Abstract
The formation of a micronucleus due to chromosome lagging is a well known mechanism of chromosomal loss. However, the post-mitotic fate of the micronucleus and the chromosomal DNA within it is poorly understood. We observed micronuclei (MN) that had multiple copies of the X chromosome (ranging from 4 to 10) when analyzing cultured human lymphocytes using fluorescence in situ hybridization (FISH). A possible mechanism for this observation is that the chromosome(s) or chromatid(s) contained within the micronuclei successfully completed one or more cycles of replication after their expulsion from the primary nucleus.
Keywords: Micronuclei, X chromosome, Fluorescence in situ hybridization, Acquired aneuploidy
1. Introduction
One mechanism for chromosome loss in peripheral blood lymphocytes is anaphase lagging with a micronucleus/micronuclei (MN) forming that contain(s) the laggard chromosome(s) [1–4]. MN are small, round, chromatin-containing structures that have a measured diameter between 1/16 and 1/3 the size of the main nucleus [5]. The fate of MN after their formation is poorly understood. Possible scenarios for the post-mitotic fate of the MN include: (1) expulsion from the cell; (2) reincorporation into the main nucleus; or (3) retention within the cell's cytoplasm as an extra-nuclear entity. In the first scenario, the DNA within the MN is not expected to be functional or capable of replication due to the absence of the necessary cytoplasmic components. In the second case, the reincorporated chromosome may be indistinguishable from those of the main nucleus and might resume normal biological activity. In the third scenario, it is not known how long a cell could retain a MN or how its DNA might function.
The existence of rare MN with three or more centromeric signals has been previously reported, although the details about the actual number of signals seen were not discussed [6,7]. The observation of two, three or four centromeric signals within a MN could be explained by loss of a mitotic chromosome(s) from a single mitotic division. For example, four centromeric signals within a MN could be explained by the presence of two replicated homologous chromosomes that were both expelled (with each signal representing one chromatid). Three centromeric signals within a MN could be due to either the presence of three chromatids or the presence of four chromatids, two of which would lie close enough that their signals might overlap. However, the presence of five or more signals requires a mechanism other than malsegregation from the previous mitotic division. In this study, as many as 10 X chromosomes were seen within a single MN. This finding leads one to speculate that the original chromosome(s) or chromatid(s) were excluded into a MN that was retained within the cytoplasm of a cell and subsequently completed one or more rounds of DNA/chromosome replication.
2. Material and methods
2.1. Lymphocyte cultures and slide preparations
The MN observations that are included in this report were collected as part of a larger study to assess acquired chromosomal changes that occur with aging in females. After obtaining their informed consent, a 10 ml heparinized blood sample was collected from 24 female donors ranging in age from 45 to 58 (CCHR #9508-3A; Virginia Commonwealth University). All of the participating females were healthy at the time of sample collection and reported no history of chronic illness. Lymphocyte cultures were established according to standard techniques [8]. Briefly, Histopaque-1077 (Sigma) was used for lymphocyte isolation. The lymphocytes were then cultured in RPMI 1640 growth media (supplemented with 15% fetal bovine serum and phytohemagglutinin [PHA]) for 72 h at 37 °C. Colcemid (Irvine Scientific-final concentration of 0.1 μg/ml) was added 20 min prior to the harvest. During the harvest, the cells were treated with hypotonic solution (20 min with 0.075 M KCl) and fixed three times in 3:1 methanol:acetic acid. After harvest, the metaphase spreads and interphase nuclei solution was dropped onto cold, wet slides followed by brief steaming and drying. In order to determine if the any of the study participants might have a constitutional chromosomal abnormality, a standard chromosomal analysis was performed for each of the 24 females [9]. A total of 10 GTG-banded metaphase spreads were analyzed and representative karyotypes prepared (using a Cytovision imaging system from Applied Imaging) for each individual.
2.2. Fluorescence in situ hybridization (FISH) studies on MN
MN identification was done following DAPI staining as described previously [10], according to the criteria of Fenech [5] and Countryman and Heddle [11]. Tri-color FISH on lymphocyte cultures was performed according to the probe manufacturer's protocol (Vysis) using combinations of chromosome-specific enumeration probes for chromosomes X, 1, 3, 5, 8, 9, 10, 13, 16, 17, and 21 (the combinations used were for X,3,17; X,9; X,16,5; X,21,13; and 8,1,10). The X chromosome probe was included in four of the five probe combinations (which were applied to separate slides) to allow for the collection of data to test for possible batch effects that might influence the hybridization results. A total of 1000 randomly selected nuclei were assessed for the presence of spontaneously occurring MN for each probe combination (for a total of 4000 nuclei scored using the X chromosome probe or 5000 total nuclei scored over the five slides evaluated per female). The MN were scored using a Zeiss Axioskop equipped with triple bandpass and single bandpass filters, with the photographic documentation being done using a Cytovision Image Analysis System (Applied Imaging).
For MN having four or more signals, additional FISH studies were done, using a chromosome-specific distally located probe (for the X chromosome the Kallmann's region probe was used, which is localized to Xp22.3), to further characterize the chromosomal contents of these MN. To initiate these serial studies, the original centromere-specific probe was removed with a 1 min wash in 70% formamide/2× SSC solution at 70 °C. After air-drying, the slides were then counterstained with DAPI and viewed to ensure that there was complete removal of the probe. After a brief wash in 2× SSC, the slides were dried and then hybridized with a distal locus-specific probe labeled in Spectrum Orange and the chromosome-specific pericentromeric probe labeled in Spectrum Green.
3. Results
The standard GTG-banding chromosomal analysis showed the chromosomal complements of the 24 females studied to be within normal limits (46,XX). The presence of MN having 4 or more centromeric signals represented less than 0.1% of all MN found in the slides from the 24 females. These atypical MN all contained multiple signals for the X chromosome and accounted for 30% (22 of 73 MN) of all X chromosome positive MN (Table 1 and Fig. 1). The number of X chromosome centromeric signals ranged from one to ten with the modal number being two. Of the MN having multiple signals, only 7 (9.6%) contained six or more signals.
Table 1.
Summary of the number of MN having one or more signal(s) for the X chromosome following FISH
| Number of X chromosome signals | Number of MN observed |
|---|---|
| 1 | 21 |
| 2 | 29 |
| 3 | 1 |
| 4 | 15 |
| 5 | 0 |
| 6 | 3 |
| 7 | 0 |
| 8 | 3 |
| 9 | 0 |
| 10 | 1 |
| Total | 73 |
Fig. 1.
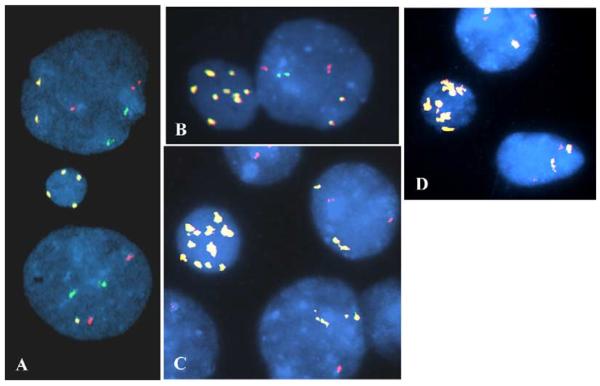
Atypical MN having multiple signals for the X chromosome. In these MN and nuclei, the enumeration probe combination used for images A and B was for chromosomes X (yellow), 3 (orange), and 17 (green). The probe combination for images C and D was for chromosomes X (yellow) and 9 (orange). The MN shown have at least four (A), six (D), eight (B) or 10 (C) signals for the pericentromeric region of the X chromosome. In addition to the X chromosomal aneuploidy that was present in the MN, loss of an X chromosome is present in the lower nucleus in image A. In image C the lower nucleus has only one signal for chromosome 9.
These multiple X chromosome centromeric signals in the MN could have resulted from two general mechanisms: (1) structural changes resulting in gains of the pericentromeric region of the X chromosome; or (2) the presence of multiple intact X chromosomes. In order to distinguish between these two scenarios, the atypical MN were also evaluated in a dual-color FISH study using the same X chromosome pericentromeric probe (Spectrum Green) and a probe that is specific for the distal region of the short arm of the X chromosome (Xp22.3 labeled in Spectrum Orange). Following hybridization, equal numbers of pericentromeric and locus-specific signals were seen in each of the MN having multiple signals (Fig. 2). The greatest number of X chromosomes that were noted to be confined within a single MN was 10.
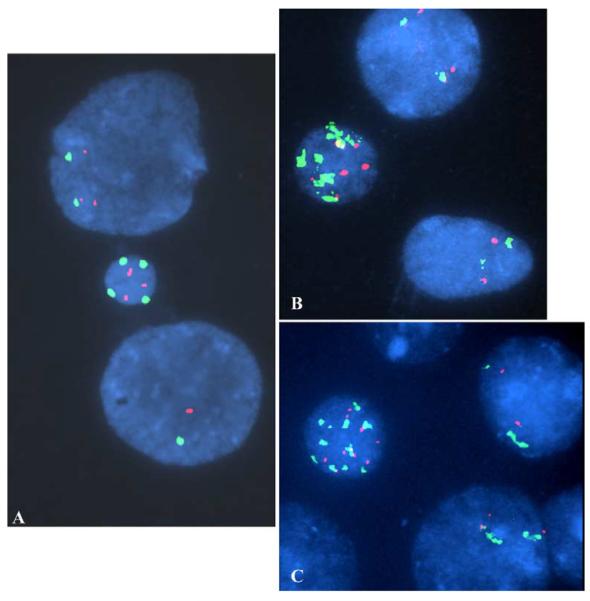
Fig. 2.
Atypical MN with multiple signals of the pericentromeric and distal short arm regions of the X chromosome. MN having multiple signals were further assessed using dual color FISH for the pericentromeric (DXZ1—Spectrum Green) and the distal short arm (Xp22.3—Spectrum Orange [Vysis]) region of the X chromosome. Three of the same MN shown in Fig. 1 (images A [previously A], B [previously D], and C [previously C]) are shown to have equal numbers of the short arm and pericentromeric probes. Furthermore, the pericentromeric probe results were reproducible in location and number. In images B and C, the yellow signals that are seen result from the juxtapositioning of orange and green signals within the MN. Also, not all signals are on the same focal plane within the MN or nuclei (for example, the MN in frame A had four short arm signals present, but one of them (the upper most bipartite signal) was in a different photographic field). Lastly, the X chromosome loss that was seen in the lower nucleus in image A was confirmed (only one X chromosome present).
4. Discussion
Although the majority of MN that arise are thought to contain a single chromosome [10], a small number of MN have been observed to have two or more chromosomes. A MN having two to four copies of the same chromosome could be explained by the lagging of one or two chromatid(s)/homologous chromosome(s) at anaphase, resulting in their malsegregation. Indeed, these MN accounted for 90.4% (66 of 73) of the X chromosome containing MN observed in this study. However, the presence of six, eight or ten copies of the same chromosome, as was observed for seven MN in this study, requires an additional mechanism(s) of formation. It is possible that multiple copies of an X chromosome pre-existed in the parental nucleus and were simultaneously sequestered into a single MN. Alternatively, the MN having four or more chromosomes/chromatids may have been retained within the cellular cytoplasm and the chromosome(s) within it replicated during the S-phase of the cell cycle either in vivo and/or in vitro, just like the chromosomes of the main nucleus. Given that there is no cellular mechanism for division of a MN into “daughter MN” during mitosis, the chromosomes or chromatids within the MN are not expected to segregate. If the MN was retained through several cellular divisions, then the DNA within it would be replicated multiple times, giving rise to atypical MN containing many copies of the same chromosome. For example, if a MN initially contained one chromatid, it would yield a MN having two or four chromosomes after one or two rounds of replication, respectively (Fig. 3). The PHA stimulated lymphoctyes evaluated in this study completed an average of two cell cycles in vitro. Thus, from the data in this investigation, we cannot clearly determine if the multiple signals arose in the MN as an in vitro finding (two rounds of replication could account for the observed numbers of signals), an in vivo event, or a combination of the two occurrences. Interestingly, the presence of 10 chromosomes within a single MN could not be explained by a replication mechanism alone, since this number is not a multiple of the replication events (Fig. 3). One explanation for the observation of atypical MN such as this one is that the replication process occurs for some but not all of the chromosomes, producing a MN with a number of X chromosomes that is not a multiple of 4. Another possible explanation for such MN is that during mitosis the MN membrane disintegrates and the multiple copies of the X chromosome that were originally confined within one MN were either sequestered into more than one newly formed MN or were reincorpo-rated into the main nucleus.
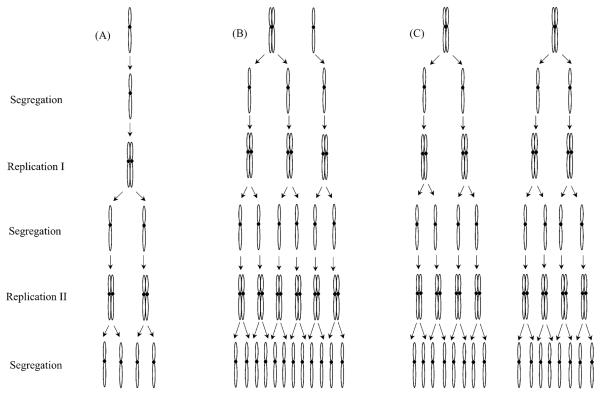
Fig. 3.
Theoretical numbers of chromosomes present in MN following chromosomal separation and replication within the parental cellular cytoplasm. Examples of the chromosomal contents of MN that formed following the nuclear extrusion of one chromatid (A); one replicated chromosome (illustrated by the right or left half of image C); one post-S replicated chromosome and a chromatid (B); and two replicated (post-S) chromosomes (C). If the MN containing a single chromatid could successfully replicate within the cell's cytoplasm, it would be expected to yield a MN having two (first replication cycle) or four (second replication cycle) chromosomes. Similarly, the MN having a replicated chromosome and a chromatid (B) could give rise to a MN having either six (first replication/separation cycle) or 12 chromosomes. In the third example, two replicated chromosomes could result in the presence of eight (first cycle of replication/segregation) or 16 chromosomes. The observation of 10 signals within a MN, as was seen in our study, cannot be explained by any of these scenarios involving just chromosomal replication.
The atypical MN we observed only contained X chromosomes, with no MN being seen having multiple signals for the other chromosomes studied (chromosomes 1, 3, 5, 8, 9, 10, 13, 16, 17, 20, 21). This finding may simply reflect the increased number of observations that our study design provided for the X chromosome, thereby increasing the chances of seeing these rare, spontaneously arising MN. Alternatively, the presence of only X chromatin in the atypical MN may reflect the finding that the X chromosome has been shown to be preferentially lost in females ([12–14], etc.) and, as a result, has been shown to be over represented in MN ([6,15], etc.). However, in addition to differences in the frequencies of loss of the X chromosome, one could speculate that this apparent non-random observation could reflect a selection difference, in that the inclusion of multiple, inactive X chromosomes within the MN of a cell would be expected to have a less detrimental effect than the inclusion of an autosome if this chromatin is actively transcribed, as well as replicated.
In summary, MN with multiple copies of the chromosome X were seen in this study of cultured lymphocytes from females. Given the number of X chromosomes observed, it seems quite possible that some MN may be retained within a cell and may be capable of functioning as an independent extra-nuclear entity capable of chromosomal replication (and possibly other cellular functions). This finding could have implications for understanding the cellular impact of MN that are present in an increased frequency in individuals as they age.
Acknowledgements
This publication was made possible by grant number R01ES12074 from the National Institute of Environmental Health Sciences (NIEHS), NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH.
References
- [1].Ford J, Schultz C, Correll A. Chromosome elimination in micronuclei: a common cause of hypoploidy. Am. J. Hum. Genet. 1988;43:733–740. [PMC free article] [PubMed] [Google Scholar]
- [2].Ford J, Correl A. Chromosome errors at mitotic anaphase. Genome. 1992;35:702–705. doi: 10.1139/g92-107. [DOI] [PubMed] [Google Scholar]
- [3].Nath J, Tucker J, Hando J. Y chromosome aneuploidy, micronuclei, kinetochores and aging in men. Chromosoma. 1995;103:725–731. doi: 10.1007/BF00344234. [DOI] [PubMed] [Google Scholar]
- [4].Catalan J, Falck G, Norppa H. The X chromosome frequently lags behind in female lymphocyte anaphase. Am. J. Hum. Genet. 2000;66:687–691. doi: 10.1086/302769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fenech M. The cytokinesis-block micronucleus technique: a detailed description of the method and its application to genotoxicity studies in human populations. Mutation Res. 1993;285:35–44. doi: 10.1016/0027-5107(93)90049-l. [DOI] [PubMed] [Google Scholar]
- [6].Catalan J, Autio K, Wessman M, Lindholm C, Knuutila S, Sorsa M, Norppa H. Age-associated micronuclei containing centromeres and the X chromosome in lymphocytes of women, Cytogenet. Cell Genet. 1995;68:11–16. doi: 10.1159/000133879. [DOI] [PubMed] [Google Scholar]
- [7].Catalan J, Autio K, Kuosma E, Norppa H. Age-dependent inclusion of sex chromosomes in lymphocyte micronuclei of man. Am. J. Hum. Genet. 1998;63:1464–1472. doi: 10.1086/302092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Clouston H. Lymphocyte culture. In: Rooney D, editor. Human Cytogenetics: Constitutional Analysis. third ed. Oxford University Press; New York: 2001. pp. 33–54. [Google Scholar]
- [9].Benn PA, Tantravahi U. Chromosome staining and banding techniques. In: Rooney D, editor. Human Cytogenetics: Constitutional Analysis. third ed. Oxford University Press; New York: 2001. pp. 99–102. [Google Scholar]
- [10].Leach NT, Jackson-Cook C. The application of spectral karyotyping (SKY) and fluorescent in situ hybridization (FISH) technology to determine the chromosomal content(s) of micronuclei. Mutation Res. 2001;495:11–19. doi: 10.1016/s1383-5718(01)00194-2. [DOI] [PubMed] [Google Scholar]
- [11].Countryman P, Heddle J. The production of micronuclei from chromosome aberration in irradiated cultures of human lymphocytes. Mutation Res. 1976;41:321–332. doi: 10.1016/0027-5107(76)90105-6. [DOI] [PubMed] [Google Scholar]
- [12].Fitzgerald P, McEwan C. Total aneuploidy and age-related sex chromosome aneuploidy in cultured lymphocytes of normal men and women. Hum. Genet. 1977;39:329–337. doi: 10.1007/BF00295428. [DOI] [PubMed] [Google Scholar]
- [13].Galloway S, Buckton K. Aneuploidy and ageing: chromosomes studies on a random sample of the population using G-banding, Cytogenet. Cell Genet. 1978;20:78–95. doi: 10.1159/000130842. [DOI] [PubMed] [Google Scholar]
- [14].Fox TD, Robertson F, Bullock I. Non-random chromosome loss in PHA-stimulated lymphocytes from normal individuals. Mutation Res. 1983;122:403–406. doi: 10.1016/0165-7992(83)90027-1. [DOI] [PubMed] [Google Scholar]
- [15].Hando J, Nath J, Tucker J. Sex chromosomes, micronuclei and aging in women. Chromosoma. 1994;103:186–192. doi: 10.1007/BF00368011. [DOI] [PubMed] [Google Scholar]