Abstract
Atrial fibrillation and obesity are increasing in prevalence and are inter-related epidemics. There has been limited assessment of how obesity and the metabolic syndrome impact P wave indices, established electrocardiographic predictors of atrial fibrillation. We conducted a cross-sectional analysis to determine the association of obesity and the components of the metabolic syndrome with P wave indices in the population-based Atherosclerosis Risk in Communities (ARIC) Study. Analyses were adjusted for demographic, anthropometric, and clinical variables and cardiovascular diseases and risk factors. Following relevant exclusions, 14,433 subjects were included (55% women and 24.7% black). In multivariable analyses, we identified significant, progressive increases in PR interval, P wave maximum duration, and P wave terminal force with BMI 25–30 kg/m2 and BMI ≥30 kg/m2 compared to the reference group <25 kg/m2 (P<0.0001 for trend for all P wave indices). These effects were present in both blacks and whites. Presence of metabolic syndrome was also associated with longer P wave indices. When components of the metabolic syndrome were examined separately, hypertension resulted in significant (P<0.001) augmentation of the three P wave indices. Similarly, waist circumference was associated with greater P wave maximum duration in both races (p<0.001). We concluded that P wave indices are significantly associated with obesity and particularly with hypertension and waist circumference. P wave indices may comprise intermediate markers, independent of age and cardiovascular risk, of the pathway linking obesity and with the risk of AF.
Keywords: P waves, obesity, metabolic syndrome, epidemiology
Atrial fibrillation (AF) and obesity have emerged as concurrent epidemics with profound societal and medical costs. The burgeoning prevalence of AF has been well documented (1;2) and highlights the necessity for aggressive interventions to target novel and established risk factors.(3) The increasing prevalence of obesity in the United States, demonstrated by findings from the National Health and Nutrition Examination Survey (NHANES) ,(4) likewise underscores the importance of broad public health interventions. Obesity is highly related to AF, increasing risk by 40–75% in men and women (5) and associated with increased transition from paroxysmal to permanent AF.(6) The metabolic syndrome has similarly been associated with AF.(7) Thus, the nation’s rising obesity rates anticipate continued escalation in the prevalence of AF.
One potential mechanism for obesity and the metabolic syndrome increasing AF risk concerns atrial electromechanical remodeling. P wave indices serve as intermediate phenotypes reflecting alterations in atrial electrophysiology and morphology. We hypothesized that obesity and metabolic syndrome’s impact on atrial electrophysiologic properties would be evident as alteration of P wave indices in the resting 12-lead ECG. Consequently, we examined the cross-sectional association of the components of the metabolic syndrome with P wave indices. Specifically, we sought to distinguish how obesity and metabolic syndrome impacted P wave indices. Second, recognizing the established racial differences between P wave indices in whites and blacks(8) and the decreased incidence of AF in blacks despite identified risk factors,(9) we examined racial differences of the impact of obesity and metabolic syndrome on P wave indices.
Methods
Study Cohort
The Atherosclerosis Risk in Communities (ARIC) Study is a longitudinal, population-based study evaluating cardiovascular epidemiology. The study consists of a cohort of 15,792 participants, aged 45 to 64 years, enrolled 1987–1989 from four U.S. locations (Forsyth County, NC; Jackson, MI; suburbs of Minneapolis, MN; and Washington Country, MD). At the initial enrollment visit, cohort participants underwent an interview, standardized exam, electrocardiography (ECG), and laboratory assessment. Evaluation was repeated at three triennial visits accompanied by on-going adjudication of cardiovascular end-points. Further details concerning the study, design, recruitment, and methodology have been described elsewhere.(10–12) The ARIC study was approved by the institutional review board at each participating institution and all participants provided informed consent.
Participant demographics, anthropometrics, medical history, ECG and medications were obtained simultaneously at the enrollment exam. Alcohol and tobacco use, education, income level, and physical activity were obtained by self-report using a standardized questionnaire. The standardized exam included three blood pressure measurements obtained while seated by trained study technicians, the last two of which were averaged and reported here. Hypertension was defined as a systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mm Hg, or use of antihypertensive medications. Diabetes was defined as having a fasting glucose ≥ 126 mg/dL, non-fasting glucose ≥ 200 mg/dL, prior reported diagnosis of diabetes, or use of hyperglycemic medications or insulin. Cholesterol measurement for determination of total cholesterol, high density lipoprotein (HDL), and triglyceride levels have been summarized previously.(13;14) Heart failure was defined by validated criteria employing cardiopulmonary signs and symptoms and heart failure medications.(15) History of myocardial infarction was obtained at the baseline interview by individuals’ report of prior myocardial infarction, adjudicated reading of the baseline ECG, and history of coronary intervention by catheter or surgical revascularization. Stroke ascertainment and validation was performed as described previously.(16) Participants with prevalent AF (n=37); lacking or unreadable ECGs (n=243); pre-excitation (Wolff-Parkinson-White), pacemakers, third degree heart block, PR intervals <= 80 or >=320 ms, were excluded (n=394); and lacking clinical covariates relevant to the current analysis (n=685) were excluded. Following exclusions, there were 14,433 subjects included in the analysis. In a secondary analysis, we excluded those taking pharmacologic therapies including beta blockers, cardiac glycosides, calcium channel blockers and antiarrhythmics (n=2010) leaving a total of 12,423 subjects in this analysis.
Electrocardiography and ascertainment of P wave indices
All ARIC participants had an ECG at the enrollment and each subsequent visit. ECGs were recorded on MAC PC Personal Cardiographs (Marquette Electronics Inc., Milwaukee, WI) and were subsequently submitted to a central reading center at the EPICORE Center (University of Alberta, Edmonton, Alberta, Canada) and thereafter to the Epidemiological Cardiology Research Center (EPICARE), Wake Forest University, Winston-Salem, NC. All ECGs were visually inspected for quality and legibility at their acquisition and by the reading centers and then stored in a digital format.
P wave indices selected for the present analysis included PR interval, maximum P wave duration, and the P wave terminal force. The methods for calculating P wave indices in ARIC study have been published elsewhere (8). In summary, the PR interval was determined as the mean duration in milliseconds from the P wave onset until the initiation of the QRS segment in the 12 ECG leads. Maximum P wave duration was defined as the longest median P wave duration in milliseconds in any of the 12 leads. P wave terminal force was calculated from the negative component of P wave in lead V1 (P prime) by multiplying the duration in seconds and voltage in microvolts. Heart rate was derived from the mean rate identified on the ECG. Electrocardiographic left ventricular hypertrophy was identified from the ECG using the Cornell criteria.(17) The QRS duration was determined as the mean on-set to off-set of the QRS segment in milliseconds.
Determination of the metabolic syndrome
Components of the metabolic syndrome were determined according to the definition adapted by the American Heart Association and the National Heart, Lung and Blood Institute(18) and include the following components: (1) waist circumference ≥ 102 cm in men or 88 cm in women; (2) presence of elevated blood pressure, defined as systolic blood pressure ≥ 130 mm Hg, diastolic blood pressure ≥ 85 mm Hg, or use of antihypertensives; (3) presence of insulin resistance, defined as fasting glucose ≥ 100 mg/dL or history of diabetes; (4) fasting triglyceride level ≥ 150 mg/dL or use of medication for dyslipidemia; and (5) HDL cholesterol < 40 mg/dL in men or < 50 mg/dL in women, or use of medication for dyslipidemia. The metabolic syndrome is defined by presence of three or more of these components.
Statistical analysis
Continuous variables were summarized to examine their mean and standard deviations. The distributions of categorical variables were obtained. Body mass index (BMI) was categorized as <25, 25–30, and >30 kg/m2. The association of BMI, metabolic syndrome, and its components with P wave indices was estimated using general linear models with each of the P wave indices as dependent variables. The differences in P wave indices associated with increasing category of BMI were estimated adjusting for age, race, and sex (Model 1). Subsequent analysis adjusted for all variables from Model 1 and included study site, alcohol, smoking status, years smoking, physical activity, heart rate, education, and income level (Model 2). The next analysis adjusted for all variables from Model 2 and included systolic blood pressure, diastolic blood pressure, diabetes, prevalent myocardial infarction, prevalent heart failure, prior stroke, glucose level, high density lipoprotein, total cholesterol, hypertensive medication, and electrocardiographic left ventricular hypertrophy. Effect estimates for each of the P wave indices were also stratified by race. Interactions between race and BMI were tested including multiplicative terms in the model. We conducted similar analysis for each component of the metabolic syndrome, treating the components as dichotomous variables. Linear trends were assessed by including BMI in the three models as a continuous variable. Interaction for race and sex was assessed. We examined the possibility of a non-linear relationship between BMI and the P wave indices using restricted cubic splines, incorporating knots at 5, 27.5, 50, 72.5 and 95 quantiles as recommended by Harrell(19). All cubic spline analyses were adjusted for race and sex and run separately by race. Analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC) and Stata v10.
The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. The authors thank the staff and participants of the ARIC study for their important contributions. The present study was supported by NHLBI grants RC1 HL099452 and American Heart Association awards 09SDG2280087 (AA) and 09FTF2190028 (JWM). The authors are solely responsible for the design and conduct of the present study, the analysis, drafting and editing of the manuscript, and its final contents.
Results
Table 1 describes the demographic, anthropometric, clinical, laboratory and electrocardiographic characteristics of the cohort and their distributions by black or white race. The sample was 55.3% women and 24.6% black race. Blacks had a greater proportion of BMI > 30 kg/m2 (40.1% versus 22.7%), higher mean systolic blood pressure (128.4 versus 118.5 mm Hg), and greater proportion with hypertension (54.2% compared to 27.0%) or diabetes (16.1% versus 8.6%). All P wave indices were greater in blacks compared to whites.
Table 1.
Baseline characteristics of the Atherosclerosis Risk in Communities Cohort.
| Entire cohort (n=14,433) |
Black Race (n=3,558) |
White Race (n=10,875) |
|
|---|---|---|---|
| Clinical characteristics* | |||
| Age, years | 54.2 (5.8) | 53.5 (5.8) | 54.4 (5.7) |
| Women, No (%) | 7981 (55.3) | 2210 (62.11) | 5771 (53.07) |
| Alcohol, g/wk, mean (SD) | 42.3 (94.7) | 31.7 (96.0) | 45.8 (94.1) |
| Smoking, Prior or current, No (%) | 8409 (58.26) | 1899 (53.37) | 6510 (59.87) |
| Body mass index, kg/m2 | |||
| <25 | 4819 (33.4) | 779 (21.9) | 4040 (37.2) |
| 25–30 | 5718 (39.6) | 1351 (38.0) | 4367 (40.2) |
| ≥ 30 | 3896 (27.0) | 1428 (40.1) | 2468 (22.7) |
| Waist Circumference, cm | 96.9 (13.9) | 98.9 (15.1) | 96.3 (13.4) |
| Systolic blood pressure, mm Hg | 120.9 (18.6) | 128.4 (21.0) | 118.5 (17.0) |
| Hypertension, No (%) | 4868 (33.7) | 1929 (54.2) | 2939 (27.0) |
| Diabetes mellitus, No (%) | 1510 (10.5) | 571 (16.1) | 939 (8.6) |
| Ratio of total to HDL cholesterol, mean (SD**) | 0.25 (0.1) | 0.27 (0.1) | 0.24 (0.1) |
| Prevalent heart failure, No (%) | 628 (4.4) | 228 (6.4) | 400 (3.7) |
| Myocardial infarction, No (%) | 561 (3.9) | 121 (3.4) | 440 (4.1) |
| AV nodal agents (beta-blockers or calcium channel blockers), No (%) | 1843 (12.8) | 450 (12.7) | 1393 (12.8) |
| Heart rate, mean (SD), beats/min | 67 (10) | 67 (11) | 67 (10) |
| Electrocardiographic LVH, No (%) | 287 (2.0) | 189 (5.3) | 98 (0.9) |
| QRS interval, mean (SD) | 97.4 (12.3) | 96.2 (12.3) | 97.8 (12.3) |
| P wave indices | |||
| Maximum P wave duration, ms | 108.3 (12.4) | 112.4 (12.2) | 107.0 (12.1) |
| Mean P wave duration, ms | 105.4 (12.8) | 109.5 (12.6) | 104.1 (12.6) |
| P wave terminal force, µV·sec | 1.74 (1.8) | 2.14 (2.1) | 1.61 (1.7) |
| PR interval, mean (SD), ms | 164.0 (25.1) | 172.5 (27.1) | 161.2 (23.8) |
Values are mean (SD) except as noted.
Abbreviations: SD, standard deviation; HDL, high-density lipoprotein; LVH, left ventricular hypertrophy.
PR interval, P maximum wave duration, and P wave terminal force increased significantly across BMI categories compared to the reference category of BMI < 25 kg/m2. The differences between each of the P wave indices by BMI category are described in Table 2 and are present even after multivariable adjustment (p<0.0001). PR interval, maximum P wave duration and P wave terminal force were 6.5 ms (95% CI 5.4–7.6), 6.6 ms (95% CI 6.0–7.1) and 0.24 µV·sec (95% CI 0.16–0.32) higher in obese individuals (BMI>30 kg/m2) than in those with normal or low BMI (< 25 kg/m2).
Table 2.
Difference (95% CI) in P wave indices by BMI categories.
| Per BMI category | |||||
|---|---|---|---|---|---|
| Model* | <25 | 25–30 | >30 | P for trend | |
| PR Interval, ms | 1 | Reference | +4.38 (3.43–5.32) | +7.35 (6.31–8.29) | P<0.0001 |
| 2 | Reference | +4.40 (3.45–5.35) | +7.94 (6.88–9.00) | P<0.0001 | |
| 3 | Reference | +3.70 (2.73–4.67) | +6.49 (5.35–7.63) | P<0.0001 | |
| P wave maximum duration, ms | 1 | Reference | +3.80 (3.35–4.24) | +7.48 (6.99–7.97) | P<0.0001 |
| 2 | Reference | +3.76 (3.31–4.20) | +7.59 (7.09–8.09) | P<0.0001 | |
| 3 | Reference | +3.33 (2.88–3.79) | +6.55 (6.02–7.09) | P<0.0001 | |
| P wave terminal force, µV·sec | 1 | Reference | +0.123 (.053–.192) | +.458 (.381–.534) | P<0.0001 |
| 2 | Reference | +.144 (.076–.212) | +.433 (.357–.509) | P<0.0001 | |
| 3 | Reference | +.064 (−.004–.133) | +.236 (.155–.317) | P<0.0001 | |
All models are general linear models. Model 1 is adjusted for age, race, and sex. Model 2 includes variables from model 1 and study site, alcohol, smoking status, years smoking, physical activity, heart rate, education and income level. Model 3 includes all variables from model 2 and systolic blood pressure, diastolic blood pressure, diabetes, prevalent myocardial infarction, prevalent heart failure, prior stroke, glucose level, high density lipoprotein, total cholesterol, hypertensive medication, and electrocardiographic left ventricular hypertrophy.
Abbreviations: ms, milliseconds; µV, microvolts.
P wave indices were stratified by race and examined according to the three BMI categories, as described in Table 3. Progressive and significant increases in each of the three P wave indices across BMI categories was observed in both black and white participants (P for trend <0.0001 for all three P wave indices). Blacks had greater increases in PR interval and P wave maximum duration than whites with increasing BMI. When P wave indices were stratified by sex, similarly significant increases in P wave indices were noted with progressive BMI groups (P for trend <0.0001; data not shown). Because atrial electrophysiology may be altered by diverse cardiovascular diseases, we conducted a secondary analyses after excluding subjects with prevalent myocardial infarction, stroke and heart failure (n=1300). Likewise, in order to examine the impact of atrial conduction without the influence of pharmacologic therapies including beta blockers, cardiac glycosides, calcium channel blockers and antiarrhythmics, we conducted a secondary analysis excluding subjects (n=2010) taking these agents. Both of these secondary analyses observed identically significant trends of a similar magnitude (P for trend P<0.0001 for all P wave indices in both secondary analyses) in increase of P wave indices with progressive BMI category. Race-BMI interactions were significant both for PR interval (p=0.027) and maximum P wave duration (p<0.0001).
Table 3.
Difference (95% CI) of BMI, stratified by white versus black race.*
| Per BMI category | Per BMI | P for trend | |||
|---|---|---|---|---|---|
| <25 | 25–30 | >30 | kg/m2 increase | ||
| White | |||||
| PR Interval, ms | Reference | +3.75 (2.73–4.78) | +7.31 (6.12–8.51) | +0.63 (0.53–0.72) | P<0.0001 |
| P wave maximum duration, ms | +3.59 (3.10–4.09) | +7.60 (7.01–8.18) | +0.63 (0.58–0.67) | P<0.0001 | |
| P wave Terminal Force, µV·sec | +.198 (.126–.270) | +.496 (.412–.580) | +.042 (.035–.048) | P<0.0001 | |
| Black | |||||
| PR Interval, ms | +6.62 (4.25–8.99) | +9.93 (7.51–12.35) | +0.45 (0.30–0.60) | P<0.0001 | |
| P wave maximum duration, ms | +4.35 (3.31–5.39) | +7.63 (6.57–8.70) | +0.43 (0.37–0.50) | P<0.0001 | |
| P-wave Terminal Force, µV·sec | +.041 (−.139–.221) | +.332 (.149–.516) | +.023 (.012–.035) | P<0.0001 | |
All analyses adjusted for age, sex, study site, alcohol, smoking status, years smoking, physical activity, heart rate, education and income level.
Figure 1 shows restricted cubic spline estimates of the PR interval by BMI. The figure identifies a rapid increase in PR interval duration with increasing BMI. In whites (shown in red), there is continued increase in PR interval with rising BMI. In contrast, blacks observe a plateau followed by gentle descent after reaching BMI>30 kg/m2. Figure 2 depicts cubic restricted spline estimates of P wave maximum duration by BMI, again stratified by race. The figure shows whites having a progressive increase in maximum P wave duration throughout the distribution of BMI even to levels beyond 30 kg/m2. In contrast, for black participants the maximum P wave duration peaks at approximately BMI 30 kg/m2 and is then followed by an extended plateau. Cubic restricted spline estimates were similarly developed to describe the PR interval and P wave maximum duration by waist circumference. These are shown as Supplementary Figure 1 and 2, respectively, and are markedly similar to those describing BMI.
Figure 1.
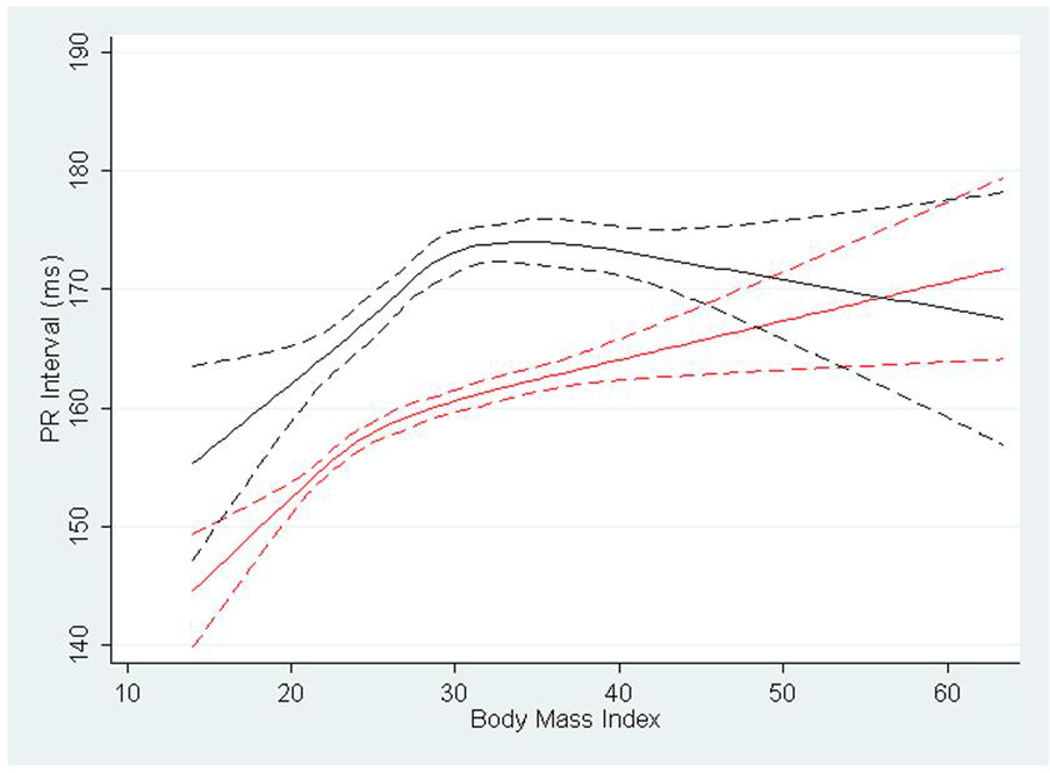
Cubic restricted splines depicting the association of PR interval and body mass index in blacks (shown in black) and whites (shown in red) in the Atherosclerosis Risk in Communities Study. Dotted lines represent 95% confidence intervals surrounding the estimate.
Figure 2.
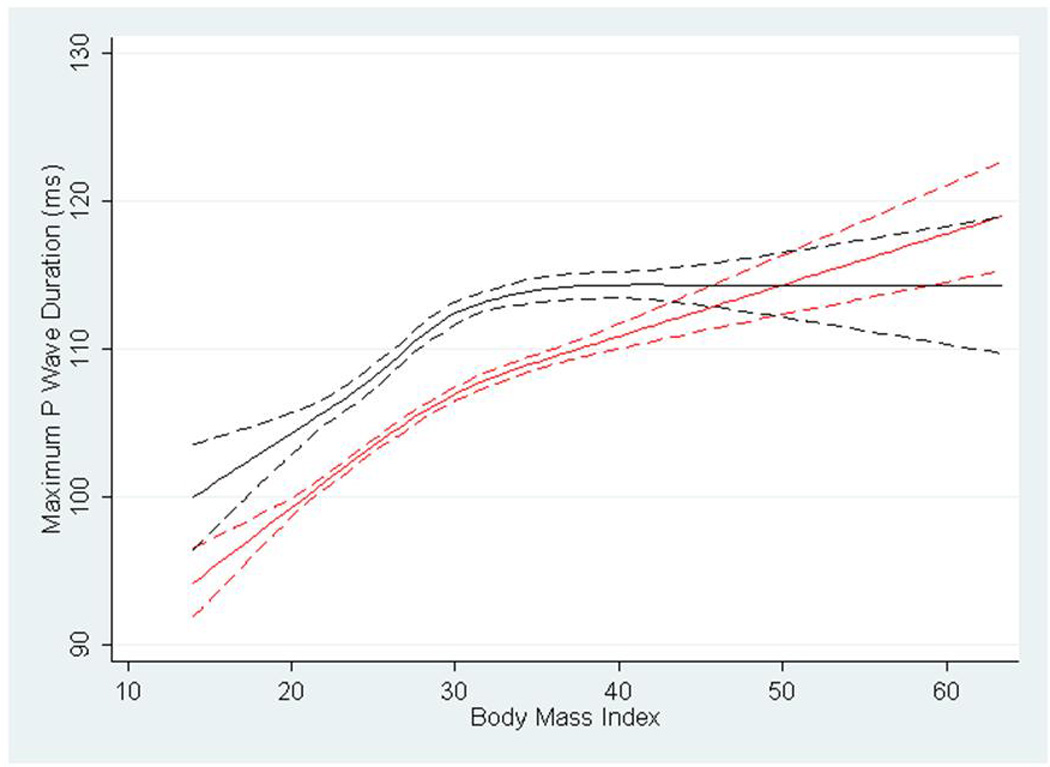
Cubic restricted splines depicting the association of P wave maximum duration and body mass index in blacks (shown in black) and whites (shown in red) in the Atherosclerosis Risk in Communities Study. Dotted lines represent 95% confidence intervals surrounding the estimate.
In multivariable analysis, individuals with metabolic syndrome, compared to those without, had longer PR interval (3.9 ms, 95% CI 3.1–4.8), longer maximum P wave maximum duration (3.5 ms, 95% CI 3.1–3.9), and higher P wave terminal force (0.27 µV·sec, 95% CI 0.21–0.32). The association of the metabolic syndrome with P wave indices was also examined according to race and sex. As in the entire sample, in both black and white race, subjects meeting criteria for the metabolic syndrome had longer PR interval and P wave maximum duration and greater P wave terminal force compared to those without the metabolic syndrome (P value for all comparisons p<0.0001; presented in Supplementary Table). Results were similarly significant for sex-stratified analyses (P value for all comparisons p<0.0001; data not shown).
We then examined the five components of the metabolic syndrome separately to determine the mean increase in each of the P wave indices with the presence of each of the distinct components. Results of the analyses for the separate components of the metabolic syndrome are described in Table 4. Participants with waist circumference ≥ 102 cm in men or 88 cm in women had significantly greater PR interval and P wave maximum duration and P wave terminal force (all analyses p<0.001) than those without. When the presence of waist circumference was stratified by race, the increase in P wave maximum duration remained significant for both black and white participants. Likewise, the presence of hypertension was associated with a significant increase in each of the P wave indices, again observed across racial strata (all analyses p<0.001). Triglycerides, HDL, and fasting glucose had more modest and variable effect on P wave indices. The mean PR intervals and P wave maximum durations stratified by race and according to progressive increases in the number of metabolic syndrome components are described by Figure 3 and Figure 4, respectively. The histograms indicate the direct increase in these P wave indices with incremental addition of metabolic syndrome components.
Table 4.
Difference (95% CI) in P wave indices with presence of individual metabolic syndrome components, stratified by race.*
| Metabolic Syndrome Components | |||||
|---|---|---|---|---|---|
| Hypertension | Waist Circumference | Triglycerides | HDL | Fasting Glucose | |
| All | |||||
| PR interval, ms | +3.09† (2.24–3.94) | +1.71†† (0.64–2.77) | +0.11 (−0.86–1.09) | +1.76† (0.86–2.66) | −0.59 (−1.43–0.24) |
| P wave maximum duration, ms | +2.81† (2.41–3.21) | +1.66† (1.16–2.16) | −0.13 (−0.59–0.33) | +0.36 (−0.06–0.78) | −0.07 (−0.46–0.33) |
| P wave terminal force µV·sec | +.468† (.407-.529) | −.009 (−.085–.067) | +.001 (−.069–.071) | +.047 (−.017–.112) | +.053 (−.007–.113) |
| White | |||||
| PR Interval, ms | +2.94† (2.00–3.88) | +1.36†† (0.19–2.53) | +0.22 (−0.83–1.27) | +1.99† (0.99–2.99) | −0.67 (−1.59–0.27) |
| P wave maximum duration, ms | +2.83† (2.37–3.29) | +1.44† (0.87–2.00) | −0.12 (−0.62–0.39) | +0.25 (−0.23–0.74) | +0.03(−0.43–0.48) |
| P wave terminal force µV·sec | +.396† (.330-.462) | −.036 (−.119–.045) | +.010 (−.063–.083) | +.049 (−.020–.119) | +.052 (−.013–.117) |
| Black | |||||
| PR Interval, ms | +3.31† (1.37–5.25) | +2.45 (−0.08–4.99) | −1.05 (−3.55–1.44) | +1.04 (−0.99–3.08) | −0.35 (−2.21–1.51) |
| P wave maximum duration, ms | +2.60† (1.75–3.45) | +1.79†† (0.68–2.90) | −0.45(−1.54–0.64) | +0.64 (−0.24–1.53) | −0.39 (−1.20–0.43) |
| P wave terminal force µV·sec | +.672† (.527-.817) | +.038 (−.151–.227) | −.038 (−.225–.149) | +.058 (−.094–.210) | +.072 (−.066–.210) |
All analyses adjusted for age, sex, study site, body mass index, alcohol, smoking status, years smoking, physical activity, heart rate, education and income level and the other four components of the metabolic syndrome.
Abbreviations: ms, milliseconds; µV, microvolts.
p<0.001;
p<0.05
Figure 3.
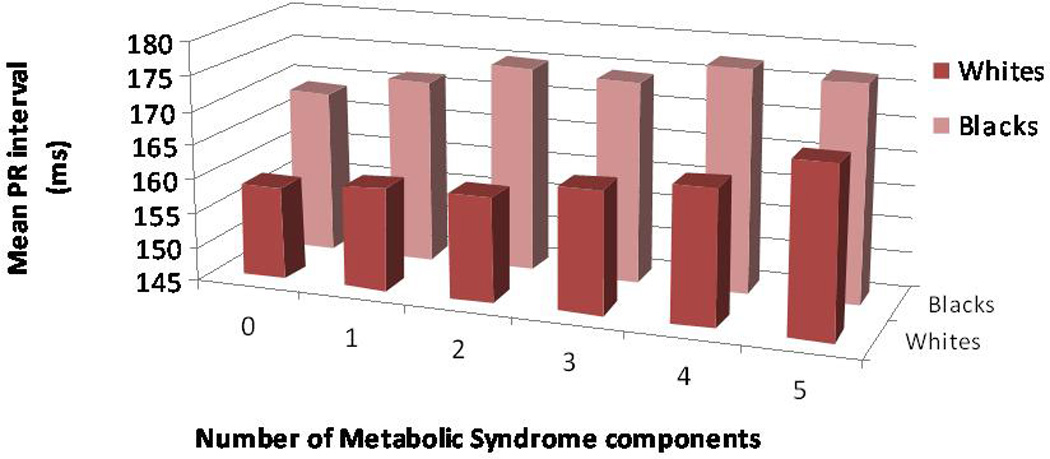
Mean PR interval (ms), stratified by race, according to progressive increases in number of metabolic syndrome components.
Figure 4.
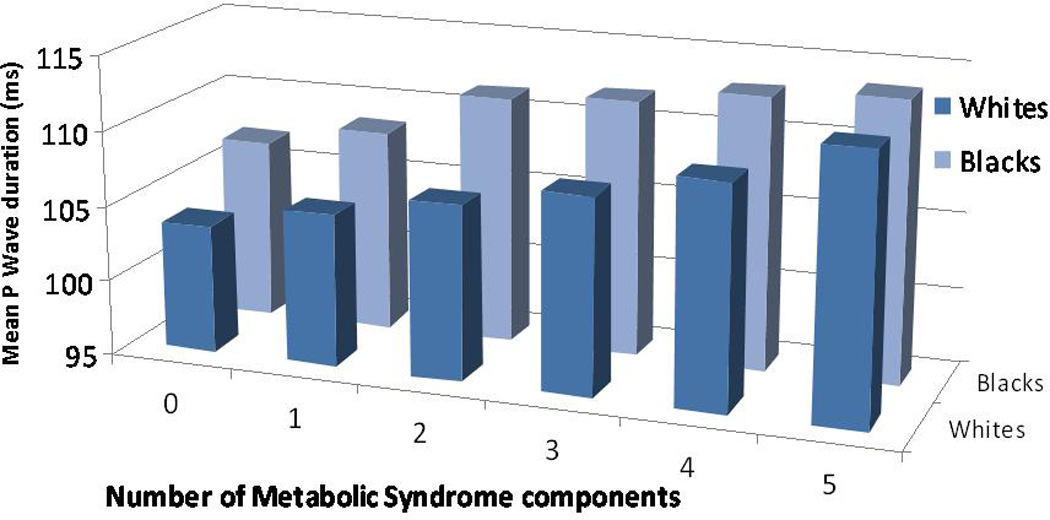
Mean P wave duration (ms), stratified by race, according to progressive increases in number of metabolic syndrome components.
Discussion
In this large, population-based cohort of black and white participants, higher BMI was associated with significant, progressive larger P wave indices of PR interval, P wave maximum duration and P wave terminal force. These differences remained after adjusting for demographic, clinical, and metabolic covariates. Indeed, all P wave indices showed a significant increase (P<0.0001) with augmentation across BMI categories which persisted after stratification by black and white race. P wave indices were further significantly longer in participants with the metabolic syndrome, again persisting after stratification by race. When the five individual components of the metabolic syndrome were examined, it became apparent that waist circumference and hypertension were the chief factors responsible for prolongation of P wave indices.
To our knowledge, this is the largest (n=14,433) assessment of the association of P wave indices with obesity and parameters of the metabolic syndrome. The analyses presented here suggest that P wave indices are progressively altered by BMI, obesity, and constituent components of metabolic syndrome, particularly blood pressure and waist circumference. The P wave indices examined in this study have been found to have limited correlation and hence do not demonstrate collinearity.(8) Their substantive modification likely reflects the alteration of various aspects of atrial electrophysiology in the setting of obesity or metabolic syndrome’s components. Our analysis therefore establishes the electrocardiographic alterations which accompany these insults and imply the occurrence of atrial electrophysiologic remodeling.
Similar findings for the relation between BMI and P wave indices were identified in a recent population-based multiethnic analysis examining pericardial fat(20) That study, conducted on a subgroup of the Multiethnic Study of Atherosclerosis (MESA), reported that increased BMI was associated with prolongation of P wave indices, consistent with our results, after adjusting for age, sex, ethnicity and pericardial fat. However, the MESA study’s examination of BMI and P wave indices did not adjust for clinical variables. In contrast, our study is strengthened by comprehensive adjustment for cardiovascular risk factors in our multivariable analyses.
Our analyses provide important insights into an intermediate step in the pathway from obesity and the other metabolic insults examined here towards increased AF risk. The metabolic syndrome has an established association with AF and a population attributable risk as elevated as 22% in a recent ARIC investigation.(7;21;22)
Insulin resistance and obesity have cellular and electrophysiologic effects which alter atrial tissue architecture and morphology. Atrial tissue of diabetic subjects demonstrates altered metabolic function, specifically with regard to impairment of mitochondrial function and resulting oxidative stress.(23) Obesity is associated with increased LA size in cross-sectional analysis and population-based longitudinal analyses.(24;25) Obesity’s association with alteration of P wave indices is likely multifactorial, stemming from the interrelated sequalae of obesity’s vascular and hormonal impacts: obesity increases cardiac loading, resulting in compensatory remodeling;(26) obesity alters the myocardial matrix, secondary to adipose-derived hormones, in turn driving electrophysiologic remodeling;(27;28) and obesity induces paracrine hormone expression with endovascular effects that may also alter atrial pressures and loading conditions.(29–31) Thus, obesity’s relation to AF is likely mediated by a complex array of structural and electrical remodeling, reflected in turn by the alteration of P wave indices seen in our study.
Prolonged P wave duration has further been associated with impaired left atrial diastolic indices.(32) Thus, P wave indices may represent an intermediate step along a pathway from obesity towards atrial remodeling. We suspect that the metabolic processes of obesity and metabolic syndrome components yield ultimately to progressive atrial fibrosis, the common pathway resulting from chronic inflammation and myocyte dysregulation.(33–35) The end result is profibrillatory modification of atrial tissue with increased risk for AF.
Our study identified different patterns in P wave indices’ response to obesity and metabolic syndrome components when we stratified by race. While both blacks and whites had greater P wave indices corresponding with progressive increase in BMI in multivariable analyses, the responses to the rise in BMI differed by race. Specifically, we found that after a BMI of approximately 30 kg/m2, the PR interval and P wave maximum duration in blacks plateaus. In contrast, whites showed a continued increase in P wave indices extending beyond BMI > 30 kg/m2. Both blacks and whites had significant (P<0.001) increases in PR interval and P wave maximum duration with hypertension and waist circumference. Further study of the demographic and racial determinants of P wave indices is essential towards understanding the results observed here.
Our study has several limitations. First, the study is cross-sectional and therefore does not examine the longitudinal associations between P wave indices and components of the metabolic syndrome. Similarly, the cross-sectional design of the study is unable to distinguish causality between BMI and metabolic syndrome and alteration of P wave indices. However, it is unlikely that P wave indices are a causative etiology for obesity or metabolic syndrome. Second, while we adjusted our analysis for multiple demographic, clinical, and laboratory variables, there may be unaccounted-for confounders, such as other disease processes (e.g. valvular disease, diastolic dysfunction, thyroid disorders) associated with alteration of P wave indices.(36) Third, while we sought to exclude subjects with prevalent AF, subjects with paroxysmal AF may have inadvertently been included. P wave indices would be expected to be longer in such individuals. We would expect the number of such cases to be minimal based upon the comprehensive screening at study enrollment and the relatively young age of study participants. Finally, while the biracial cohort is a tremendous strength of this study, the generalizability to other ethnic groups aside from those studied here remains limited.
In conclusion, we demonstrated the impact of obesity and metabolic syndrome and its components on P wave indices in a large community-based cohort. Our results indicate that obesity and hypertension impact P wave indices; prolong the PR interval, P wave maximum duration, and P wave terminal force. Our results contribute to the epidemiology of P wave indices, and elucidate intermediate mechanisms relating obesity to substantive AF risk.
Supplementary Material
Acknowledgments
Funding: The Atherosclerosis Risk in Communities Study is a collaborative study with the following NHLBI contract support: N01-HC-55015, N01-HC55016, N01-HC-55018, N01-HC-55019, N01-HC-55022. The present study was additionally supported by NHLBI grants RC1 HL099452 and American Heart Association awards 09SDG2280087 (AA, RFM) and 09FTF2190028 (JWM).
Footnotes
Disclosures: None
Reference List
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, et al. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation. 2009;119(4):606–618. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 5.Frost L, Hune LJ, Vestergaard P. Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med. 2005;118(5):489–495. doi: 10.1016/j.amjmed.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 6.Tsang TS, Barnes ME, Miyasaka Y, Cha SS, Bailey KR, Verzosa GC et al. Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: a longitudinal cohort study of 21 years. Eur Heart J. 2008 doi: 10.1093/eurheartj/ehn324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe H, Tanabe N, Watanabe T, Darbar D, Roden DM, Sasaki S, et al. Metabolic syndrome and risk of development of atrial fibrillation: the Niigata preventive medicine study. Circulation. 2008;117(10):1255–1260. doi: 10.1161/CIRCULATIONAHA.107.744466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC., Jr Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40(4):1204–1211. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soliman EZ, Alonso A, Goff DC., Jr Atrial fibrillation and ethnicity: the known, the unknown and the paradox. Future Cardiol. 2009;5(6):547–556. doi: 10.2217/fca.09.49. [DOI] [PubMed] [Google Scholar]
- 10.The Atherosclerosis Risk in Communities (ARIC) Study: design objectives The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 11.Jackson R, Chambless LE, Yang K, Byrne T, Watson R, Folsom A, et al. Differences between respondents, nonrespondents in a multicenter community-based study vary by gender ethnicity The Atherosclerosis Risk in Communities (ARIC) Study Investigators. J Clin Epidemiol. 1996;49(12):1441–1446. doi: 10.1016/0895-4356(95)00047-x. [DOI] [PubMed] [Google Scholar]
- 12.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49(2):223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 13.Chamberlain AM, Folsom AR, Schreiner PJ, Boerwinkle E, Ballantyne CM. Low-density lipoprotein and high-density lipoprotein cholesterol levels in relation to genetic polymorphisms and menopausal status: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 2008;200(2):322–328. doi: 10.1016/j.atherosclerosis.2007.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasmussen-Torvik LJ, Yatsuya H, Selvin E, Alonso A, Folsom AR. Demographic and cardiovascular risk factors modify association of fasting insulin with incident coronary heart disease and ischemic stroke (from the Atherosclerosis Risk In Communities Study) Am J Cardiol. 2010;105(10):1420–1425. doi: 10.1016/j.amjcard.2009.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eriksson H, Caidahl K, Larsson B, Ohlson LO, Welin L, Wilhelmsen L, et al. Cardiac and pulmonary causes of dyspnoea--validation of a scoring test for clinical-epidemiological use: the Study of Men Born in 1913. Eur Heart J. 1987;8(9):1007–1014. doi: 10.1093/oxfordjournals.eurheartj.a062365. [DOI] [PubMed] [Google Scholar]
- 16.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30(4):736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 17.Casale PN, Devereux RB, Kligfield P, Eisenberg RR, Miller DH, Chaudhary BS, et al. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol. 1985;6(3):572–580. doi: 10.1016/s0735-1097(85)80115-7. [DOI] [PubMed] [Google Scholar]
- 18.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 19.Harrell FE., Jr . Regression Modeling Strategies with Applications to Linear Models, Logistic Regression, and Survival Analysis. New York, NY: Springer-Verlag; 2001. [Google Scholar]
- 20.Babcock MJ, Soliman EZ, Ding J, Kronmal A, Goff DC., Jr Pericardial Fat and Atrial Conduction Abnormalities in the Multiethnic Study of Atherosclerosis (MESA) Obesity (Silver Spring) 2010 doi: 10.1038/oby.2010.121. [DOI] [PubMed] [Google Scholar]
- 21.Chamberlain AM, Agarwal SK, Ambrose M, Folsom AR, Soliman EZ, Alonso A. Metabolic syndrome and incidence of atrial fibrillation among blacks and whites in the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2010;159(5):850–856. doi: 10.1016/j.ahj.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Echahidi N, Mohty D, Pibarot P, Despres JP, O’Hara G, Champagne J, et al. Obesity and metabolic syndrome are independent risk factors for atrial fibrillation after coronary artery bypass graft surgery. Circulation. 2007;116(11 Suppl):I213–I219. doi: 10.1161/CIRCULATIONAHA.106.681304. [DOI] [PubMed] [Google Scholar]
- 23.Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, Neufer PD. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol. 2009;54(20):1891–1898. doi: 10.1016/j.jacc.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McManus DD, Xanthakis V, Sullivan LM, Zachariah J, Aragam J, Larson MG, et al. Longitudinal tracking of left atrial diameter over the adult life course: Clinical correlates in the community. Circulation. 2010;121(5):667–674. doi: 10.1161/CIRCULATIONAHA.109.885806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ybarra J, Resmini E, Planas F, Navarro-Lopez F, Webb S, Pou JM, et al. Relationship between adiponectin and left atrium size in uncomplicated obese patients: adiponectin, a link between fat and heart. Obes Surg. 2009;19(9):1324–1332. doi: 10.1007/s11695-009-9924-5. [DOI] [PubMed] [Google Scholar]
- 26.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88(2):389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.You T, Nicklas BJ, Ding J, Penninx BW, Goodpaster BH, Bauer DC, et al. The metabolic syndrome is associated with circulating adipokines in older adults across a wide range of adiposity. J Gerontol A Biol Sci Med Sci. 2008;63(4):414–419. doi: 10.1093/gerona/63.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schram K, Sweeney G. Implications of myocardial matrix remodeling by adipokines in obesity-related heart failure. Trends Cardiovasc Med. 2008;18(6):199–205. doi: 10.1016/j.tcm.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Qasim A, Mehta NN, Tadesse MG, Wolfe ML, Rhodes T, Girman C, et al. Adipokines, insulin resistance, and coronary artery calcification. J Am Coll Cardiol. 2008;52(3):231–236. doi: 10.1016/j.jacc.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52(15):1201–1210. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sweeney G. Cardiovascular effects of leptin. Nat Rev Cardiol. 2010;7(1):22–29. doi: 10.1038/nrcardio.2009.224. [DOI] [PubMed] [Google Scholar]
- 32.Goyal SB, Spodick DH. Electromechanical dysfunction of the left atrium associated with interatrial block. Am Heart J. 2001;142(5):823–827. doi: 10.1067/mhj.2001.118110. [DOI] [PubMed] [Google Scholar]
- 33.Kourliouros A, Savelieva I, Kiotsekoglou A, Jahangiri M, Camm J. Current concepts in the pathogenesis of atrial fibrillation. Am Heart J. 2009;157(2):243–252. doi: 10.1016/j.ahj.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51(8):802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 35.Casaclang-Verzosa G, Gersh BJ, Tsang TS. Structural and functional remodeling of the left atrium: clinical and therapeutic implications for atrial fibrillation. J Am Coll Cardiol. 2008;51(1):1–11. doi: 10.1016/j.jacc.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 36.Magnani JW, Williamson MA, Monahan KM, Ellinor PT, Benjamin EJP. Wave Indices: Current Status and Future Directions in Epidemiology, Clinical and Research Applications. Circulation: Arrhythmia and Electrophysiology. 2009;2:72–79. doi: 10.1161/CIRCEP.108.806828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


