Abstract
There has been scant evidence for a phase-shifting effect of melatonin in shift-work or jet-lag protocols. This study tested whether melatonin can facilitate phase shifts in a simulated night-work protocol. Subjects (n = 32) slept in the afternoons/evenings before night work (a 7-h advance of the sleep schedule). They took melatonin (0.5 mg or 3.0 mg) or placebo before the first four of eight afternoon/evening sleep episodes at a time when melatonin has been shown to phase advance the circadian clock. Melatonin produced larger phase advances than placebo in the circadian rhythms of melatonin and temperature. Average phase advances (±SD) of the dim light melatonin onset were 1.7 ± 1.2 h (placebo), 3.0 ± 1.1 h (0.5 mg), and 3.9 ± 0.5 h (3.0 mg). A measure of circadian adaptation, shifting the temperature minimum enough to occur within afternoon/evening sleep, showed that only subjects given melatonin achieved this goal (73% with 3.0 mg, 56% with 0.5 mg, and 0% with placebo). Melatonin could be used to promote adaptation to night work and jet travel.
Keywords: body temperature, jet lag, sleep, sleepiness, shift work, work-schedule tolerance, humans, constant routine
Exogenous administration of the pineal hormone melatonin can produce phase shifts in circadian rhythms, e.g., in the firing rates of suprachiasmatic nuclei neurons in vitro (36), in the activity rhythms (e.g., Refs. 6, 10, 43, 51) and patterns of c-fos expression in rodents (54), and in the rhythms of melatonin secretion (e.g., Refs. 30, 31, 34, 57) and core body temperature (e.g., Refs. 2, 18, 49) in humans. Melatonin can entrain the free-running circadian rhythms of blind people (33, 46) and has been used to treat the symptoms of circadian maladaptation associated with delayed sleep phase syndrome (e.g., Refs. 13, 40, 41, 55). Lewy and colleagues (32) generated a phase-response curve (PRC) to melatonin in humans that demonstrated circadian phase advances with melatonin administration in the late afternoon and evening and circadian phase delays with administration in the later hours of sleep and morning.
Circadian adaptation to jet travel across many time zones or to night-shift work requires that circadian rhythms phase shift to reentrain to the new light-dark (LD) and sleep-wake cycle. There is currently a great deal of interest in whether properly timed melatonin administration can facilitate circadian phase shifting in these situations. Melatonin has been administered to treat the symptoms associated with night-shift work (e.g., Refs. 22, 27, 28) and jet lag (e.g., Refs. 1, 11, 42, 52, 53), yet few studies have actually measured the phase shifts in circadian rhythms.
In two laboratory studies, circadian rhythms were measured before and after a large shift in the sleep-wake schedule. In one, subjects took melatonin for 7 days to adapt to a 9-h advance of the sleep-wake schedule (49). Melatonin (5 mg) was administered during the phase-advance portion of the melatonin PRC and produced larger circadian phase shifts (by ∼2.5 h) than placebo. The other study was a comparison of melatonin administration and bright light for improving circadian adaptation to a 9-h delay in the sleep-wake schedule (16). During three night-work episodes, subjects in the bright light group were exposed to 4 h of bright light timed to phase delay circadian rhythms. Bright light produced a significantly larger phase delay in circadian rhythms than placebo (8.8 vs. 4.2 h). Melatonin subjects took a total of 4 mg melatonin (or placebo) before and during their three daytime sleep episodes (2 mg at 0800, 1 mg at 1100, and 1 mg at 1400). Melatonin did not produce a larger phase delay than placebo (4.7 vs. 4.2 h). It is likely that melatonin administration occurred during both the phase-delay and phase-advance portions of the melatonin PRC (32), accounting for the minimal effect melatonin had in shifting the circadian clock. Thus only the first of these studies produced evidence that melatonin can help circadian rhythms reentrain after a large shift in the sleep-wake schedule. However, because that study took place in indoor laboratory conditions, it was not a good model for night-shift work. The circadian rhythms of night workers must phase shift in the face of powerful zeitgebers that keep circadian rhythms entrained to the 24-h day.
A study by Sack et al. (44, 45, 47) is the only night-shift field study of melatonin in which circadian rhythm phase shifts were measured. In this study, night-shift workers alternated between 1 wk of consecutive 10-h night shifts and 1 wk off. Melatonin (0.5 mg) or placebo was administered at bedtime for 2 consecutive wk in a crossover design, and phase was measured at the end of each week. Melatonin produced larger circadian phase shifts than placebo in only 7 of the first 24 subjects studied (47). Thus this study did not provide strong evidence that melatonin can help phase shift the circadian rhythms of night-shift workers. One problem was the lack of control over the time of melatonin administration and over the subjects’ sleep schedules. The workers took melatonin whenever they decided to go to bed, and they did not always start the study in the same circadian phase position. Nevertheless, these data are exciting because melatonin helped phase shift circadian rhythms in a few workers in a real-world setting.
In the present study, we controlled the timing of melatonin administration, the sleep-wake schedule, and, to some extent, the light-dark cycle in a field setting. Subjects slept in the afternoons and evenings before their simulated night shifts, and melatonin was administered during the phase-advance portion of the melatonin PRC. The objective of the study was to determine whether appropriately timed melatonin can help produce phase shifts in individuals who must adapt to a large phase advance in their sleep schedule while exposed to the conflicting 24-h zeitgebers.
METHODS
Participants
Thirty-two healthy young adults (13 women, 19 men) between the ages of 19 and 35 yr (mean age ± SD = 24.2 ± 4.8 yr) completed the study. Participants had no medical, psychiatric, or sleep disorders as assessed from telephone and in-person interviews, medical history, and several screening questionnaires. They were free from prescription medications, including oral contraceptives. To minimize any effect of irregular sleep schedules on entrained circadian phase, we excluded individuals who had worked a night-shift job during the 3 mo before the study or who had traveled across more than two time zones during the month before the study. To restrict the dose of melatonin (mg/kg) within a relatively narrow range, we excluded individuals with body mass indexes >29 kg/m2 or who weighed >100 kg. As part of the prestudy screening, each subject completed the Horne and Östberg Morningness-Eveningness Questionnaire (26). The protocol was approved by the Rush-Presbyterian-St. Luke’s Medical Center Institutional Review Board. All subjects gave written informed consent and were paid for their participation.
Design
This was a between-subjects design in which dose of melatonin (placebo, 0.5 mg, or 3.0 mg) was the independent variable. Twelve subjects received placebo, 9 received 0.5 mg melatonin, and 11 subjects received 3.0 mg melatonin under double-blind conditions. Assignment to groups was balanced for sex and Morningness-Eveningness score. Two to three subjects, assigned to different groups, were run simultaneously.
Dark/Sleep and Night-Work Schedules
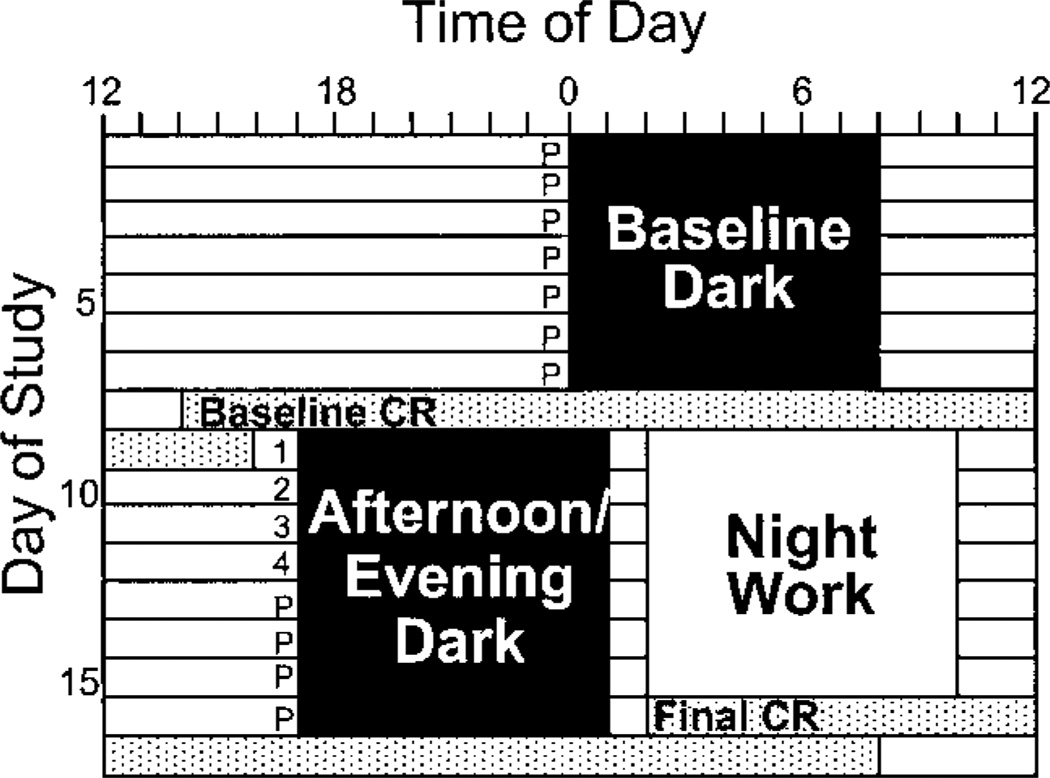
Subjects adhered to a strict protocol with daily 8-h dark periods designated for sleep (see Fig. 1). For 7 baseline days, subjects were scheduled to sleep during fixed dark episodes at night. Next there was a 26-h constant routine (baseline CR) in which subjects were kept awake in a semirecumbent position in dim light <10 lx. After the baseline CR, the dark/sleep schedule was advanced abruptly 7 h from baseline; subjects slept during fixed afternoon/evening dark periods and stayed awake for simulated night-work episodes in ordinary room light (<250 lx). A final CR (26–30 h) followed after 8 days of advanced dark/sleep. Subjects took placebo, 0.5 mg melatonin, or 3.0 mg melatonin capsules 30 min before bedtime on the first 4 days of afternoon/evening dark (7.5 h before the time of scheduled bedtime during baseline) under double-blind conditions. Thus melatonin or placebo was administered during the phase-advance portion of the melatonin PRC (32). All subjects took placebo capsules 30 min before bedtime on all other study days.
Fig. 1.
Sample protocol for a participant with baseline dark scheduled from 0000 to 0800. The numbers (1–4) before the first 4 afternoon/evening dark episodes indicate times when participants in the melatonin group took a melatonin pill. Placebo subjects took placebos at those times, and all subjects took placebos at the times indicated P. CR, constant routine.
Subjects slept at home in bedrooms that we darkened by covering the windows with black plastic; in the summer months, we also installed air conditioners in their bedrooms. All dark/sleep periods were 8 h in duration, and subjects were required to remain in bed and attempt to sleep for the full 8 h. The baseline sleep periods were assigned on the basis of subjects’ typical routines as documented in sleep diaries that they kept for 1 wk before beginning the study. The average scheduled baseline bedtime (±SD) was 0016 (±0:50) Daylight Savings Time. The study was conducted June-October 1997, July-September 1998, and July-September 1999. Hereinafter, clock time will be reported in Central Standard Time.
Subjects spent the first simulated night-work episode in the laboratory where they played games and watched movies under the supervision of a research assistant. The remaining six night shifts were spent at home. Subjects were allowed to move about their homes freely but were required to remain indoors in ordinary room light <250 lx. Light levels in subjects’ homes were verified with a light meter (Minolta model TL-1, Ramsey, NJ).
To mimic the behavior of real night workers who travel home from work in the morning, subjects were required to go outside for at least 5 min during the first hour after the end of their scheduled night-work episodes. Subjects wore a photosensor around the neck during their waking hours during the night-work portion of the study so that we could monitor compliance. The photosensor connected to a small monitor (AMS-1000, Consumer Sensory Products) that recorded light intensity once per minute. To minimize the effect of bright light on circadian rhythms, subjects wore dark welders goggles with top and side shields (Cricket Frames, no. 5 lenses, ∼3% transmission, Uvex Safety, Smithfield, RI) whenever they went outside into daylight during the night-work portion of the study.
Subjects wore a Mini Motionlogger actigraph (BMA-32 model, Ambulatory Monitoring, Ardsley, NY) on the non-dominant wrist continuously throughout the study. Estimated sleep times were also reported by subjects using daily sleep logs. On awakening from each sleep episode, subjects estimated the times of sleep onset and wake time and awakenings during the sleep period that lasted >5 min. Although subjects were asked to refrain from napping during the study, they were not penalized for napping unintentionally and were required to fill out a sleep log for any unscheduled sleep period.
CRs
Each subject participated in a baseline 26-h CR after the baseline week. A final CR (26 h in 8 subjects, 30 h in 24 subjects) followed the 8 days of afternoon/evening dark. The CR procedure was a modification of the CR methods of others (12, 38). During the CRs, subjects remained awake in dim light of <10 lx (verified at the level of the subjects’ foreheads with Minolta TL-1 light meter). They sat in comfortable recliners and were allowed to change posture for bathroom trips only.
Subjects gave a 2-ml saliva sample every 30 min using salivette collection devices (Sarstedt, Newton, NC) and were offered a small isocaloric snack every 2 h. Water was available ad libitum, except for the 10 min before a sample time. To avoid contamination of the saliva, no caffeine, chocolate, bananas, toothpaste, or lipstick were allowed. Saliva samples were centrifuged immediately after collection and frozen at −9°C. The samples were later packed in dry ice and shipped overnight to DiagnosTech (Osceola, WI) to be radioimmuno-assayed for melatonin. All samples from an individual subject were assayed in the same batch. The sensitivity of the assay was 0.7 pg/ml, and the intra- and interassay variations were 7.5 and 9.6%, respectively.
Subjects watched movies, played games, read, and talked with other participants and the supervising research assistant during the CRs.
Body Temperature
Core body temperature was monitored throughout the 17 days of the study using a flexible, disposable rectal thermistor (YSI, Yellow Springs, OH) connected to a small portable monitor (AMS-1000, Consumer Sensory Products) that recorded temperature once per minute. Temperature probes were inserted to maintain a constant depth of 10 cm and were secured with tape and latex tubing. Subjects were allowed to remove the probe for up to 3 h/day.
Sleepiness and Mood Ratings
Subjects completed the Stanford Sleepiness Scale (SSS) (25) twice a day: 30 min after scheduled wake time and 30 min before scheduled bedtime. To assess mood, subjects completed the Profile of Mood States (POMS) (37), a 65-item adjective checklist, twice a day. The first daily POMS (morning POMS) was completed 9 h after scheduled wake time, and subjects were instructed to rate how they felt since awakening; during the night-work portion of the study, this time block corresponded to the simulated night-work episode. The second daily POMS (evening POMS) was completed 30 min before scheduled bedtime; for this rating, subjects were asked to consider the preceding 6.5 h (the time since they had filled out the morning POMS form). The POMS forms were analyzed to produce scores for six factors: tension-anxiety, fatigue-inertia, depression-dejection, vigor-activity, anger-hostility, and confusion-bewilderment.
Other Procedures
Because alcohol consumption can distort core body temperature recordings (20), subjects were instructed to abstain from alcohol and were warned that they might be visited at home and given an unscheduled breathalyzer test; 50% were given a breathalyzer test during the evening hours on a baseline day, and all passed (0.000 reading, Alco-Sensor III, Intoximeters, St. Louis, MO). Caffeine intake was restricted such that subjects who regularly drank caffeinated beverages could consume caffeine during the study, but they were required to drink the same amount every day and only within the first hour after their scheduled wake time. Subjects were instructed to refrain from ingesting ibuprofen and other nonsteroidal anti-inflammatory drugs because they have been shown to decrease melatonin secretion (39).
To encourage compliance with the protocol, subjects were required to telephone the laboratory voice mail system at scheduled wake time, 30 min after scheduled wake time, after they took their pill (30 min before bedtime), and every 2 h during the night-work episodes at home. The voice mail system recorded the time and date of each call, and subjects were docked $5 from their compensation for every call time they missed. Every 2–3 days, subjects came to the laboratory to have their data downloaded. We checked their temperature, photosensor, wrist activity, and questionnaire data in their presence to verify that they were adhering to the dark/ sleep and night-work schedules. Two subjects were dropped from the study for noncompliance.
Data Analysis
Circadian rhythm phase shifts
Circadian rhythm phase shifts were derived from three methods: temperature minimum (Tmin) from core temperature rhythm recorded continuously throughout the study and then mathematically de-masked [home temperature (Home-T)]; Tmin from core temperature rhythm recorded during the CRs [laboratory temperature (Lab-T)]; and the dim light melatonin onset (DLMO) from saliva samples collected during the CRs.
Home-T
To reveal the endogenous component of the temperature rhythm, the raw temperature data were demasked by adding a constant (a demasking factor, DF) to compensate for the exogenous increases and decreases in body temperature associated with activity and bedrest/sleep (4, 5, 9, 21, 35). The DFs were tailored for each individual based on his or her baseline temperature amplitude. First, we averaged the consecutive temperature values into 60-min bins and calculated the difference between each day’s minimum and maximum 60-min bin to obtain the daily range. Then we averaged the range of the last 5 baseline days and used 20% of that average as the DF. (In 6 subjects, fewer days were used because of missing data or changes in menstrual phase.) The temperature data were demasked according to the scheduled dark/sleep times by adding a factor with a trapezoidal function. The factor increased linearly from 0 to DF during the first 60 min of scheduled dark/sleep to compensate for the gradual cooling on going to bed and decreased from DF to 0 during the first 60 min after scheduled wake time to offset the gradual warming after rising.
For female subjects, we accounted for the effect of menstrual cycle phase on body temperature amplitude by calculating a DF for each menstrual phase. We determined whether women were follicular or luteal by counting forward and backward from menses onset and by noting the day of the temperature rise from the follicular to luteal phase. In two female subjects, the menstrual phase changed during baseline, and both DFs were determined from baseline data; in seven women, one DF was calculated from baseline data, and the DF for the other menstrual phase was determined algebraically using the data of Kattapong et al. (29). The four remaining women did not change menstrual phase during the study. The DFs ranged from 0.19 to 0.32°C (mean ± SD = 0.25 ± 0.04°C) in women and 0.22 to 0.40°C (mean ± SD = 0.28 ± 0.04°C) in men.
Daily Tmins were estimated by fitting a cosine curve to each 24-h section of demasked data. During baseline, the 24-h sections began at 1400; during the night-work portion of the protocol, each 24-h section was centered around the previous day’s Tmin to follow the gradual changes in phase. The Home-T phase shift was the difference between the average Tmin of the last 5 days of baseline (days 3–7 on Fig. 1) and the average Tmin of the 3 days near the end of night work (days 13–15). Day 16 was not used because it included the unusually low activity of the final CR.
CR temperature (Lab-T)
Lab-T Tmins were estimated by fitting a complex cosine curve (the 24-h fundamental plus the 12-h harmonic) to the last 24 h of the CRs. The amplitude and phase of both the 24-h and 12-h components were varied to produce a fitted composite curve. The Tmin was the minimum of the composite curve. Lab-T phase shifts were the differences between the Tmin during the final and baseline CRs.
Melatonin (DLMO)
The phase marker from the circadian rhythm of melatonin was the DLMO. We defined DLMO as the first time that the melatonin level reached 20% of each individual’s maximum level and continued to rise. In our subjects, the DLMO thresholds ranged from 1.7 to 13.3 pg/ml (mean = 4.0 ±2.5 pg/ml). DLMO time was calculated by interpolating linearly between the times of the samples before and after the subject’s melatonin levels reached the threshold. Melatonin phase shifts were the differences between DLMO during the final and baseline CRs.
Sleep, sleepiness, and mood
The wrist activity monitors were set to collect activity counts in 1-min bins using zero-crossing mode. Sleep times were estimated by scoring the actigraphy data using the algorithm of Sadeh et al. (48), which has been validated with polysomnography in a similar sample of young adults. The algorithm was applied to intervals of data between the scheduled in-bed and wake times to obtain an objective estimate of total sleep time and sleep efficiency during scheduled dark/sleep. Total sleep times within the scheduled dark/sleep episodes and number and duration of unscheduled naps were calculated from the sleep logs. Sleep times during scheduled dark/sleep episodes from the wrist monitors and sleep logs were averaged over three sections of the protocol: the last 5 baseline days (days 3–7), the first 4 days of afternoon/evening dark (days 9–12, corresponding to the days of melatonin administration in the melatonin groups), and the last 4 days of afternoon/evening dark (days 13–16).
The bedtime and wake time SSS scores were averaged over the same sections of the protocol as the sleep times with one exception: we excluded the bedtime sleepiness rating made immediately after the baseline CR on day 9.
For each subject, the morning and evening POMS scales were averaged over three sections of the protocol: the last 5 baseline days, the first 4 days of afternoon/evening dark (corresponding to the days of melatonin administration in the melatonin groups), and the last 3 days of afternoon/evening dark.
Statistics
Data were analyzed using SPSS for Windows (version 8.0, Evanston, IL). We used ANOVA to analyze the effect of melatonin dose on circadian phase shifts and multivariate ANOVA (MANOVA) to examine the effects of melatonin dose on sleep estimates, sleepiness, and mood ratings made at multiple times during the protocol. We used a χ2 analysis to compare the proportions of subjects in the three groups whose Tmin phase advanced enough to occur during the afternoon/evening dark episode. A probability value of 0.05 was considered statistically significant. Data are presented as means ± SD unless otherwise specified.
RESULTS
There were no differences among the three groups in Morningness-Eveningness score [F2,29 = 0.109, not significant (NS)] or sex (χ2 = 0.425, NS).
Circadian Rhythm Phase Shifts
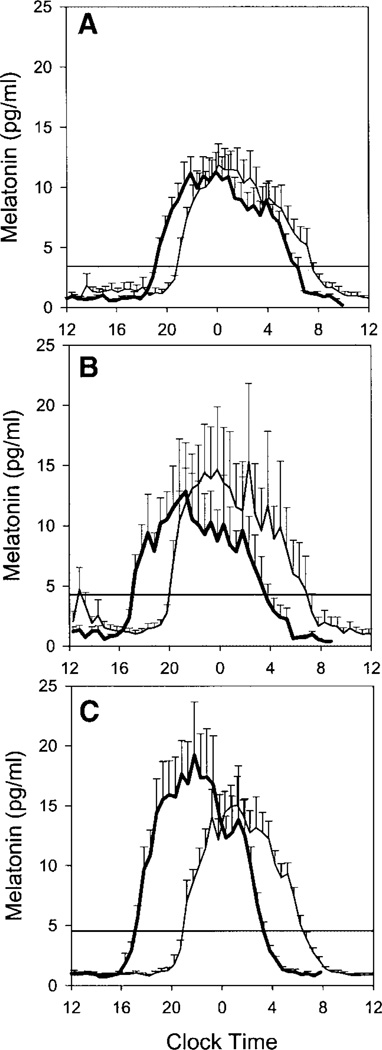
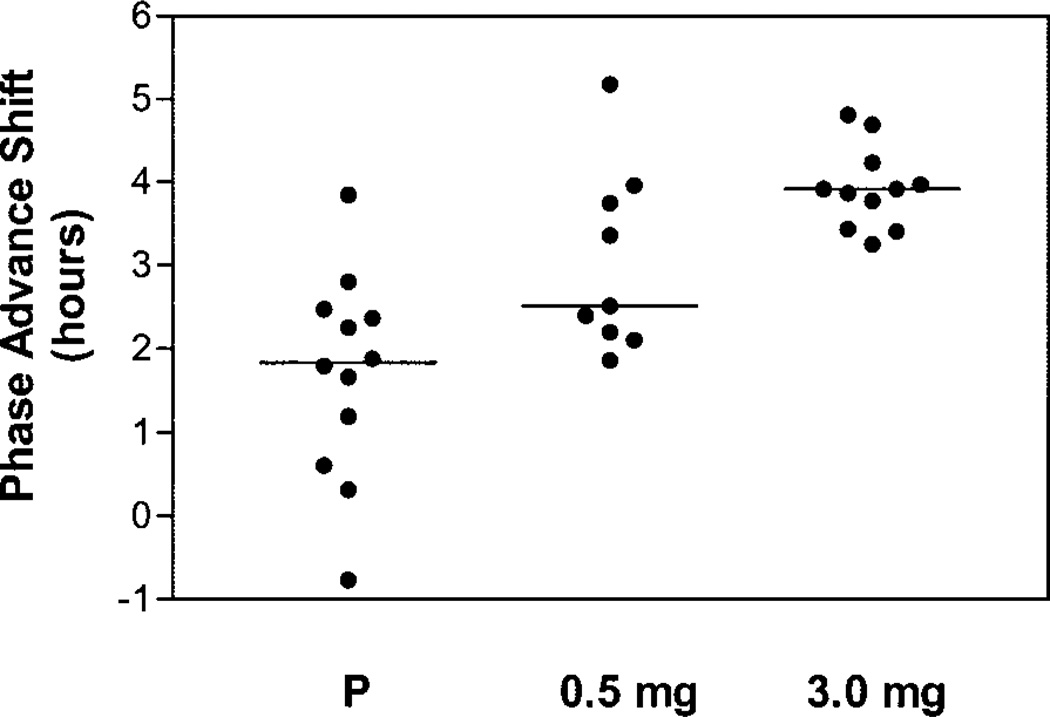
The magnitude of the circadian rhythm phase-advance shifts increased with larger doses of melatonin. Figure 2 shows average salivary melatonin profiles from the three groups. The phase advance of the DLMO was 1.7 ± 1.2 h for the placebo group, 3.0 ± 1.1 h for the 0.5-mg melatonin group, and 3.9 ± 0.5 h for the 3.0-mg melatonin group. Figure 3 shows the DLMO phase-advance shifts for each individual subject. The median phase advance increased, and the within-group variability decreased, with larger doses of melatonin. All subjects in the 3.0-mg group had phase shifts of 3–5 h.
Fig. 2.
Mean salivary melatonin profiles for the placebo (A), 0.5-mg melatonin (B), and 3.0-mg melatonin groups (C). In A–C, the mean melatonin profile during the final CR is indicated by the bold line; the other line illustrates the mean melatonin profile during the baseline CR. Horizontal lines indicate the average dim light melatonin onset (DLMO) threshold for each group. Error bars show SEs. To compile the mean profiles, the individual subjects’ melatonin profiles were aligned with respect to each individual’s DLMO.
Fig. 3.
Scatter histogram of DLMO phase-advance shifts for each individual subject. Horizontal lines show the median phase shift for each group.
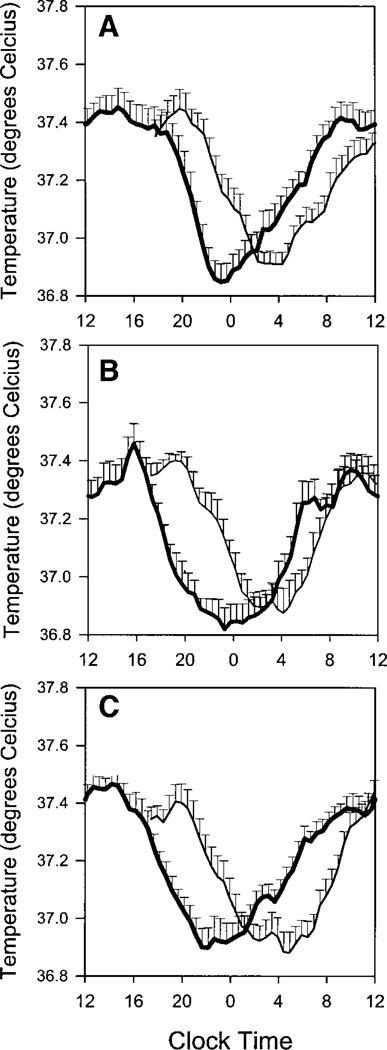
Larger phase-advance shifts with increasing doses of melatonin were also seen in temperature. Figure 4 shows average temperature curves for the three groups during the CRs (Lab-T). The phase advances of the Lab-T Tmins were 3.1 ± 1.5 h for the placebo group, 3.4 ± 1.5 h for the 0.5-mg melatonin group, and 4.8 ± 1.5 h for the 3.0-mg melatonin group.
Fig. 4.
Mean core body temperature curves for the placebo (A), 0.5-mg melatonin (B), and 3.0-mg melatonin groups (C). In A–C, the average core temperature profile during the final CR is indicated by the bold line; the other line indicates the core temperature profile during the baseline CR. Error bars show SEs. To compile the mean profiles, the core temperature recordings of individual subjects were averaged into 30-min bins and aligned with respect to the time of each individual’s fitted temperature minimum (Tmin).
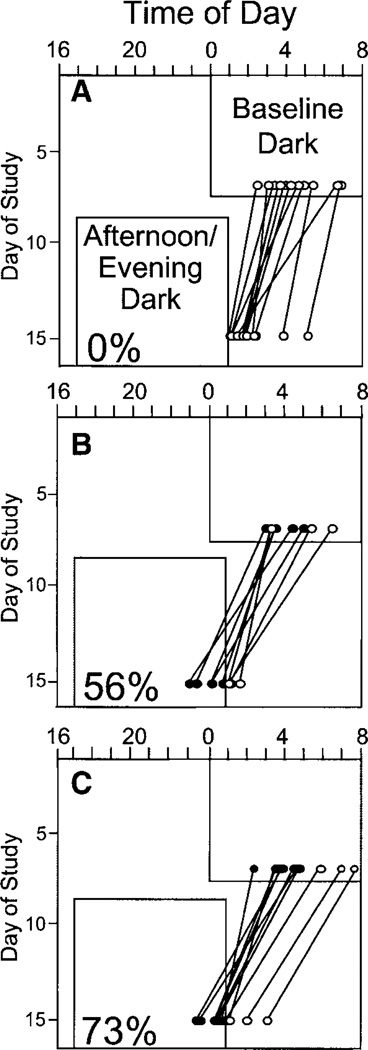
Figure 5 shows the individual phase shifts in the Tmin using the Home-T method. Phase advances were 2.4 ± 1.2 h for the placebo group, 3.8 ± 1.4 h for the 0.5-mg melatonin group, and 4.0 ± 1.2 h for the 3.0-mg melatonin group. Although the largest individual phase shifts were less than the full 7-h advance of dark/sleep, some were large enough to bring the Tmin into the afternoon/evening dark period. As shown in Fig. 5, 0% of placebo subjects, 56% of 0.5-mg melatonin subjects, and 73% of 3.0-mg subjects had average Tmins that occurred during dark/sleep during the last few days of night work and afternoon/evening dark (days 13–15). Significantly more melatonin subjects from both the 0.5-mg and 3.0-mg groups had phase shifts large enough to move their Tmins into dark/sleep than placebo subjects (χ2 = 13.742, P = 0.001).
Fig. 5.
Home temperature (Home-T) Tmins for subjects in the placebo group (A; n = 12), 0.5-mg melatonin group (B; n = 9), and 3.0-mg melatonin group (C; n = 11). Baseline Tmin circles indicate the average of the last 5 days of baseline, and afternoon/evening Tmin circles mark the average of days 13–15 of night work. Lines connect each individual’s Tmins at the 2 time points. Subjects who phase advanced enough for their Tmin to occur during afternoon/evening dark are indicated with filled circles, and percentages indicate the proportion of subjects in each group whose Tmins occurred during the afternoon/evening dark period. All times were standardized to a 0000 to 0800 baseline sleep period.
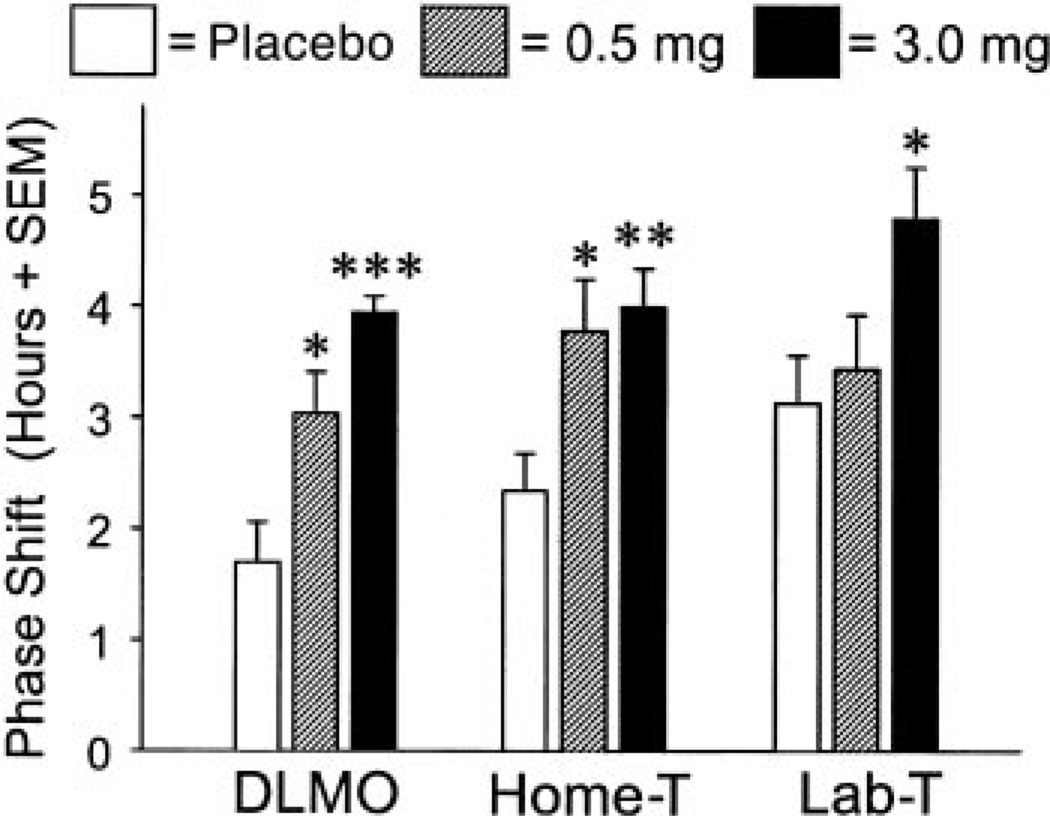
Figure 6 illustrates the mean phase advances for all three methods of measuring circadian rhythm phase shifts. ANOVA showed a significant main effect of melatonin dose for all three methods of measuring phase shifts (DLMO: F2,29 = 14.64, P = 0.001; Lab-T: F2,29 = 3.97, P = 0.03; Home-T: F2,29 = 6.11, P = 0.006). Post hoc comparisons using Tukey’s honestly significant difference test revealed significantly larger phase advances in the 3.0-mg group compared with the placebo group for all three methods (DLMO: P = 0.001; Lab-T: P = 0.03; Home-T: P = 0.009), as well as significantly larger phase advances in the 0.5-mg group compared with the placebo group for DLMO (P = 0.01) and Home-T (P = 0.03). The differences between the 0.5-mg group and the 3.0-mg group did not reach statistical significance for any measure.
Fig. 6.
Magnitude of circadian rhythm phase-advance shifts. Doses were placebo and 0.5 and 3.0 mg melatonin. Measurement methods: DLMO, from saliva samples collected during the CRs; Home-T, Tmin from core temperature rhythm recorded continuously throughout the study and then mathematically demasked; laboratory temperature (Lab-T), Tmin from core temperature rhythm recorded during the CRs. *P < 0.05, **P < 0.01, ***P < 0.001 compared with placebo.
We attempted to enroll subjects within a relatively narrow weight range so that the dose of melatonin (mg/kg) would not be vastly different among individuals. Nevertheless, it was of interest to know whether phase-shift magnitude was related to weight in subjects from the two melatonin groups, i.e., whether a smaller person would have a larger response because of a higher dose (mg/kg) of melatonin. We performed Pearson correlations between weight and the three phase measures for each melatonin group. Surprisingly, in the 0.5-mg group, weight was positively correlated with phase shift for two of the three phase measures, indicating that subjects who weighed more (and therefore had smaller mg/kg melatonin doses) had larger phase shifts (DLMO: r = 0.50; Home-T: r = 0.66; Lab-T: r = 0.12). In the 3.0-mg melatonin group, the correlations were not as consistent (DLMO: r = −0.26; Home-T: r = 0.26, Lab-T: r = −0.10). Thus, overall, we did not find a strong relationship between body size and phase-shift magnitude.
Actigraphic Estimates of Sleep
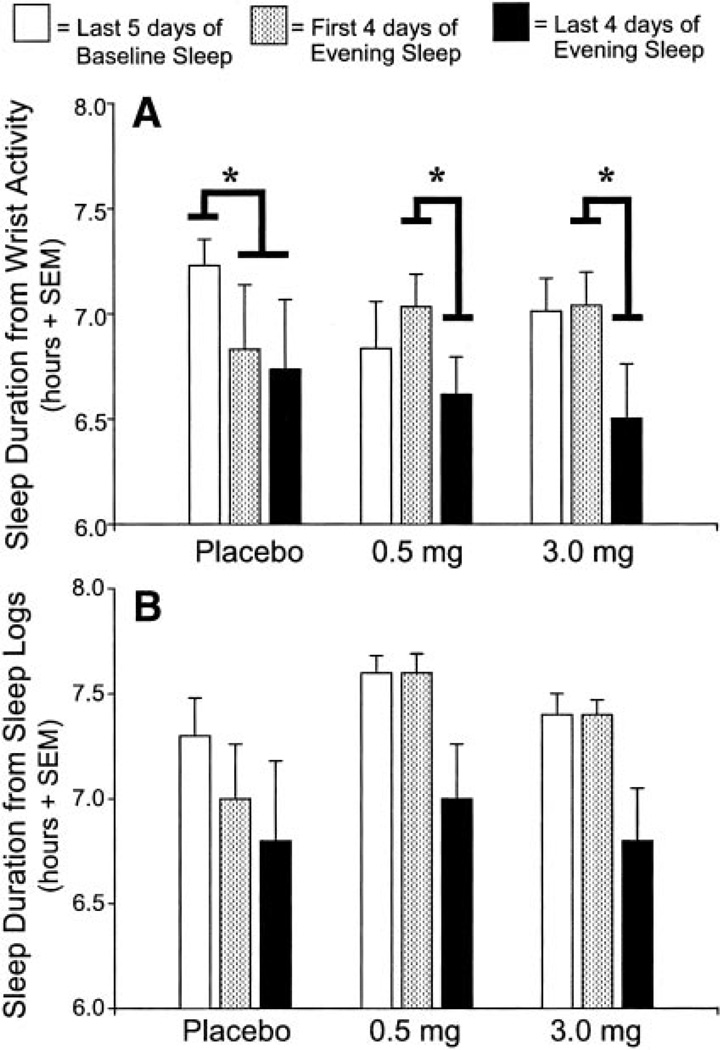
Figure 7A illustrates the average sleep durations estimated from wrist actigraphy for the three groups across three segments of the protocol: the last 5 days of baseline, the first 4 days of afternoon/evening dark (when subjects in the melatonin groups took melatonin), and the last 4 days of afternoon/evening dark. MANOVA revealed a main effect of protocol segment ( F2,28 = 9.34, P = 0.001) and a significant protocol segment-by-group interaction (F4,58 = 2.78, P = 0.04).
Fig. 7.
Average sleep durations within the 8-h dark/sleep episodes estimated with wrist actigraphy (A) and sleep logs (B). Bold black lines indicate the significant Helmert contrasts. *P < 0.01.
To interpret the interaction effects, we ran simple main effects with Helmert contrasts (56) for the three groups across the three protocol segments. For each group, there were two contrasts: 1) baseline night sleep vs. shifted afternoon/evening sleep (first 4 days of afternoon/evening dark and last 4 days of afternoon/ evening dark combined), and 2) first 4 days of afternoon/evening sleep vs. last 4 days of afternoon/evening sleep. Because the data failed Mauchly’s test of sphericity, we used a Geisser-Greenhouse correction for a more conservative estimate of degrees of freedom.
The contrasts (as indicated in Fig.7A) showed that placebo subjects had significantly shorter sleep durations during the eight afternoon/evening dark episodes compared with baseline (corrected F1,48 = 13.16, P < 0.01). Sleep did not differ between the first 4 days of afternoon/evening dark and the last 4 days of afternoon/evening dark (corrected F1,48 = 0.60, NS). In both groups of melatonin subjects, however, contrasts showed significantly longer sleep durations during the first 4 days of afternoon/evening dark (when they took melatonin) compared with the last 4 days of afternoon/ evening dark (when they took placebo) (0.5 mg: corrected F2,48 = 8.65, P < 0.01; 3.0 mg: corrected F1,48 = 17.449, P < 0.01). There was no difference between baseline and all eight afternoon/evening sleep episodes combined (0.5 mg: corrected F2,48 = 0.004, NS; 3.0 mg: corrected F1,48 = 3.53, NS).
Sleep Reported on Daily Sleep Logs
Sleep durations reported by subjects on daily sleep logs were similar to actigraphic estimates (Fig. 7B). MANOVA showed a main effect of protocol segment ( F2,28 = 7.91, P < 0.01), but the protocol segment-by-group interaction was not statistically significant (F4,58 = 1.89, NS).
During baseline, 33% of placebo subjects, 33% of 0.5-mg melatonin subjects, and 36% of 3.0-mg melatonin subjects reported unscheduled naps. There were a total of 14 naps reported during baseline with an average nap length of 56 ± 25 min. During the night-work portion of the study, 58% of placebo subjects, 66% of 0.5-mg melatonin subjects, and 18% of 3.0-mg melatonin subjects reported napping. The difference between groups in the tendency to nap during the night-work portion of the study showed only a trend for more napping in 0.5-mg melatonin and placebo groups (χ2 = 5.69, P = 0.058). Nevertheless, subjects in the placebo and 0.5-mg melatonin groups were about three times more likely to report napping during the night-work portion of the study than subjects in the 3.0-mg melatonin group. The average length of the 29 naps reported during the night-work portion of the study was 80 ± 46 min.
Subjective Ratings of Sleepiness and Mood
MANOVA analysis of the SSS ratings showed a main effect of protocol segment (morning SSS: F2,28 = 6.88, P < 0.01; evening SSS: F2,28 = 13.5, P < 0.001) but no protocol segment-by-group interaction (morning SSS: F4,58 = 0.47, NS; evening SSS: F4,58 = 1.07, NS). Overall, compared with baseline, subjects reported feeling sleepier immediately after waking and less sleepy right before bedtime during the night-work portion of the study.
MANOVA analysis of the POMS ratings showed a main effect of protocol segment for four of the scales: fatigue-inertia (morning: F2,28 = 18.41, P < 0.001; evening: F2,28 = 3.83, P < 0.05), vigor-activity (morning: F2,28 = 19.96, P < 0.001; evening: F2,28 = 3.67, P < 0.05), confusion-bewilderment (morning: F2,28 = 9.87, P < 0.001; evening: F2,28 = 3.49, P < 0.05), and tension-anxiety (evening rating only; morning: F2,28 = 0.82, NS; evening: F2,28 = 3.98, P < 0.05). There were no protocol segment-by-group interactions. Overall, subjects reported more negative mood during the night-work portion of the study than during baseline.
DISCUSSION
Melatonin administration enhanced the phase shifting of circadian rhythms after a 7-h advance of the sleep schedule. After 8 days of afternoon/evening sleep, the endogenous circadian rhythm of melatonin (marked by the DLMO) advanced by ∼4 h in the group that received 3.0 mg of melatonin, whereas it advanced <2 h in the group that received placebo. Similar results were obtained using the circadian rhythm of core body temperature.
In our study, the phase-shifting effects of melatonin appeared to be dose dependent, with the 3.0-mg dose producing the largest average phase advance. This is consistent with findings in humans (17) and rodents (51). For instance, Deacon and Arendt (17) demonstrated a graded phase-shifting response in six human subjects who took 0.05, 0.5, or 5 mg melatonin (or placebo) for 1 day at 1700. Circadian phase advances were 0.4 ± 0.1 h for the 0.05-mg dose, 0.7 ± 0.2 h for the 0.5-mg dose, and 1.4 ± 0.2 h for the 5-mg dose. The phase shifts in our study were larger than these and larger than those in the melatonin PRC generated by Lewy et al. (32). The circadian rhythms of our subjects shifted more because our design was a reentrainment protocol in which sleep was displaced from its usual time at night, whereas in the other studies sleep was held constant. (For a further discussion of this issue and a comparison of the phase shifts facilitated by melatonin in this study with phase shifts facilitated by bright light in a similar reentrainment study, see Ref. 7.)
A few subjects in the placebo group had substantial (>2 h) shifts in their DLMO, which shows that some humans are able to phase advance their circadian rhythms simply through prolonged, consistent exposure to an advanced dark/sleep schedule (which creates an advanced LD cycle). The use of dark goggles while outside could have made subjects more sensitive to the dim (<250 lx) light during the night shift, producing a more effective shifted LD cycle. Nevertheless, even after 8 days of advanced dark/sleep, the majority of placebo subjects had very small DLMO phase shifts of 1–2 h. There were also substantial individual differences in the magnitude of the phase shifts in the melatonin groups. The size of these phase shifts did not appear to be related to the dose (mg/kg) in the individual; in fact, in the 0.5-mg group, the weak correlations between weight and phase-shift magnitude suggested that larger subjects (who received lower melatonin doses in mg/kg) had larger phase advances. Alternately, the different magnitudes of the phase shifts within the melatonin groups could be related to differences in melatonin metabolism (which we did not measure). Different magnitudes of phase shifts within all three groups could be related to differences in sensitivity to the dim (<250 lx) light during the night shifts (23, 24), subtle differences in the 24-h pattern of light exposure, or other individual differences.
This was a simulated night-work field study. It has been suggested that one indicator of circadian adaptation to night work is a phase shift large enough to bring subjects’ Tmins into the shifted sleep period (14, 19). During the last few days of night work and afternoon/ evening sleep, none of the placebo subjects had Tmins that phase advanced enough to occur during the sleep period, whereas more than one-half of the 0.5-mg melatonin subjects and almost three-quarters of 3.0-mg subjects had Tmins that occurred during afternoon/ evening sleep. Thus, although the magnitude of the phase-advance shifts with melatonin was smaller than the advance of the sleep schedule, in many subjects the shifts were large enough to achieve this degree of realignment between circadian rhythms and sleep.
During the first four afternoon/evening sleep episodes, while subjects in the melatonin groups were taking the melatonin, sleep duration was high, comparable to that of baseline. Thus melatonin prevented the well-known decrease in sleep duration due to sleeping at the wrong circadian phase, as illustrated by our placebo group. During the last four sleep episodes, however, sleep duration in the melatonin groups decreased by ∼30 min, and they had about the same amount of sleep as placebo subjects. Thus, despite larger phase-advance shifts and Tmins that occurred during the sleep episodes in many subjects, sleep duration was still shorter than during baseline.
One explanation for the finding that melatonin did not improve sleep during the last four afternoon/ evening sleep episodes is that the phase-advance shifts in the melatonin groups were not large enough for sufficient realignment of circadian rhythms with the sleep episode. Although the majority of melatonin subjects had phase advances large enough to shift their Tmins into the sleep episode, some subjects’ Tmins occurred at the very end of the sleep episode, just meeting criteria for this measure of adaptation. The goal of shifting the Tmin into sleep to achieve circadian adaptation makes only a rough distinction between very poor alignment and better alignment. Furthermore, use of this simple dichotomy as a marker of circadian adaptation was originally proposed for phase-delayed sleep, when the Tmin delays into the beginning of sleep (14, 19). Thus it may be that a greater amount of realignment is necessary to improve sleep when the Tmin advances into the end of the sleep period. In any case, melatonin improved sleep more during the first four sleep episodes, even though there was less realignment, than during the last four sleep episodes when there was more realignment. Thus the benefit of mel-atonin during the first four afternoon/evening sleep episodes was more likely due to its soporific effects (e.g., Ref. 50) and not its phase-shifting effects. It is also possible that a withdrawal or rebound effect occurred when subjects stopped taking melatonin and that this counteracted the benefit of the partial circadian realignment that did occur. However, we are not aware of any investigations of withdrawal or rebound effects after individuals discontinue melatonin administration.
Although our protocol involved 8 days of afternoon/ evening sleep preceding night work, melatonin was administered only before the first four of these sleep episodes. We did not give subjects melatonin before the last four sleep episodes because the direct temperature-lowering effect of melatonin (3, 8, 15) would have interfered with our Home-T measure of circadian phase. We suggest that to protect daytime sleep quality in night workers, melatonin should be taken before all daytime sleep episodes. For practical application in real night-shift workers, we predict that daily melatonin treatment would not only improve sleep but could also help to produce more complete circadian reen-trainment.
Although subjects in this study were asked to refrain from napping, several subjects reported napping unintentionally during baseline and during the night-work portion of the study. Subjects in the 3.0-mg group (who exhibited the largest phase shifts) were less likely to nap during the night-work portion of the study than the subjects in the 0.5-mg group and the placebo group. One interpretation of these data is that the large cir-cadian phase shifts, and thus greater realignment between circadian rhythms and the sleep-wake schedule, made subjects more alert during their wake time. An alternate explanation is that the slightly larger number of naps by subjects in the 0.5-mg and placebo groups prevented them from achieving larger phase shifts. However, we are not aware of any studies that tested whether napping retards circadian rhythm phase shifting. In any case, the data indicate that even in situations where napping is prohibited, unintentional sleep episodes can occur. In the present study, the naps most likely occurred because the subjects were sleepy, because they were not penalized for napping, and because they had long bouts of time when they were not directly observed. These data highlight the fact that unintentional sleep episodes are a risk in real night workers, where accidentally falling asleep can contribute to increased safety hazards and decreased job performance. Our data suggest that melatonin could help prevent unintentional sleep episodes in real night workers.
In our study, subjects did not follow the typical schedule of a real night-shift worker. It is more common for night-shift workers to sleep after their night shifts, thereby producing a phase delay of the sleep/ wake schedule, rather than sleeping before their night shift as was done in this study. We chose to study phase advances because at the time we started the study, the available PRCs for melatonin in humans had much more robust phase-advance portions than phase-delay portions (31). Now there are better data describing the phase-delay portion of the melatonin PRC (32), and we are optimistic about the possibility that melatonin could help produce phase delays in a field study such as this one. This question certainly deserves empirical study in the future. In the meantime, the present findings may offer another option to night-shift workers who would prefer to sleep in the afternoon and evening before work and thus follow the traditional day-work pattern of “sleep, work, leisure” rather than the typical night-shift work pattern of “sleep, leisure, work.” Scheduling night workers’ sleep immediately before the night shift could also reduce the number of hours that the workers are awake before starting work and therefore reduce the homoeostatic pressure for sleep. This could reduce the incidence of unintentional sleep episodes and fatigue-related accidents both on the job and traveling to and from work.
In addition to the timing of sleep in the afternoon and evening, our protocol differed from real night work in other ways, including the mandatory use of dark sunglasses to shield subjects from the effects of the environmental light-dark cycle, the darkening of subjects’ bedrooms, and the requirement that subjects adhere to a regular sleep-wake schedule. These factors likely contributed to the phase shifts that we observed in our study and could be adapted by real night workers to enhance their adaptation to night work in the real-world setting.
Perspectives.
Night-shift work is an important health and safety problem. In addition to the decrease in quality of life for the individual shift workers, the consequences of night-shift work impinge on public safety. Our data indicate that melatonin helped produce larger phase shifts than placebo when measured a few days after administration. Melatonin also prevented the decrease in sleep time due to sleeping at the wrong circadian phase on the days it was administered. These data suggest that melatonin treatment may prove to be a useful strategy for helping real night workers adapt to working night shifts.
Acknowledgments
We thank S. Allen, V. P. Asokan, M. Fleck, S. Martin, and C. P. Stewart for assistance with data collection. We thank L. Fogg for help with statistical analyses and J. K. Wyatt for comments on the manuscript. We are grateful to the volunteers who participated in this study.
Melatonin and matching placebo were donated by Ecological Formulas, Concord CA.
This research was supported by National Institute of Mental Health Grant MH-11239 to K. M. Sharkey and National Institute of Neurological Disorders and Stroke Grant NS-35695 to C. I. Eastman
REFERENCES
- 1.Arendt J, Aldhous M, Marks V. Alleviation of jet lag by melatonin: preliminary results of controlled double blind trial. Br Med J. 1986;292:1170. doi: 10.1136/bmj.292.6529.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attenburrow MEJ, Dowling BA, Sargent PA, Sharpley AL, Cowen PJ. Melatonin phase advances circadian rhythm. Psychopharmacology. 1995;121:503–505. doi: 10.1007/BF02246501. [DOI] [PubMed] [Google Scholar]
- 3.Badia P, Myers B, Boecker M, Culpepper J, Harsh JR. Bright light effects on body temperature, alertness, EEG and behavior. Physiol Behav. 1991;50:583–588. doi: 10.1016/0031-9384(91)90549-4. [DOI] [PubMed] [Google Scholar]
- 4.Baehr EK, Fogg LF, Eastman CI. Intermittent bright light and exercise to entrain human circadian rhythms to night work. Am J Physiol Regulatory Integrative Comp Physiol. 1999;277:R1598–R1604. doi: 10.1152/ajpregu.1999.277.6.R1598. [DOI] [PubMed] [Google Scholar]
- 5.Barrett J, Lack L, Morris M. The sleep-evoked decrease of body temperature. Sleep. 1993;16:93–99. [PubMed] [Google Scholar]
- 6.Benloucif S, Dubocovich ML. Melatonin and light induce phase shifts of circadian activity rhythms in the C3H/HeN mouse. J Biol Rhythms. 1996;2:113–125. doi: 10.1177/074873049601100204. [DOI] [PubMed] [Google Scholar]
- 7.Burgess HJ, Sharkey KM, Eastman CI. Bright light, dark and melatonin can promote circadian adaptation in night shift workers. Sleep Med Rev. In press [PubMed] [Google Scholar]
- 8.Cagnacci A, Elliott JA, Yen SSC. Melatonin: a major regulator of the circadian rhythm of core temperature in humans. J Clin Endocrinol Metab. 1992;75:447–452. doi: 10.1210/jcem.75.2.1639946. [DOI] [PubMed] [Google Scholar]
- 9.Carrier J, Monk TH. Estimating the endogenous circadian temperature rhythm without keeping people awake. J Biol Rhythms. 1997;12:266–277. doi: 10.1177/074873049701200308. [DOI] [PubMed] [Google Scholar]
- 10.Cassone VM, Chesworth MJ, Armstrong SM. Dose-dependent entrainment of rat circadian rhythms by daily injection of melatonin. J Biol Rhythms. 1986;1:219–229. doi: 10.1177/074873048600100304. [DOI] [PubMed] [Google Scholar]
- 11.Claustrat B, Brun J, David M, Sassolas G, Chazot G. Melatonin and jet lag: confirmatory result using a simplified protocol. Biol Psychiatry. 1992;32:705–711. doi: 10.1016/0006-3223(92)90300-o. [DOI] [PubMed] [Google Scholar]
- 12.Czeisler CA, Allan JS, Strogatz SH, Ronda JM, Sanchez R, Rios CD, Freitag WO, Richardson GS, Kronauer RE. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;233:667–671. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- 13.Dahlitz M, Alvarez B, Vignau J, English J, Arendt J, Parkes JD. Delayed sleep phase syndrome response to melatonin. Lancet. 1991;337:1121–1124. doi: 10.1016/0140-6736(91)92787-3. [DOI] [PubMed] [Google Scholar]
- 14.Dawson D, Campbell SS. Timed exposure to bright light improves sleep and alertness during simulated night shifts. Sleep. 1991;14:511–516. doi: 10.1093/sleep/14.6.511. [DOI] [PubMed] [Google Scholar]
- 15.Dawson D, Encel N. Melatonin and sleep in humans. J Pineal Res. 1993;15:1–12. doi: 10.1111/j.1600-079x.1993.tb00503.x. [DOI] [PubMed] [Google Scholar]
- 16.Dawson D, Encel N, Lushington K. Improving adaptation to simulated night shift: timed exposure to bright light versus daytime melatonin administration. Sleep. 1995;18:11–21. doi: 10.1093/sleep/18.1.11. [DOI] [PubMed] [Google Scholar]
- 17.Deacon S, Arendt J. Melatonin-induced temperature suppression and its acute phase-shifting effects correlate in a dose-dependent manner in humans. Brain Res. 1995;688:77–85. doi: 10.1016/0006-8993(95)96872-i. [DOI] [PubMed] [Google Scholar]
- 18.Deacon S, English J, Arendt J. Acute phase-shifting effects of melatonin associated with suppression of core body temperature in humans. Neurosci Lett. 1994;178:32–34. doi: 10.1016/0304-3940(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 19.Eastman CI, Martin SK. How to use light and dark to produce circadian adaptation to night shift work. Ann Med. 1999;31:87–98. doi: 10.3109/07853899908998783. [DOI] [PubMed] [Google Scholar]
- 20.Eastman CI, Stewart KT, Weed MR. Evening alcohol consumption alters the circadian rhythm of body temperature. Chronobiol Int. 1994;11:141–142. doi: 10.3109/07420529409055901. [DOI] [PubMed] [Google Scholar]
- 21.Folkard S. The pragmatic approach to masking. Chronobiol Int. 1989;6:55–64. doi: 10.3109/07420528909059141. [DOI] [PubMed] [Google Scholar]
- 22.Folkard S, Arendt J, Clark M. Can melatonin improve shift workers’ tolerance of the night shift? Some preliminary findings. Chronobiol Int. 1993;10:315–320. doi: 10.3109/07420529309064485. [DOI] [PubMed] [Google Scholar]
- 23.Hebert M, Fleck MS, Martin S, Fishman GA, Eastman CI. Melatonin suppression by light depends on light history. Sleep. 1999;22:S136–S137. [Google Scholar]
- 24.Hebert M, Martin SK, Eastman CI. Effect of light history on light sensitivity in humans (Abstract) Proc 7th Mtg Soc Res Biol Rhythms. 2000;30 [Google Scholar]
- 25.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 26.Horne JA, Östberg O. Self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 27.James M, Tremea MO, Jones JS, Krohmer JR. Can melatonin improve adaptation to night shift? Am J Emerg Med. 1998;16:367–370. doi: 10.1016/s0735-6757(98)90129-2. [DOI] [PubMed] [Google Scholar]
- 28.Jorgensen KM, Witting MD. Does exogenous melatonin improve day sleep or night alertness in emergency physicians working night shifts? Ann Emerg Med. 1998;31:699–704. doi: 10.1016/s0196-0644(98)70227-6. [DOI] [PubMed] [Google Scholar]
- 29.Kattapong KR, Fogg LF, Eastman CI. Effect of sex, menstrual cycle phase and oral contraceptive use on circadian temperature rhythms. Chronobiol Int. 1995;12:257–266. [Google Scholar]
- 30.Krauchi K, Cajochen C, Mori D, Graw P, Wirz-Justice A. Early evening melatonin and S-20098 advance circadian phase and nocturnal regulation of core body temperature. Am J Physiol Regulatory Integrative Comp Physiol. 1997;272:R1178–R1188. doi: 10.1152/ajpregu.1997.272.4.R1178. [DOI] [PubMed] [Google Scholar]
- 31.Lewy AJ, Ahmed S, Jackson JML, Sack RL. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol Int. 1992;9:380–392. doi: 10.3109/07420529209064550. [DOI] [PubMed] [Google Scholar]
- 32.Lewy AJ, Bauer VK, Ahmed S, Thomas KH, Cutler NL, Singer CM, Moffit MT, Sack RL. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int. 1998;15:71–83. doi: 10.3109/07420529808998671. [DOI] [PubMed] [Google Scholar]
- 33.Lockley SW, Skene DJ, James K, Thapan K, Wright J, Arendt J. Melatonin administration can entrain the free-running circadian system of blind subjects. J Endocrinol. 2000;164:R1–R6. doi: 10.1677/joe.0.164r001. [DOI] [PubMed] [Google Scholar]
- 34.Mallo C, Zaidan R, Faure A, Brun J, Chazot G, Claustrat B. Effects of a four-day nocturnal melatonin treatment on the 24 h plasma melatonin, cortisol and prolactin profiles in humans. Acta Endocrinol. 1988;119:474–480. doi: 10.1530/acta.0.1190474. [DOI] [PubMed] [Google Scholar]
- 35.Martin SK, Eastman CI. Medium-intensity light produces circadian rhythm adaptation to simulated night-shift work. Sleep. 1998;21:154–165. [PubMed] [Google Scholar]
- 36.McArthur AJ, Gillette MU, Prosser RA. Melatonin directly resets the rat suprachiasnmatic circadian clock in vitro. Brain Res. 1991;565:158–161. doi: 10.1016/0006-8993(91)91748-p. [DOI] [PubMed] [Google Scholar]
- 37.McNair DM, Lorr M, Droppleman LF. Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 38.Mills JN, Minors DS, Waterhouse JM. Adaptation to abrupt time shifts of the oscillator(s) controlling human circa-dian rhythms. J Physiol. 1978;285:455–470. doi: 10.1113/jphysiol.1978.sp012582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy PJ, Myers BL, Badia P. Nonsteroidal anti-inflammatory drugs alter body temperature and suppress mela-tonin in humans. Physiol Behav. 1996;59:133–139. doi: 10.1016/0031-9384(95)02036-5. [DOI] [PubMed] [Google Scholar]
- 40.Nagtegaal JE, Kerkhof GA, Smits MG, Swart ACW, Van der meer YG. Delayed sleep phase syndrome: a placebo-controlled cross-over study on the effects of melatonin administered five hours before the individual dim light melatonin onset. J Sleep Res. 1998;7:135–143. doi: 10.1046/j.1365-2869.1998.00102.x. [DOI] [PubMed] [Google Scholar]
- 41.Oldani A, Ferini-Strambi L, Zucconi M, Stankov B, Fra-schini F, Smirne S. Melatonin and delayed sleep phase syndrome: ambulatory polygraphic evaluation. Neuroreport. 1994;6:132–134. doi: 10.1097/00001756-199412300-00034. [DOI] [PubMed] [Google Scholar]
- 42.Petrie K, Dawson AG, Thompson L, Brook R. A double-blind trial of melatonin as a treatment for jet lag in international cabin crew. Biol Psychiatry. 1993;33:526–530. doi: 10.1016/0006-3223(93)90007-z. [DOI] [PubMed] [Google Scholar]
- 43.Redman J, Armstrong S, Ng KT. Free-running activity rhythms in the rat: entrainment by melatonin. Science. 1983;219:1089–1091. doi: 10.1126/science.6823571. [DOI] [PubMed] [Google Scholar]
- 44.Sack RL, Blood M, Lewy AJ. Melatonin administration promotes circadian adaptation to night-shift work. Sleep Res. 1994;23:509. [Google Scholar]
- 45.Sack RL, Blood M, Lewy AJ. Melatonin administration to night-shift workers: an update. Sleep Res. 1995;24:539. [Google Scholar]
- 46.Sack RL, Brandes RW, Kendall AR, Lewy AJ. Entrain-ment of free-running circadian rhythms by melatonin in blind people. N Engl J Med. 2000;343:1070–1077. doi: 10.1056/NEJM200010123431503. [DOI] [PubMed] [Google Scholar]
- 47.Sack RL, Lewy AJ. Melatonin as a chronobiotic: treatment of circadian desynchrony in night workers and the blind. J Biol Rhythms. 1997;12:595–603. doi: 10.1177/074873049701200615. [DOI] [PubMed] [Google Scholar]
- 48.Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17:201–207. doi: 10.1093/sleep/17.3.201. [DOI] [PubMed] [Google Scholar]
- 49.Samel A, Wegmann HM, Vejvoda M, Maab H, Gundel A, Schutz M. Influence of melatonin treatment on human circadian rhythmicity before and after a simulated 9-hr time shift. J Biol Rhythms. 1991;6:235–248. doi: 10.1177/074873049100600304. [DOI] [PubMed] [Google Scholar]
- 50.Sharkey KM, Fogg LF, Eastman CI. Effects of melatonin administration on daytime sleep after simulated night shift work. J Sleep Res. 2001;10:181–192. doi: 10.1046/j.1365-2869.2001.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma VK, Singaravel M, Subbaraj R, Chandrashekaran MK. Locomotor activity rhythm in the field mouse Mus booduga phase-shifts to melatonin injections in a dose-dependent manner. Biol Rhythm Res. 1999;30:313–320. [Google Scholar]
- 52.Spitzer RL, Terman M, Williams JB, Terman JS, Malt UF, Singer F, Lewy AJ. Jet lag: clinical features, validation of a new syndrome-specific scale, and lack of response to melatonin in a randomized, double-blind trial. Am J Psychiatry. 1999;156:1392–1396. doi: 10.1176/ajp.156.9.1392. [DOI] [PubMed] [Google Scholar]
- 53.Suhner A, Schlagenhauf P, Johnson R, Tschopp A, Steffen R. Comparative study to determine the optimal mela-tonin dosage form for the alleviation of jet lag. Chronobiol Int. 1998;15:655–666. doi: 10.3109/07420529808993201. [DOI] [PubMed] [Google Scholar]
- 54.Sumova A, Illnerova H. Melatonin instantaneously resets intrinsic circadian rhythmicity in the rat suprachiasmatic nucleus. Neurosci Lett. 1996;218:181–184. doi: 10.1016/s0304-3940(96)13159-1. [DOI] [PubMed] [Google Scholar]
- 55.Tzischinsky O, Dagan Y, Lavie P. The effects of melato-nin on the timing of sleep in patients with delayed sleep phase syndrome. In: Touitou Y, Arendt J, Pevet P, editors. Melatonin and Pineal Gland: From Basic Science to Clinical Application. Amsterdam, The Netherlands: Medica Excerpta; 1993. pp. 351–354. [Google Scholar]
- 56.Winer BJ. Statistical Principles in Experimental Design. New York: McGraw-Hill; 1971. [Google Scholar]
- 57.Zaidan R, Geoffriau M, Brun J, Taillard J, Bureau C, Chazot G, Claustrat B. Melatonin is able to influence its secretion in humans: description of a phase-response curve. Neuroendocrinology. 1994;60:105–112. doi: 10.1159/000126726. [DOI] [PubMed] [Google Scholar]