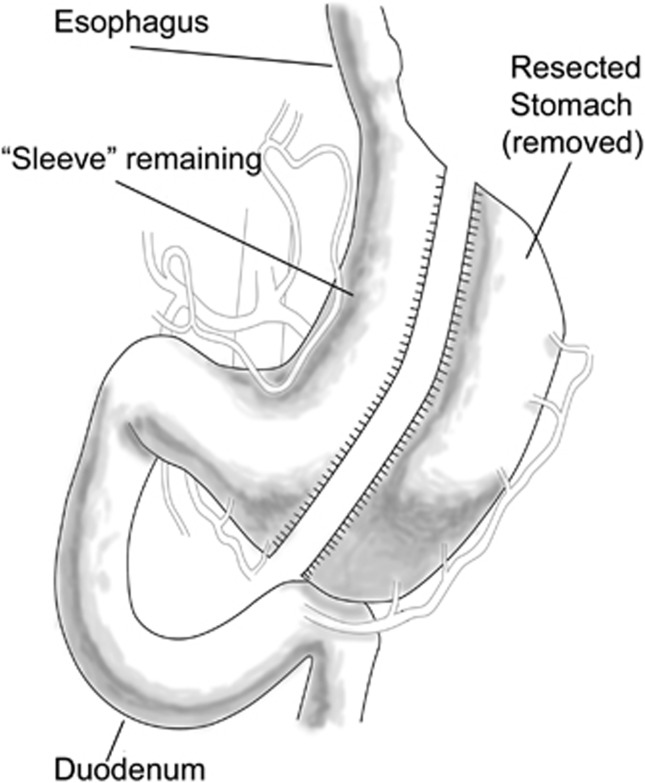
Figure 3.
Sleeve gastrectomy. A long linear staple line is created from the proximal stomach to the antrum leaving a linear tube conduit from gastroesophageal junction to pylorus. The disconnected stomach is removed leaving a small lumen behind restricting the gastric capacity.
Malabsorptive surgical procedures
These malabsorptive procedures were prototyped over 40 years ago by the jejuno-ileal (JI) bypass, which was abandoned over a decade ago.13 The operative technique for this procedure consisted of connecting a short length of proximal jejunum (∼4–14 inches beyond the ligament of Treitz) to the distal ileum ∼4–12 inches proximal to the ileocecal valve. The weight loss mechanism created by this procedure was primarily malabsorption, as the majority of the absorptive surface of the small intestine was bypassed. Unfortunately, multiple complications were associated with this procedure, including most seriously hepatic steatonecrosis (which occurred with the prevalence of up to 30%) and hepatic cirrhosis (with a prevalence of ∼7%). Nephrolithiasis with oxalate stones occurs in ∼21% of the patients following the JI bypass. This procedure has been largely abandoned and many patients have had the procedure reversed.
The modern equivalent of the JI bypass is the biliopancreatic diversion in which a small gastric pouch is connected to an alimentary limb of jejunum. The biliopancreatic limb of small bowel from the proximal duodenum to about the distal jejunum carries only bile and pancreatic secretions but no chyme, while the alimentary limb has only food substances but no pancreatic or biliary secretion. There is a very short common limb of distal ileum. This is a very effective procedure but produces profound malabsorption. The duodenal switch procedure is quite similar to biliopancreatic diversion, however the gastric pouch is created by a sleeve gastrectomy.
Restrictive surgical procedures
The first of the restrictive bariatric procedures was the vertical-banded gastroplasty in which a small gastric pouch was created by a double linear row of staples in the cardia. The outlet from this small pouch is constricted by a band. A current variation of the banded gastroplasty is the sleeve gastrectomy, wherein the entire greater curvature of the stomach from fundus to antrum is resected17, 18, 19, 20 (Figure 3). The gastric tube created is merely 3–4 cms in diameter from cardia to antrum.
A new putative replacement for the sleeve gastrectomy or vertical-banded gastroplasty is the very popular Lap-Band21, 22, 23, 24 (Figure 2). Food passes in the Lap-Banded stomach through the outlet from the upper gastric pouch to the lower portion of the stomach much more slowly because of this circumferential water-filled collar. The patient feels full for a longer period of time. The major advantage of the Lap-Band is its limited invasive approach, as most of these are placed laparoscopically. There is no stomach or bowel stapling or division, or intestinal routing. The band is adjustable; it is reversible and appears to have the lowest operative complication rate of all bariatric procedures, as well as the lowest associated mortality and a very low risk of malnutrition. The disadvantage is that patients are required to have regular outpatient clinic follow-up visits to provide band adjustments for optimal weight-loss results. The gastric band has slower initial weight loss than the gastric bypass.25, 26, 27
Combined restrictive and malabsorptive surgical procedures
The most popular, and in fact, the current gold standard for bariatric surgery is the Roux-en-Y gastric bypass28, 29, 30, 31, 32, 33, 34 (Figure 1). These procedures are done in almost all cases laparoscopically and they have combined restrictive and malabsorptive components. A small gastric pouch with a 30-ml capacity is anastomosed to a loop of jejunum transected distal to the ligament of Treitz. The first of these procedures was performed by Mason in 1966 and the first laparoscopic gastric bypass by Wittgrove in 1994. There is however a large bypassed portion of the stomach and the entire duodenum.
Considerations for the gastroenterologist
It is important for the clinical gastroenterologist to recognize that even with the most popular of the surgical bariatric procedures, namely the Roux-en-Y gastric bypass, and indeed for all procedures with a malabsorptive component, there are profound nutritional alterations and potential serious complications that can occur in both an early and late post-operative setting.35, 36, 37, 38 Remember that the gastric capacity is reduced from nearly 2 l to 30 ml. The gastric stoma leads directly into the mid jejunum in the Roux-en-Y configuration. It is important to understand that the gastrojejunostomy is an end-to-side anastomosis, with a very small afferent limb of jejunum, so when an endoscope is passed through the stoma into the efferent jejunum there will often be a small blind afferent limb that had been closed off proximally by a surgical staple line. Roux-en-Y gastric bypass patients will also have a second anastomosis, namely a jejunojejunostomy, ∼60–80 cm distal to the gastrojejunostomy. It may not be possible to reach the jejunojejunostomy using a standard endoscope, and to get to this level one may in fact require the use of an enteroscope or a sterilized pediatric colonoscope. Also, the gastroenterologist must be mindful of the fact that virtually the entire remnant stomach, duodenum, pancreatic and biliary systems are remote to and virtually impossible to approach by standard endoscopy. There have only been a few isolated case reports of clinicians entering the bypassed stomach, duodenum and proximal jejunum via retrograde passage of an endoscope through the jejunojejunostomy.
The gastroenterologist (indeed all physicians caring for patients following bariatric surgery) must recognize that there are potential profound consequences of bariatric procedures that bypass the duodenum and/or shorten the absorptive surface of the small bowel.35, 38 This is most important with the essential vitamins and minerals.39, 40 The most serious potential problems are definitely associated with malabsorption of the fat-soluble vitamins: A, D, E and K, as well as iron and calcium. There are also instances of thiamine, folate, vitamin B12, riboflavin, copper and zinc deficiency. Patients therefore must always remain on a liquid or chewable multi-vitamin diet and must also have their nutritional status assessed and monitored regularly, including specific laboratory testing every 3–4 months while losing weight, and at least annually thereafter. Given the potential for profound malabsorption of multiple vitamins and minerals, the gastroenterologist must always be alert to the unique signs and symptoms of these deficiencies. When in doubt, test and/or seek consultation.
Endoscopic intervention in patients undergoing gastric surgery for morbid obesity
The most common of the post-operative complications that can occur in patients undergoing bariatric surgery include anastomotic strictures, anastomotic bleeding, dumping syndrome and vitamin malabsorption.41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56 Strictures at the gastrojejunal anastomosis are not uncommon, as by definition the stoma is designed to be relatively small to provide restriction of food intake.41, 43, 49 For the first month or so, patients may have surgical edema and the anastomosis will expand once the edema resolves; only if a patient cannot tolerate liquids should dilation under these circumstances occur. In general, stomal dilation should only occur after consulting the attending surgeon. As the gastrojejunal anastomosis is eccentric in most circumstances when created by hand sewing or with the use of an end-to-end stapling device, great care must be taken when attempting to pass a critically narrowed anastomosis. The technique for safely performing endoscopic balloon dilation of an anastomotic stricture is as follows. Indeed it is preferable to first pass any endoscope, even if pediatric sized, under direct vision into the efferent limb. The balloon dilation catheter should be advanced directly under direct visualization into the more distal jejunum, after which it is safe to withdraw both the endoscope and balloon together, positioning the balloon across the anastomosis so that dilation can occur with care using manufacturer-recommended pressures of the balloon under direct observation. Surprisingly, most patients with an anastomotic stricture require only one, or infrequently two, dilations. Again, it is worth emphasizing the importance of consulting with a bariatric surgeon when proposing stricture dilation of a bariatric anastomotic stricture to understand the size of the anastomosis, the maximum dilation desired, and balloon size to be used.
Anastomotic bleeding may occur post-operatively.46, 47, 48 It is important to remember that there are multiple staple lines in the traditional Roux-en-Y gastric bypass, and usually two anastomoses, which can be created either using a stapling device or with suture. The gastrojejunostomy is visible to the endoscopist typically without difficulty, and as mentioned, the jejunojejunostomy is also accessible endoscopically, perhaps necessitating an enteroscope or pediatric colonoscope. The gastric remnant, entire duodenum, pancreatic duct and biliary tree are, of course, not available to gastroenterologist unless extraordinary maneuvers are undertaken. Bleeding in a post-operative bariatric surgery patient must always be assessed initially by the bariatric surgeon. Once intraperitoneal bleeding has been ruled out, it may be necessary to perform upper endoscopy to identify the site of bleeding and provide hemostatic treatment. For endoscopic treatment of post-operative bleeding from either the gastrojejunal or jejunojejunal anastomotis, and/or any other gastrointestinal source (i.e., ulceration) that may be readily seen using standard endoscopy, techniques including injection, argon plasma coagulation, heater probe, bipolar electro coagulation, or clipping are acceptable. Once again, the gastroenterologist must be aware of the surgical anatomy and consultation with the attending surgeon is essential to provide optimal patient care.
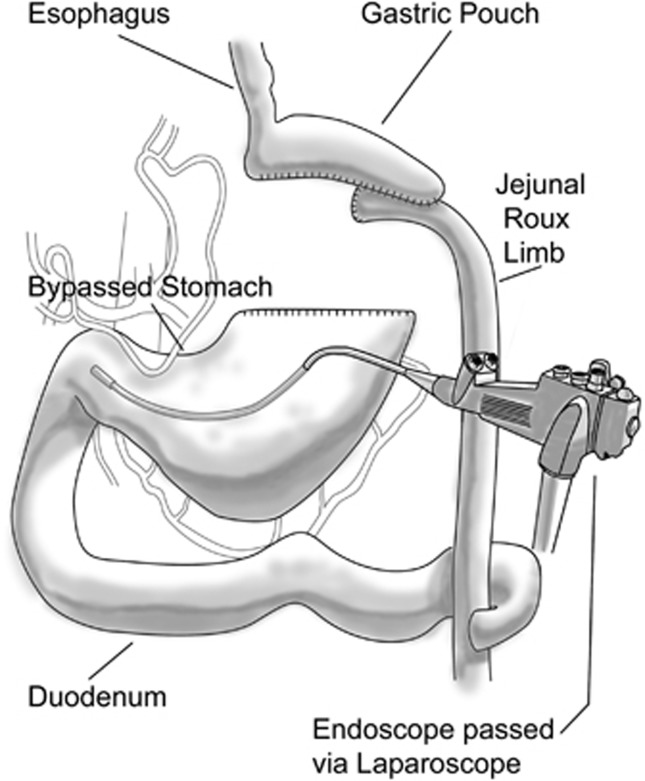
To evaluate the remnant stomach in a patient with suspected bleeding, techniques such as nuclear scanning, CT scanning or angiography may be used. Access to the remnant stomach is possible using intraoperative direct access, either via laparoscopic or open technique, with direct insertion of the endoscope through the abdominal wall and the gastric wall, secured with a purse-string suture placed by the surgeon to allow insufflation of the gastric lumen and minimize insufflation leakage around the endoscope. This novel technique may be utilized in patients with no evidence of blood loss from either the upper, lower or mid-bowel based upon standard imaging, and allows visualization of the remnant stomach, duodenum and proximal jejunum.50, 51 Intraoperative direct gastric remnant endoscopy, via laparoscopy or open surgery, allows reliable access to these areas of the proximal bowel not accessable using routine endoscopy (Figure 4). In this technique, a laparoscope is placed in the abdomen and the remnant stomach cannulated directly by trocar. The obturator is removed and the endoscope (previously sterilized) is passed in the operative field directly into the remnant stomach. The view and approach to the remnant stomach, pylorus and duodenum up to the jejunojejunostomy is relatively normal. This will allow for visualization, clearing and if necessary coagulation or clipping hemostatic procedures for any visible likely source of hemorrhage. This approach is to be recommended over heroic efforts to gain access to the duodenum and afferent limb by endoscopic passage through the jejunojejunostomy. In the latter procedure, considerable effort must be made to telescope the bowel unto the advancing instrument preferably a pediatric colonoscope.
Figure 4.
Translaparoscopic endoscopy. To visualize the remnant stomach and duodenum (following a gastric bypass), an endoscope is introduced by the surgeon through a laparoscopic trochar placed directly in to the stomach. Endoscopic examination of the gastric remnant and duodenum is thereby possible at the time of laparoscopy.
Dumping Syndrome is common initially in patients undergoing the Roux-en-Y gastric bypass.52, 53, 54, 55, 56 This can usually be treated by the careful selection of diet including avoidance of high-carbohydrate liquid diets as well as separation of solids and liquids in a meal. As stated above, vitamin deficiency can usually be avoided by the life-long maintenance of liquid or chewable multivitamins.
Given the restriction and small capacity of the gastric pouch, internists and gastroenterologist must be mindful of the fact that prolonged retention of food and drugs in the pouch is part of the restriction. One must particularly avoid pharmaceutical agents that have a propensity for ulceration including tetracycline, potassium chloride, aspirin or non-steroidal anti-inflammatory drugs. Once again, preference should be given to the administration of non-ulcerating chewable or liquid pharmaceutical agents.
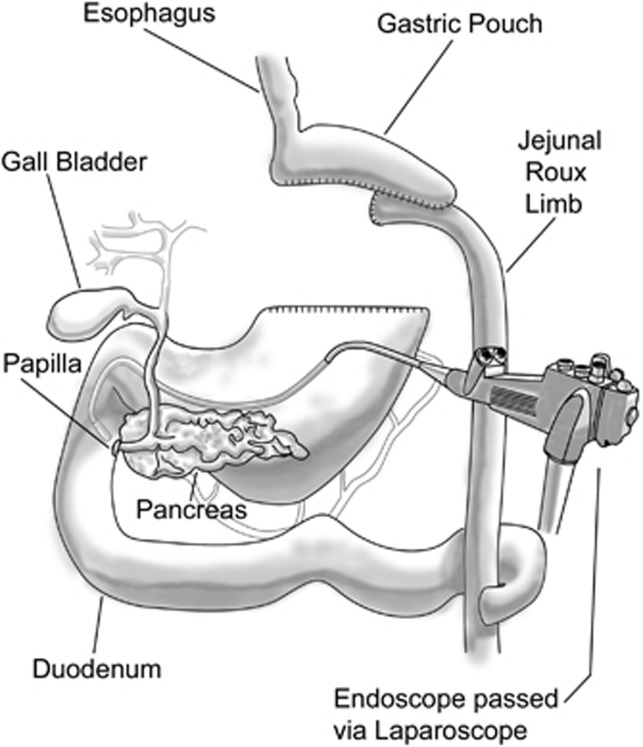
A unique problem occurs in evaluating and treating patients with gallstone-induced cholangitis, pancreatitis or biliary obstruction from strictures or tumors.57, 58, 59, 60, 61, 62, 63 Once again the optimal approach is via translaparoscopic gastroscopic passage through an operatively place trocar directly into the remnant stomach.57, 61 In this instance, a sterilized duodenoscope is passed under observation in the operative field directly through the trocar and rapidly through the pylorus into the second portion of the duodenum (Figure 5). If necessary, the patient can be placed transiently in a decubitus position although with minor modification a supine position on the operative table is satisfactory. The views of the periampullary area and the major and minor papillae are quite normal and the approach via endoscopic retrograde cholangiopancreatography (ERCP) should be considered identical to that encountered in standard ERCP under moderate sedation or general anesthesia. In this instance however, special attention must be made to having routine fluoroscopic imagery together with all necessary ERCP supply items and adequate personnel to assist in the often delicate maneuvering necessary to gain access to the bile duct and pancreatic duct.
Figure 5.
Translaparoscopic endoscopic retrograde cholangiopancreatography (ERCP). As in Figure 4, the appropriate endoscope (this time a side-viewing ERCP endoscope) is introduced translaparoscopically directly into the remnant stomach. The ERCP endoscope is positioned at the papilla for cannulation of the pancreatic and bile ducts.
In a few instances, others have gained access to the duodenal sweep via a retrograde passage through the mouth and jejunojejunostomy.63 If necessary this route can be facilitated by advancing a transhepatic guide wire to the jejunojejunostomy, which is then grabbed by the duodenoscope and the instrument assisted in advancement in a retrograde fashion to the papilla. Once again, routine ERCP techniques can be applied, however in this instance just as in the case of retrograde passage through a standard Bilroth II anastomosis, proper reverse sphincterotomes should be available, as the view through the duodenoscope is 180 degrees opposite that routinely encountered through standard ERCP techniques. Though this latter technique, i.e., passing a duodenoscope retrograde through the duodenum to the papilla may have the advantage of avoiding a translaparoscopic approach, a standard approach through the pylorus into the duodenum at laparoscopy renders the visualization of the papilla completely normal and avoids the necessity for non-standard techniques.
SUMMARY AND CONCLUSION
Bariatric surgery is now extremely common in the United States and indeed in much of the northern hemisphere. Profound changes in the anatomy and physiology do occur that are responsible to the dramatic loss of weight desired by patients. The clinician must be available that during the early and late post-operative period there can be serious consequences by virtue of the altered anatomy and physiology for nutrient absorption.
The authors declare no conflict of interest.
References
- Cheung WW, Mao P. Recent advances in obesity: genetics and beyond. Endocrinology. 2012;2247 doi: 10.5402/2012/536905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samper-Ternent R, Al Snih S. Obesity in older adults: epidemiology and implications for disability and disease. Rev Clin Gerontol. 2012;22:10–34. doi: 10.1017/s0959259811000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea J, Diamandis EP, Sharma AM, et al. The obesity epidemic. Clin Chem. 2012;58:968–973. doi: 10.1373/clinchem.2011.180976. [DOI] [PubMed] [Google Scholar]
- Noël PH, Copeland LA, Pugh MJ, et al. Obesity diagnosis and care practices in the Veterans Health Administration. J Gen Intern Med. 2010;25:510–516. doi: 10.1007/s11606-010-1279-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockman J, Steelman J. Diagnoses of overweight children and adolescents seen in a pediatric endocrinology clinic. J Pediatr Endocrinol Metab. 2008;21:221–224. doi: 10.1515/jpem.2008.21.3.221. [DOI] [PubMed] [Google Scholar]
- Chugh PK, Sharma S. Recent advances in the pathophysiology and pharmacological treatment of obesity. J Clin Pharm Ther. 2012;10:1365–2710. doi: 10.1111/j.1365-2710.2012.01347.x. [DOI] [PubMed] [Google Scholar]
- Schamroth CL. The perils of pharmacological treatment for obesity: a case of sibutramine-associated cardiomyopathy and malignant arrhythmias. Cardiovasc J Afr. 2012;12:23. doi: 10.5830/CVJA-2011-003. [DOI] [PubMed] [Google Scholar]
- Derosa G, Maffioli P. Anti-obesity drugs: a review about their effects and their safety. Expert Opin Drug Saf. 2012;11:459–471. doi: 10.1517/14740338.2012.675326. [DOI] [PubMed] [Google Scholar]
- Burch J, McKenna C, Palmer S, et al. Rimonabant for the treatment of overweight and obese people. Health Technol Assess. 2009;13 (Suppl 3:13–22. doi: 10.3310/hta13suppl3/03. [DOI] [PubMed] [Google Scholar]
- Li M, Cheung BM. Pharmacotherapy for obesity. Br J Clin Pharmacol. 2009;68:804–810. doi: 10.1111/j.1365-2125.2009.03453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florentin M, Kostapanos MS, Nakou ES, et al. Efficacy and safety of ezetimibe plus orlistat or rimonabant in statin-intolerant nondiabetic overweight/obese patients with dyslipidemia. J Cardiovasc Pharmacol Ther. 2009;14:274–282. doi: 10.1177/1074248409343935. [DOI] [PubMed] [Google Scholar]
- Johansson K, Neovius K, DeSantis SM, et al. Discontinuation due to adverse events in randomized trials of orlistat, sibutramine and rimonabant: a meta-analysis. Obes Rev. 2009;10:564–575. doi: 10.1111/j.1467-789X.2009.00581.x. [DOI] [PubMed] [Google Scholar]
- Buchwald H, Buchwald JN. Evolution of operative procedures for the management of morbid obesity 1950-2000. Obes Surg. 2002;12:705–717. doi: 10.1381/096089202321019747. [DOI] [PubMed] [Google Scholar]
- Kendrick ML, Dakin GF. Surgical approaches to obesity. Mayo Clin Proc. 2006;81:18–24. doi: 10.1016/s0025-6196(11)61177-4. [DOI] [PubMed] [Google Scholar]
- Buchwald H, Williams SE. Bariatric surgery worldwide 2003. Obes Surg. 2004;14:1157–1164. doi: 10.1381/0960892042387057. [DOI] [PubMed] [Google Scholar]
- Jones KB., Jr Bariatric surgery--where do we go from here. Int Surg. 2004;89:51–57. [PubMed] [Google Scholar]
- Victorzon M. An update on sleeve gastrectomy. Minerva Chir. 2012;67:153–164. [PubMed] [Google Scholar]
- Helmiö M, Victorzon M, Ovaska J, et al. SLEEVEPASS: A randomized prospective multicenter study comparing laparoscopic sleeve gastrectomy and gastric bypass in the treatment of morbid obesity: preliminary results. Surg Endosc. 2012;26:2521–2526. doi: 10.1007/s00464-012-2225-4. [DOI] [PubMed] [Google Scholar]
- Lemanu DP, Srinivasa S, Singh PP, et al. Single-stage laparoscopic sleeve gastrectomy: Safety and efficacy in the super-obese. J Surg Res. 2012;177:49–54. doi: 10.1016/j.jss.2012.01.011. [DOI] [PubMed] [Google Scholar]
- ASMBS Clinical Issues Committee Updated position statement on sleeve gastrectomy as a bariatric procedure. Surg Obes Relat Dis. 2012;8:e21–e26. doi: 10.1016/j.soard.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Arthurs S, Abrahamian Y, Loughren EL, et al. New technology review process: the laparoscopic adjustable gastric band. Perm J. 2011;15:54–60. doi: 10.7812/tpp/11-095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cywes R, Bhoyrul S, Billy H, APEX Study Group et al. Interim results at 48 weeks of LAP-BAND AP experience (APEX) study: prospective, multicenter, open-label longitudinal patient observational study. Surg Obes Relat Dis. 2011;8:741–746. doi: 10.1016/j.soard.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Ponce J, Lindsey B, Pritchett S, et al. New adjustable gastric bands available in the United States: a comparative study. Surg Obes Relat Dis. 2011;7:74–79. doi: 10.1016/j.soard.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Ayloo S, Bueno R. Band erosion: laparoscopic removal of lap-band. Surg Endosc. 2009;23:657–658. doi: 10.1007/s00464-008-0194-4. [DOI] [PubMed] [Google Scholar]
- Cunneen SA. Review of meta-analytic comparisons of bariatric surgery with a focus on laparoscopic adjustable gastric banding. Surg Obes Relat Dis. 2008;4:S47–S55. doi: 10.1016/j.soard.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Busetto L, Angrisani L, Basso N, et al. Italian Group for Lap-Band. Safety and efficacy of laparoscopic adjustable gastric banding in the elderly. Obesity (Silver Spring) 2008;16:334–338. doi: 10.1038/oby.2007.85. [DOI] [PubMed] [Google Scholar]
- Chakravarty PD, McLaughlin E, Whittaker D, et al. Comparison of laparoscopic adjustable gastric banding (LAGB) with other bariatric procedures; a systematic review of the randomised controlled trials. Surgeon. 2012;10:172–182. doi: 10.1016/j.surge.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Ramón JM, Salvans S, Crous X, et al. Effect of Roux-en-Y gastric bypass vs sleeve gastrectomy on glucose and gut hormones: a prospective randomised trial. J Gastrointest Surg. 2012;16:1116–1122. doi: 10.1007/s11605-012-1855-0. [DOI] [PubMed] [Google Scholar]
- Sugerman HJ. Bariatric surgery for severe obesity. Proc Am Philos Soc. 2011;155:263–275. [PubMed] [Google Scholar]
- Herron D, Roohipour R. Complications of Roux-en-Y gastric bypass and sleeve gastrectomy. Abdom Imaging. 2012;37:712–718. doi: 10.1007/s00261-012-9866-6. [DOI] [PubMed] [Google Scholar]
- Boza C, Gamboa C, Salinas J, et al. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy: a case-control study and 3 years of follow-up. Surg Obes Relat Dis. 2011;8:243–249. doi: 10.1016/j.soard.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Romy S, Donadini A, Giusti V, et al. Roux-en-Y gastric bypass vs gastric banding for morbid obesity: a case-matched study of 442 patients. Arch Surg. 2012;147:460–466. doi: 10.1001/archsurg.2011.1708. [DOI] [PubMed] [Google Scholar]
- Spivak H, Abdelmelek MF, Beltran OR, et al. Long-term outcomes of laparoscopic adjustable gastric banding and laparoscopic Roux-en-Y gastric bypass in the United States. Surg Endosc. 2012;26:1909–1919. doi: 10.1007/s00464-011-2125-z. [DOI] [PubMed] [Google Scholar]
- Frank P, Crookes PF. Short- and long-term surgical follow-up of the postbariatric surgery patient. Gastroenterol Clin North Am. 2010;39:135–146. doi: 10.1016/j.gtc.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Coupaye M, Puchaux K, Bogard C, et al. Nutritional consequences of adjustable gastric banding and gastric bypass: a 1-year prospective study. Obes Surg. 2009;19:56–65. doi: 10.1007/s11695-008-9571-2. [DOI] [PubMed] [Google Scholar]
- Lynch RJ, Eisenberg D, Bell RL. Metabolic consequences of bariatric surgery. J Clin Gastroenterol. 2006;40:659–668. doi: 10.1097/00004836-200609000-00001. [DOI] [PubMed] [Google Scholar]
- Mango VL, Frishman WH. Physiologic, psychologic, and metabolic consequences of bariatric surgery. Cardiol Rev. 2006;14:232–237. doi: 10.1097/01.crd.0000223656.06812.ae. [DOI] [PubMed] [Google Scholar]
- Ledoux S, Msika S, Moussa F, et al. Comparison of nutritional consequences of conventional therapy of obesity, adjustable gastric banding, and gastric bypass. Obes Surg. 2006;16:1041–1049. doi: 10.1381/096089206778026415. [DOI] [PubMed] [Google Scholar]
- Richardson DW, Vinik AI. Metabolic implications of obesity: before and after gastric bypass. Gastroenterol Clin North Am. 2005;34:9–24. doi: 10.1016/j.gtc.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Rojas P, Carrasco F, Codoceo J, et al. Trace element status and inflammation parameters after 6 months of Roux-en-Y gastric bypass. Obes Surg. 2011;21:561–568. doi: 10.1007/s11695-011-0368-3. [DOI] [PubMed] [Google Scholar]
- Takata MC, Ciovica R, Cello JP, et al. Predictors, treatment, and outcomes of gastrojejunostomy stricture after gastric bypass for morbid obesity. Obes Surg. 2007;17:878–884. doi: 10.1007/s11695-007-9163-6. [DOI] [PubMed] [Google Scholar]
- Carter JT, Tafreshian S, Campos GM, et al. Routine upper GI series after gastric bypass does not reliably identify anastomotic leaks or predict stricture formation. Surg Endosc. 2007;21:2172–2177. doi: 10.1007/s00464-007-9326-5. [DOI] [PubMed] [Google Scholar]
- Al Harakeh AB. Complications of laparoscopic Roux-en-Y gastric bypass. Surg Clin North Am. 2011;91:1225–1237. doi: 10.1016/j.suc.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Azagury DE, Abu Dayyeh BK, Greenwalt IT, et al. Marginal ulceration after Roux-en-Y gastric bypass surgery: characteristics, risk factors, treatment, and outcomes. Endoscopy. 2011;43:950–954. doi: 10.1055/s-0030-1256951. [DOI] [PubMed] [Google Scholar]
- Hamdan K, Somers S, Chand M. Management of late postoperative complications of bariatric surgery. Br J Surg. 2011;98:1345–1355. doi: 10.1002/bjs.7568. [DOI] [PubMed] [Google Scholar]
- Heneghan HM, Meron-Eldar S, Yenumula P, et al. Incidence and management of bleeding complications after gastric bypass surgery in the morbidly obese. Surg Obes Relat Dis. 2011;8:729–735. doi: 10.1016/j.soard.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Ferreira LE, Song LM, Baron TH. Management of acute postoperative hemorrhage in the bariatric patient. Gastrointest Endosc Clin N Am. 2011;21:287–294. doi: 10.1016/j.giec.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Rabl C, Peeva S, Prado K, et al. Early and late abdominal bleeding after Roux-en-Y gastric bypass: sources and tailored therapeutic strategies. Obes Surg. 2011;21:413–420. doi: 10.1007/s11695-011-0354-9. [DOI] [PubMed] [Google Scholar]
- Cingi A, Yavuz Y. Intraoperative endoscopic assessment of the pouch and anastomosis during laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2011;21:1530–1534. doi: 10.1007/s11695-011-0355-8. [DOI] [PubMed] [Google Scholar]
- Puri V, Alagappan A, Rubin M, et al. Management of bleeding from gastric remnant after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2012;8:3–5. doi: 10.1016/j.soard.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Dick A, Byrne TK, Baker M, et al. Gastrointestinal bleeding after gastric bypass surgery: nuisance or catastrophe. Surg Obes Relat Dis. 2010;6:643–647. doi: 10.1016/j.soard.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Frantzides CT, Carlson MA, Shostrom VK, et al. A survey of dumping symptomatology after gastric bypass with or without lesser omental transection. Obes Surg. 2011;21:186–193. doi: 10.1007/s11695-010-0124-0. [DOI] [PubMed] [Google Scholar]
- Hejazi RA, Patil H, McCallum RW. Dumping syndrome: establishing criteria for diagnosis and identifying new etiologies. Dig Dis Sci. 2010;55:117–123. doi: 10.1007/s10620-009-0939-5. [DOI] [PubMed] [Google Scholar]
- Padoin AV, Galvão Neto M, Moretto M, et al. Obese patients with type 2 diabetes submitted to banded gastric bypass: greater incidence of dumping syndrome. Obes Surg. 2009;19:1481–1484. doi: 10.1007/s11695-009-9943-2. [DOI] [PubMed] [Google Scholar]
- Deitel M. The change in the dumping syndrome concept. Obes Surg. 2008;18:1622–1624. doi: 10.1007/s11695-008-9756-8. [DOI] [PubMed] [Google Scholar]
- Z'graggen K, Guweidhi A, Steffen R, et al. Severe recurrent hypoglycemia after gastric bypass surgery. Obes Surg. 2008;18:981–988. doi: 10.1007/s11695-008-9480-4. [DOI] [PubMed] [Google Scholar]
- Falcão M, Campos JM, Neto MG, et al. Transgastric endoscopic retrograde cholangiopancreatography for the management of biliary tract disease after Roux-en-Y gastric bypass treatment for obesity. Obes Surg. 2012;22:872–876. doi: 10.1007/s11695-012-0635-y. [DOI] [PubMed] [Google Scholar]
- Madan A, Urayama S. Successful ERCP in a Roux-en-Y gastric bypass patient, performed via a small remnant of gastrogastric communication. Endoscopy. 2011;43 (Suppl:E73–E74. doi: 10.1055/s-0030-1256038. [DOI] [PubMed] [Google Scholar]
- Geert P, Jacques H, Guido L. ERCP by laparoscopic transgastric access and cholecystectomy at the same time in a patient with gastric bypass who was seen with choledocholithiasis. Gastrointest Endosc. 2010;72:1115–1116. doi: 10.1016/j.gie.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Malherbe V, Badaoui A, Huybrecht H, et al. Management of common bile duct stone late after laparoscopic Roux-en-Y gastric bypass for obesity. Acta Chir Belg. 2009;109:820–823. doi: 10.1080/00015458.2009.11680549. [DOI] [PubMed] [Google Scholar]
- Badaoui A, Malherbe V, Rosiere A, et al. ERCP by laparoscopic transgastric access and cholecystectomy at the same time in a patient with gastric bypass who was seen with choledocholithiasis. Gastrointest Endosc. 2010;71:212–214. doi: 10.1016/j.gie.2009.06.031. [DOI] [PubMed] [Google Scholar]
- Peeters G, Himpens J. A hybrid endo-laparoscopic therapy for common bile duct stenosis of a choledocho-duodenostomy after a Roux-en-Y gastric bypass. Obes Surg. 2009;19:806–808. doi: 10.1007/s11695-009-9829-3. [DOI] [PubMed] [Google Scholar]
- Ahmed AR, Husain S, Saad N, et al. Accessing the common bile duct after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2007;3:640–643. doi: 10.1016/j.soard.2007.06.004. [DOI] [PubMed] [Google Scholar]