Abstract
Purpose
Pancreatic adenocarcinoma is an untreatable cancer with a 5-year survival rate of about 6 % or less for the past 35 years. This lack of significant progress is largely due to the lack of elucidation and understanding of the factors involved in the development of this cancer. Recent studies identified and implicated zinc in the development and progression of pancreatic cancer. This study was conducted to establish the changes in zinc, ZIP3 zinc transporter, and Ras-responsive element-binding protein 1 (RREB-1) transcription factor as early events in the development of malignancy.
Methods
In situ relative zinc determination and immunohistochemical analysis of ZIP3 and RREB-1 were performed on archived human pancreatic tissue sections and tissue micro-arrays. Normal/benign versus adenocarcinoma pancreas was compared. Panc1 cells were employed to determine the influence of RREB-1 on ZIP3 expression.
Results
Zinc levels of normal ductal and acinar epithelium were markedly and consistently decreased in adenocarcinoma. Pancreatic intraepithelial neoplasia (PanIN) lesions also exhibited a loss of zinc. ZIP3 and RREB-1 were also markedly downregulated. Initial results indicate that RREB-1 regulates ZIP3 expression.
Conclusions
These results corroborate the earlier report that zinc, ZIP3, and RREB-1 are markedly decreased in early stage adenocarcinoma. Additionally and most importantly, these changes occur in PanIN, which are thought to be precancerous lesions leading to ductal adenocarcinoma. These results support a concept that downregulation of RREB-1 causes downregulation of ZIP3, which results in decreased zinc in premalignant and carcinoma cells. The decrease in zinc is essential to remove its cytotoxic effects on malignant cells. This relationship constitutes a new concept of early genetic/metabolic events in the progressive transformation of normal cells to premalignant cells to malignant cells in the development of pancreatic cancer.
Keywords: Pancreatic cancer, Zinc, ZIP3, RREB-1, PanIN
Introduction
Pancreatic cancer (adenocarcinoma) is a highly lethal disease with the worst prognosis of all the major malignancies. Of the ~37,000 new cases/year, 33,000 will result in death [1]. The lack of significant progress in dealing with pancreatic cancer is evident from the 5-year survival rate, which has remained at ~6 % or less for the past 35 years. Such statistics demonstrate the absence of and need for effective treatment of pancreatic cancer once it has been diagnosed and for effective biomarkers for early detection. A major contributing factor for this lack of significant progress is the lack of elucidation and poor understanding of the factors and events involved in the development of malignancy and its progression to advanced stages.
Recent studies [2] have implicated zinc as an important factor in the development and progression of pancreatic cancer. The cellular zinc levels are markedly decreased in ductal and acini epithelium in adenocarcinoma as compared to the higher zinc levels in the normal ductal and acini epithelium. The decrease in zinc is evident in well-differentiated malignancy and persists in the advancement to moderately and poorly differentiated malignancy. Concurrent with the decrease in zinc, ZIP3 zinc uptake transporter and RREB-1 (Ras-responsive element-binding protein 1) transcription factor are down-regulated. The fact that these changes are evident in the early stage adenocarcinoma is indicative of the likelihood that the changes are initiated in the transformation of the normal cell to the malignant cell. As such, one could expect that the decrease in zinc and the downregulation of ZIP3 and RREB-1 will be evident in premalignant cells/lesions that lead to the development of malignant cells. Presently, pancreatic intraepithelial neoplasia (PanIN) is considered to be precancerous lesions that give rise to the malignant cells [3–5].
In this report, we have focused on the identification of decreased zinc and downregulation of ZIP3 and RREB-1 in the transformation of the normal epithelium to early stage malignancy, with focus on PanIN lesions. We also further corroborate the decrease in zinc in adenocarcinoma as compared to normal/benign pancreatic epithelium.
Material and Methods
Zinc, Zip3, and RREB-1 levels were determined and compared in archived pancreatic tissue sections provided by Dr. Desouki and tissue micro-array (TMA) slides (obtained from US Biomax, Inc). The study included a total of 26 cancer cases and 18 normal/benign cases. The results presented below are representative of the status that exists in these cases.
The dithizone (DTZ) staining for zinc and the immunohistochemistry (IHC) for ZIP3 and RREB-1 are described in [2]. For capturing and storing digital images of the slide sections and cores, we employed the Aperio ScanScope Digital Slide Scanner system and/or the Reichert Omega Model #4000 microscopy system equipped with high-definition, two-megapixel cameras and a desktop computer [2]. Because the intensity of the staining methods has considerable variability, all studies were conducted simultaneously with a normal/benign tissue and an adenocarcinoma tissue to establish relative differences under the same staining conditions.
Panc1 cells obtained from ATCC were cultured under standard conditions in DMEM medium supplemented with 2 % FBS and 1 % penicillin–streptomycin mixture [2]. RREB-1 siRNA and non-targeting siRNAwere obtained from Dharmacon RNAi Technologies. Twenty-four hours after plating in 12-well plates, the Panc1 cells were transfected with 50 nM RREB-1 siRNA or non-targeting siRNA using DharmaFECT 2 Transfection Reagent (Dharmacon) following the manufacturer’s instructions. Forty-eight hours after the siRNA transfections, the cells were either fixed and stained for ZIP3 or RREB-1 by IHC or collected, and whole cell lysates were prepared for western blot analysis of ZIP3 and RREB-1 abundance.
For western blot analysis, proteins in the whole cell lysates were separated by SDS-PAGE gel electrophoresis, transferred to nitrocellulose membranes, and incubated overnight with ZIP3 antibody. The nitrocellulose membranes were then stripped of antibody and re-probed using RREB-1 antibody. The same membranes were then re-probed with β-actin antibody for loading controls (ICN Biomedicals).
Results
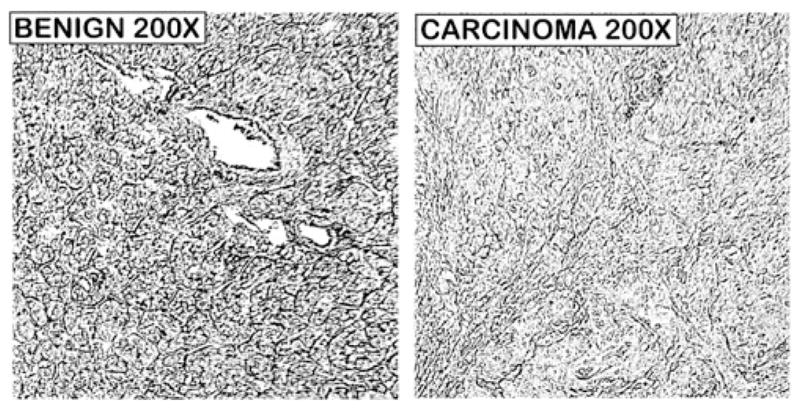
Figure 1 is a typical representation of the comparative in situ DTZ staining for relative levels of zinc in normal/benign versus adenocarcinoma tissue sections. The Zn-DTZ complex is visualized mainly as black pigments. Figure 1 compares a tissue section from a non-cancer case versus a tissue section from a confirmed pancreatic adenocarcinoma case. The benign tissue section exhibits high Zn-DTZ staining of the acini and ductal epithelium. In contrast, a major loss of zinc is apparent in the adenocarcinoma section. In addition, the PanIN lesion epithelium as well as the carcinoma cells exhibits the loss of zinc. This specific loss of detectable zinc is further confirmed by the retention of high Zn-DTZ staining of the islet cells in the adenocarcinoma field. Islet beta cells are known to contain high zinc levels.
Fig. 1.
DTZ staining of relative zinc levels in unmatched pancreatic benign versus adenocarcinoma tissue sections
Figure 2 compares zinc levels of pancreatic adenocarcinoma and an adjacent matched benign tissue. The benign tissue section exhibited ducts and acini that contained relatively high Zn-DTZ staining in contrast to the loss of zinc in the adenocarcinoma tissue section.
Fig. 2.
DTZ staining of relative zinc levels in adjacent pancreatic benign versus adenocarcinoma tissue sections obtained from a confirmed cancer case
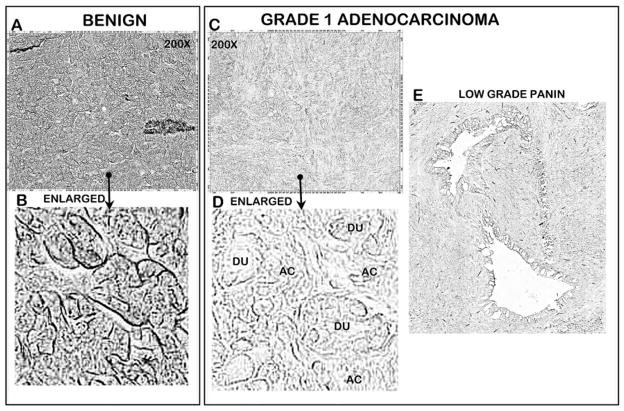
Figure 3 shows the loss of zinc in a very early well-differentiated adenocarcinoma. The section shows the presence of well populated acini along with well-differentiated malignant ducts (compare Fig. 3b with d), all of which exhibit zinc loss. Also apparent is the loss of zinc in a very early low-grade PanIN epithelium.
Fig. 3.
DTZ staining of relative zinc levels in a benign tissue section versus a well-differentiated (grade 1) adenocarcinoma. DU duct, AC acini
Another case (Fig. 4) provided an archived tissue section that had been confirmed as adenocarcinoma. However, upon applying DTZ staining, a small region was noticeably heavily stained as compared to the expected very low Zn-DTZ staining of the remainder of the cancer tissue section. Higher magnification revealed that the heavily stained region was populated with “normal appearing acini and ducts”, which would be consistent with its higher Zn levels as compared to the very low Zn level in the main adenocarcinoma region of the tissue section.
Fig. 4.
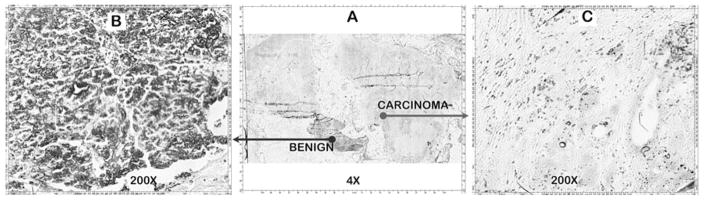
DTZ staining of relative zinc levels in an adenocarcinoma tissue section that included an adjacent benign tissue area
We then performed ZIP3 IHC on serial sections of the normal and adenocarcinoma sections employed for DTZ staining in Fig. 1. Figure 5 shows the high abundance of ZIP3 transporter with localization at the plasma membrane of the ductal and acinar epithelium. In contrast, ZIP3 transporter is virtually absent in adenocarcinoma and in PanIN. Similarly, ZIP3 IHC of normal pancreas and adenocarcinoma TMA cores (Fig. 6) also reveals high abundance of ZIP3 transporter, especially basilar membrane localization, in the normal epithelium. In contrast, ZIP3 transporter is virtually absent in carcinoma and PanIN epithelium. Figure 7 also shows the loss of basal membrane ZIP3 transporter in malignant duct and in the epithelium of a PanIN-1A early lesion. The consistent loss of ZIP3 is especially important because the basilar transporter in normal ductal and acinar epithelium is responsible for the cellular uptake and accumulation of zinc. Therefore, the absence of the transporter is likely an important factor in the decrease of zinc in carcinoma and PanIN.
Fig. 5.
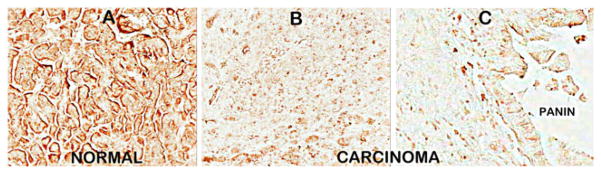
ZIP3 transporter IHC of the same benign and carcinoma tissue sections that were employed in Fig. 1. Note the distinct basal membrane localization of transporter in the normal ductal/acini epithelium, which is absent in adenocarcinoma cells and PanIN epithelium
Fig. 6.
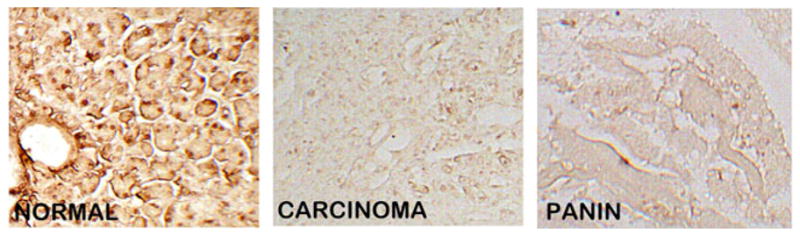
ZIP3 transporter IHC of TMA normal versus carcinoma cores. Note the distinct basal membrane localization of transporter in the normal ductal/acini epithelium, which is absent in adenocarcinoma cells and PanIN epithelium
Fig. 7.
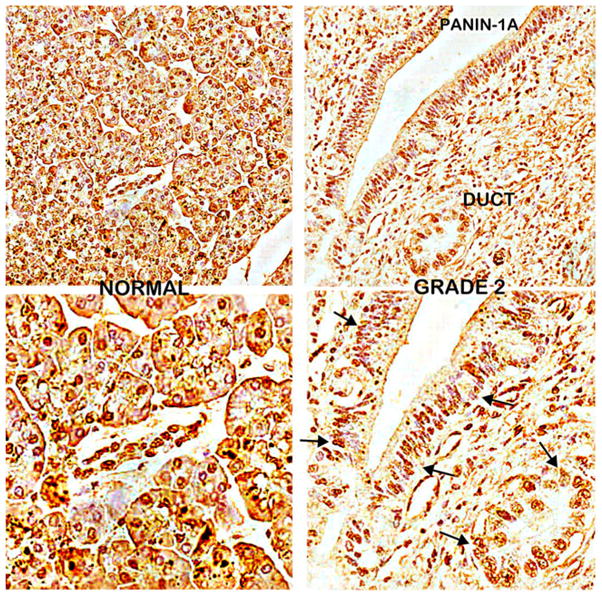
ZIP3 transporter IHC of normal epithelium as compared to ductal carcinoma and an early PanIN-1A lesion. Note the loss of basal membrane ZIP3 transporter in malignant duct and in the epithelium of the PanIN-1A lesion
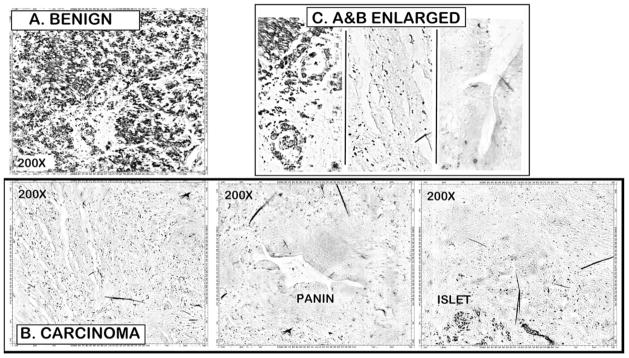
Figure 8 shows the RREB-1 IHC of serial sections of the DTZ-stained slides shown in Fig. 2. The benign tissue section from this adenocarcinoma case shows distinct nuclear staining of the ductal and acinar epithelium. In contrast, the carcinoma tissue section shows a marked decrease or absence of nuclear REBB-1 transcription factor. Figure 9 compares RREB-1 in cases of cancer adjacent normal cores versus adenocarcinoma cores. Nuclear RREB-1 staining is prominent in the normal ductal and acinar epithelium. In contrast, RREB-1 is markedly decreased in adenocarcinoma and in PanIN lesions.
Fig. 8.
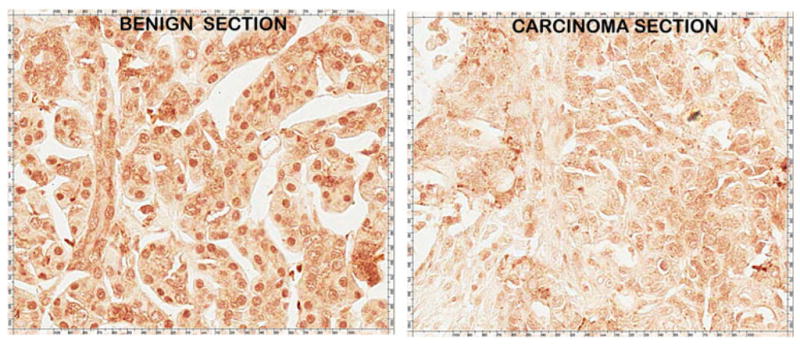
RREB-1 IHC of serial sections of the adjacent pancreatic benign versus adenocarcinoma tissue sections that were employed in Fig. 2. Note the distinct heavy nuclear staining of the benign epithelium, which is lost in adenocarcinoma
Fig. 9.
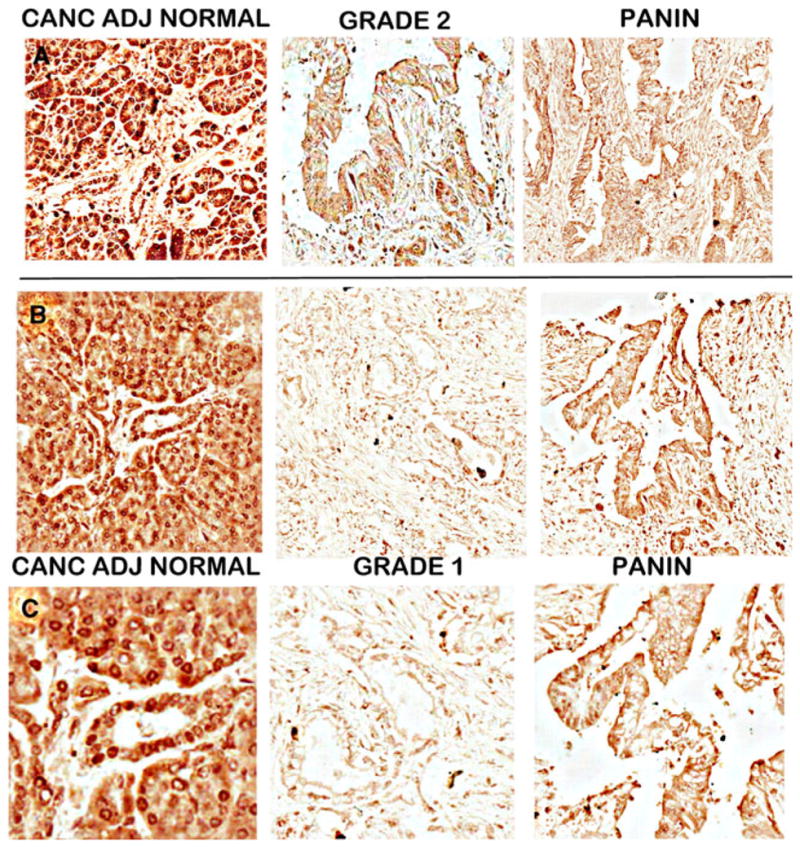
RREB-1 IHC of TMA normal versus carcinoma cores. A and B are different cases of cancer adjacent normal and carcinoma cores. C is an enlargement of B to show detail of RREB-1 nuclear staining. Note the distinct heavy nuclear staining of the normal epithelium, which is absent in adenocarcinoma and PanIN epithelium
Figure 10 compares the RREB-1 abundance in tissue sections from a benign case with chronic pancreatitis evidenced by fibrous stroma, paucity of ductal and acinar epithelium as well as infiltration with mononuclear inflammatory cells versus a adenocarcinoma case. The benign ductal and acinar epithelium exhibited strong REBB-1 nuclear staining. In contrast, the adenocarcinoma and intermediate grade PanIN epithelium exhibit marked loss of RREB-1. It is especially important to note that the benign acini as well as ductal epithelium are architecturally distorted and markedly atrophic due to the chronic pancreatitis. Despite this, the expression of RREB-1 is retained as in normal pancreas. This provides evidence that the down-regulation of RREB-1 in carcinoma and in PanIN is a cancer-specific effect.
Fig. 10.
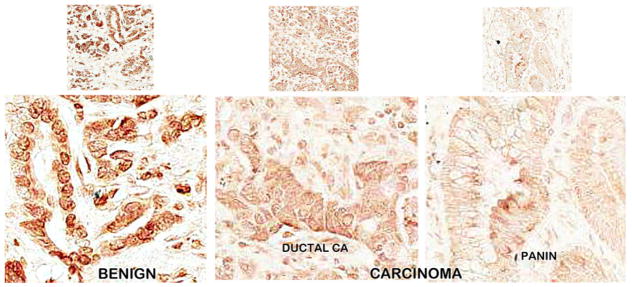
RREB-1 IHC of benign versus adenocarcinoma tissue sections. The benign section is from a chronic pancreatitis case and exhibits some pathological appearance. Note the distinct heavy nuclear staining of the benign epithelium, which is lost in adenocarcinoma and PanIN epithelium
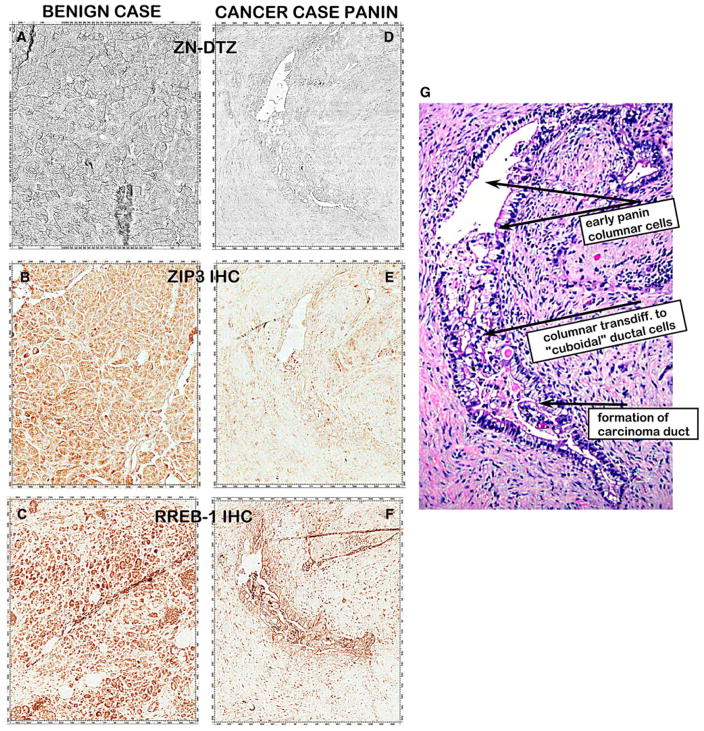
Figure 11 shows the corresponding decrease in zinc, ZIP3, and RREB-1 in the same PanIN lesion from a carcinoma case versus the levels in a benign tissue. This was possible since we had serial sections of this carcinoma tissue. The PanIN DTZ and ZIP3 IHC staining was performed on the same tissue section for the same PanIN lesion. The PanIN RREB-1 staining was performed on the next serial section that was stained for Zn-DTZ and ZIP3 shown in Fig. 1d, e. The results show that the distinct decrease in zinc, ZIP3, and RREB-1 exists simultaneously in the same PanIN lesion. Also evident is the apparent decreases in the initial PanIN columnar cells, in the transdifferentiated “cuboidal-like” cells, and in the released cuboidal adenocarcinoma cells that are forming adenocarcinoma ducts.
Fig. 11.
H&E, DTZ stain and ZIP3 and RREB-1 IHC of the same PanIN lesion from a carcinoma tissue versus benign tissue section. D and E are the same tissue section; F and G are adjacent serial sections of the D and E section
The consistent concurrent decrease in RREB-1 and ZIP3 is suggestive of the possible involvement of RREB-1 in the silencing of ZIP3 in the development of adenocarcinoma. We previously reported [2] that Panc1 cells, a cell line developed from ductal adenocarcinoma, exhibit ZIP3 expression although ZIP3 is downregulated in situ. Figure 12 shows the nuclear staining of RREB-1, which demonstrates that it is also re-expressed in Panc1 cells along with ZIP3, and this is corroborated by the western blot demonstration of RREB-1 and ZIP3. We then determined if downregulation of RREB-1in the Panc1 cells would result in a corresponding decrease in ZIP3, and this was confirmed by the corresponding decreases in REBB-1 and ZIP3 (Fig. 12b). Moreover, we have identified RREB-1 consensus sequences in the ZIP3 gene (unpublished information). Collectively, the clinical studies and these initial experimental results provide evidence that the concurrent decrease in RREB-1 in PanIN and in adenocarcinoma contributes to the silencing of ZIP3.
Fig. 12.
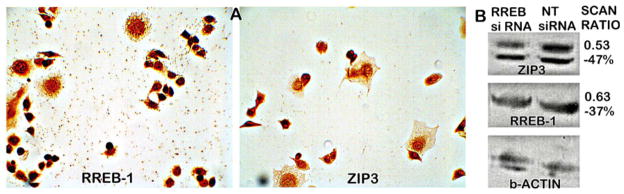
A RREB-1 and ZIP3 IHC of Panc1 cells. Note the heavy nuclear staining of RREB-1 and the membrane and cytosol staining of ZIP3. B Western blot of Panc1 cells showing downregulation by RREB-1 siRNA compared to the non-target (NT) siRNA control. “Scan ratio” is determined from the optical density of the blots adjusted to b-actin. The ratio is the adjusted RREB siRNA/non-target siRNA
Discussion
These results corroborate our earlier report that cellular zinc levels are markedly decreased in pancreatic adenocarcinoma as compared to normal ductal and acinar epithelium. Additionally and most importantly, we now show that the decrease in zinc is also evident in PanIN epithelium through all stages of PanIN development including the putative invasive ductal adenocarcinoma cells.
These combined studies of relative zinc levels in pancreatic tissues establish the new relationship that zinc levels are decreased in pancreatic adenocarcinoma. Most surprisingly, our studies represent the only reports of direct determination of zinc levels in pancreatic normal exocrine and adenocarcinoma tissues. The lone exception is a preliminary study with only one tissue sample, which suggested that a decrease in zinc level might be associated with pancreatic adenocarcinoma [6]. However, another report [7] concluded that zinc levels are increased in pancreatic adenocarcinoma, but no direct measurements of the zinc levels in human pancreatic tissues were performed or provided. Our combined reports that collectively involved zinc level determinations in numerous pancreatic tissue samples have not shown any evidence of an increase in zinc levels in adenocarcinoma.
The results of this study also corroborate the down-regulation of ZIP3 transporter and RREB-1 transcription factor concurrently with the decrease in zinc and that these changes are evident in early stage malignancy and persist in advancing malignancy. This indicated that these concurrent events must have been initiated prior to their appearance in well-differentiated malignancy, most likely in premalignant lesions/cells that progress to malignant cells. For this reason, we focused on the identification of these changes in PanIN lesions. It is now apparent that the decrease in zinc and down-regulation of ZIP3 and RREB-1 exist in PanIN epithelium as well as in early stage malignancy. The concurrent loss of zinc, ZIP3, and REBB-1 is evident in the early stage PanIN epithelium and persists though the PanIN transformation to the putative invasive ductal adenocarcinoma cells and ultimately persists in early stage malignancy through the advanced stage malignancy. A prevailing view is that PanINs, especially PanIN 2 and PanIN 3, are precancer lesions that give rise to ductal adenocarcinoma (for reviews, see [3–5]). Scarlett et al. [3] have presented the concept of the involvement of PanIN lesions in the transformation of normal ductal epithelium to ductal adenocarcinoma, which is shown in Fig. 13. The results that we have achieved mimic their concept and implicate the loss of zinc and downregulation of ZIP3 and RREB-1 at the initial transformation of the normal ductal epithelium to the early PanIN development, which is sustained through the PanIN transformation to ductal adenocarcinoma.
Fig. 13.
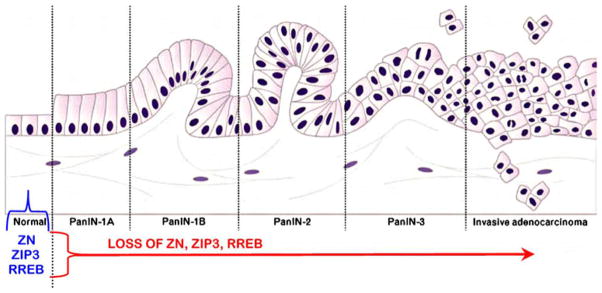
The relationship of zinc, ZIP3, and RREB-1 changes to the concept of PanIN as the precancer lesion in the development of ductal carcinoma [3]. Modified from [3] with permission of the publisher
It is now apparent that the decrease in zinc and the down-regulation of ZIP3 and RREB-1 are early pancreatic carcinogenic events that occur in the progressive transformation of the normal cell to the malignant cell. The consistency of these concurrent changes supports the concept that they are related events. ZIP3 transporter localization at the basilar membrane of the normal ductal and acinar epithelium is compelling evidence that it functions to take up zinc from circulation to maintain the required normal level of cellular zinc. Its down-regulation in PanIN and in adenocarcinoma results in decreased zinc uptake and cellular accumulation. Therefore, the silencing of ZIP3 expression resulting in the loss of the transporter is an important factor that results in the decrease in zinc in adenocarcinoma. RREB-1 is a zinc finger transcription factor that has been described either as an activator of gene transcription [8–11], or as a repressor of gene expression [12–15], the action of which is dependent upon the gene and cell type [16]. Our clinical and experimental evidence leads to the expectation that RREB-1 is a positive modulator of ZIP3 gene expression in normal ductal and acinar epithelium, and the downregulation of REBB-1 in PanIN and adenocarcinoma contributes to the silencing of ZIP3 expression. We are now proceeding with studies to establish the role of RREB-1 in the regulation of ZIP3 expression.
The decrease in zinc in PanIN lesions and adenocarcinoma is likely required to prevent the cytotoxic effects that normal cellular levels of zinc impose on malignant cells. Exposure of malignant pancreatic cells to physiological levels of zinc under conditions that increase the cellular accumulation of zinc results in inhibition of cell growth [2, 17, 18]. Therefore, it is plausible to expect that in situ restoration of increased zinc levels in precancerous lesions and in carcinoma cells will prevent and/or abort pancreatic malignancy. However, a critical revelation of this study and our previous report is the in situ silencing of ZIP3 in the development and progression of adenocarcinoma. This condition likely necessitates that any potential zinc treatment or zinc prevention approach will require a vehicle or process that facilitates the uptake of zinc by the malignant or premalignant cells. This relationship could lead to the development of a zinc-associated chemotherapeutic agent to combat, and possibly prevent, this untreatable deadly cancer.
Acknowledgments
We thank Lippincott Williams and Wilkins for granting permission to reproduce and modify Fig. 13 taken from Scarlett et al. [3].
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Contributor Information
Leslie C. Costello, Email: lcostello@umaryland.edu, Department of Oncology and Diagnostic Sciences, University of Maryland Dental School, 650 West Baltimore Street, Baltimore, MD 21201, USA. The University of Maryland Greenebaum Cancer Center, Baltimore, MD 21201, USA
Jing Zou, Department of Oncology and Diagnostic Sciences, University of Maryland Dental School, Baltimore, MD 21201, USA.
Mohamed Mokhtar Desouki, Department of Pathology (Division of Breast/Ob/Gyn), Magee-Womens Hospital, University of Pittsburgh School of Medicine, Pittsburgh, PA 15213, USA.
Renty B. Franklin, Department of Oncology and Diagnostic Sciences, University of Maryland Dental School, 650 West Baltimore Street, Baltimore, MD 21201, USA. The University of Maryland Greenebaum Cancer Center, Baltimore, MD 21201, USA
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Costello LC, Levy B, Desouki M, Zou J, Bagasra O, Johnson L, Hanna N, Franklin RB. Decreased zinc and down regulation of ZIP3 zinc uptake transporter in the development of pancreatic adenocarcinoma. Canc Biol Ther. 2011;12:297–303. doi: 10.4161/cbt.12.4.16356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scarlett CJ, Salisbury EL, Biankin AV, Kench J. Precursor lesions in pancreatic cancer: morphological and molecular pathology. Pathol. 2011;43:183–200. doi: 10.1097/PAT.0b013e3283445e3a. [DOI] [PubMed] [Google Scholar]
- 4.Liszka L, Pająk J, Mrowiec S, Zielińska-Pająk E, Gołka D, Lampe P. Precursor lesions of early onset pancreatic cancer. Virchows Arch. 2011;4:439–51. doi: 10.1007/s00428-011-1056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Recavarren C, Labow DM, Liang J, Zhang L, Wong M, Zhu H, Wang J, Francis F, Xu R. Histologic characteristics of pancreatic intraepithelial neoplasia associated with different pancreatic lesions. Hum Pathol. 2011;42:18–24. doi: 10.1016/j.humpath.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 6.Danielsen A, Steinnes E. A study of some selected trace elements in normal and cancerous tissue by neutron activation analysis. J Nucl Med. 1970;11:260–4. [PubMed] [Google Scholar]
- 7.Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H, Wang X, Zhang S, Liuzzi JP, Chang SM, Cousins RJ, Fisher WE, Brunicardi FC, Logsdon CD, Chen C, Yao Q. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci U S A. 2007;104:18636–41. doi: 10.1073/pnas.0709307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thiagalingam A, De Bustros A, Borges M, Jasti R, Compton D, Diamond L, Mabry M, Ball DW, Baylin SB, Nelkin BD. RREB-1, a novel zinc finger protein, is involved in the differentiation response to Ras in human medullary thyroid carcinomas. Mol Cell Biol. 1996;16:5335–45. doi: 10.1128/mcb.16.10.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujimoto-Nishiyama A, Ishii S, Matsuda S, Inoue J, Yamamoto T. A novel zinc finger protein, Finb, is a transcriptional activator and localized in nuclear bodies. Gene. 1997;195:267–75. doi: 10.1016/s0378-1119(97)00172-8. [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Hew HC, Lu ZG, Yamaguchi T, Miki Y, Yoshida K. DNA damage signaling recruits RREB-1 to the p53 tumour suppressor promoter. Biochem J. 2009;422:543–51. doi: 10.1042/BJ20090342. [DOI] [PubMed] [Google Scholar]
- 11.Jiang W, Sequeira JM, Nakayama Y, Lai SC, Quadros EV. Characterization of the promoter region of TCblR/CD320 gene, the receptor for cellular uptake of transcobalamin-bound cobalamin. Gene. 2010;15:49–55. doi: 10.1016/j.gene.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukhopadhyay NK, Cinar B, Mukhopadhyay L, Lutchman M, Ferdinand AS, Kim J, Chung LW, Adam RM, Ray SK, Leiter AB, Richie JP, Liu BC, Freeman MR. The zinc finger protein ras-responsive element binding protein-1 is a coregulator of the androgen receptor: implications for the role of the Ras pathway in enhancing androgenic signaling in prostate cancer. Mol Endocrinol. 2007;21:2056–70. doi: 10.1210/me.2006-0503. [DOI] [PubMed] [Google Scholar]
- 13.Flajollet S, Poras I, Carosella ED, Moreau P. RREB-1 is a transcriptional repressor of HLA-G. J Immunol. 2009;183:6948–59. doi: 10.4049/jimmunol.0902053. [DOI] [PubMed] [Google Scholar]
- 14.Milon BC, Agyapong A, Bautista R, Costello LC, Franklin RB. (2010) Ras responsive element binding protein-1 (RREB-1) down-regulates hZIP1 expression in prostate cancer cells. Prostate. 2010;70:118–29. doi: 10.1002/pros.21063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou J, Milon BC, Desouki MM, Costello LC, Franklin RB. hZIP1 zinc transporter down-regulation in prostate cancer involves the overexpression of ras responsive element binding protein-1 (RREB-1) Prostate. 2012 doi: 10.1002/pros.21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oxford G, Smith SC, Hampton G, Theodorescu D. Expression profiling of Ral-depleted bladder cancer cells identifies RREB-1 as a novel transcriptional Ral effector. Oncogene. 2007;26:7143–752. doi: 10.1038/sj.onc.1210521. [DOI] [PubMed] [Google Scholar]
- 17.Jayaraman AK, Jayaraman S. Increased level of exogenous zinc induces cytotoxicity and up-regulates the expression of the ZnT-1 zinc transporter gene in pancreatic cancer cells. J Nutr Biochem. 2011;22:79–88. doi: 10.1016/j.jnutbio.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Donadelli M, Dalla Pozza E, Scupoli MT, Costanzo C, Scarpa A, Palmieri M. Intracellular zinc increase inhibits p53(−/−) pancreatic adenocarcinoma cell growth by ROS/AIF-mediated apoptosis. Biochim Biophys Acta. 2009;1793:273–80. doi: 10.1016/j.bbamcr.2008.09.010. [DOI] [PubMed] [Google Scholar]