Abstract
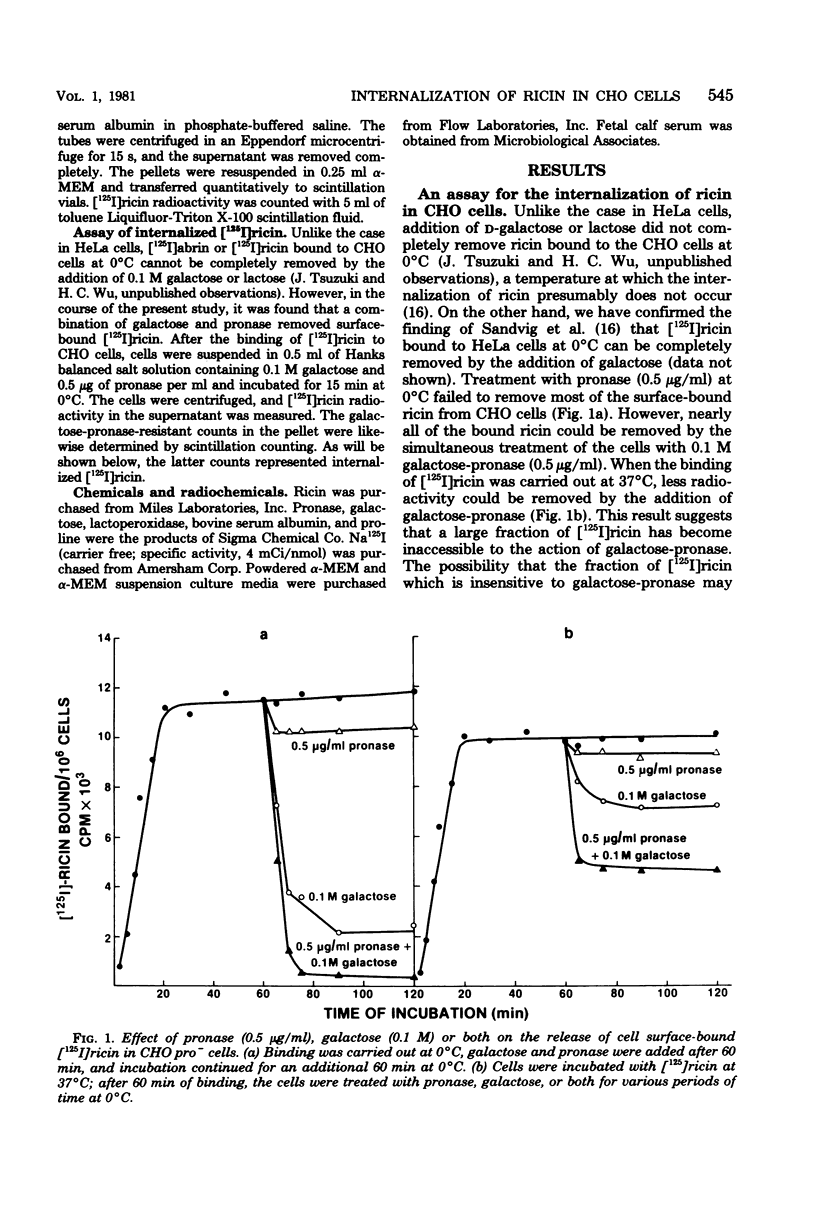
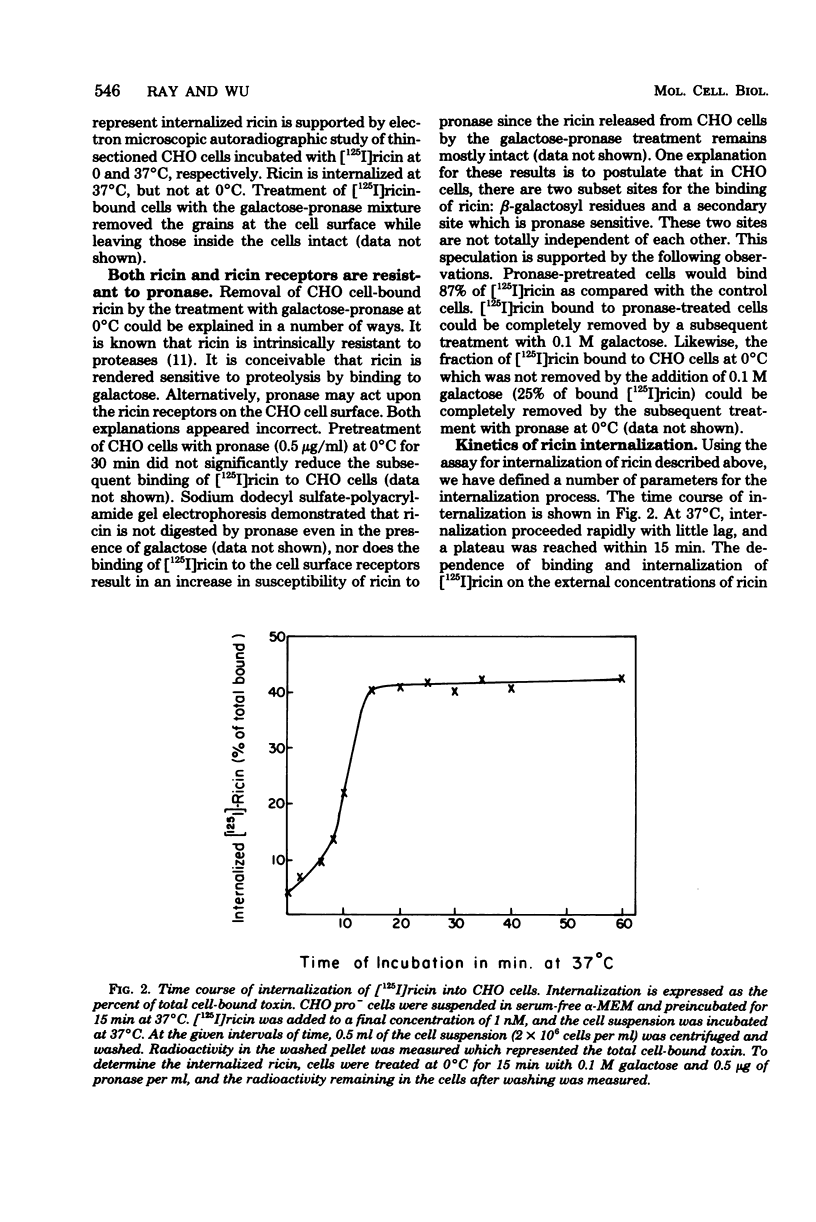
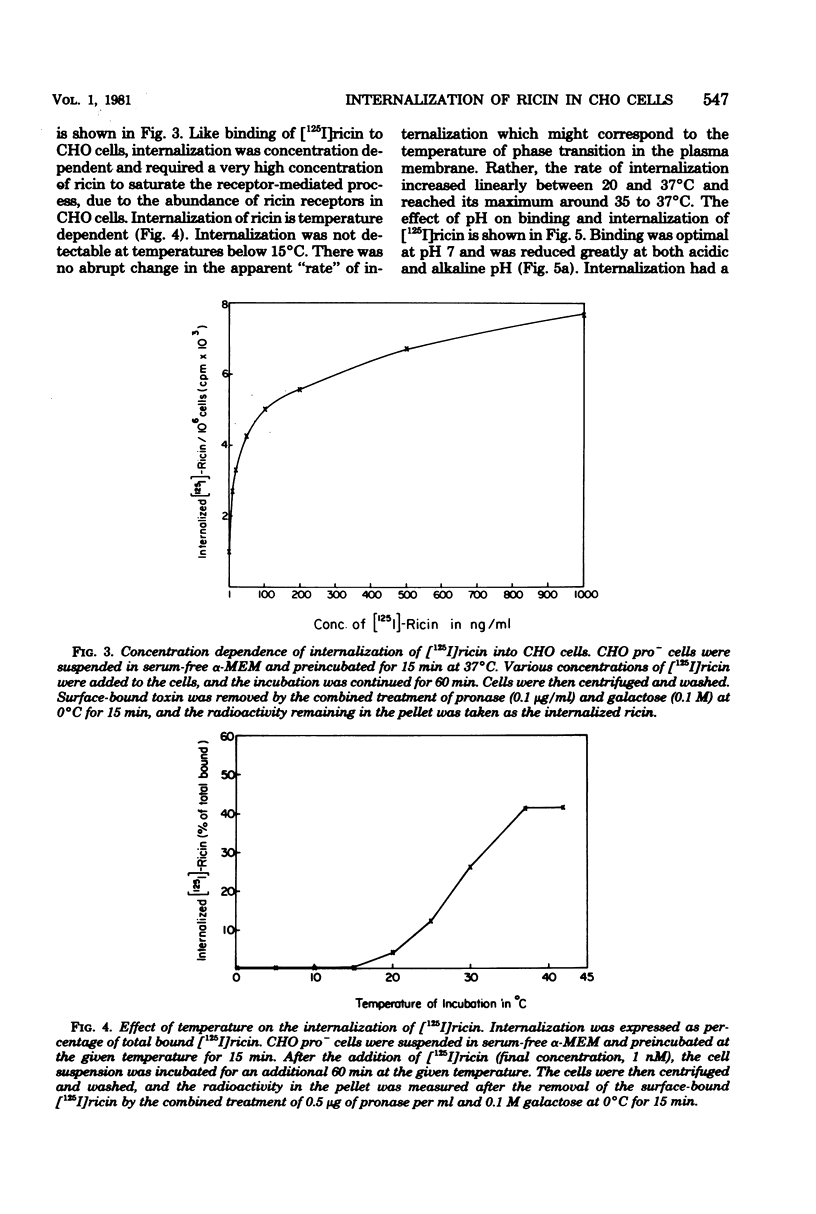
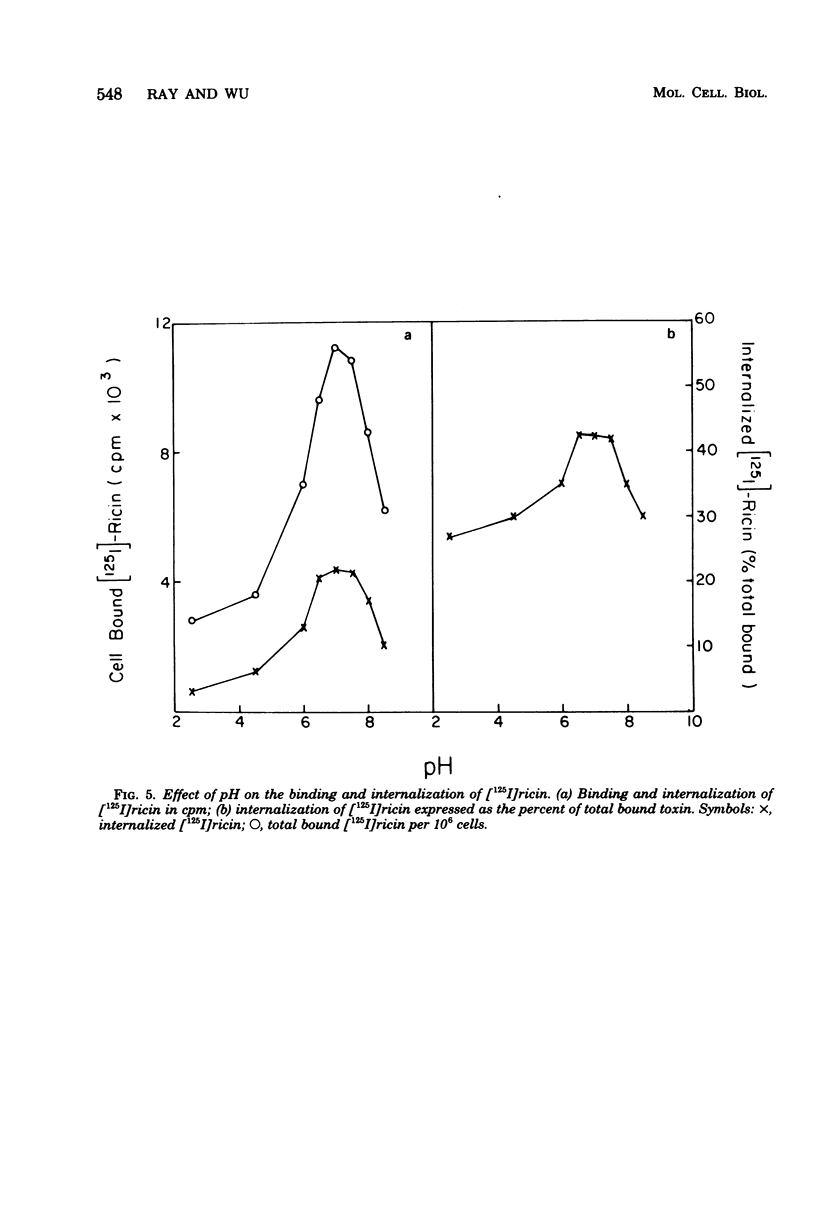
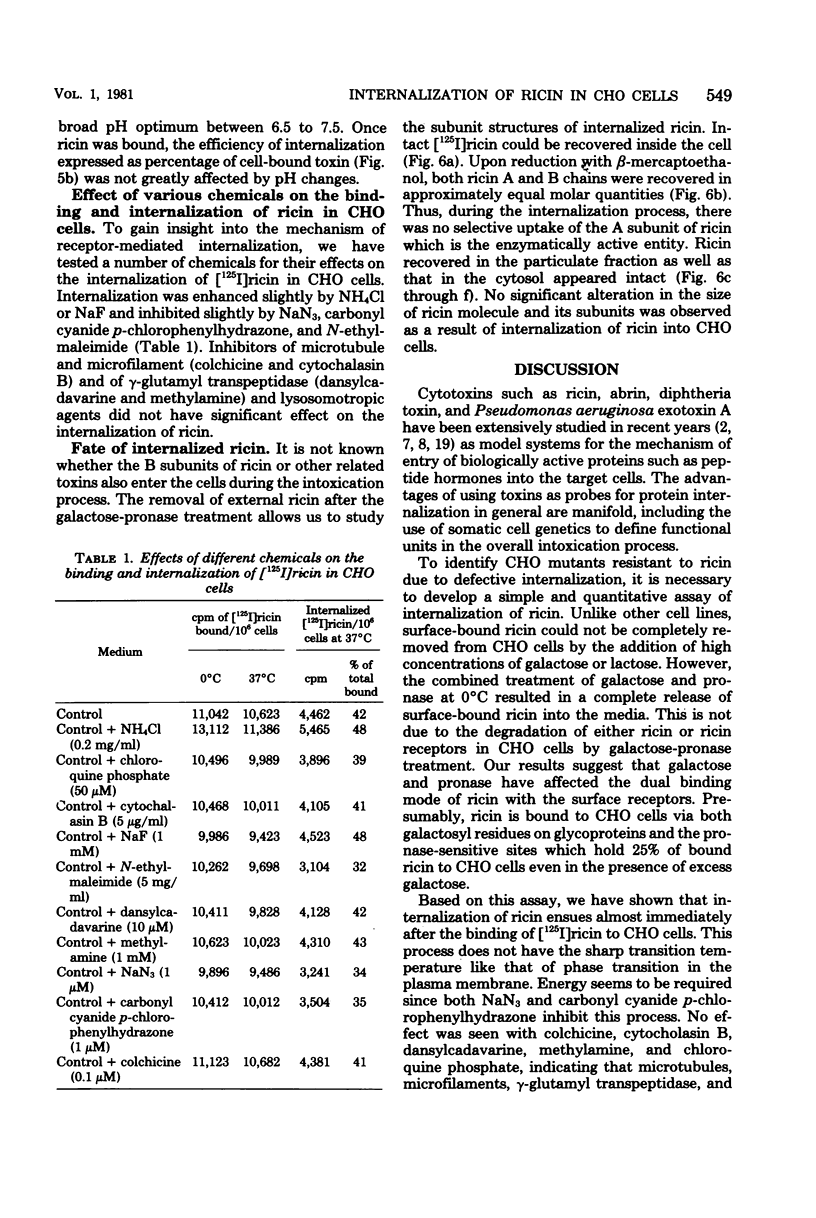
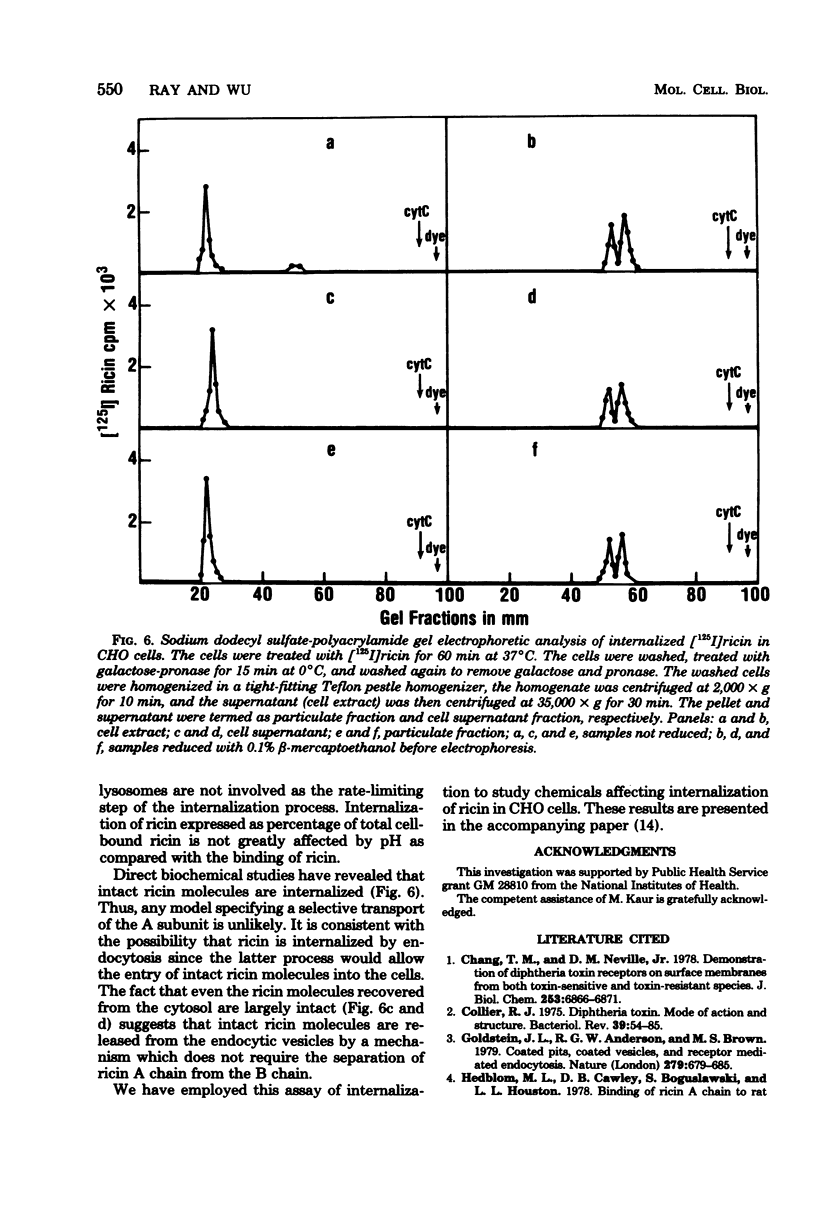
Internalization of ricin into Chinese hamster ovary cells has been investigated. Combined treatment with galactose and pronase at 0 degrees C resulted in a complete release of surface-bound [125I]ricin into the media. Galactose-pronase-resistant cell-bound [125I]ricin represents internalized ricin molecules inside the cells. The internalization process is time, temperature, and concentration dependent. The pH optimum of internalization of ricin is about pH 7. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis has revealed that intact ricin molecules are internalized. Neither reduction nor proteolytic processing of ricin is required for the entry of ricin into Chinese hamster ovary cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang T., Neville D. M., Jr Demonstration of diphtheria toxin receptors on surface membranes from both toxin-sensitive and toxin-resistant species. J Biol Chem. 1978 Oct 10;253(19):6866–6871. [PubMed] [Google Scholar]
- Collier R. J. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975 Mar;39(1):54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Hedblom M. L., Cawley D. B., Boguslawski S., Houston L. L. Binding of ricin A chain to rat liver ribosomes: relationship to ribosome inactivation. J Supramol Struct. 1978;9(2):253–268. doi: 10.1002/jss.400090210. [DOI] [PubMed] [Google Scholar]
- Lugnier A. A., Creppy E. E., Le Meur M. A., Gerlinger P., Dirheimer G. Reciprocal interactions of ricin from Ricinus communis L. seeds with eukaryote ribosomes. FEBS Lett. 1977 Apr 15;76(2):166–172. doi: 10.1016/0014-5793(77)80144-0. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Differential chemical protection of mammalian cells from the exotoxins of Corynebacterium diphtheriae and Pseudomonas aeruginosa. Infect Immun. 1977 Apr;16(1):232–239. doi: 10.1128/iai.16.1.232-239.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Lacorbiere M., Hunter T. R. Mechanism of cell entry and toxicity of an affinity- purified lectin from Ricinus communis and its differential effects on normal and virus-transformed fibroblasts. Cancer Res. 1975 Jan;35(1):144–155. [PubMed] [Google Scholar]
- Olsnes S., Pihl A. Isolation and properties of abrin: a toxic protein inhibiting protein synthesis. Evidence for different biological functions of its two constituent-peptide chains. Eur J Biochem. 1973 May;35(1):179–185. doi: 10.1111/j.1432-1033.1973.tb02823.x. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Refsnes K., Christensen T. B., Pihl A. Studies on the structure and properties of the lectins from Abrus precatorius and Ricinus communis. Biochim Biophys Acta. 1975 Sep 9;405(1):1–10. doi: 10.1016/0005-2795(75)90308-6. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Saltvedt E., Pihl A. Isolation and comparison of galactose-binding lectins from Abrus precatorius and Ricinus communis. J Biol Chem. 1974 Feb 10;249(3):803–810. [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- Ray B., Wu H. C. Enhanced internalization of ricin in nigericin-pretreated Chinese hamster ovary cells. Mol Cell Biol. 1981 Jun;1(6):560–567. doi: 10.1128/mcb.1.6.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refsnes K., Olsnes S., Pihl A. On the toxic proteins abrin and ricin. Studies of their binding to and entry into Ehrlich ascites cells. J Biol Chem. 1974 Jun 10;249(11):3557–3562. [PubMed] [Google Scholar]
- Sandvig K., Olsnes S., Pihl A. Kinetics of binding of the toxic lectins abrin and ricin to surface receptors of human cells. J Biol Chem. 1976 Jul 10;251(13):3977–3984. [PubMed] [Google Scholar]
- Tomita M., Kurokawa T., Onozaki K., Ichiki N., Osawa T., Ukita T. Purification of galactose-binding phytoagglutinins and phytotoxin by affinity column chromatography using sepharose. Experientia. 1972 Jan 15;28(1):84–85. doi: 10.1007/BF01928278. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wei C. H., Hartman F. C., Pfuderer P., Yang W. K. Purification and characterization of two major toxic proteins from seeds of Abrus precatorius. J Biol Chem. 1974 May 25;249(10):3061–3067. [PubMed] [Google Scholar]



