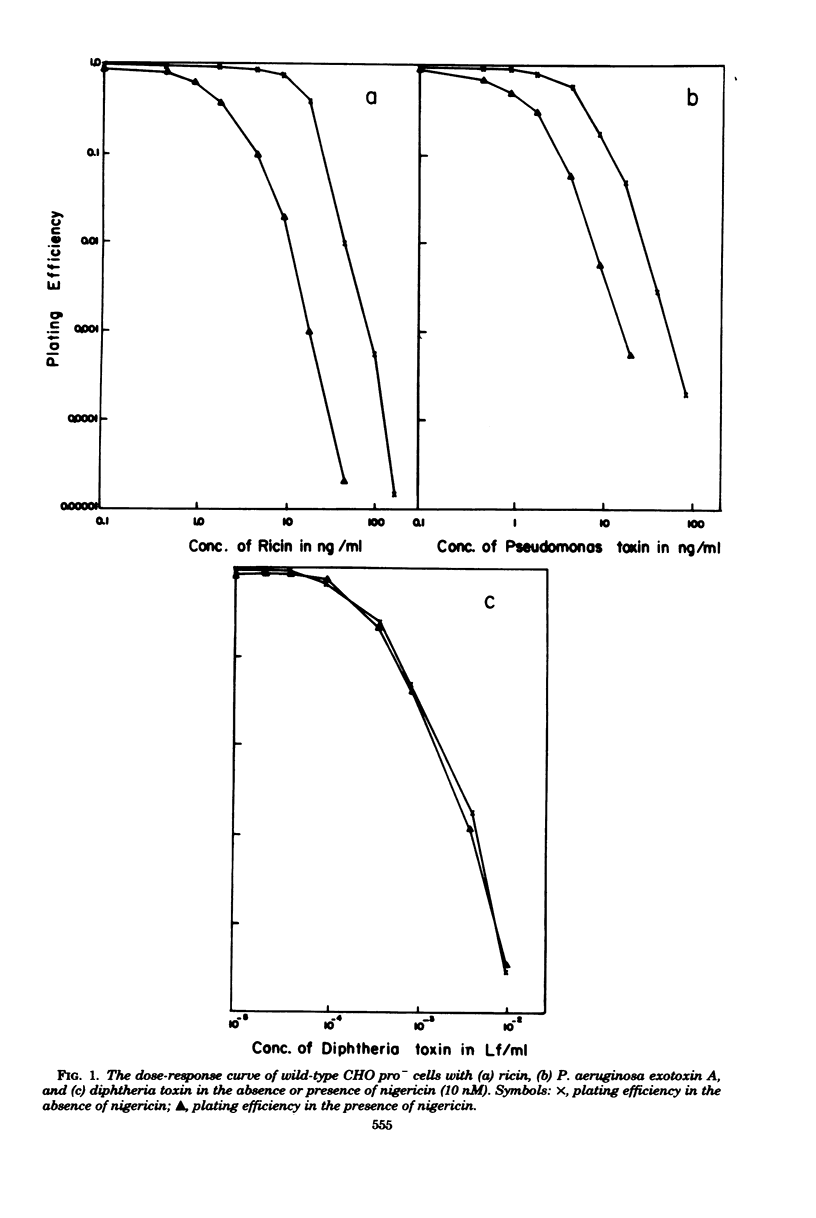
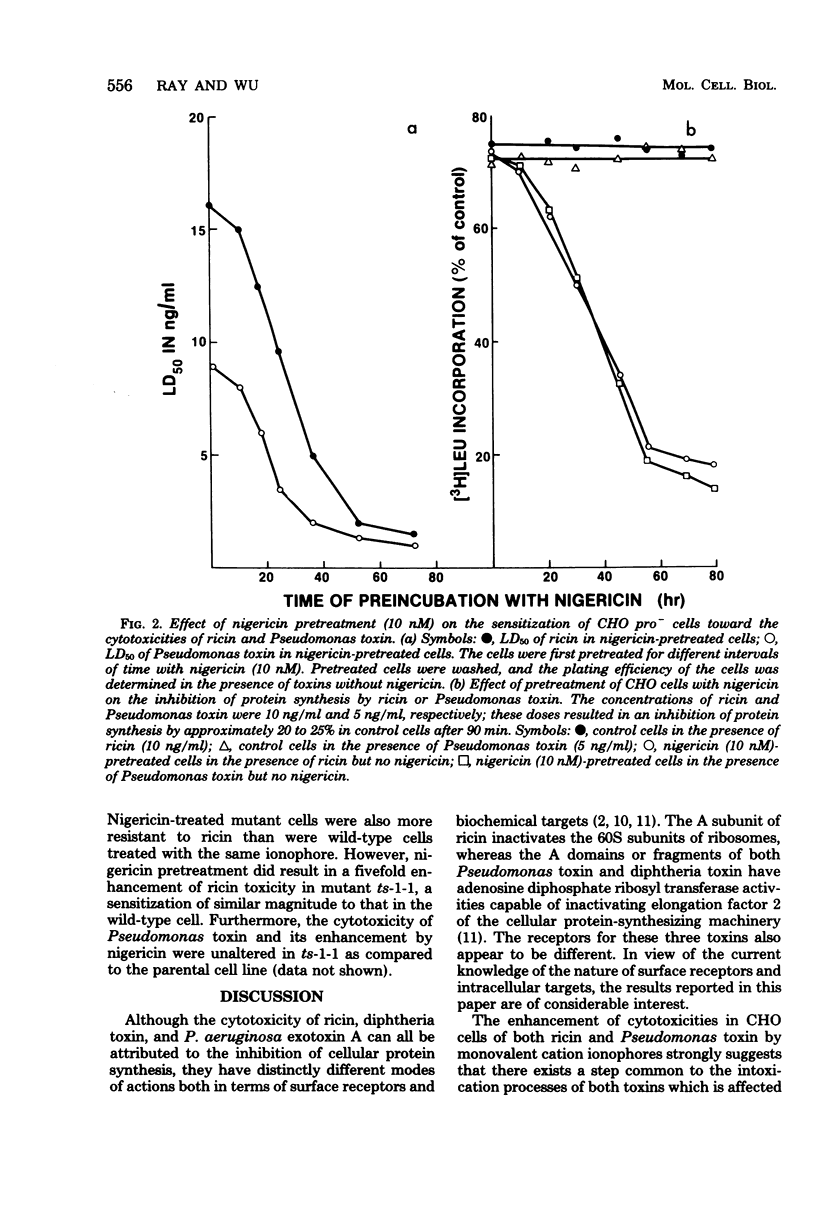
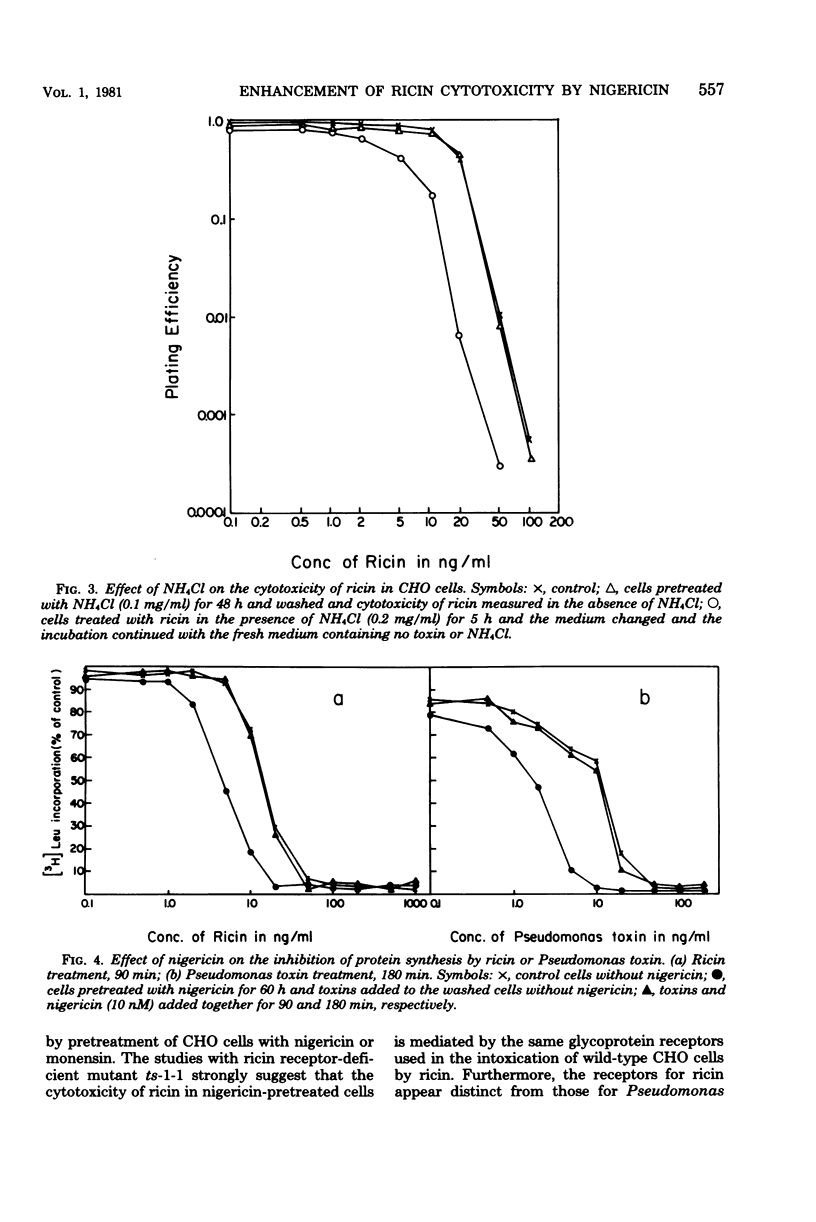
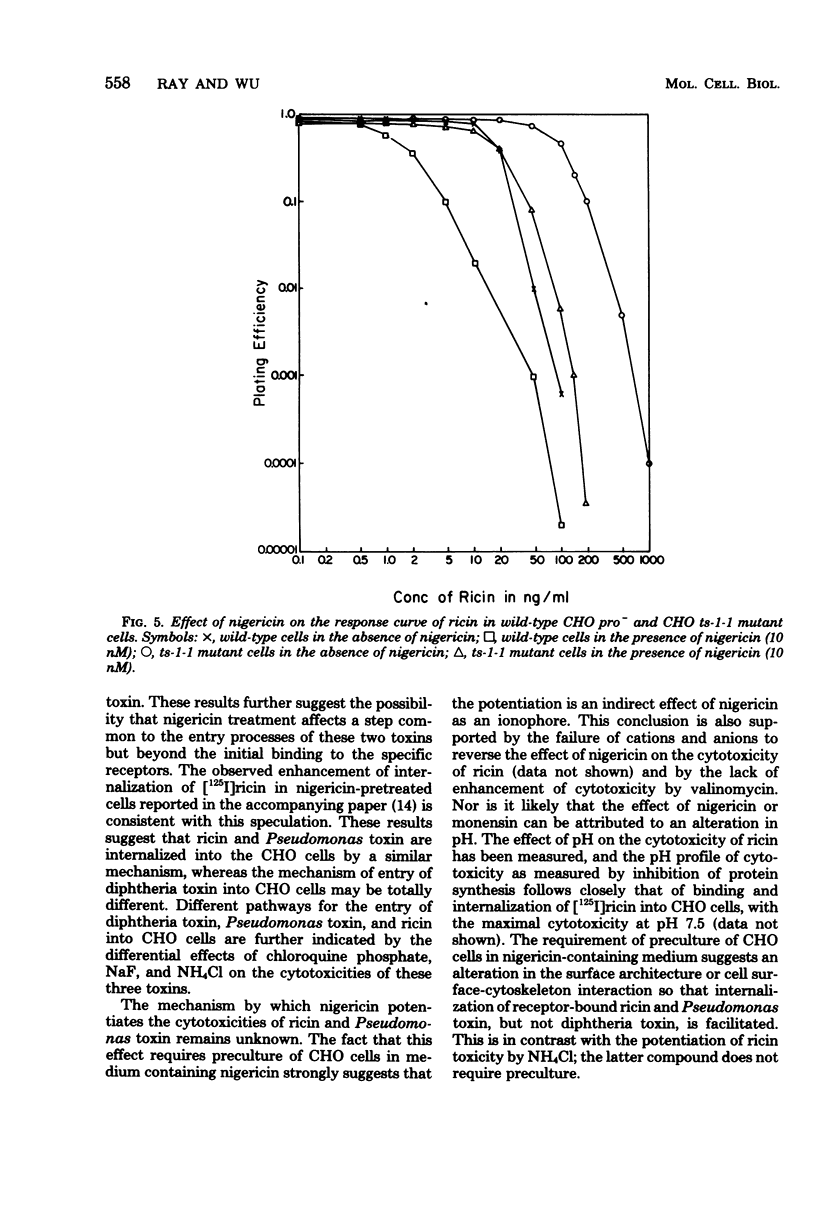
Abstract
Nigericin and monensin, ionophores for Na+ and K+, have been found to enhance the cytotoxicities of abrin, ricin, and Pseudomonas aeruginosa exotoxin A in Chinese hamster ovary (CHO) cells. They do not affect the cytotoxicity of diphtheria toxin in the same cell line. Maximal sensitization of the CHO cells toward ricin and Pseudomonas toxin requires preculture of CHO cells in the presence of nigericin. Inhibition of protein synthesis in CHO cells by ricin or Pseudomonas toxin is also enhanced by preculture of CHO cells in the presence of nigericin. These results suggest a common step in the intoxication process of ricin and Pseudomonas toxin, the rate of which is facilitated by pretreatment with nigericin. This step is, however, not shared by the intoxication of CHO cells with diphtheria toxin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Everse J., Lappi D. A., Beglau J. M., Lee C. L., Kaplan N. O. Investigations into the relationship between structure and function of diphtheria toxin. Proc Natl Acad Sci U S A. 1977 Feb;74(2):472–476. doi: 10.1073/pnas.74.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedblom M. L., Cawley D. B., Boguslawski S., Houston L. L. Binding of ricin A chain to rat liver ribosomes: relationship to ribosome inactivation. J Supramol Struct. 1978;9(2):253–268. doi: 10.1002/jss.400090210. [DOI] [PubMed] [Google Scholar]
- Kim K., Groman N. B. Mode of inhibition of diphtheria toxin by ammonium chloride. J Bacteriol. 1965 Dec;90(6):1557–1562. doi: 10.1128/jb.90.6.1557-1562.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. C., Hernaez-Davis L., Cuatrecasas P. Lysomotropic amines cause intracellular accumulation of receptors for epidermal growth factor. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3283–3287. doi: 10.1073/pnas.77.6.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppla S. H. Large-scale purification and characterization of the exotoxin of Pseudomonas aeruginosa. Infect Immun. 1976 Oct;14(4):1077–1086. doi: 10.1128/iai.14.4.1077-1086.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppla S., Dorland R. B., Middlebrook J. L. Inhibition of diphtheria toxin degradation and cytotoxic action by chloroquine. J Biol Chem. 1980 Mar 25;255(6):2247–2250. [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Differential chemical protection of mammalian cells from the exotoxins of Corynebacterium diphtheriae and Pseudomonas aeruginosa. Infect Immun. 1977 Apr;16(1):232–239. doi: 10.1128/iai.16.1.232-239.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld E. F., Sando G. N., Garvin A. J., Rome L. H. The transport of lysosomal enzymes. J Supramol Struct. 1977;6(1):95–101. doi: 10.1002/jss.400060108. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Fernandez-Puentes C., Carrasco L., Vazquez D. Ribosome inactivation by the toxic lectins abrin and ricin. Kinetics of the enzymic activity of the toxin A-chains. Eur J Biochem. 1975 Dec 1;60(1):281–288. doi: 10.1111/j.1432-1033.1975.tb21001.x. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Refsnes K., Christensen T. B., Pihl A. Studies on the structure and properties of the lectins from Abrus precatorius and Ricinus communis. Biochim Biophys Acta. 1975 Sep 9;405(1):1–10. doi: 10.1016/0005-2795(75)90308-6. [DOI] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- Ray B., Wu H. C. Enhanced internalization of ricin in nigericin-pretreated Chinese hamster ovary cells. Mol Cell Biol. 1981 Jun;1(6):560–567. doi: 10.1128/mcb.1.6.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilowitz H. Monovalent ionophores inhibit acetylcholinesterase release from cultured chick embryo skeletal muscle cells. Mol Pharmacol. 1979 Jul;16(1):202–214. [PubMed] [Google Scholar]
- Tartakoff A. M., Vassalli P. Plasma cell immunoglobulin secretion: arrest is accompanied by alterations of the golgi complex. J Exp Med. 1977 Nov 1;146(5):1332–1345. doi: 10.1084/jem.146.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N., Smilowitz H., Tanzer M. L. Monovalent ionophores inhibit secretion of procollagen and fibronectin from cultured human fibroblasts. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1868–1872. doi: 10.1073/pnas.76.4.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]



