Abstract
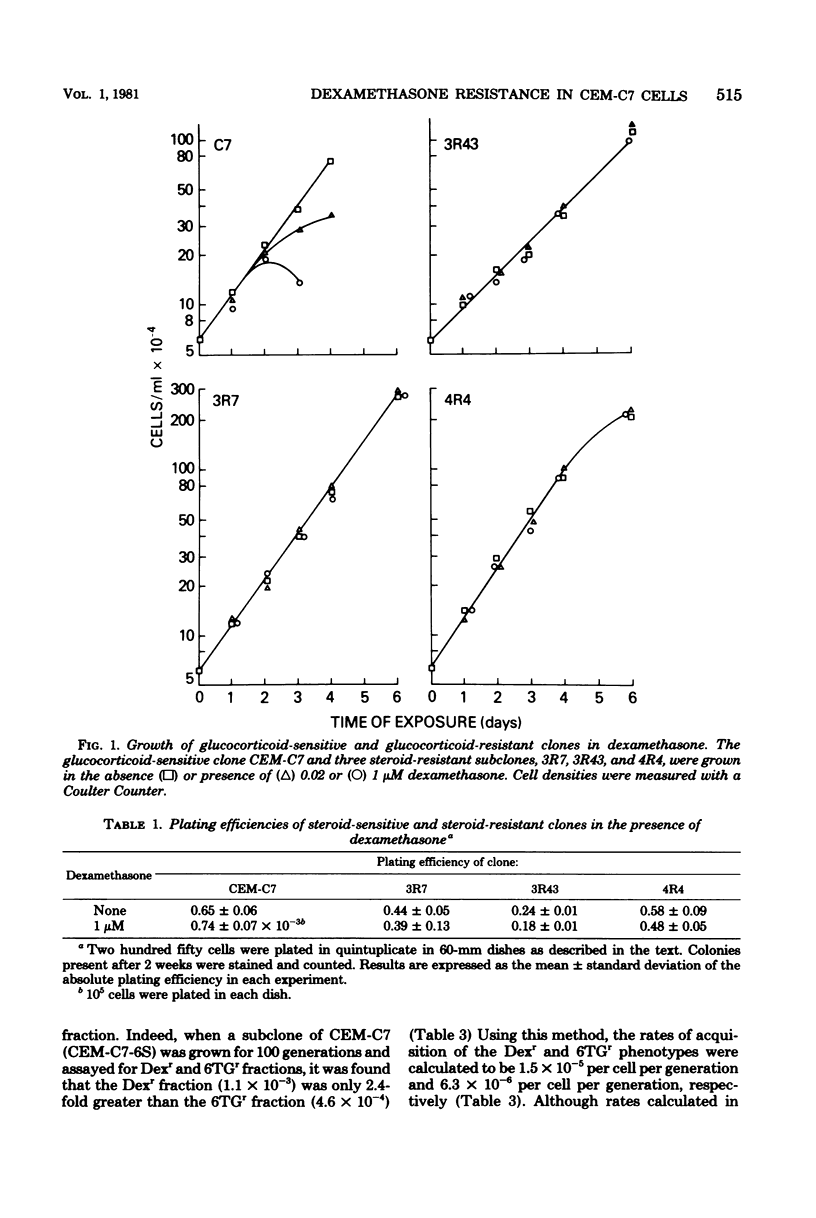
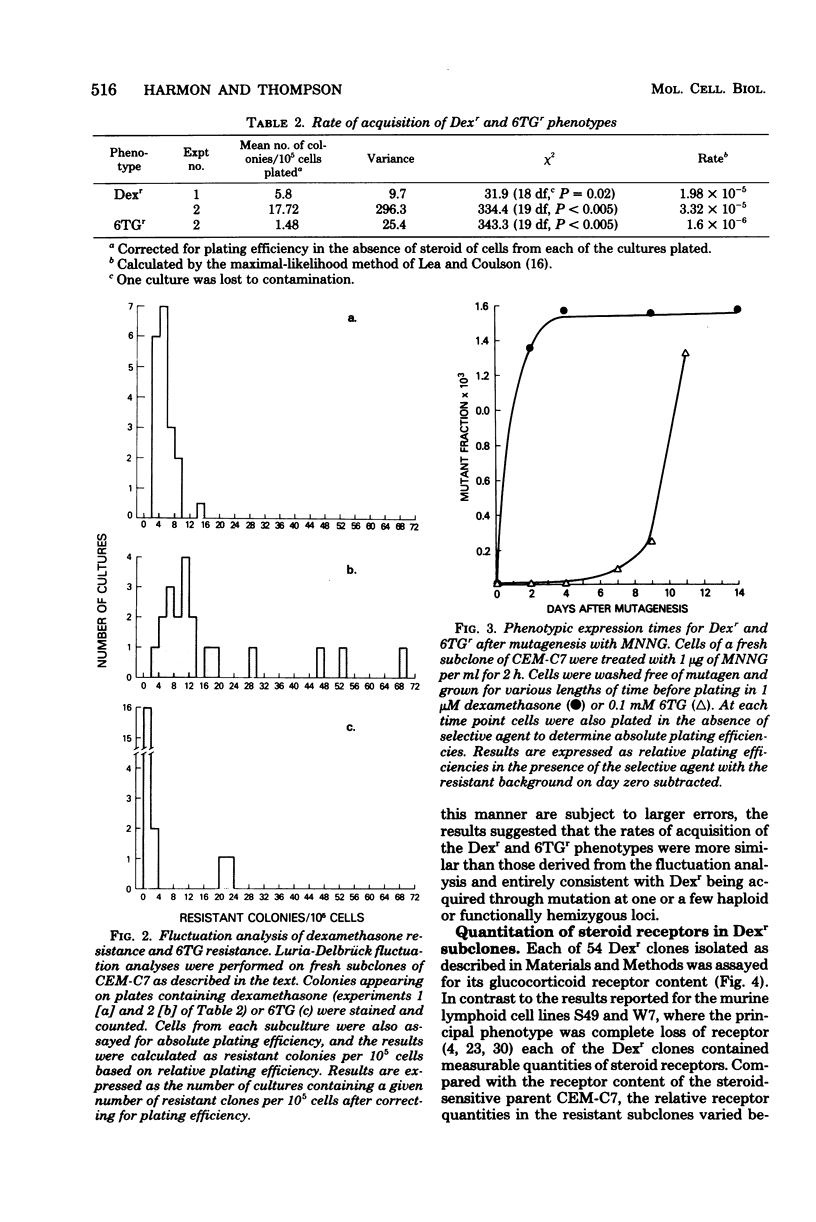
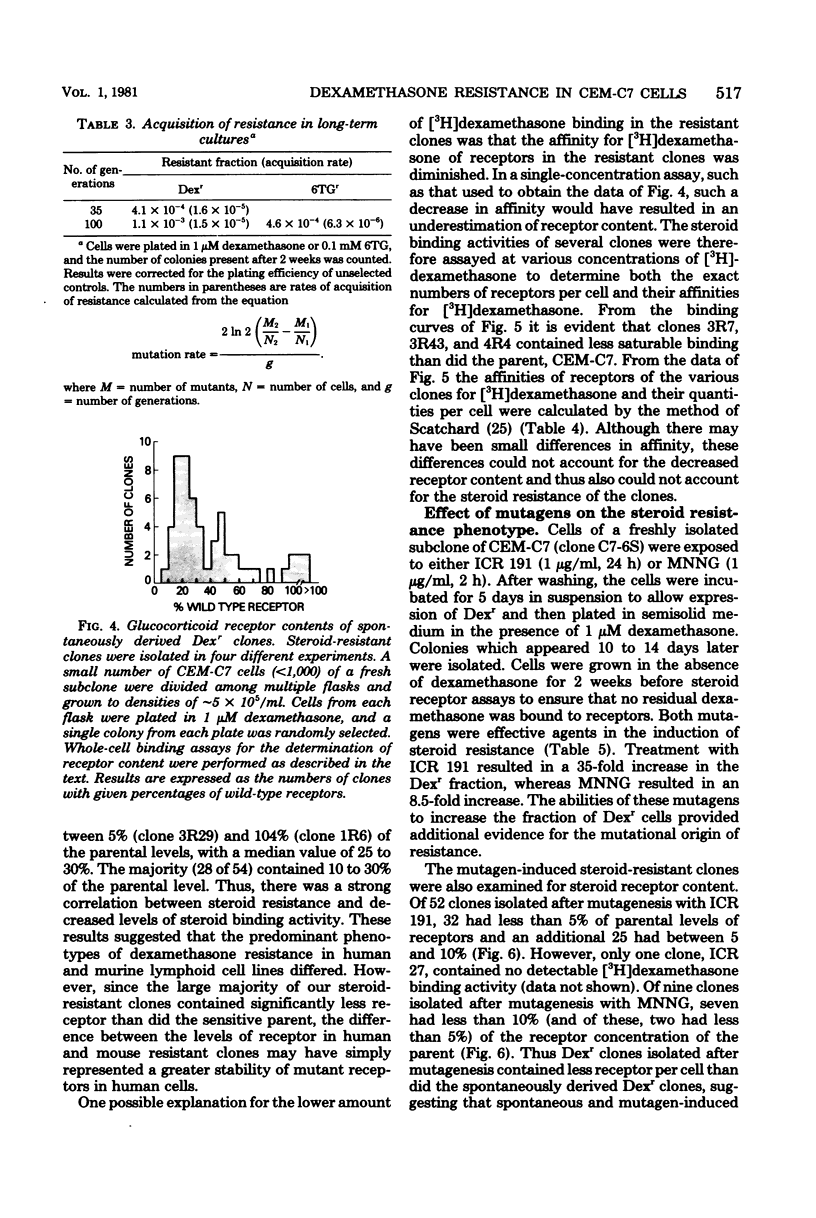
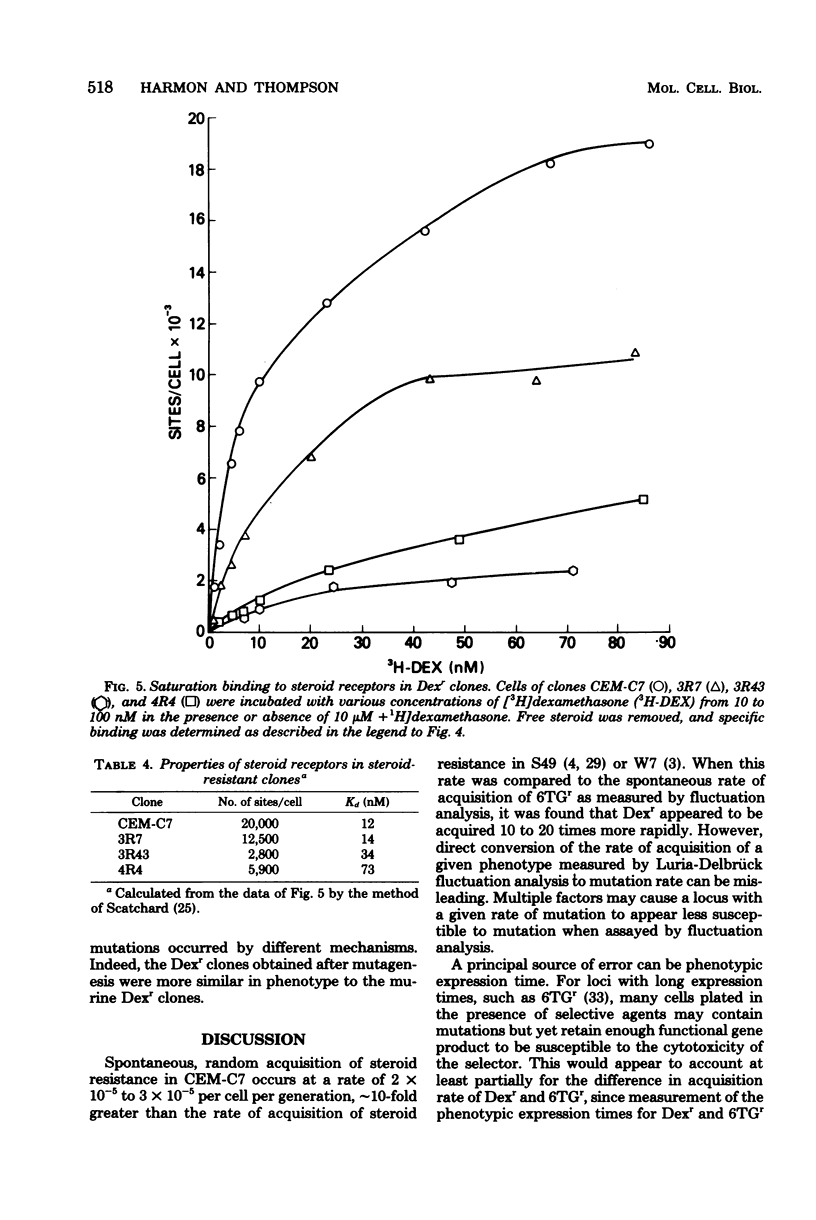
Fifty-four independent dexamethasone-resistant clones were isolated from the clonal, glucocorticoid-sensitive human leukemic T-cell line CEM-C7. Resistance to 1 microM dexamethasone was acquired spontaneously at a rate of 2.6 X 10(-5) per cell per generation as determined by fluctuation analysis. After mutagenesis with N-methyl-N'-nitro-N-nitrosoguanidine (MNNG), the phenotypic expression time for dexamethasone resistance was determined to be 3 days. Spontaneous acquisition of resistance to 0.1 mM 6-thioguanine appeared to occur at a much slower rate, 1.6 X 10(-6) per cell per generation. However, the expression time after MNNG mutagenesis for this resistant phenotype was greater than 11 days, suggesting that the different rates of acquisition for the two phenotypes measured by fluctuation analysis were the results of the disparate expression times. The mutagens ICR 191 and MNNG were effective in increasing the dexamethasone-resistant fraction of cells in mutagenized cultures; ICR 191 produced a 35.6-fold increase, and MNNG produced an 8.5-fold increase. All the spontaneous dexamethasone-resistant clones contained glucocorticoid receptors, usually less than half of the amount found in the parental clone. They are therefore strikingly different from dexamethasone-resistant clones derived from the mouse cell lines S49 and W7. Dexamethasone-resistant clones isolated after mutagenesis of CEM-C7 contained, on the average, lower concentrations of receptor than did those isolated spontaneously, and one clone contained no detectable receptor. These results are consistent with a mutational origin for dexamethasone resistance in these human cells at a haploid or functionally hemizygous locus. They also suggest that this is a useful system for mutation assay.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini R. J., DeMars R. Somatic cell mutation. Detection and quantification of x-ray-induced mutation in cultured, diploid human fibroblasts. Mutat Res. 1973 May;18(2):199–224. doi: 10.1016/0027-5107(73)90037-7. [DOI] [PubMed] [Google Scholar]
- Baxter J. D., Harris A. W., Tomkins G. M., Cohn M. Glucocorticoid receptors in lymphoma cells in culture: relationship to glucocorticoid killing activity. Science. 1971 Jan 15;171(3967):189–191. doi: 10.1126/science.171.3967.189. [DOI] [PubMed] [Google Scholar]
- Bourgeois S., Newby R. F. Diploid and haploid states of the glucocorticoid receptor gene of mouse lymphoid cell lines. Cell. 1977 Jun;11(2):423–430. doi: 10.1016/0092-8674(77)90060-5. [DOI] [PubMed] [Google Scholar]
- Bourgeois S., Newby R. F., Huet M. Glucorticoid resistance in murine lymphoma and thymoma lines. Cancer Res. 1978 Nov;38(11 Pt 2):4279–4284. [PubMed] [Google Scholar]
- Buchwald M. Mutagenesis at the ouabain-resistance locus in human diploid fibroblasts. Mutat Res. 1977 Sep;44(3):401–411. doi: 10.1016/0027-5107(77)90098-7. [DOI] [PubMed] [Google Scholar]
- Carlson S. A., Gelehrter T. D. Hormonal regulation of membrane phenotype. J Supramol Struct. 1977;6(3):325–331. doi: 10.1002/jss.400060305. [DOI] [PubMed] [Google Scholar]
- Caskey C. T., Kruh G. D. The HPRT locus. Cell. 1979 Jan;16(1):1–9. doi: 10.1016/0092-8674(79)90182-x. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R., Smith K. A., Munck A. Glucocorticoid receptors and sensitivity of isolated human leukemia and lymphoma cells. Cancer Res. 1978 Nov;38(11 Pt 2):4268–4272. [PubMed] [Google Scholar]
- Gehring U., Tomkins G. M. A new mechanism for steroid unresponsiveness: loss of nuclear binding activity of a steroid hormone receptor. Cell. 1974 Nov;3(3):301–306. doi: 10.1016/0092-8674(74)90145-7. [DOI] [PubMed] [Google Scholar]
- Griffin J. E., Wilson J. D. The syndromes of androgen resistance. N Engl J Med. 1980 Jan 24;302(4):198–209. doi: 10.1056/NEJM198001243020404. [DOI] [PubMed] [Google Scholar]
- Grove J. R., Dieckmann B. S., Schroer T. A., Ringold G. M. Isolation of glucocorticoid-unresponsive rat hepatoma cells by fluorescence-activated cell sorting. Cell. 1980 Aug;21(1):47–56. doi: 10.1016/0092-8674(80)90113-0. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. Isolation and characterization of mutants of human diploid fibroblasts resistant to diphtheria toxin. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3337–3340. doi: 10.1073/pnas.75.7.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney J. F., Gross S. R., Aronow L., Pratt W. B. Specific glucocorticoid-binding macromolecules from mouse fibroblasts growing in vitro. A possible steroid receptor for growth inhibition. Mol Pharmacol. 1970 Sep;6(5):500–512. [PubMed] [Google Scholar]
- Harmon J. M., Norman M. R., Fowlkes B. J., Thompson E. B. Dexamethasone induces irreversible G1 arrest and death of a human lymphoid cell line. J Cell Physiol. 1979 Feb;98(2):267–278. doi: 10.1002/jcp.1040980203. [DOI] [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- Lippman M. E., Thompson E. B. Steroid receptors and the mechanism of the specificity of glucocorticoid responsiveness of somatic cell hybrids between hepatoma tissue culture cells and mouse fibroblasts. J Biol Chem. 1974 Apr 25;249(8):2483–2488. [PubMed] [Google Scholar]
- Lippman M. E., Yarbro G. K., Leventhal B. G. Clinical implications of glucocorticoid receptors in human leukemia. Cancer Res. 1978 Nov;38(11 Pt 2):4251–4256. [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankovitz R., Buchwald M., Baker R. M. Isolation of ouabain-resistant human diploid fibroblasts. Cell. 1974 Nov;3(3):221–226. doi: 10.1016/0092-8674(74)90135-4. [DOI] [PubMed] [Google Scholar]
- Moehring T. J., Moehring J. M. Selection and characterization of cells resistant to diphtheria toxin and pseudomonas exotoxin A: presumptive translational mutants. Cell. 1977 Jun;11(2):447–454. doi: 10.1016/0092-8674(77)90063-0. [DOI] [PubMed] [Google Scholar]
- Norman M. R., Thompson E. B. Characterization of a glucocorticoid-sensitive human lymphoid cell line. Cancer Res. 1977 Oct;37(10):3785–3791. [PubMed] [Google Scholar]
- Pfahl M., Kelleher R. J., Jr, Bourgeois S. General features of steroid resistance on lymphoid cell lines. Mol Cell Endocrinol. 1978 Apr;10(2):193–207. doi: 10.1016/0303-7207(78)90125-9. [DOI] [PubMed] [Google Scholar]
- Pious D., Soderland C. HLA variants of cultured human lymphoid cells: evidence for mutational origin and estimation of mutation rate. Science. 1977 Aug 19;197(4305):769–771. doi: 10.1126/science.70075. [DOI] [PubMed] [Google Scholar]
- Schmidt T. J., Harmon J. M., Thompson E. B. 'Activation-labile' glucocorticoid-receptor complexes of a steroid-resistant variant of CEM-C7 human lymphoid cells. Nature. 1980 Jul 31;286(5772):507–510. doi: 10.1038/286507a0. [DOI] [PubMed] [Google Scholar]
- Seifert S. C., Gelehrter T. D. Isolation of rat hepatoma cell variants selectively resistant to dexamethasone inhibition of plasminogen activator. J Cell Physiol. 1979 Jun;99(3):333–341. doi: 10.1002/jcp.1040990308. [DOI] [PubMed] [Google Scholar]
- Seifert S. C., Gelehrter T. D. Mechanism of dexamethasone inhibition of plasminogen activator in rat hepatoma cells. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6130–6133. doi: 10.1073/pnas.75.12.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley C. H., Tomkins G. M. Isolation of lymphoma cell variants resistant to killing by glucocorticoids. Cell. 1974 Aug;2(4):213–220. doi: 10.1016/0092-8674(74)90013-0. [DOI] [PubMed] [Google Scholar]
- Sibley C. H., Tomkins G. M. Mechanisms of steroid resistance. Cell. 1974 Aug;2(4):221–227. doi: 10.1016/0092-8674(74)90014-2. [DOI] [PubMed] [Google Scholar]
- Sibley C. H., Yamamoto K. R. Mouse lymphoma cells: mechanisms of resistance to glucocorticoids. Monogr Endocrinol. 1979;12:357–376. doi: 10.1007/978-3-642-81265-1_20. [DOI] [PubMed] [Google Scholar]
- Stevens J., Stevens Y. W. Physicochemical differences between glucocorticoid-binding components from the corticoid-sensitive and -resistant strains of mouse lymphoma P1798. Cancer Res. 1979 Oct;39(10):4011–4021. [PubMed] [Google Scholar]
- Thilly W. G., DeLuca J. G., Hoppe H., 4th, Penman B. W. Mutation of human lymphoblasts by methylnitrosourea. Chem Biol Interact. 1976 Sep;15(1):33–50. doi: 10.1016/0009-2797(76)90126-5. [DOI] [PubMed] [Google Scholar]
- Thompson E. B., Granner D. K., Gelehrter T., Erickson J., Hager G. L. Unlinked control of multiple glucocorticoid-induced processes in HTC cells. Mol Cell Endocrinol. 1979 Sep;15(3):135–150. doi: 10.1016/0303-7207(79)90034-0. [DOI] [PubMed] [Google Scholar]
- Venetianer A., Bajnoczky K., Gal A., Thompson E. B. Isolation and characterization of L-cell variants with altered sensitivity to glucocorticoids. Somatic Cell Genet. 1978 Sep;4(5):513–530. doi: 10.1007/BF01542923. [DOI] [PubMed] [Google Scholar]
- Wolff J. A., Brubaker C. A., Murphy M. L., Pierce M. I., Severo N. Prednisone therapy of acute childhood leukemia: prognosis and duration of response in 330 treated patients. J Pediatr. 1967 Apr;70(4):626–631. doi: 10.1016/s0022-3476(67)80052-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Stampfer M. R., Tomkins G. M. Receptors from glucocorticoid-sensitive lymphoma cells and two clases of insensitive clones: physical and DNA-binding properties. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3901–3905. doi: 10.1073/pnas.71.10.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]



