Abstract
Background
Congenital diaphragmatic hernia (CDH) is a common birth defect with significant morbidity and mortality. Although the etiology of CDH remains poorly understood, studies from animal models and patients with CDH suggest that genetic factors play an important role in the development of CDH. Chromosomal anomalies have been reported in CDH.
Methods
In this study, the authors investigate the frequency of chromosomal anomalies and copy number variants in 256 parent-child trios of CDH using clinical conventional cytogenetic and microarray analysis. The authors also selected a set of CDH related training genes to prioritize the genes in those segmental aneuploidies and identified the genes and gene sets that may contribute to the etiology of CDH.
Results
The authors identified chromosomal anomalies in 16 patients (6.3 %) of the series including 3 aneuploidies, 2 unbalanced translocation, and 11 patients with de novo CNVs ranging in size from 95 kb to 104.6 Mb. The authors prioritized the genes in the CNV segments and identified KCNA2, LMNA, CACNA1S, MYOG, HLX, LBR, AGT, GATA4, SOX7, HYLS1, FOXC1, FOXF2, PDGFA, FGF6, COL4A1, COL4A2, HOMER2, BNC1, BID, and TBX1 as genes that may be involved in diaphragm development. Gene enrichment analysis identified the most relevant gene ontology (GO) categories as those involved in tissue development (p=4.4×10−11) or regulation of multicellular organismal processes (p=2.8×10−10) and “receptor binding” (p = 8.7×10−14) and “DNA binding transcription factor activity” (p= 4.4×10−10).
Conclusions
Our findings support the role of chromosomal anomalies in CDH and provide a set of candidate genes including FOXC1, FOXF2, PDGFA, FGF6, COL4A1, COL4A2, SOX7,BNC1, BID, and TBX1 for further analysis in CDH.
Keywords: Congenital diaphragmatic hernia (CDH), copy number variant (CNV), chromosomal anomalies, gene priority, gene enrichment
Introduction
Congenital diaphragmatic hernia (CDH) is a failure of complete diaphragm formation which allows abdominal organs to herniate into the thoracic cavity and compromises lung development. It is a common birth defect with an incidence of approximately one in 3000 live births.[1] Newborns with CDH often have severe life-threatening pulmonary hypoplasia and pulmonary hypertension. CDH mortality varies between centers (20% to 40%), and long term morbidity remains relatively high despite advances in therapy for treatment of the pulmonary disease.[2, 3] Approximately 40% of cases with CDH are associated with an additional anomaly, most commonly congenital heart, brain, renal, and genitourinary malformations.[4] The etiology of CDH is complex and poorly understood and likely involves both environmental and genetic factors. Studies showed that vitamin A homeostasis and retinoic acid contribute to the development of CDH.[5] Identical twin studies and incomplete penetrance of CDH suggest the possibility of epigenetic modifications in CDH development.[6] In addition to environmental factors, single gene knock out mouse models, rare single gene disorders, familial aggregation, and chromosome disorders associated with CDH support the contribution of genetics in disease etiology.[7–9]
Few disease-causing genes have been identified in CDH because of the small number of large CDH pedigrees due to the high mortality rate which limits the access to affected individuals and makes classical genetic approaches of linkage analysis more challenging. Chromosomal anomalies have been reported in as many as 30% of cases of CDH.[4] Karyotypes and/or fluorescence in situ hybridization (FISH) have identified many recurrent complete or partial aneuploidies in CDH such as trisomy 21, trisomy 18, 15q26 (OMIM 142340, DIH1), 8p23.1 (OMIM 222400, DIH2) and 1q41-q42 (OMIM 612530).[8, 10–13] Recently, new genomic technologies including array-based comparative genomic hybridization (aCGH) and single nucleotide polymorphism (SNP) microarrays have been widely used to identify copy number variants (CNVs) associated with CDH at 15q26 and 1q41-q42.[14, 15] De novo events have been found to be significantly enriched in CDH cases compared to controls or the unaffected siblings[16] and are often large and may alter one or more genes in dosage[17] and/or expression.[18]
In this study, we investigated the frequency and distribution of cytogenetic anomalies and de novo CNVs in a large series of CDH parent-proband trios. We also conducted a gene enrichment analysis for all the genes within the de novo CNVs and prioritized genes for future investigation.
Methods
Subjects
Patients were recruited as part of the DHREAMS (Diaphragmatic Hernia Research & Exploration; Advancing Molecular Science) study (http://www.cdhgenetics.com/). The DHREAMS study is a birth cohort of infants with a diaphragm defect identified at one of seven collaborating medical centers. Columbia University began enrollment in January, 2005, and recruitment at six other sites (Washington University Medical Center/St. Louis Children’s Hospital, University of Pittsburgh, Cincinnati Children’s Hospital and Medical Center/University of Cincinnati, Omaha Children’s Hospital/University of Nebraska, University of Michigan/CS Mott Children’s Hospital and Vanderbilt University) began from 2009 to 2010. All neonates with a diagnosis of a diaphragm defect were eligible for the study. Infants born at the study sites and those transferred into the study sites were eligible. Blood samples were collected from the affected neonate and both parents. Skin and diaphragm biopsies were collected at the time of the diaphragm repair. The birth cohort consists of 170 neonates born during the enrollment period.
In addition, we included a retrospective cohort of 71 patients opportunistically identified through CDH follow up clinics at the DHREAMS study sites, CDH patient support group networks and our research website. Eligible participants had to have a documented personal history of a diaphragm defect confirmed by medical record review. Deceased individuals were included if a post mortem tissue sample was available. A blood, saliva and/or tissue sample was collected from the affected patient and both parents.
Our series also includes 15 fetal cases recruited from Columbia University with a diaphragm defect confirmed by ultrasound and/or fetal MRI and electively terminated. Skin, diaphragm or other tissues or placenta were collected from fetal cases and blood or saliva samples were collected from parents.
The diaphragm defect was classified as an eventration, posteriolateral (Bochdalek) hernia, anterior hernia (Morgagni-Larrey) or agenesis of the hemidiaphragm and classified as left, right or central. Patients with no structural anomalies other than the diaphragm defect were classified as isolated and patients with at least one additional of the following birth defects as non-isolated: congenital heart disease, central nervous system defect, pyloric stenosis, omphalocele, polysplenia, asplenia, gastric dysmotility, Hirschprung disease, intestinal malrotation, situs inversus, genital urinary defect, skeletal anomalies, cleft lip/palate, abnormal new born hearing screen, dysmorphic features, club foot, congenital cystic adenomatoid malformation (CCAM), or bronchopulmonary sequestration (BPS). Pulmonary hypoplasia, cardiac displacement and intestinal herniation were considered to be part of the diaphragm defect sequence and were not considered to be an additional malformation. Clinical data were abstracted from the medical chart by study personnel at each site and included data on prenatal history, neonatal outcome, and longitudinal follow up data including Bayley III and Vineland II developmental assessments and a parent interview about the patient’s health since discharge at 2 years of age. A complete family history including history of diaphragm defects and major malformations was collected on all patients by a single genetic counselor. All the individuals with CDH and their parents provided informed consent/assent for participation in this study, which was performed in accordance with institutional review board approved protocols at the study site of enrollment.
Cytogenetic and clinical chromosome microarray analyses
Test reports were collected and reviewed for clinical conventional cytogenetic and clinical chromosome microarray analysis (karyotype, FISH; Array-CGH: NimbleGen CGX-3 array, SignatureChipOS 135K oligonucleotide array; Affymetrix SNP6.0 at LabCorp). DNA was available from diaphragm, blood, skin, amniocentesis, amniocytes, chorionic villus sampling (CVS) or other available tissues.
CNVs characterization
All probands who did not have a clinical chromosome microarray were analyzed with their parents using Affymetrix Human Genome-Wide SNP Array 6.0 (Santa Clara, CA). DNA was extracted from blood, diaphragm, saliva or other available tissues using Gentra PUREGENE DNA Blood and Tissue Isolation Kit (Qiagen, Valencia, CA). DNA sources available for genotyping are summarized in Table S1. Samples were prepared according to the manufacturer’s protocol (Affymetrix, Santa Clara, CA). The arrays were scanned by the GeneChip Scanner 3000 7G, following by the genotyping quality control (QC) using Affymetrix Genotyping Console (GTC) version 4.0.
We used both PennCNV [19]and Nexus Copy Number software (V6.0) (BioDiscovery Inc., El Segundo, CA) to call CNVs. In PennCNV copy number states with 1 and 2 were assigned as loss, 3 as normal and 4 or 5 as gain. In Nexus all the parameters thresholds were set to default values (segment mean log2 ratio of >0.23 or −0.37 were called gain or loss event, respectively). CNVs that were called in both algorithms with at least one base pair overlap were counted as the same segment. The Human NCBI Build 36.1 was used as reference genome. CNVs identified in the proband alone and absent from both parents were considered putative de novo CNVs. Putative de novo CNVs were then compared to the public databases such as Toronto Database of Genomic Variation (DGV) and the Copy Number Variation project at the Children’s Hospital Philadelphia (CHOP). Those CNVs that were present in normal controls with similar length were excluded from further analysis. To identify the potentially pathogenic variations, the remaining de novo CNVs were compared to the Database of Genomic Structural Variation (dbVar), DECIPHER database and the International Standards for Cytogenomic Arrays Consortium (ISCAC) databases. Gene functions were considered when there is no similar length of pathogenic CNVs in the databases.
Quantitative real-time PCR Confirmations
To independently confirm CNVs, real-time quantitative PCR (qPCR) analysis was performed for all the putative rare, de novo CNVs. Primer assays were either designed by primer3 or directly purchased from Applied Biosystems. Quantitative PCRs were carried out in a reaction volume of 10 μl with 20 ng genomic DNA in LightCycler 480 (Roche Diagnostics), using Lightcycler® Faststart DNA MasterPLUS SYBR Green I or USB® VeriQuest™ Probe qPCR master mix. The conditions used for amplification were: one cycle of 95 °C for 10 min, followed by 50 cycles of 95 °C for 15 s and 59 °C for 1 min. All samples were run in triplicate, and data were normalized against the reference gene RNase P. Relative copy number was determined by the relative standard curve method. The relative copy number values for each interested region between 0.8–1.2 were considered normal while values ≥1.3 and ≤0.7 were considered evidence of duplication and deletion, respectively.
Gene prioritization and enrichment analysis
To determine the genes that were more likely to be involved in the pathogenesis of CDH, we performed gene prioritization analysis with the genes in the confirmed, de novo pathogenic CNVs. We composed a list of genes related to the development of the diaphragm through a comprehensive PubMed literature search using the keywords “diaphragm” and “gene” and put into ToppGene Suite[20] to make a comprehensive list of genes associated with human or mouse diaphragmatic hernia or diaphragm abnormality (Table S2). We use it as a training set to prioritize genes that may lead to an increased risk for CDH in our CNV intervals. We then tested the functional enrichment of those prioritized genes to identify the potential biological pathways involved in CDH. Gene prioritization was analyzed using ToppGene Suite[20] and Endeavour [21]. The p value in the ToppGene Suite was set as a default threshold 0.05 with false discovery rate correction. For better visualization, enriched gene sets were organized into a network. The network was generated by the plugin “Enrichment Map”[22] using Cytoscape[23] network software v.2.8.3 with moderately conservative parameters.
Statistics
The statistical analyses were performed using the R program. Chi-square test was used to assess the gene ontology enrichment and the association between de novo CNVs and survive rate and clinical syndromes.
Results
We studied 256 CDH trios composed of birth, retrospective and terminated fetal cohorts (Table 1). There are 143 males (55.8%) and 146 (57.0%) isolated CDH cases. Two hundred seventeen (84.8%) cases occurred on the left side. Among the 256 cases, 165 cases had either karyotypes (106) or clinical chromosome microarray (6) or both (53) and 91 cases had no genetic evaluation as part of routine clinical care. Except for two probands with Trisomy 21, all the other 254 cases had either clinical chromosome microarray (59) or Affymetrix 6.0 arrays (195) (Table 1).
Table 1.
Description of the CDH cohort
| Prospective Birth cohort | Retrospective cohort | Terminated fetal cases | Total | |
|---|---|---|---|---|
| Number (256 in total) | 170 | 71 | 15 | 256 |
| Male/Female | 97/73 | 41/30 | 5/10 | 143/113 |
| Isolated/Non-Isolated | 93/77 | 47/24 | 6/9 | 146/110 |
| Left/Right/Other | 141/23/6 | 63/4/4 | 13/2/0 | 217/29/10 |
| White NH*/White H*/Black/Asian/Other | 100/32/6/12/20 | 59/8/0/2/2 | 3/6/1/2/3 | 162/46/7/16/25 |
| Karyotypes | 109 | 35 | 15 | 159 |
| Clinical Chromosome Microarrays | 43 | 9 | 7 | 59 |
| Research Chromosome Microarray | 125 | 62 | 8 | 195 |
Note:
White NH refers to White non-Hispanic; White H refers to White Hispanic
Cytogenetic calls
A karyotype was completed on 159 of the 256 total patients (109/170 birth patients, 35/71 of the retrospective patients and 15/15 fetal patients). Eight of the 159 cases (5.0%) had chromosomal anomalies (Table 2). Two patients (04-0030 and 05-0007) had trisomy 21, one patient (05-0004) had mosaic trisomy 18 (50% cells had T18). Two patients (01-0118 and 01-437) had unbalanced translocations inherited from one parent with a balanced translocation. Two patients (01-0162 and 07-0010) had deletions at 8p23.1, and one patient (01-0024) had a deletion at 13q34.
Table 2.
Abnormal chrosomomal results in individuals with CDH
| Patient ID | Gender | Cohort | Side of Lesion | Isolated/Non-isolated | Discharge status | Source | Result | detect method | Inheritance | Additional anomalies |
|---|---|---|---|---|---|---|---|---|---|---|
| 04-0030 | F | prospective | Left | Non-isolated | Died | Amniocentesis | 47, XX +21 | Karyotype | de novo | ASD, VSD |
| 05-0007 | M | prospective | morgagni hernia | Non-isolated | alive | Blood | 47, XY +21 | Karyotype | de novo | dysmorphic facial features consistent with Down syndrome |
| 05-0004 | M | prospective | Left | Non-isolated | alive | Blood | 47, XY, +18[20]/46, XY[20]; 18p11.32-q23 (138,763-76,111,164)x3 | Karyotype/clinical chromosomal microarray | de novo | VSD |
| 01-0118 | M | prospective | Left | Non-isolated | Died | Blood | 46 XY, +der(22)t(11;22)(q23.3;q11.2)mat; 11q23.1-q25(116187461-134452384)x3; 22q11.1-q11.21(14432516-18709056)x3 | Karyotype/research chromosomal microarray | Maternal | Dandy Walker malformation, Hirschprung’s disease, Preauricular pits, bilateral malformed thumbs, Pulm HTN |
| 01-0437 | F | prospective | Left | Non-isolated | alive | Blood | 46, XX, add(7)(p22).ish der(7)t(7;12)(p22;p11.2); 7p22.3(52899-2004194)x1; 12p13.33-p11.22(20691-28622103)x3 | Karyotype/clinical chromosomal microarray | Paternal | bilateral postaxial polydactyly, dysmorphic facial features |
| 01-0162 | F | prospective | Left | Non-isolated | alive | Diaphragm | 46 XX, del(8)(p23.1p23.1).ish del(8)(pter+); 8p23.1(8043620-11883409)x1 | Karyotype/research chromosomal microarray | de novo | ASD, VSD, Incomplete myelination in anterior limb of right and left |
| 07-0010 | M | prospective | Left | Isolated | Died | Amniocytes | 46, XY, del(8)(p23.1); 8p23.2-p22 (192262-15227167)x1 | Karyotype/clinical chromosomal microarray | de novo | None |
| 01-0024 | M | prospective | Left | Non-isolated | Died | Blood | 46, XY, .ish del(13)(q34)(subtel13-, ZIC2+); 13q33.3-q34(107348518-114142980)x1 | Karyotype/research chromosomal microarray | de novo | ASD, VSD, AV Canal, Absent right kidney with low-lying left kidney, left thumb aplasia, right talipes equinovarus, vertebral anomalies - gracile ribs and fusion of multiple vertebral bodies, scoliosis, contractures of right hand, Pulm HTN |
| 01-0581 | F | fetal | Left | Non-isolated | pregnancy termination | liver | 1q12-qter(142693887-247249716)x3 | clinical chromosomal microarray | de novo | CHD, agenesis of the corpus callosum, bilatera; ventriculomegaly, micrognathia, retrognathi posterior displacement of the hard and soft palate, posterior displacement of the tounge, bilateral low set ears, left sided microtia (small vestigial structure and absence of external ear canal), right sided underdevelopment of the helix, bilateral finger contractures |
| 01-0375 | M | fetal | Left | Non-isolated | pregnancy termination | CVS | 1p13.3-p12 (107379866-119388162)X1 | clinical chromosomal microarray | de novo | VSD, Abnormal posterior fossa with inferior splaying of cerebellar vermis, thickened nuchal fold (0.7 cm), no measurable cisterna magna, left absence of lens and orbit, left club foot, shorten long bones, flatten face, umbilical cord cyst |
| 01-0536 | F | retrospective | Left | Isolated | alive | Blood | 15q25.2(80999115-82497270)x1 | research chromosomal microarray | de novo | None |
| 01-0454 | F | retrospective | Left | Isolated | alive | Blood | 17q12(32058213-33668463)x1 | research chromosomal microarray | de novo | None |
| 02-0008 | F | retrospective | Left | Non-isolated | alive | Blood | 17q12(31897638-33362422)x1 | research chromosomal microarray | de novo | abnormal newborn hearing screen |
| 05-0005 | F | retrospective | Left | Non-isolated | alive | Diaphragm | 6p25.3-p25.2(1190603-2762165)x1 | research chromosomal microarray | de novo | VSD, Dandy walker malformation, cleft palate, optic nerve hypoplasia |
| 07-0005 | F | prospective | Right | Isolated | Died | Diaphragm | 11p15.4(6733461-6850699)x1 | research chromosomal microarray | de novo | None |
| 01-0015 | F | retrospective | Left | Isolated | alive | Blood | 14q24.1(68911762-69007402)x3 | research chromosomal microarray | de novo | None |
Abbreviation: F, female; M, male; NA, not available; CVS, chorionic villus sampling; ASD, atrial septal defect; VSD, ventricular septal defect; AV Canal, Atrioventricular canal; Pulm HTN, pulmonary hypertension; CHD, congenital heart disease.
A clinical chromosome microarray was completed on 59 of the 256 total patients (43/170 birth patients, 9/71 retrospective patients, 7/15 fetal patients) (Table 1). Of the 59 cases, 53 also had karyotypes. Five patients had an abnormal chromosome microarray which included three patients who also had an abnormal karyotype (05-0004, 01-0437, and 07-0010). One patient (01-0581) had a mosaic duplication of 1q12-qter in 20% cells not observed by karyotype. The other patient (01-0375) had a 12 Mb deletion at 1p13.3-1p12 that was not detected on CVS karyotype. 9/10 patients with chromosomal anomalies detected by karyotype and/or clinical chromosome microarrays were non-isolated CDHs (Table 2).
Research chromosome microarray results
A total of 195 patients with CDH and their available parents were analyzed with Affymetrix SNP 6.0 arrays. Three probands had been initially reported with chromosomal anomalies by karyotypes and were analyzed to improve resolution of the breakpoints. We evaluated 54 putative de novo CNVs not found in normal controls using qPCR to independently confirm the results. Eight CNVs were inherited from one of the parents, and 36 did not confirm by qPCR. Forty-four (81.5%) of the 54 CNVs were false positives. Six CNVs were independently confirmed using qPCR and were de novo (Table 2), and ranged in size from 95 kb to 1.6 Mb. One CNV was duplication (01-0015); all others were deletions. The number of genes in these CNVs ranged from 2 to 22. Two of 6 patients with de novo CNVs were non-isolated CDHs. In addition, the breakpoints were refined for the four chromosomal anomalies which were detected by karyotype alone (Table 2). Patient 01-0118 had an 18.3 Mb duplication at 11q23.1-q25 and a 4.3 Mb length of duplication at 22q11.1-q11.21. Patient 01-0024 had a 6.8 Mb deletion at 13q33.3-q34. Patient 01-0162 had a 3.8 Mb deletion at 8p23.1.
When the chromosomal anomalies were identified in diaphragm, we tested all DNA from the other available tissues. No difference was found between DNA from diaphragm or other tissues. In summary, there were 16 CDH patients with cytogenetic anomalies including 3 patients with aneuploidies, two patients with unbalanced translocations inherited from a parent with a balanced translocation, and 11 patients with de novo CNVs ranging in size from 96 kb to 104.6Mb at 1p13.3-p12, 1q12-qter, 6p25.3-p25.2, 8p23.1, 8p23.2-p22, 11p15.4, 13q33.3-q34, 14q24.1, 15q25.2, and 17q12. There were 9/170 (5.3%) patients from the birth cohort, 5/71 (7.0%) patients from retrospective cohort and 2/15 (13%) patients from fetal cohort with cytogenetic anomalies. There is an association of chromosomal anomalies and survival rate (4/9 (44.4%) vs. 141/161(87.6%)) in the birth cohort (p=0.004). The participants with abnormal cytogenetic or CNV results were more likely to be non-isolated CDHs (7/9 (77.8%) vs. 70/161 (43.5%)) (p=0.05) in the birth cohort.
Prioritized genes
To further characterize the relevant genes in the CNVs, we performed gene prioritization analysis. Trisomy18 and 21 were excluded for this analysis. A training set of 112 genes was used to test the genes in the pathogenic CNV regions (Table S2). There are 1769 protein-coding genes within the CNVs. Seven hundred and ten genes were predicted to be significant based on the all parameter sets in Toppgene. KCNA2 at 1p13.3-1p12; LMNA, CACNA1S, MYOG, HLX, and AGT at 1q12-qter; GATA4 at 8p23.1 or 8p23.2-p22; HYLS1 at 11q23.1-q25 were already included in our training gene set, and were designated as priorities. Studies in a CDH cohort suggested DISP1 at 1q41q42.12 as a candidate CDH gene.[24] We also include this as our priority gene. FOXC1, FOXF2 at 6p25.3-p25.2; PDGFA at 7p22.3; FGF6 at 12p13.33-p11.22; COL4A1, COL4A2 at 13q33.3-q34; HOMER2, BNC1 at 15q25.2; LHX1 at 17q12; BID, TBX1 at 22q11.1-q11.21 were the top novel genes predicted in each CNV interval by ToppGene and Endeavour (Table 4). No significant genes were found in regions of 11p15.4 (07-0005) and 14q24.1 (01-0015); these two regions were then excluded for following analysis.
Gene ontology enrichment analysis
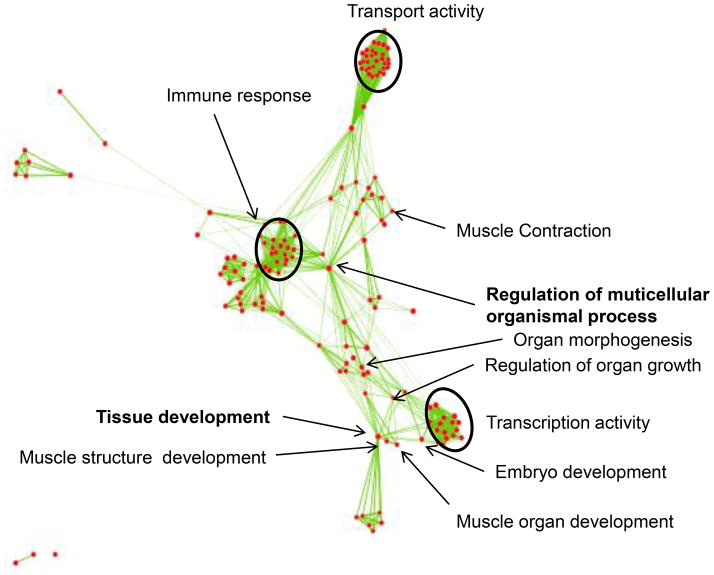
Besides the top predicted priority genes, there were 1,769 genes in the CNV regions and 710 genes were predicted to be significant. To systematically investigate the connections among those 710 genes in CNVs, we performed gene ontology (GO) enrichment for all the 710 significance genes (p<0.05) and 8 priority training genes in CNV regions. The 718 genes were enriched in 238 biological process and 46 molecular function GO categories with significance (p<0.05). For better visualization, we generated a functional map using enrichment map plugin in cytoscape (Figure 1). We found the most significant biological process are tissue development (p=4.4×10−11) and regulation of multicellular organismal processes (p=2.8×10−10), which are the most likely relevant biological processes to CDH (Table S3). The 162 genes in these GO categories are distributed in all CDH CNV regions, including diaphragm hernia related or predicted genes LMNA, CACNA1S, MYOG, HLX, AGT, GATA4, FOXC1, FOXF2, PDGFA, FGF6, LHX1 and TBX1. The most significant molecular functions for these 162 genes are “receptor binding” (p = 8.7×10−14) and “DNA binding transcription factor activity” (p = 4.4×10−10). Figure S1 shows the top 20 molecular function gene enrichment set for the 162 genes.
Figure 1.
Gene enrichment sets were mapped as a network. Gene enrichment analysis results in 238 biological process and 46 molecular function gene ontology (GO) categories. Each node represents a GO. The GO categories are related by mutual overlap (edges). The major functional enrichment sets are highlighted by text. The two GO categories with bold fonts are the most significant ones.
Discussion
CDH is a multifactorial birth defect with a significant genetic contribution. In our study of 256 parent child trios with CDH, we identified a total of 16 of 256 (6.3%) patients with aneuploidies, unbalanced translocations, or de novo copy number variations which is consistent with the previous studies with a prevalence of cytogenetic anomalies ranging from 2%–31%.[4] No tissue specific cytogenetic anomalies were found, suggesting that genetic anomalies isolated to the diaphragm are not common. Since pleuroperitoneal folds are thought to be the origin of posterolateral diaphragmatic hernia forms,[25] our method to screen for mosaic CNVs in diaphragm muscle could still have missed CNVs isolated to the pleuroperitoneal folds.
We identified aneuplodies in three patients (trisomy 18 and 21). We identified two patients with unbalanced translocations inherited from parents with balanced translocations which significantly increase the risk of recurrence for these two families. In addition, we identified 11 patients with de novo CNVs on chromosome microarray ranging in size from 96 kb to 104.56 Mb. The chromosome microarrays were useful in defining the breakpoints of the cytogenetically visible anomalies to precisely map the genes contained within these regions of gain or loss. We observed losses more frequently than gains (9 losses and 2 gains in the segmental aneuploidies). In general, deletions are generally more deleterious than their corresponding duplications. In our birth cohort, we found that the CDH patients with chromosomal anomalies were more likely to be non-isolated and to have other additional birth defects and had an increased mortality suggesting a worse prognosis for those CDH cases with cytogenetic anomalies.
Our study is the largest CDH series evaluating cytogenetic abnormalities. Our prospectively collected CDH birth cohort is also the least biased series to analyze the association between chromosomal anomalies and mortality associated anomalies in CDH patients compared with the previous reported series which were retrospectively collected. Our rate of 6.3% visible cytogenetic anomalies and microdeletions/microduplications is lower than many of the previously reported series (Table S3), but many of the previous series were based on small numbers of cases with as few as 12 patients and oversampled cases with known cytogenetic anomalies. Our series may have some bias of ascertainment as CDH is increasingly diagnosed prenatally and in some cases chromosome prenatal microarrays are performed with elective termination of some fetuses with cytogenetic anomalies. We observed a larger fraction of chromosomal anomalies in patients with non-isolated compared with isolated patients in our birth cohort (9.1% vs. 2.1%), consistent with prior studies.[26, 27]
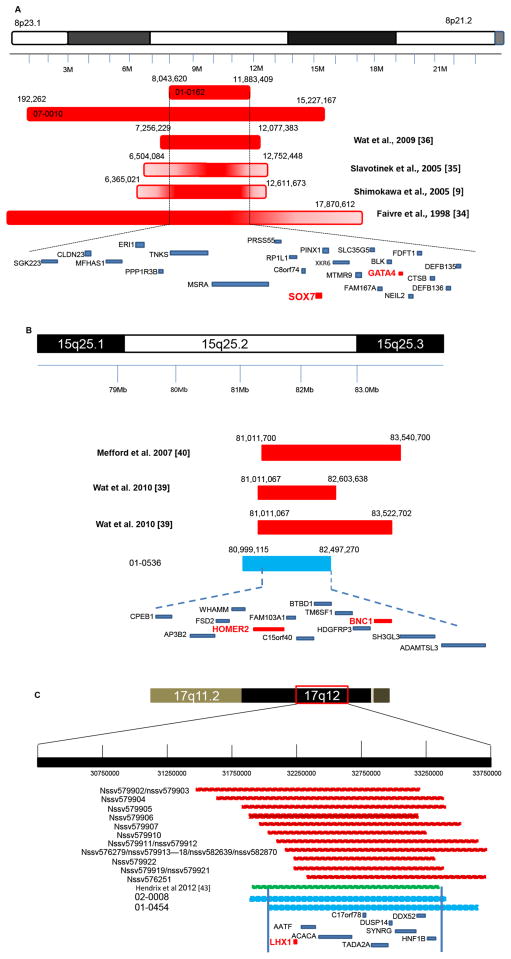
We identified recurrent deletions of 8p23.1 in two patients (01-0162 and 07-0010) a deletion previously reported in CDH patients.[12, 28–30] Overlapping 3.8 Mb and 15.1 Mb de novo deletions were identified in a non-isolated (01-0162) and isolated (07-0010) CDH patient, respectively (Figure 2A). Our results narrowed down the 8p23.1 critical region to 3.8 Mb, and further strengthen the association of haploinsufficiency of one or more genes in 8p23.1 with CDH. Twenty-three genes map to the 3.8 Mb critical region including GATA4, a zinc finger transcription factor. The majority of patients with CDH with deletions of 8p23.1 also have cardiac anomalies, and deletions or loss-of-function mutations in GATA4 have been identified in individuals with structural congenital heart disease. Heterozygous Gata4+/Δex2 mice display cardiac, lung and diaphragm defects.[31] However, no point mutations in GATA4 have been identified in any humans with CDH to date. One explanation is that CDH may require altered expression of more than one gene in the deletion interval to affect development of the diaphragm. Alternatively, the number of CDH patient screened for GATA4 mutations has been quite modest [30]. Gene prioritization and network results demonstrated that SOX7 is an additional candidate gene in this minimal deletion region. SOX7 is a transcription factor involved in the regulation of embryonic development. The gene plays crucial roles in parietal endoderm differentiation through regulating GATA4.[32] Wat MJ et al suggested that haploinsufficiency of SOX7 may increase the severe cardiac phenotype in individuals with a GATA4 deletion.[30]
Figure 2.
The recurrent CDH regions. A. The deletions of 8p23.1 associated with CDH. The red bars with solid or gradient shape are the maximally deleted regions for each patient. There are two deletions identified in our patients and 01-0162 deletion narrowed to the minimum critical 8p23.1 CDH CNV region. Genes in the critical CNV are shown with blue bars. Priority genes are shown as red font and bar. B. The deletions of 15q25.2 associated with CDH. The minimum deleted regions for each patient are shown with solid bars. The light blue bar is the 1.5 Mb deletion in 01-0536. Thirteen genes in the narrowed critical 15q25.2 CDH region are indicated with blue bars. Priority genes are shown as red font and bar. C. The recurrent pathogenic 17q12 deletions among ISCAC database and CDH patients. Red squares indicate the data from ISCAC; green square represents a 1.4 Mb 17q12 deletion reported recently; light blue squares indicate the deletions of our CDH patients (02-0008 and 01-0454). Genes in the overlapped deletion regions are indicated with blue bar. LHX1 is the predicted priority gene in this region shown as red font and bar.
Another recurrent deletion is the de novo 1.5 Mb microdeletion at 15q25.2 in patient (01-0536). Three other patients with CDH have been reported with 15q25.2 deletions [33, 34] (Figure 2B). A fetus with CDH and mild hydrocephalus with a ~2.5 Mb deletion [34] and two 1.6 Mb and 2.5 Mb non-isolated CDH patients were reported.[33] Other phenotypes associated with 15q25.2 deletions include intellectual disabilities, congenital heart disease, cryptorchidism and short stature.[33–35] The deletion in our patient is within the reported CDH 15q25.2 deletion region, and narrows the critical CDH regions to 13 protein coding genes in this region. HOMER2 and BNC1 were predicted to be one of the priority genes mapping to this region. HOMER2 is a scaffolding protein that links carious synaptic signaling proteins. Homer proteins and their mRNAs expression in mouse heart, skeletal muscle and diaphragm.[36] Basonuclin (BNC1) as a transcription factor that performs a regulatory function in the cell growth and differentiation may also plays a role in the development of diaphragm since studies showed that impaired cell proliferation might contribute to the abnormal diaphragm development.[25]
Microdeletion of 17q12 has been frequently reported to be associated with psychiatric abnormality, macrocephaly, developmental delay or renal disease (data from ISCAC, Figure 2C). Recently, Hendrix et al found a 1.4 Mb deletion at 17q12 in a CDH fetus.[37] As shown in Figure 2C, our two patients and this CDH fetus had overlapping 17q12 deletions and narrowed the critical region to 1.39 Mb containing 9 protein-coding genes. Gene enrichment analysis predicts Lim Homeobox1 (LHX1) as a candidate gene for CDH. LHX1 has a unique cysteine-rich zinc-binding domain, and chromatin immunoprecipitation analysis found that Lhx1 promoter region is directly bound by Dlx5, a candidate gene for split-hand/split-foot type 1 malformation associated with sensorineural hearing loss.[38] One of our patients (02-0008) had a mild hearing loss. Dlx5 also plays a key role in development of many organs. The balance of antagonistic interaction between Lhx1 and Dlx5[38] may be disrupted by haploinsufficiency of LHX1leading to CDH.
There are several previously reported CDH patients with duplication of 1q.[39, 40] Most of these duplications are the consequence of a balanced parental translocation. No patients including our patient with a large 1q duplication have survived for long periods except one case with CDH and multiple anomalies.[41]
Three de novo deletions at 13q33.3-q34, 6p25.3-p25.2 and 1p13.3-p12 are novel CDH deletions. There are two genes (ERH, SLC39A9) in 95 kb duplication region at 14q24.1 and no similar pathogenic CNVs found in other databases. In analyzing the 117 kb deletion of 11p15.4, we identified there are two similar size CNVs (nsv467700 and nsv467701) in dbVar and one (chr11:6710956-6762848) in DECIPHER. CNVs nsv467700 and nsv467701 are from a control set study. The CNV in DECIPHER is inherited. There are no similarly sized CNVs in ISCAC. There are five protein coding genes in the 11p15.4 interval; all of them are olfactory receptors. The gene priority analysis did not identify significant genes that related to CDH at 11p15.4 and 14q24.1 intervals. All the evidence suggests these two variants may not be pathogenic although they are de novo.
Gene prioritization and enrichment analysis identified the most significant biological processes: tissue development/organ regulation categories. Genes within these two categories were significantly enriched in transcription factor molecular functions, consistent with findings that transcription factor genes such as GATA4 (OMIM: 600576)[31] and MYOG (OMIM: 159980)[42] are implicated in diaphragm development. Immune response related gene sets were frequently included in the aneuploid segments, consistent with previous studies that genes involved in immune and environment response are frequently included in pathogenic CNVs.[43]
A limitation of our study is that the training CDH gene list is based on the limited knowledge of the implicated relationship of these genes with diaphragm development. However, there is little direct genetic evidence showing that mutations in these genes are implicated in CDH. Our enrichment and prioritization studies are based on these genes and an assumption of association with CDH; thus there may be a bias toward detecting similar genes based upon our training list genes. Although the genes we selected based on the above analyses were most likely involved in CDH, we did not establish a direct relationship between these genes and CDH. Ultimately, it will be important to identify the mutations in individual genes to definitely demonstrate association with CDH. Russell et al.[44] prioritized CDH candidate genes based upon primordial/mature diaphragm transcriptome data and identified Crabp2 and Pbx1 which map to our 1q12-qter deletion region as highly expressed in the pluriperitoneal folds and potentially relevant to human CDH. Our study may have underestimated the overall frequency of CNVs associated with CDH. We could have eliminated some CNVs by filtering with DGV since not all the CNVs in DGV have been verified or are necessarily benign. We could have missed some CNVs called by only one of the two algorithms but believes this is unlikely given the high percentage of CNVs that were not confirmed by qPCR. Most of the chromosomal anomalies were identified in non-isolated CDH patients, and there may be other genetic or non-genetic causes of isolated CDH. Since the CNVs alter the copy number of several genes in these intervals, we cannot determine if the same or different genes in these intervals cause all the structural anomalies. Although the number of patients included in our series was the largest CDH series reported for CNV analysis, the number of patients was modest and replication of these results and identification of other patients with overlapping CNVs will confirm and further define the relevant intervals.
In summary, using high resolution copy number variation analysis and network analysis, we have identified 16 CNVs in 256 CDH parents trios, of which 14 are likely to be pathogenic. The genes we have identified provide a set of high priority genes for further analysis in CDH studies.
Supplementary Material
The top 20 molecular function gene ontology categories generated in the 162 genes enriched "tissue development" and "regulation of multicellular organismal process". Y axis is the name of each GO, X axis is the number of genes that enriched in each GO.
Table 3.
Candidate genes in pathogenic CNVs
| Chromosome Location | Coordinates (hg18; bp)
|
Size (bp) | Proband | Event | Genes | |
|---|---|---|---|---|---|---|
| Start | End | |||||
| 1p13.3-p12 | 107379866 | 119388162 | 12008296 | 01-0375 | Loss | KCNA2 |
| 1q12-qter | 142693887 | 247249716 | 104555829 | 01-0581 | gain | LMNA, CACNA1S, MYOG, HLX, AGT, DISP1 |
| 6p25.3-p25.2 | 1190603 | 2762165 | 1571562 | 05-0005 | Loss | FOXC1, FOXF2 |
| 7p22.3 | 52899 | 2004194 | 1951295 | 01-0437 | Loss | PDGFA |
| 8p23.1 | 8043620 | 11883409 | 3839789 | 01-0162 | Loss | GATA4, SOX7 |
| 8p23.2-8p22 | 192262 | 15227167 | 15034905 | 07-0010 | Loss | GATA4, SOX7 |
| 11q23.1-q25 | 116187461 | 134452384 | 18264924 | 01-0118 | Gain | HYLS1 |
| 12p13.33-p11.22 | 20691 | 28622103 | 28601412 | 01-0437 | Gain | FGF6 |
| 13q33.3-q34 | 107348518 | 114142980 | 6794462 | 01-0024 | Loss | COL4A1, COL4A2 |
| 15q25.2 | 80999115 | 82497270 | 1498156 | 01-0536 | Loss | HOMER2, BNC1 |
| 17q12 | 31897638 | 33362422 | 1464784 | 02-0008 | Loss | LHX1 |
| 17q12 | 32058213 | 33668463 | 1610250 | 01-0454 | Loss | LHX1 |
| 22q11.1-q11.21 | 14432516 | 18709056 | 4276541 | 01-0118 | Gain | BID, TBX1 |
Acknowledgments
We greatly appreciate the families who participated in this study and all the clinical care teams who assisted with study coordination. We are grateful for the technical assistance provided by Patricia Lanzano, Jiancheng Guo and Liyong Deng and discussion with Richard Gill from Columbia University. We also thank Jeannie Kreutzman, and Robert Drongowski from University of Michigan; Gina R Miller, Trish Burns from Cincinnati Children’s Hospital Medical Center; Sheila Horak from University of Nebraska; Mary Dabrowiak from Monroe Carell Jr Children’s Hospital at Vanderbilt; Laurie Luther from University of Pittsburgh.
Study data were collected and managed using REDCap electronic data capture tools hosted at Columbia University.[45] REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
Funding: This work was supported by NIH grant HD057036 and was supported in part by Columbia University’s CTSA grant UL1 RR024156 from NCATS-NCRR/NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Web Resources
Database of Genomic Variation (DGV), http://projects.tcag.ca/variation/
CHOP, http://cnv.chop.edu/
Database of Genomic Structural Variation (dbvar), http://www.ncbi.nlm.nih.gov/dbvar/
DECIPHER database, https://decipher.sanger.ac.uk/application/
ISCA Consortium database, www.iscaconsortium.org
Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim
UCSC Genome Browser, http:/genome.ucsc.edu
Genedistiller, http://www.genedistiller.org/
Primer3, http://frodo.wi.mit.edu/primer3/
ToppGene Suite, http://toppgene.cchmc.org
Endeavour, http://homes.esat.kuleuven.be/~bioiuser/endeavour/tool/endeavourweb.php
EnrichmentMap, http://baderlab.org/Software/EnrichmentMap
Contributors
LY, LM, SG, WKC conceived and designed the experiments. LY, CL, KC, SG, LM performed the experiments. LY, CL, JW analyzed the data. MA, GA, CS, GM, TC, KA, FYL, DC, DP, BW, BB contributed reagents/materials. LY, JW, WKC wrote the paper. All authors read, revised and approved the final version of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Langham MR, Kays DW, Ledbetter DJ, Frentzen B, Sanford LL, Richards DS. Congenital diaphragmatic hernia - Epidemiology and outcome. Clinics in Perinatology. 1996;23:671. [PubMed] [Google Scholar]
- 2.Skari H, Bjornland K, Haugen G, Egeland T, Emblem R. Congenital diaphragmatic hernia: A meta-analysis of mortality factors. Journal of Pediatric Surgery. 2000;35:1187–97. doi: 10.1053/jpsu.2000.8725. [DOI] [PubMed] [Google Scholar]
- 3.Sluiter I, van de Ven CP, Wijnen RMH, Tibboel D. Congenital diaphragmatic hernia: Still a moving target. Seminars in Fetal & Neonatal Medicine. 2011;16:139–44. doi: 10.1016/j.siny.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Pober BR. Overview of epidemiology, genetics, birth defects, and chromosome abnormalities associated with CDH. American Journal of Medical Genetics Part C-Seminars in Medical Genetics. 2007;145C:158–71. doi: 10.1002/ajmg.c.30126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kling DE, Schnitzer JJ. Vitamin A deficiency (VAD), teratogenic, and surgical models of congenital diaphragmatic hernia (CDH) Am J Med Genet C Semin Med Genet. 2007;145C:139–57. doi: 10.1002/ajmg.c.30129. [DOI] [PubMed] [Google Scholar]
- 6.Pober BR, Lin A, Russell M, Ackerman KG, Chakravorty S, Strauss B, Westgate MN, Wilson J, Donahoe PK, Holmes LB. Infants with Bochdalek diaphragmatic hernia: sibling precurrence and monozygotic twin discordance in a hospital-based malformation surveillance program. Am J Med Genet A. 2005;138A:81–8. doi: 10.1002/ajmg.a.30904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brady PD, Srisupundit K, Deyriendt K, Fryns JP, Deprest JA, Vermeesch JR. Recent Developments in the Genetic Factors Underlying Congenital Diaphragmatic Hernia. Fetal Diagn Ther. 2011;29:25–39. doi: 10.1159/000322422. [DOI] [PubMed] [Google Scholar]
- 8.Holder AM, Klaassens M, Tibboel D, de Klein A, Lee B, Scott DA. Genetic factors in congenital diaphragmatic hernia. American Journal of Human Genetics. 2007;80:825–45. doi: 10.1086/513442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding DC, Hsu S, Chu TW, Chen WH. Congenital diaphragmatic hernia with familial occurrence in a Taiwanese pedigree. J Chin Med Assoc. 2005;68:484–6. doi: 10.1016/S1726-4901(09)70079-6. [DOI] [PubMed] [Google Scholar]
- 10.Lurie IW. Where to look for the genes related to diaphragmatic hernia? Genetic Counseling. 2003;14:75–93. [PubMed] [Google Scholar]
- 11.Biggio JR, Descartes MD, Carroll AJ, Holt RL. Congenital diaphragmatic hernia: Is 15q26.1-26. 2 a candidate locus. American Journal of Medical Genetics Part A. 2004;126A:183–5. doi: 10.1002/ajmg.a.20464. [DOI] [PubMed] [Google Scholar]
- 12.Shimokawa O, Miyake N, Yoshimura T, Sosonkina N, Harada N, Mizuguchi T, Kondoh S, Kishino T, Ohta T, Remco V, Takashima T, Kinoshita A, Yoshiura K, Niikawa N, Matsumoto N. Molecular characterization of del(8)(p23.1p23. 1) in a case of congenital diaphragmatic hernia. Am J Med Genet A. 2005;136:49–51. doi: 10.1002/ajmg.a.30778. [DOI] [PubMed] [Google Scholar]
- 13.Youssoufian H, Chance P, Tuck-Muller CM, Jabs EW. Association of a new chromosomal deletion [del(1)(q32q42)] with diaphragmatic hernia: assignment of a human ferritin gene. Hum Genet. 1988;78:267–70. doi: 10.1007/BF00291674. [DOI] [PubMed] [Google Scholar]
- 14.Scott DA, Klaassens M, Holder AM, Lally KP, Fernandes CJ, Galjaard RJ, Tibboel D, de Klein A, Lee B. Genome-wide oligonucleotide-based array comparative genome hybridization analysis of non-isolated congenital diaphragmatic hernia. Human Molecular Genetics. 2007;16:424–30. doi: 10.1093/hmg/ddl475. [DOI] [PubMed] [Google Scholar]
- 15.Wat MJ, Veenma D, Hogue J, Holder AM, Yu ZY, Wat JJ, Hanchard N, Shchelochkov OA, Fernandes CJ, Johnson A, Lally KP, Slavotinek A, Danhaive O, Schaible T, Cheung SW, Rauen KA, Tonk VS, Tibboel D, de Klein A, Scott DA. Genomic alterations that contribute to the development of isolatedand non-isolated congenital diaphragmatic hernia. Journal of Medical Genetics. 2011;48:299–307. doi: 10.1136/jmg.2011.089680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itsara A, Wu H, Smith JD, Nickerson DA, Romieu I, London SJ, Eichler EE. De novo rates and selection of large copy number variation. Genome Res. 2010;20:1469–81. doi: 10.1101/gr.107680.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lupski JR, Stankiewicz P. Genomic disorders: Molecular mechanisms for rearrangements and conveyed phenotypes. Plos Genetics. 2005;1:627–33. doi: 10.1371/journal.pgen.0010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleinjan DA, van Heyningen V. Long-range control of gene expression: Emerging mechanisms and disruption in disease. American Journal of Human Genetics. 2005;76:8–32. doi: 10.1086/426833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang K, Li M, Hadley D, Liu R, Glessner J, Grant SF, Hakonarson H, Bucan M. PennCNV: an integrated hidden Markov model designed for high-resolution copy number variation detection in whole-genomeSNP genotyping data. Genome Res. 2007;17:1665–74. doi: 10.1101/gr.6861907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Bardes EE, Aronow BJ, Jegga AG. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009;37:W305–11. doi: 10.1093/nar/gkp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aerts S, Lambrechts D, Maity S, Van Loo P, Coessens B, De Smet F, Tranchevent LC, De Moor B, Marynen P, Hassan B, Carmeliet P, Moreau Y. Gene prioritization through genomic data fusion. Nat Biotechnol. 2006;24:537–44. doi: 10.1038/nbt1203. [DOI] [PubMed] [Google Scholar]
- 22.Merico D, Isserlin R, Stueker O, Emili A, Bader GD. Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS One. 2010;5:e13984. doi: 10.1371/journal.pone.0013984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cline MS, Smoot M, Cerami E, Kuchinsky A, Landys N, Workman C, Christmas R, Avila-Campilo I, Creech M, Gross B, Hanspers K, Isserlin R, Kelley R, Killcoyne S, Lotia S, Maere S, Morris J, Ono K, Pavlovic V, Pico AR, Vailaya A, Wang PL, Adler A, Conklin BR, Hood L, Kuiper M, Sander C, Schmulevich I, Schwikowski B, Warner GJ, Ideker T, Bader GD. Integration of biological networks and gene expression data using Cytoscape. Nat Protoc. 2007;2:2366–82. doi: 10.1038/nprot.2007.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kantarci S, Ackerman KG, Russell MK, Longoni M, Sougnez C, Noonan KM, Hatchwell E, Zhang X, Pieretti Vanmarcke R, Anyane-Yeboa K, Dickman P, Wilson J, Donahoe PK, Pober BR. Characterization of the chromosome 1q41q42. 12 region, and the candidate gene DISP1, in patients with CDH. Am J Med Genet A. 2010;152A:2493–504. doi: 10.1002/ajmg.a.33618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clugston RD, Zhang W, Greer JJ. Early development of the primordial mammalian diaphragm and cellular mechanisms of nitrofen-induced congenital diaphragmatic hernia. Birth Defects Res A Clin Mol Teratol. 2010;88:15–24. doi: 10.1002/bdra.20613. [DOI] [PubMed] [Google Scholar]
- 26.Witters I, Legius E, Moerman P, Deprest J, Van Schoubroeck D, Timmerman D, Van Assche FA, Fryns JP. Associated malformations and chromosomal anomalies in 42 cases of prenatally diagnosed diaphragmatic hernia. Am J Med Genet. 2001;103:278–82. [PubMed] [Google Scholar]
- 27.Kantarci S, Casavant D, Prada C, Russell M, Byrne J, Haug LW, Jennings R, Manning S, Blaise F, Boyd TK, Fryns JP, Holmes LB, Donahoe PK, Lee C, Kimonis V, Pober BR. Findings from aCGH in patients with congenital diaphragmatic hernia (CDH): a possible locus for Fryns syndrome. Am J Med Genet A. 2006;140:17–23. doi: 10.1002/ajmg.a.31025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faivre L, Morichon-Delvallez N, Viot G, Narcy F, Loison S, Mandelbrot L, Aubry MC, Raclin V, Edery P, Munnich A, Vekemans M. Prenatal diagnosis of an 8p23. 1 deletion in a fetus with a diaphragmatic hernia and review of the literature. Prenat Diagn. 1998;18:1055–60. doi: 10.1002/(sici)1097-0223(1998100)18:10<1055::aid-pd405>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 29.Slavotinek A, Lee SS, Davis R, Shrit A, Leppig KA, Rhim J, Jasnosz K, Albertson D, Pinkel D. Fryns syndrome phenotype caused by chromosome microdeletions at 15q26.2 and 8p23. 1. J Med Genet. 2005;42:730–6. doi: 10.1136/jmg.2004.028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wat MJ, Shchelochkov OA, Holder AM, Breman AM, Dagli A, Bacino C, Scaglia F, Zori RT, Cheung SW, Scott DA, Kang SH. Chromosome 8p23. 1 deletions as a cause of complex congenital heart defects and diaphragmatic hernia. Am J Med Genet A. 2009;149A:1661–77. doi: 10.1002/ajmg.a.32896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jay PY, Bielinska M, Erlich JM, Mannisto S, Pu WT, Heikinheimo M, Wilson DB. Impaired mesenchymal cell function in Gata4 mutant mice leads to diaphragmatic hernias and primary lung defects. Dev Biol. 2007;301:602–14. doi: 10.1016/j.ydbio.2006.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Futaki S, Hayashi Y, Emoto T, Weber CN, Sekiguchi K. Sox7 plays crucial roles in parietal endoderm differentiation in F9 embryonal carcinoma cells through regulating Gata-4 and Gata-6 expression. Mol Cell Biol. 2004;24:10492–503. doi: 10.1128/MCB.24.23.10492-10503.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wat MJ, Enciso VB, Wiszniewski W, Resnick T, Bader P, Roeder ER, Freedenberg D, Brown C, Stankiewicz P, Cheung SW, Scott DA. Recurrent microdeletions of 15q25. 2 are associated with increased risk of congenital diaphragmatic hernia, cognitive deficits and possibly Diamond--Blackfan anaemia. J Med Genet. 2010;47:777–81. doi: 10.1136/jmg.2009.075903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mefford HC, Clauin S, Sharp AJ, Moller RS, Ullmann R, Kapur R, Pinkel D, Cooper GM, Ventura M, Ropers HH, Tommerup N, Eichler EE, Bellanne-Chantelot C. Recurrent reciprocal genomic rearrangements of 17q12 are associated with renal disease, diabetes, and epilepsy. Am J Hum Genet. 2007;81:1057–69. doi: 10.1086/522591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagenstaller J, Spranger S, Lorenz-Depiereux B, Kazmierczak B, Nathrath M, Wahl D, Heye B, Glaser D, Liebscher V, Meitinger T, Strom TM. Copy-number variations measured by single-nucleotide-polymorphism oligonucleotide arrays in patients with mental retardation. Am J Hum Genet. 2007;81:768–79. doi: 10.1086/521274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soloviev MM, Ciruela F, Chan WY, McIlhinney RA. Mouse brain and muscle tissues constitutively express high levels of Homer proteins. Eur J Biochem. 2000;267:634–9. doi: 10.1046/j.1432-1327.2000.01078.x. [DOI] [PubMed] [Google Scholar]
- 37.Hendrix NW, Clemens M, Canavan TP, Surti U, Rajkovic A. Prenatally Diagnosed 17q12 Microdeletion Syndrome with a Novel Association with Congenital Diaphragmatic Hernia. Fetal Diagn Ther. 2012;31:129–33. doi: 10.1159/000332968. [DOI] [PubMed] [Google Scholar]
- 38.Sajan SA, Rubenstein JL, Warchol ME, Lovett M. Identification of direct downstream targets of Dlx5 during early inner ear development. Hum Mol Genet. 2011;20:1262–73. doi: 10.1093/hmg/ddq567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahn HY, Shin JC, Kim YH, Ko HS, Park IY, Kim SJ, Rha JG, Kim SP. Prenatal diagnosis of congenital diaphragmatic hernia in a fetus with 46, XY/46, X,-Y,+der(Y)t(Y;1)(q12; q12) mosaicism: a case report. J Korean Med Sci. 2005;20:895–8. doi: 10.3346/jkms.2005.20.5.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng S, Patil SR, Yankowitz J. Prenatal detection of mosaic trisomy 1q due to an unbalanced translocation in one fetus of a twin pregnancy following in vitro fertilization: a postzygotic error. Am J Med Genet A. 2003;120A:464–9. doi: 10.1002/ajmg.a.20189. [DOI] [PubMed] [Google Scholar]
- 41.Otake K, Uchida K, Inoue M, Koike Y, Matsushita K, Miki C, Sugiyama T, Kusunoki M. Congenital diaphragmatic hernia with a pure duplication of chromosome 1q: report of the first surviving case. Pediatr Surg Int. 2009;25:827–31. doi: 10.1007/s00383-009-2421-z. [DOI] [PubMed] [Google Scholar]
- 42.Knapp JR, Davie JK, Myer A, Meadows E, Olson EN, Klein WH. Loss of myogenin in postnatal life leads to normal skeletal muscle but reduced body size. Development. 2006;133:601–10. doi: 10.1242/dev.02249. [DOI] [PubMed] [Google Scholar]
- 43.Cooper GM, Nickerson DA, Eichler EE. Mutational and selective effects on copy-number variants in the human genome. Nat Genet. 2007;39:S22–9. doi: 10.1038/ng2054. [DOI] [PubMed] [Google Scholar]
- 44.Russell MK, Longoni M, Wells J, Maalouf FI, Tracy AA, Loscertales M, Ackerman KG, Pober BR, Lage K, Bult CJ, Donahoe PK. Congenital diaphragmatic hernia candidate genes derived from embryonic transcriptomes. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2978–83. doi: 10.1073/pnas.1121621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The top 20 molecular function gene ontology categories generated in the 162 genes enriched "tissue development" and "regulation of multicellular organismal process". Y axis is the name of each GO, X axis is the number of genes that enriched in each GO.