Abstract
Several cost-effectiveness models of disease-modifying treatments (DMTs) for multiple sclerosis (MS) have been developed for different populations and different countries. Vast differences in the approaches and discrepancies in the results give rise to heated discussions and limit the use of these models. Our main objective is to discuss the methodological challenges in modelling the cost effectiveness of treatments for MS. We conducted a review of published models to describe the approaches taken to date, to identify the key parameters that influence the cost effectiveness of DMTs, and to point out major areas of weakness and uncertainty. Thirty-six published models and analyses were identified. The greatest source of uncertainty is the absence of head-to-head randomized clinical trials. Modellers have used various techniques to compensate, including utilizing extension trials. The use of large observational cohorts in recent studies aids in identifying population-based, ‘real-world’ treatment effects. Major drivers of results include the time horizon modelled and DMT acquisition costs. Model endpoints must target either policy makers (using cost-utility analysis) or clinicians (conducting cost-effectiveness analyses). Lastly, the cost effectiveness of DMTs outside North America and Europe is currently unknown, with the lack of country-specific data as the major limiting factor. We suggest that limited data should not preclude analyses, as models may be built and updated in the future as data become available. Disclosure of modelling methods and assumptions could improve the transferability and applicability of models designed to reflect different healthcare systems.
1 Introduction
Until the 1990s, there was no specific therapy for the treatment of multiple sclerosis (MS). Management consisted of symptom control, physiotherapy, psychiatric and social support, and disability aids. In the USA, there are currently six products that are licensed as disease-modifying treatments (DMTs) in relapsing-remitting MS (RRMS): interferon beta-1a intramuscular (IM) [Avonex], interferon beta-1a subcutaneous (SC) [Rebif], interferon beta-1b (Betaseron, Extavia), glatiramer acetate (Copaxone), natalizumab (Tysabri), and the more recently approved fingolimod (Gilenya)1–9. In addition, mitoxantrone (Novantrone) is US FDA approved as a DMT for secondary progressive MS (SPMS). Interferons and glatiramer acetate are typically used as first-line DMTs. Natalizumab and fingolimod are more potent immunomodulators but carry additional risks and are typically reserved for MS refractory to first-line treatments. The use of mitoxantrone is even more limited by its potential for cardiac toxicity and leukaemia. These second-line DMTs can effectively reduce relapse rates but may impose additional costs due to monitoring for and treating their complications.
Models to assess the cost effectiveness of MS DMTs have been developed for various populations in the USA, Canada, the UK and many countries in Europe. However, the use of models for the assessment of the cost effectiveness of MS DMTs has been the subject of considerable debate, both because of the data used (or not used) and because of the methodology chosen for modelling and parameter estimation10–15. A recent review of cost-of-illness studies and cost-effectiveness analyses is available elsewhere, including a breakdown of results by DMT type16. In this review, we will discuss the methodological challenges in modelling the cost effectiveness of treatments for MS. Our approach consisted of identifying previously published models, analysing the approaches taken to date, identifying the assumptions and parameters likely to have the greatest influence on cost effectiveness, and discussing where the major areas of uncertainty lie.
2 Approach
We searched Ovid MEDLINE (keywords “multiple sclerosis” and “costs and cost analysis”, including all subheadings) on 26 April 2012, which resulted in 386 studies. PubMed was also used (("multiple sclerosis"[Title/Abstract]) AND (cost[Title/Abstract]) searched on 30 April 2012), which resulted in 443 publications. Titles and abstracts were reviewed and 31 analyses were identified that reported cost plus a quality-of-life metric or measure of disease activity as a combined outcome. Four additional studies were identified by reviewing the references of published studies, resulting in a total of 35 studies identified (summarized in Table 1).
Table 1.
Summary of the cost-effectiveness modelling studies of multiple sclerosis DMTs
| Study | Country | Population | Base year | Time horizon |
Perspective | DMT | Treatment effects source data |
Health outcome | Base-case resulta | Sensitivity (improving cost- effectiveness ratio) |
|---|---|---|---|---|---|---|---|---|---|---|
| Otten84 | Canada | RRMS, SPMS | 1992 | 40 years | Healthcare systemg | IFNβ-1b | RCT | Cost per DYA | Can$219,000 per normalized DYA | Increased treatment efficacy, higher compliance |
| Brown et al.88 | Canada | RRMS | Not reported | 40 years | Healthcare systemg | IFNβ-1b | RCT | Cost per relapse avoided, cost per DYA | Can$31,000+ per relapse avoided Can$306,000 per normalized EDSS DYA | Increased treatment efficacy, higher compliance, lower treatment cost |
| Parkin et al.80 | UK | RRMS (IFNβ-1b, IFNβ-1a and GA) | 1996 | 2, 5 and 10 years | Societalg | IFNβ-1a, IFNβ-1b, GA | RCT | QALY | 2 years: £327,000 (IFNβ-1b) to £434,000 (GA) per QALY gained 5 years: £328,000 (IFNβ-1b) per QALY gained 10 years: £228,000 (IFNβ-1b) per QALY gained | Increased number of relapses, decreased drug costs |
| Otten85 | Canada | RRMS, SPMS | 1996 | 2 years | Healthcare systemg | IFNβ-1a | RCT | QALY | Can$406,000 per QALY gained | Increased disease progression |
| Forbes et al.89 | UK | SPMS | 1995 | 3 years | Healthcare systemn | IFNβ-1b | RCT | QALY | £1,025,000 per QALY gained | Not sensitive to changes in drug cost |
| Brown et al.17 | Canada | RRMS | 1997 | 40 years | Healthcare systemg | IFNβ-1b | RCT | Cost per DYA | Can$275,000 per DYA | Treatment efficacy |
| Kobelt et al.90 | Sweden | SPMS | 2000 | 10 years | Societalg | IFNβ-1b | RCT | QALY | SEK343,000 per QALY gained | Lower utility for severe disability state, longer relapse duration, exclusion of extra monitoring costs of treatment |
| Kendrick and Johnson11 | UK | RRMS | 1995 | 20 years | Healthcare system and societaln | IFNβ-1a | RCT | QALY | Healthcare system: £27,000 (2 years' treatment) to £38,000 (20 years' treatment) per QALY gained Societal: cost saving | Not done |
| Parkin et al.91 | UK | RRMS | 1997 | 5 and 10 years | Societalg | IFNβ-1b | RCT | QALY | £328,300 (5 years) and £228,300 (10 years) per QALY gained | Change in range of conditions |
| Bose et al.92 b | UK | RRMS | 2000 | 8 years | Unknownn | GA | RCT | Cost per relapse avoided, cost per disability unitavoided, QALY | £11,000 per relapse avoided, £9,000 per disability unit avoided, and £23,000–65,000 per QALY gained (depending on relapse disutility) | Unknown |
| Phillips et al.93 b | UK | RRMS | 1999 | 10 and 20 years | Societaln | IFNβ-1b | RCT | QALY | £8,000 per QALY gained. | Unknown |
| Kobelt et al.94 | Sweden | SPMS | 2000 | 10 years | Societaln | IFNβ-1b | RCT | QALY | SEK257,000 per QALY gained | None reported |
| Nuijten and Hutton10 | UK | Initial RRMS (allowing for development of SPMS) vs. usual care | 1998 | Pt's lifetime | Insurern | IFNβ-1b | RCT | QALY | £52,000 per QALY gained | Inclusion of relapses, decreased drug cost, increased disability progression |
| Chilcott et al.64 | UK | RRMS, SPMS | 2001 | 20 years | Healthcare systemg | IFNβ-1a, IFNβ-1b, GA | RCT, commercial in-confidence data | QALY | £42,000–98,000 per QALY gained. | Longer time horizon, incorporating disability progression after stopping treatment, decreased drug cost |
| Lepen et al.95 | UK and France | RRMS | 2000 | 10 and 20 years | Societali | IFNβ-1a | RCT, prospective extension study | Cost per EDSS-month saved | UK: £453 per EDSS-month saved over 10 years; £222 per EDSS-month saved over 20 years France: €712 per EDSS-month saved over 10 years; €374 per EDSS-month saved over 20 years | None reported |
| Kobelt at al.14 | Sweden | RRMS, SPMS | 1999 | 10 years | Societalg | IFNβ-1b | RCT, prospective extension study | QALY | €7,800 (over 36 months of treatment) and €38,700 (over 54 months of treatment) per QALY gained | Longer time horizon, increased MS mortality, treatment at higher disability levels (SPMS) |
| Rubio-Terres et al.12 | Spain | RRMS | 2001 | Pt's lifetime | Societalg | IM IFNβ-1a, SC IFNβ-1a, SC IFNβ-1b, GA | RCT | Average lifetime cost per pt | Dominant (GA) | Increased disability progression, increased cost of disease |
| Touchette et al.96 | USA | SPMS | 2000 | 10 years | Insurer and societali | IFNβ-1b | RCT | QALY | US$395,000 (insurer) and US$86,000 (societal) per QALY gained | Decreased drug cost, increased treatment efficacy |
| Prosser et al.15 | USA | RRMS, SPMS | 1999 | 10 years | Societalg | IFNβ-1a, IFNβ-1b, GA | RCT | QALY | US$1,838,000 per QALY gained (men taking IFNβ-1a) to dominated (IFNβ-1b, GA) | Shorter treatment duration, starting treatment earlier, increased disease progression, decreased drug costs |
| Iskedjian et al.97 | Canada | Pts who experienced a single demyelinating event | 2002 | 15 years | Healthcare systemi | IFNβ-1a | RCT | QAMLY | Can$189,000 (IFNβ-1a) per QAMLY gained | Longer time horizon, increasing relapse disutility |
| Rubio-Terres and Dominguez-Gil13 | Spain | RRMS | 2003 | Pt's lifetime | Societalg | Azathioprine, IM IFNβ-1a, SC IFNβ-1a, SC IFNβ-1b, GA | RCT | QALY | €413,000–1,308,000 (all interferons) per QALY gained compared with azathioprine | Increased cost of disease, increased disability progression, increased treatment efficacy |
| Bell et al.28 | USA | RRMS | 2005 | Pt's lifetime | Societali | IM IFNβ-1a, SC IFNβ-1a, SC IFNβ-1b, GA | RCT, prospective extension studies, long-term follow-up | QALY | US$258,000 (GA) to US$416,000 (SC IFNβ-1a) per QALY gained | Increased disease progression, larger range of health state utilities, decreased treatment costs, longer time horizon |
| Gani et al.98 | UK | Highly active RRMS | 2006 | 30 years | Societali | NAT, GA, IFNβ (combined) | Post hoc analysis of RCT population subset | QALY for NAT | NAT: £2,000 (compared with GA) to £8,200 (compared with best supportive care) per QALY gained | Longer time horizon |
| Kobelt et al.46 | Sweden | RRMS, SPMS | 2005 | 20 years | Societali | NAT, mixture of currently prescribed DMTs | RCT, observational cohort | QALY | Dominant (NAT) | Longer time horizon |
| Janković et al.75 | Serbia | RRMS | 2008 | 40 years | Societalg | GA, IM IFNβ-1a, SC IFNβ-1a, IM IFNβ-1b | RCT, prospective extension studies, long-term follow-up | QALY | >1 billion Serbian dinars (GA) to >4 billion Serbian dinars (all IFNβs) per QALY gained | Increased drug effectiveness, increased indirect costs (wages lost) |
| Guo et al.35 | USA | RRMS | 2006 | 4 years | Payeri | High-dose SC IFNβ-1a, low-dose IM IFNβ-1a | RCT, prospective extension study | Cost per relapse prevented; cost per relapse-free day gained | US$11,000 (SC IFNβ-1a) per relapse prevented and US$232 per relapse-free day gained compared with IM IFNβ-1a | Increased treatment efficacy, longer time horizon, lower drug costs |
| Chiao and Meyer99 | USA | RRMS | 2008 | 2 years | Payeri | NAT, IM IFNβ-1a, IFNβ-1b, GA, SC IFNβ-1a | RCT | Cost per relapse avoided | US$56,594 (NAT) to US$103,665 (GA) per relapse avoided | Increased treatment efficacy |
| Earnshaw et al.29 | USA | RRMS | 2007 | Pt's lifetime | Societali | GA, NAT | RCT, prospective extension studies | QALY | US$496,222 (GA) to US$606,228 (NAT) per QALY gained | Longer time horizon, increased disease progression, lower drug costs, increased treatment efficacy, higher compliance |
| Goldberg et al.54 | USA | RRMS | 2008 | 2 years | Payeri | IM IFNβ-1a, SC IFNβ-1a, SC IFNβ-1b, GA | RCT | Cost per relapse avoided | US$81,000 (SC IFNβ-1a) to US$142,000 (IM IFNβ-1a) per relapse avoided | Increased relapse rate, increased treatment efficacy, higher compliance |
| Tappenden et al.36 | USA | RRMS, SPMS | 2005 | 50 years | Payerg | INFβ-1a, INFβ-1b | RCT | QALY | US$104,000 (SC IFNβ-1a) to US$312,000 (IFNβ-1b) per QALY gained | Not including head-to-head trials, stopping treatment at an EDSS score of 7, including nursing home costs |
| Nuijten and Mittendorf100 | Germany | RRMS | 2008 | 4 years | Societali | IM IFNβ-1a, SC IFNβ-1a, SC IFNβ-1b, GA | RCT | Cost per relapse avoided | €51,000 (SC IFNβ-1a) to €134,000 (IM IFNβ-1a) per relapse avoided | Increased treatment efficacy |
| Becker and Dembeck101 | USA | RRMS | 2008 | 2 years | Payeri | IM IFNβ-1a, SC IFNβ-1a, SC IFNβ-1b, GA | RCT | Cost per relapse avoided | US$78,000 (IM IFNβ-1a) to US$88,000 (GA) per relapse avoided | None |
| O'Day et al.102 | USA | RRMS | 2000 | 2 years | Payeri | NAT, fingolimod | RCT | Cost per relapse avoided | US$117,164 (NAT) and US$168,754 (fingolimod) per relapse avoided | Higher willingness-to-pay threshold |
| Noyes et al.47 | USA | RRMS, SPMS | 2005 | 10 years | Societalg | IM IFNβ-1a, SC IFNβ-1a, SC IFNβ-1b, GA | RCT, observational cohort | QALY | US$901,000 (IM IFNβ-1a) to US$2,179,000 (GA) per QALY gained | Lower drug cost, early treatment initiation |
| Sánchez-de la Rosa et al.69 | Spain | RRMS | 2010 | 10 years | Societali | IM IFNβ-1a, SC IFNβ-1a, SC IFNβ-1b, GA | RCT | QALY | €118,000 (GA) to €1,005,194 (SC IFNβ-1a) per QALY gained | Decrease in NAbs, higher productivity loss, longer time horizon |
The cost per health outcome compared with supportive treatment only
Based on abstracts only
Can$ Canadian dollars, DMT disease-modifying treatment, DYA disability year avoided, EDSS Expanded Disability Status Scale, g government/academic/agency sponsorship, GA glatiramer acetate, i industry sponsorship, IFNβ interferon beta, IM intramuscular, n no sponsorship or sponsorship unknown, NAb neutralizing antibody, NAT natalizumab, pt(s) patient(s), QAMLY quality-adjusted monosymptomatic life-year, RCT randomized clinical trial, RRMS relapsing-remitting multiple sclerosis, SC subcutaneous, SEK Swedish kronor, SPMS secondary progressive multiple sclerosis
3 Study Characteristics
The majority of the studies used a Markov model approach, which allows for the transfer between different health states over a period of time (e.g. progression of disability or presence of relapses). Only two models used non-Markov approaches, such as individual patient-level simulation17 or direct costs and effects estimation18. The length of the model cycle varied from 1 month15 to 3 years10.
The country with the most studies was the USA (11 studies), followed by the UK (10 studies), even though US-based models were not published until 2003 or later. The time horizon used in models ranged from 2 years to a patient’s lifetime. The majority of the studies reported base-case results from a societal perspective. We recognize that a cost-effectiveness analysis from the societal perspective may not seem practical in countries that have numerous healthcare stakeholders with conflicting agendas (like the USA) and that many high-quality cost-effectiveness evaluations from a non-societal perspective have been published19, 20. However, the societal perspective is the only approach that allows decision makers to make cross-country comparisons and to incorporate consequences to all conceivable stakeholders21.
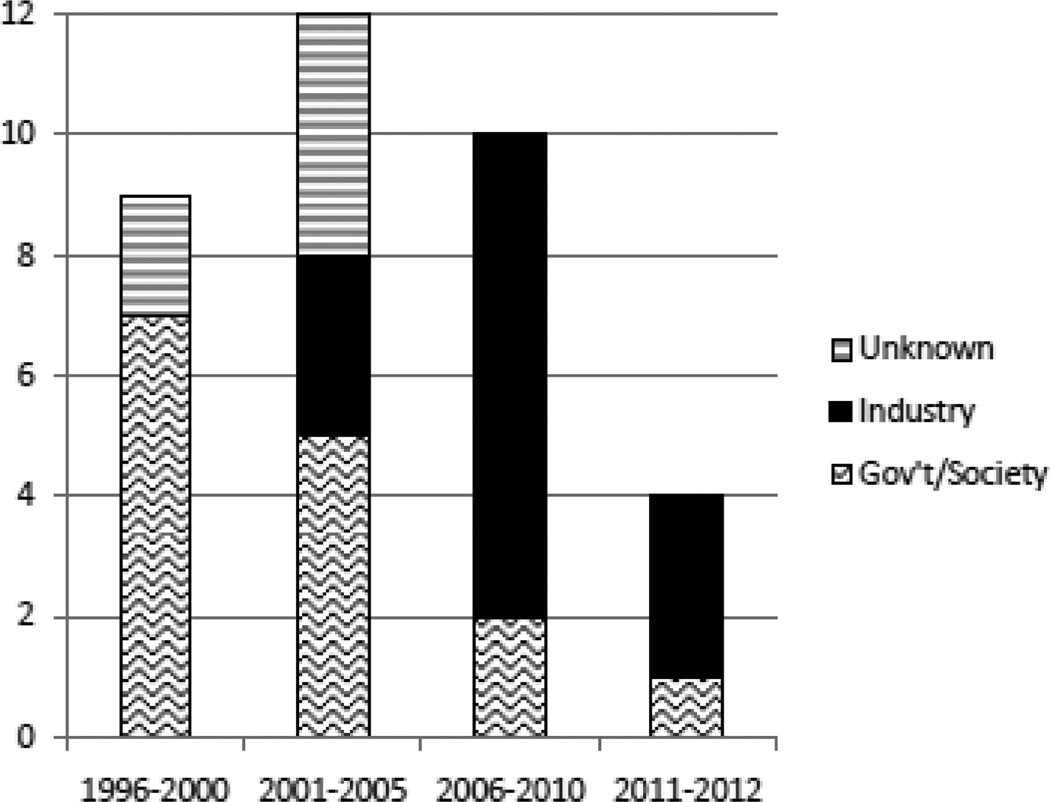
For modelling treatment effects, most studies used natural history of disease data combined with treatment effects from randomized clinical trials (RCTs) and extension studies, although two recent studies also utilized large MS patient registries46,47. Outcomes included incremental cost per QALY gained (the cost-utility endpoint), as well as cost-effectiveness outcomes such as cost per relapse avoided. Outcomes varied widely between countries and also between studies within the same country. In general, outcomes were sensitive to the DMT acquisition cost, the time horizon of the analysis, and the estimation of the treatment effects. Studies with longer treatment duration reported worse (higher) incremental cost-effectiveness ratios (ICERs)28. Lastly, there is a trend towards industry-sponsored studies (Fig. 1), especially in the USA where eight of eleven published studies were industry sponsored. There is also a trend towards cost-effectiveness endpoints (i.e. cost per relapse avoided).
Fig. 1. Number of published studies on Cost-Effectiveness Modeling Studies of MS DMTs by year of publications and by sponsor.
The number of published studies on the cost-effectiveness modelling of multiple sclerosis disease-modifying treatments by year of publication and by sponsor, 1996–2012. Gov’t government
4 Discussion
Overall, we identified the following major sources of variation and uncertainty: (1) uncertainty in the estimation of DMT effectiveness in the absence of head-to-head high-quality RCTs, including the modelling of long-term treatment effects; (2) variation in the characteristics of the included populations (age, gender, country); (3) variations in modelling assumptions (definition of health states, choice and duration of treatment, time horizon); (4) differences in the perspective and the target audience; (5) and wide disparities in the acquisition costs of DMTs between countries. An additional area of uncertainty is the cost effectiveness of DMTs outside North America and Europe.
4.1 The Importance of Head-to-Head RCTs of MS DMTs
The greatest source of bias and uncertainty in earlier models comparing DMTs was the absence of head-to-head RCTs23. Comparison across clinical trials may lead to errors and incorrect conclusions due to differences in study populations (due to varying inclusion and exclusion criteria), definitions of disease activity (e.g. relapse) and a shift towards recruitment of subjects with more benign disease in more recent clinical trials24. Early models projected treatment effects from pivotal RCTs onto natural history data, but implicit in this approach is the comparison of risk ratios, absolute treatment effects or relative treatment effects between DMTs and clinical trials. Additional drawbacks to this method include the reliance on assumptions for the durability of treatment effects after 2 years and assumptions on the applicability of treatment effects to populations not studied in the RCT. Furthermore, due to the wide acceptance of DMTs (about 50 % of all MS patients in the USA take at least one DMT in any given month)25, treatment-naïve (previously untreated) patients selected for RCT inclusion no longer represent the general population1–9.
Due mainly to the uncertainty of treatment effects after 2 years, modellers began to supplement pivotal RCT treatment effects with data from extension studies. Extension studies may be open label, non-randomized and un-blinded, which may limit the interpretation of results. However, well-designed observational studies can produce results that match those from pivotal clinical trials26, 27. Data from observational studies are currently most available for glatiramer acetate and interferons, and data for natalizumab will likely be made available in the future due to the increased surveillance associated with its use (due to the risk of progressive multifocal leukoencephalopathy [PML]). One approach to reduce the differences between RCT designs was published by Bell et al.28 in 2007, in which all treatments modelled were given identical treatment effects for the first 2 years. However, this assumption is not in line with results from the head-to-head trials that have been conducted (as explained further on in this section).
Another model by Earnshaw et al.29 compared glatiramer acetate and natalizumab utilizing RCT data for the first 2 years, followed by available trial extension data for glatiramer acetate. The treatment effects for both glatiramer acetate and natalizumab were adjusted in parallel fashion for the remainder of the model (the lifetime of a patient). However, while the incremental QALYs gained compared with supportive care were about equal for these DMTs, the results were very sensitive to changes in disability progression when tested using sensitivity analysis. Of note, the results were not sensitive to adding in the incidence of neutralizing antibodies associated with natalizumab.
There are four trials comparing interferons and glatiramer acetate directly30–33, although most trials had small cohorts (fewer than 250 participants) and not all trials reported primary results in terms of disease activity (i.e. relapse rate reduction). The EVIDENCE (EVidence of Interferon Dose-response: European North American Comparative Efficacy) trial is the largest randomized, controlled, single-blinded trial to date, and compared brands of interferon beta-1a34. The only model to utilize only data from a head-to-head trial (the EVIDENCE trial) was published by Guo et al.35 and compared SC with IM interferon beta-1a. While this approach removes the biases of comparing across clinical trials, there was no placebo arm in the EVIDENCE trial, so the cost per relapse prevented was only available for SC interferon beta-1a (since IM interferon beta-1a was the comparator). Tappenden et al.36 utilized all available trial data and combined the data with placebo-controlled RCT treatment effects using mixed-treatment comparison models. The re-calculated treatment effects on Expanded Disability Status Scale (EDSS) progression differed from the RCT-derived treatment effects, but relative risks of relapse were unchanged due to a lack of published evidence outside the RCTs.
4.2 The Role of Observational Data
An emerging potential solution to the comparison of treatment effects from different RCTs is the incorporation of data from observation cohorts, which enables generalizing results to a broader population outside clinical trial monitoring and to real-life clinical practice settings37,38. A pivotal example was recently published, bringing into question DMT effects on MS disability progression39. Another advantage is that observational data are likely to be more timely, decreasing the need to compare studies that may have been conducted almost a decade apart. However, determination of the natural history of disease progression (i.e. untreated population or control) with observational data is often complicated by concerns of selection bias, if those progressing the fastest are most likely to be treated, and the fact that there may be few remaining untreated subjects. While several analytic techniques have been developed to minimize the error of estimation due to selection bias, they are complex and not without limitations40–45.
Kobelt et al.46 published a recent model utilizing mixed-treatment effects of interferons and glatiramer acetate from a Swedish MS registry. Disease progression rates for the combined treatment population were then compared with both a natural history cohort and a clinical trial population. The combined treatment population results were compared with clinical trial results for natalizumab under the assumption that new treatments should be compared with current standard treatment. In order to place the registry patient cohort in context, a third patient cohort was modelled using RCT data for the first 2 years, followed by disease activity from a natural history patient cohort. However, the patient registry differs from the natalizumab RCT population in both known and unknown characteristics, with known variables including differing patient populations (inclusion of SPMS patients in the registry and not in the RCT) and a decreased level of monitoring in the registry (and thus likely underestimating the relapse rate and increasing the likelihood of capturing early effects on disease progression)46. Consequently, the comparison between these different patient groups likely does not decrease errors associated with comparing across clinical trials.
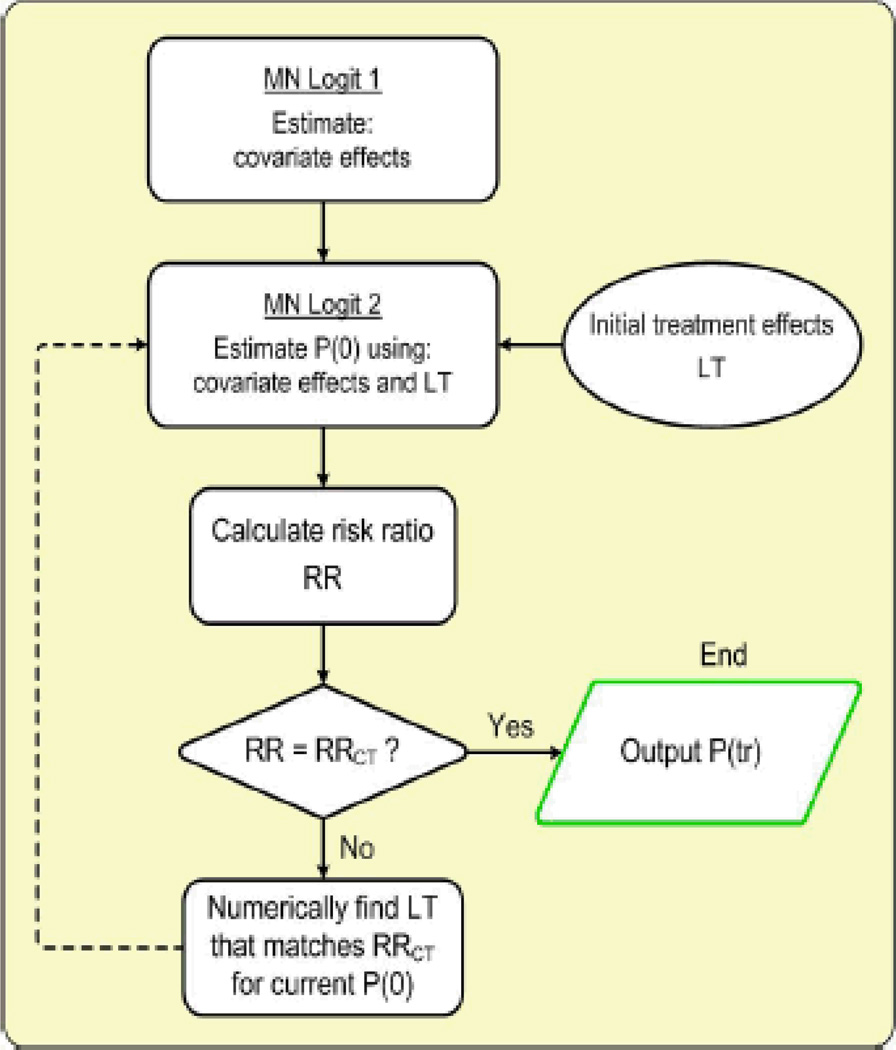
In a recently published analysis, Noyes et al.47 also utilized data from an observational cohort in the attempt to reduce the impact of some of the biases associated with using RCT data for cost-effectiveness assessment. Untreated progression rates were developed by using data from a national observational cohort (Sonya Slifka Longitudinal MS study48), and by correcting for the expected effects of patients’ DMTs as reported by the pivotal trials. Using a heterogeneous sample of MS patients representative of the entire US population of MS patients, rather than RCT subjects only, improved the generalizability of the study results by reducing selection bias47. An overview of the iterative approach for estimating DMT effects used by Noyes et al.47 is shown in Fig. 2. This approach may provide a wide application for population-based comparative effectiveness studies and economic policy assessments.
Fig. 2. Algorithm for estimating the disease state transition probabilities.
To estimate the multiplicative treatment effect coefficients [P(tr)] that would produce the same RR ratios of progression probabilities as reported by pivotal RCTs (RRCT), we kept the progression probabilities without DMT constant (MN Logit 1) while modifying the treatment factors (LT, individual dummy variable for each specific DMT). We implemented an iterative approach by using a numerical grid search algorithm to find a new set of treatment factors that match the published RRs. Next, we re-estimated no DMT transition probabilities using an MN logit model (MN Logit 2) with new treatment effects, calculated post-estimation RR ratios and modified the treatment effects if necessary. By iteratively adjusting transition probabilities (in MN Logit 2) without DMT and treatment effects, we eventually approached the values that best match the RRs of disease progression from the literature (e < 0.001). DMT disease-modifying treatment, LT treatment effect, MN multinomial, P(O) probability of progressing from current disease state, RCT randomized clinical trial, RR relative risk
4.3 Time Horizon Modelled
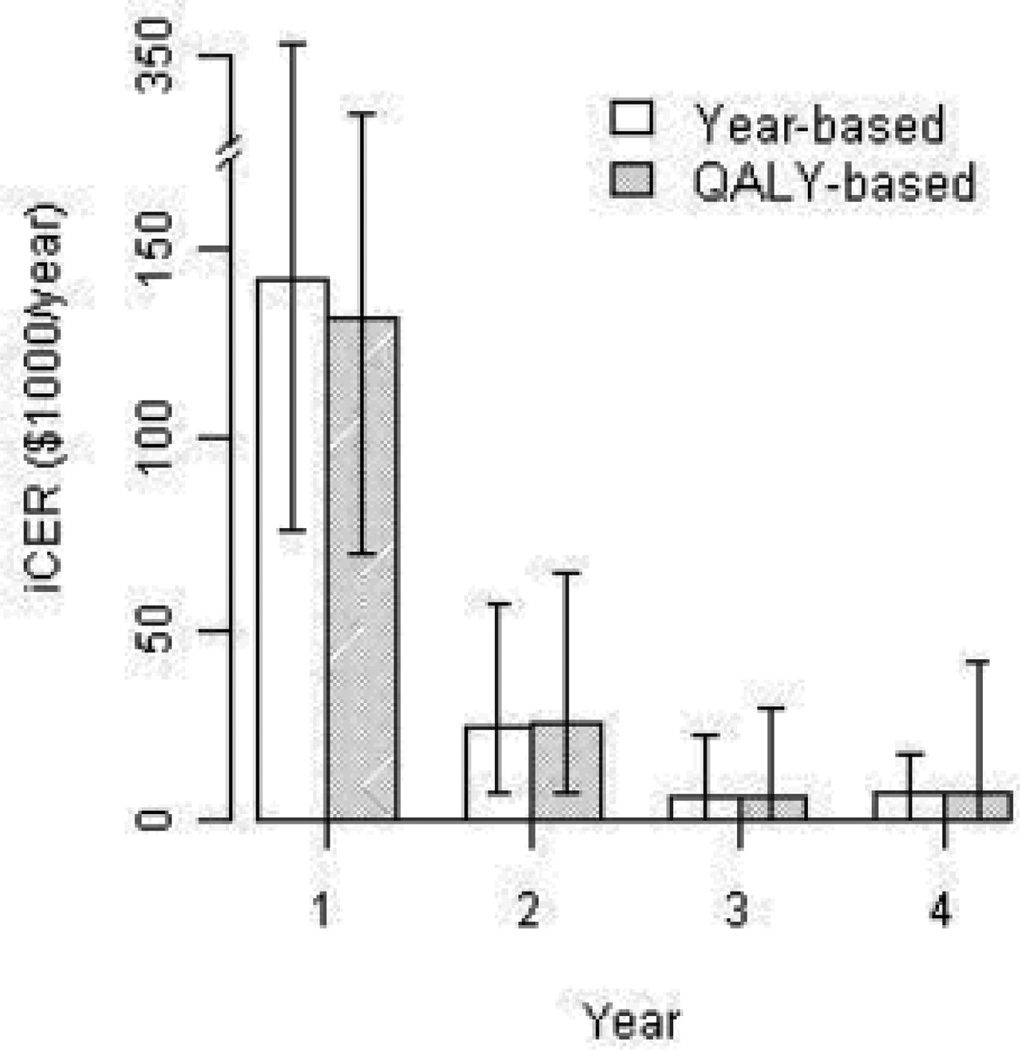
Cost-effectiveness analysis based on the in-trial information alone has limited usefulness for health policy and decision making because of its relatively short duration compared with the length of life with MS49. For this reason, the Panel on Cost-Effectiveness in Medicine recommends using a lifetime horizon for cost-effectiveness evaluations19. However, growing healthcare costs, the fast pace of technology development and innovation, and limited societal resources have shifted the priorities of the cost-effectiveness research paradigm in favour of pragmatic studies and a ‘value-of-information’ approach50–52. For this purpose, many studies present cost-effectiveness trends by presenting several ICERs for different time horizons (Fig. 3)53, in addition to the pattern of healthcare use associated with the DMT (high costs in the earlier years and benefits acquired over time). The time horizon of a model will also affect the cost-effectiveness ratio, with longer time horizons producing more favourable ICERs and greater sensitivity to treatment effects on disease progression28, 47. Shorter time horizons show greater sensitivity to treatment effects on relapse reduction46, 54, 55. Changes in medical technology, its diffusion over time, and increases in the co-morbidities in an ageing population also raise a number of significant methodological challenges for lifelong cost-effectiveness assessment52, 56–58.
Fig. 3. ICERs presented by the length of the time horizon modelled.
Reproduced from Noyes et al.53, with permission. ICER incremental cost-effectiveness ratio
4.4 Perspective and Audience
Despite the large body of research focusing on comparative effectiveness, methodologies of decision analysis and economic evaluation, the question that concerns most providers involved with MS patients is ‘what, if any, relevance does this research have for clinical practice?’59, 60. Many clinicians in the USA would say it has none61. However, in the current US marketplace, cost-effectiveness and comparative effectiveness evaluations may benefit as well as harm providers (by reducing revenue and requiring behaviour or organizational change), depending on whether these studies confirm the appropriateness of current practice (i.e. start DMT early vs. later) or indicate a need for a major change (i.e. the risks of natalizumab or superiority of comprehensive MS care vs. single neurologist-driven care). Hence, rather than ignoring or discounting the results of cost-effectiveness studies, clinicians who are truly interested in providing a high quality of care to their patients may take an active interest in the design, interpretation and application of cost-effectiveness and comparative effectiveness evaluations61. At the organizational level, aligning provider reimbursement incentives and performance incentives (like in some dual-capitated long-term care programmes)62 has a potential for achieving this goal.
Our results suggest a trend towards the greater prevalence of positive industry-sponsored cost-effectiveness studies in the USA, likely in order to improve the market share of their products (Table 1)63, 64. Industry-sponsored studies have previously been associated with more favourable ICERs22.
4.5 Drug Pricing and Prescription Medication Coverage Policies
Making decisions based on the comparative value of prescription drugs, whether or not they formally incorporate the results of cost-effectiveness research or not, is something managed care pharmacists do every day65. For MS DMTs in particular, the greatest driver of the ICER is likely to be the drug acquisition cost, which varies greatly between countries. For instance, the annual cost of interferon beta-1a IM in the UK is about £8,000 (US$12,000) compared with ~US$25,000 (US$34,000 in 2010 values) in the USA66. Drug acquisition costs are also on the rise, with a compounded annual growth rate of 8.2 % in the USA between 2006 and 200967. Noyes et al.47 recently demonstrated that if current DMT costs in the USA were reduced by two-thirds (which would match the prices in other industrialized countries), the cost effectiveness of DMTs would become comparable with the cost effectiveness of other accepted interventions20. Studies outside the USA have also shown that drug prices are a key driver of total costs69.
We also would like to highlight the fact that the additional risks of the newer DMTs may impose a great deal of extra cost due to monitoring for and treating complications70; for example, in the USA, MRI scans for PML surveillance are required for all natalizumab patients in the higher risk groups for PML at least every 3 months, not to mention the very high costs of treating natalizumab-induced PML (long hospitalizations, plasmapheresis and severe long-term disability in many cases). These adverse events and increased clinical vigilance are currently not included in the cost-effectiveness studies of natalizumab29, 46. Extra testing also needs to be carried out for fingolimod (ophthalmological and dermatological screening) and mitoxantrone (serial ECGs). The associated expenses might still be a small fraction of the drug acquisition costs, but with the ever-increasing trend in utilization and focus on patient safety, it is an important category to account for. While in the USA the main focus of cost-containment activities has traditionally been on quality improvement and waste reduction71, other countries (e.g. Australia, Canada, Sweden and the UK) have appropriately and advantageously incorporated cost effectiveness into the coverage decision-making process at the regional and national level. This decision-making process includes pre- and post-marketing authorization by implementing risk-sharing schemes (not only ‘yes’ and ‘no’ but also ‘yes, but …’72). Such an approach informs research priority decisions using a value-of-information approach,73 while also changing providers’ and consumers’ perceptions from ‘rationing’ healthcare (a tool to restrict freedom) to an approach for fair prioritization74.
4.6 Country-Specific Models Outside North America and Europe
A major area of uncertainty also lies in the need for country-specific models in areas outside Europe and North America. Our literature review revealed no studies in countries outside Europe and North America and only one study in a country in socio-economic transition75. In many countries or regions, cost data from national health systems or government contracts with pharmaceutical companies are available, and at least one cost-of-illness study has already been conducted in South America76. The prevalence of MS may also be obtained. However, quality-of-life data, the prevalence of MS disability states and healthcare utilization within MS disability states are likely not known.
There is one currently published cost-effectiveness analysis in a country in socio-economic transition that we are aware of75. In their analysis, Jankovic and colleagues75 utilized a previously published model from the USA28, supplemented with Serbian healthcare utilization by EDSS score (obtained from a retrospective chart review of randomly selected patients in a clinical centre), Serbian healthcare costs, drug acquisition costs and wages for lost productivity calculation. The unfavourable ICER in this analysis was driven by high drug acquisition costs and a low QALY gain from disease-modifying agents.
Converting a model from one country to another requires more than changing the costs. Jankovic et al.75 were able to introduce Serbian healthcare utilization into the model to more accurately identify cost savings or expenditures in that country. However, other model assumptions should also be considered when a model is tailored for another country. Initial patient distribution among health states would ideally also use country-specific data; this would account for any differences between countries in MS stage at the time of diagnosis, which is more likely to occur earlier in areas where advanced MRI techniques are available77. In addition, differences between countries in patient disability states when initiating or terminating treatment with a DMT should also be reflected in the model. Later diagnosis of MS or initiation of treatment would decrease potential health gains realized by DMTs.
The applicability of transporting utility values for health states between countries or sub-populations is another area that should be considered. The vast majority of models for MS group disability states by EDSS score and assign utilities to each score or score grouping. It is generally thought that tariffs should be used when transferring utility values from one country to another. Tariffs reflect both differences in methodology when measuring EQ-5D states between studies and cultural differences between countries or populations78, 79. Cultural differences include the willingness to trade quantity for quality of life and the weight communities place on each of the dimensions of the EQ-5D (mobility, self-care, usual activities, pain/discomfort and anxiety/depression)79. However, tariffs for the EQ-5D are available for only 17 countries, and many other survey instruments have not had tariffs calculated.
There are three potential solutions to this problem for countries for which no original survey data or tariffs are available. The first is to use the utilities incorporated into the original model, with the limitation that differences between countries and cultures are not being incorporated into the analysis. As stated in the 2003 ISPOR guidelines, “a model should not be faulted because existing data fall short of ideal standards of scientific rigor”59. One benefit of a model is that it can be updated as new data become available. A second solution is to perform a survey to collect data on quality of life and resource utilization. Early cost-effectiveness analysis studies used small surveys of 60–400 patients80–82. Over the past decade, sample sizes have increased to over 2,000 patients in the US-based Sonya Slifka database48; over 1,800 patients in France’s European Database for MUltiple Sclerosis (EDMUS) cohort83; and almost 7,000 patients in the Swedish MS registry46. A third potential solution is to forgo quality-of-life assessment and report cost-effectiveness endpoints. The earliest MS cost-effectiveness analysis identified reported ‘normalized disability years avoided’84. Recent studies have also reported endpoints such as cost per relapse avoided, cost per relapse-free years gained, and cost per years of an EDSS score of 0–5.5 gained. These endpoints are increasingly being utilized by industry-sponsored studies, likely due to the small QALY gains associated with DMT use reported in many studies.
One last difficulty faced by modellers outside North America and Europe is that there is little opportunity for cross-validation of results due to a lack of previous country-specific models. Therefore, increased testing to ensure internal validity (model structure and calculations are correct), calibration (inputs are consistent with available data) and face validity (results make intuitive sense) should be used59, 60.
5 Conclusion
With the growing focus on evidence-based medicine and on enhancing the quality and efficiency of healthcare delivery systems, the need for information about comparative effectiveness of alternative treatment strategies is increasing74, 86, 87. However, with the increase in the number of available treatments in the market as well as the growing cost of clinical trials designed to test health interventions, more researchers turn to decision analytic modelling to make decisions in the presence of uncertainty21. Our review summarized the key issues regarding modelling disease and treatment progression in MS, in particular, for the purpose of economic evaluation. We also try to provide practical solutions to some of these problems, such as combining several sources of data when calculating DMT effectiveness to improve the inherent weaknesses of each individual data source. We also emphasize that the lack of a perfect available dataset should not be used as an excuse for avoiding decisions about the costs and benefits of health interventions. Instead, we encourage investigators and decision makers to provide a complete disclosure of modelling methods and assumptions and a careful discussion of the study limitations and implications in the face of patient and physician preferences.
Key Points for Decision Makers.
While data on multiple sclerosis (MS) disease-modifying treatments (DMTs) that are currently available to decision makers have substantial limitations, this should not preclude clinicians, healthcare administrators and payers from incorporating this information into decision making, as decisions made based on real evidence tend to be more comprehensive and better reflect the stakeholder’s perspective
Researchers and decision makers could substantially improve the transferability and applicability of models designed to reflect different healthcare systems by providing complete disclosure of modelling methods and assumptions
The greatest source of uncertainty is the absence of head-to-head randomized clinical trials. Modellers have used various techniques as well as non-randomized data, such as extension trials and observation data, to compensate
The use of large observational cohorts in recent studies aids in identifying population-based, ‘real-world’ treatment effects
The major drivers of DMT cost effectiveness include time (time of DMT initiation, duration of DMT and overall study time horizon) and DMT acquisition costs
The cost effectiveness of DMTs outside North America and Europe is currently unknown, with the lack of country-specific data as the major limiting factor
Acknowledgments
The authors would like to express sincere gratitude and appreciation to Dr. Matthew Bellizzi for his clinical consultations and thoughtful suggestions. In the past, Joel Thompson and Katia Noyes have received research grants from Biogen Idec for updating a previously published risk-benefit assessment of natalizumab.
Funding Contract HC 0103 from the National Multiple Sclerosis Society (Programme Officer: Nicholas LaRocca, PhD); Clinical and Translational Science Award (CTSA) [UL1 RR024160] from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and the NIH Roadmap for Medical Research.
Footnotes
Author Contributions Joel Thompson contributed to the concept and design, the acquisition of data, the analysis and interpretation of data, drafting the manuscript, critical revision of the manuscript, and study supervision. Amir Abdolahi contributed to the acquisition of data, drafting the manuscript, and critical revision of the manuscript. Katia Noyes contributed to the concept and design, the acquisition of data, the analysis and interpretation of data, drafting the manuscript, critical revision of the manuscript, and study supervision, and obtained funding for the study. Joel Thompson acts as guarantor for the overall content of this article.
Reference List
- 1.Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG) Annals of Neurology. 1996;39(3):285–294. doi: 10.1002/ana.410390304. [DOI] [PubMed] [Google Scholar]
- 2.PRISMS Study Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet. 1998;352(9139):1498–1504. [PubMed] [Google Scholar]
- 3.Rudick RA, Goodkin DE, Jacobs LD, et al. Impact of interferon beta-1a on neurologic disability in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG) Neurology. 1997;49(2):358–363. doi: 10.1212/wnl.49.2.358. [DOI] [PubMed] [Google Scholar]
- 4.The IFNB Multiple Sclerosis Study Group. Interferon beta-1b delays progression of disability in secondary progressive multiple sclerosis: results of a European multicenter randomised study. Lancet. 1998;352:1491–1497. [PubMed] [Google Scholar]
- 5.IFNB Multiple Sclerosis Study Group. Interferon beta-lb is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43:655–661. doi: 10.1212/wnl.43.4.655. [DOI] [PubMed] [Google Scholar]
- 6.PRISMS Study Group. PRISMS-4: Long-term efficacy of interferon-beta-1a in relapsing MS. Neurology. 2001;56(12):1628–1636. doi: 10.1212/wnl.56.12.1628. [DOI] [PubMed] [Google Scholar]
- 7.Johnson KP, Brooks BR, Cohen JA, et al. Extended use of glatiramer acetate (Copaxone) is well tolerated and maintains its clinical effect on multiple sclerosis relapse rate and degree of disability. Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1998;50(3):701–708. doi: 10.1212/wnl.50.3.701. [DOI] [PubMed] [Google Scholar]
- 8.Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. The IFNB Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Neurology. 1995;45(7):1277–1285. [PubMed] [Google Scholar]
- 9.Miller DH, Khan OA, Sheremata WA, et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348(1):15–23. doi: 10.1056/NEJMoa020696. [DOI] [PubMed] [Google Scholar]
- 10.Nuijten MJ, Hutton J. Cost-effectiveness analysis of interferon beta in multiple sclerosis: a Markov process analysis. Value Health. 2002;5(1):44–54. doi: 10.1046/j.1524-4733.2002.51052.x. [DOI] [PubMed] [Google Scholar]
- 11.Kendrick M, Johnson KI. Long-term treatment of multiple sclerosis with interferon-beta may be cost effective. Pharmacoeconomics. 2000;18(1):45–53. doi: 10.2165/00019053-200018010-00005. [DOI] [PubMed] [Google Scholar]
- 12.Rubio-Terres C, Aristegui RI, Medina RF, Izquierdo AG. Cost-utility analysis of multiple sclerosis treatment with glatiramer acetate or interferon beta in Spain [in Spanish] Farm Hosp. 2003;27(3):159–165. [PubMed] [Google Scholar]
- 13.Rubio-Terres C, Dominguez-Gil HA. Cost-utility analysis of relapsing-remitting multiple sclerosis treatment with azathioprine or interferon beta in Spain [in Spanish] Rev Neurol. 2005;40(12):705–710. [PubMed] [Google Scholar]
- 14.Kobelt G, Jonsson L, Fredrikson S. Cost-utility of interferon beta1b in the treatment of patients with active relapsing-remitting or secondary progressive multiple sclerosis. Eur J Health Econ. 2003;4(1):50–59. doi: 10.1007/s10198-002-0163-0. [DOI] [PubMed] [Google Scholar]
- 15.Prosser LA, Kuntz KM, Bar-Or A, Weinstein MC. Cost-effectiveness of interferon beta-1a, interferon beta-1b, and glatiramer acetate in newly diagnosed non-primary progressive multiple sclerosis. Value Health. 2004;7(5):554–568. doi: 10.1111/j.1524-4733.2004.75007.x. [DOI] [PubMed] [Google Scholar]
- 16.Sharac J, McCrone P, Sabes-Figuera R. Pharmacoeconomic considerations in the treatment of multiple sclerosis. Drugs. 2010;70(13):1677–1691. doi: 10.2165/11538000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Brown MG, Murray TJ, Sketris IS, et al. Cost-effectiveness of interferon beta-1b in slowing multiple sclerosis disability progression. First estimates. International Journal of Technology Assessment in Health Care. 2000;16(3):751–767. doi: 10.1017/s026646230010203x. [DOI] [PubMed] [Google Scholar]
- 18.Forbes RB, Swingler RJ. An epidemiologic study of multiple sclerosis in Northern Ireland. Neurology. 1999;52(1):215–216. doi: 10.1212/wnl.52.1.214-a. [DOI] [PubMed] [Google Scholar]
- 19.Gold MR, Siegel J, Russell L, Weinstein MC. Cost-effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 20.Tufts Medical Center. Cost-Effectiveness Analysis Registry. [Accessed 22 Apr 2012]; https://research.tufts-nemc.org/cear4/default.aspx. [Google Scholar]
- 21.Culyer AJ. Perspective and desire in comparative effectiveness research. Pharmacoeconomics. 2010;28:889–897. doi: 10.2165/11535270-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Bell CM, Urbach DR, Ray JG, et al. Bias in published cost effectiveness studies: systematic review. BMJ. 2006;332:699. doi: 10.1136/bmj.38737.607558.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bell CF. The pursuit of transparency and quality improvement in cost-effectiveness analysis: a case study in disease-modifying drugs for the treatment of multiple sclerosis. Journal of Managed Care Pharmacy. 2011;17:463–468. doi: 10.18553/jmcp.2011.17.6.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klawiter EC, Cross AH, Naismith RT. The present efficacy of multiple sclerosis therapeutics. Neurology. 2009;73:983–990. doi: 10.1212/WNL.0b013e3181b9c8f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minden S, Hoaglin D, Jureidini S, et al. Disease-modifying agents in the Sonya Slifka Longitudinal Multiple Sclerosis Study. Mult Scler. 2008;14(5):640–655. doi: 10.1177/1352458507086463. [DOI] [PubMed] [Google Scholar]
- 26.Khan OA, Zabad R, Caon C, et al. Comparative assessment of immunomodulating therapies for relapsing-remitting multiple sclerosis. CNS Drugs. 2002;16:563–578. doi: 10.2165/00023210-200216080-00005. [DOI] [PubMed] [Google Scholar]
- 27.Khan O. What can be learned from open direct comparative trials in multiple sclerosis. J Neurol Sci. 2009;277(Suppl. 1):S25–S28. doi: 10.1016/S0022-510X(09)70008-5. [DOI] [PubMed] [Google Scholar]
- 28.Bell C, Graham J, Earnshaw S, et al. Cost-effectiveness of four immunomodulatory therapies for relapsing-remitting multiple sclerosis: a Markov model based on long-term clinical data. J Manag Care Pharm. 2007;13(3):245–261. doi: 10.18553/jmcp.2007.13.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Earnshaw SR, Graham J, Oleen-Burkley MK, et al. Cost effectiveness of glatiramer acetate and natalizumab in relapsing-remitting multiple sclerosis. Appl Health Econ Policy. 2009;7(2):91–108. doi: 10.1007/BF03256144. [DOI] [PubMed] [Google Scholar]
- 30.Khan OA, Tselis AC, Kamholz JA, et al. A prospective, open-label treatment trial to compare the effect of IFNbeta-1a (Avonex), IFNbeta-1b (Betaseron), and glatiramer acetate (Copaxone) on the relapse rate in relapsing-remitting multiple sclerosis: results after 18 months of therapy. Mult Scler. 2001;7:349–353. doi: 10.1177/135245850100700601. [DOI] [PubMed] [Google Scholar]
- 31.Deisenhammer F, Mayringer I, Harvey J, et al. A comparative study of the relative bioavailability of different interferon beta preparations. Neurology. 2000;54:2055–2060. doi: 10.1212/wnl.54.11.2055. [DOI] [PubMed] [Google Scholar]
- 32.Durelli L, Verdun E, Barbero P, et al. Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN) Lancet. 2002;359:1453–1460. doi: 10.1016/s0140-6736(02)08430-1. [DOI] [PubMed] [Google Scholar]
- 33.Sturzebecher S, Maibauer R, Heuner A, et al. Pharmacodynamic comparison of single doses of IFN-beta1a and IFN-beta1b in healthy volunteers. J Interferon Cytokine Res. 1999;19:1257–1264. doi: 10.1089/107999099312920. [DOI] [PubMed] [Google Scholar]
- 34.Panitch H, Goodin DS, Francis G, et al. Randomized, comparative study of interferon beta-1a treatment regimens in MS: the EVIDENCE Trial. Neurology. 2002;59(10):1496–1506. doi: 10.1212/01.wnl.0000034080.43681.da. [DOI] [PubMed] [Google Scholar]
- 35.Guo S, Bozkaya D, Ward A, et al. Treating relapsing multiple sclerosis with subcutaneous versus intramuscular interferon-beta-1a. Pharmacoeconomics. 2009;27(1):39–53. doi: 10.2165/00019053-200927010-00005. [DOI] [PubMed] [Google Scholar]
- 36.Tappenden P, McCabe C, Chilcott JB, et al. Cost-Effectiveness of Disease-Modifying Therapies in the Management of Multiple Sclerosis for the Medicare Population. Value Health. 2009;12(5):657–665. doi: 10.1111/j.1524-4733.2008.00485.x. [DOI] [PubMed] [Google Scholar]
- 37.Mullins CD, Whicher D, Reese ES, et al. Generating evidence for comparative effectiveness research using more pragmatic randomized controlled trials. Pharmacoeconomics. 2010;28(10):969–976. doi: 10.2165/11536160-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 38.Patient-Centered Outcomes Research Institute (PCORI) Methodology report: Our questions, our decisions—standards for patient-centered outcomes research. [Accessed 15 Jul 2012];2012 Jun 4; http://www.pcori.org/assets/Preliminary-Draft-Methodology-Report.pdf. [Google Scholar]
- 39.Shirani A, Zhao Y, Karim ME, et al. Association between use of interferon beta and progression of disability in patients with relapsing-remitting multiple sclerosis. JAMA. 2012;308(3):247–256. doi: 10.1001/jama.2012.7625. [DOI] [PubMed] [Google Scholar]
- 40.Heckman J. Sample selection bias as a specification error. Econometrica. 1979;47:153–161. [Google Scholar]
- 41.Heckman J, Ichimura H, Todd PE. Matching as an econometric evaluation estimator: evidence from evaluating a job training programme. Review of Economic Studies. 1997;64:605–654. [Google Scholar]
- 42.Heckman JJ, Vytlacil E. Structural equations, treatment effects, and econometric policy evaluation. Econometrica. 2005;73(3):669–738. [Google Scholar]
- 43.Dehejia R, Wahba S. Propensity score-matching methods for nonexperimental causal studies. Review of Economics and Statistics. 2002;84(1):151–161. [Google Scholar]
- 44.Newhouse JP, McClellan M. Econometrics in outcomes research: the use of instrumental variables. Annual Review of Public Health. 1998;19:17–34. doi: 10.1146/annurev.publhealth.19.1.17. [DOI] [PubMed] [Google Scholar]
- 45.Duan N, Manning WG, Morris CN, et al. Choosing Between the Sample Selection Model and Multi-Part Model. Journal of Business and Economic Statistics. 1984;2:283–289. [Google Scholar]
- 46.Kobelt G, Berg J, Lindgren P, et al. Modeling the cost-effectiveness of a new treatment for MS (natalizumab) compared with current standard practice in Sweden. Multiple Sclerosis. 2008;14:679–690. doi: 10.1177/1352458507086667. [DOI] [PubMed] [Google Scholar]
- 47.Noyes K, Bajorska A, Chappel A, et al. Cost-effectiveness of disease-modifying therapy for multiple sclerosis: A population-based study. Neurology. 2011;77:355–363. doi: 10.1212/WNL.0b013e3182270402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minden SL, Frankel D, Hadden LS, Perloff JN, Srinath KP, Hoaglin DC. The Sonya Slifka Longitudinal Multiple Sclerosis Study: Methods and Sample Characteristics. Multiple Sclerosis. 2006;12:24–38. doi: 10.1191/135248506ms1262oa. [DOI] [PubMed] [Google Scholar]
- 49.Sculpher M, Claxton K, Drummond MF, et al. Whither trial-based economic evaluation for health care decision making? Health Econ. 2006;15(7):677–687. doi: 10.1002/hec.1093. [DOI] [PubMed] [Google Scholar]
- 50.Sculpher M, Fenwick E, Claxton K. Assessing quality in decision analytic cost-effectiveness models: a suggested framework and example of application. Pharmacoeconomics. 2000;17(5):461–477. doi: 10.2165/00019053-200017050-00005. [DOI] [PubMed] [Google Scholar]
- 51.Griffin S, Claxton K, Sculpher M. Decision analysis for resource allocation in health care. J Health Serv Res Policy. 2008;(Suppl. 3):23–30. doi: 10.1258/jhsrp.2008.008017. [DOI] [PubMed] [Google Scholar]
- 52.Philips Z, Ginnelly L, Sculpher M, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess. 2004;8(36):iii–xi. 1. doi: 10.3310/hta8360. [DOI] [PubMed] [Google Scholar]
- 53.Noyes K, Veazie P, Hall WJ, et al. Cost-effectiveness of cardiac resynchronization therapy in the MADIT-CRT trial. J Cardiovasc Electrophysiol. 2013;24(1):66–74. doi: 10.1111/j.1540-8167.2012.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldberg LD, Edwards NC, Fincher C, et al. Comparing the cost-effectiveness of disease-modifying drugs for the first-line treatment of relapsing-remitting multiple sclerosis. Journal of Managed Care Pharmacy. 2009;15:543–555. doi: 10.18553/jmcp.2009.15.7.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson JP, Noyes K, Dorsey ER, et al. Quantitative risk-benefit analysis of natalizumab. Neurology. 2008;71:357–364. doi: 10.1212/01.wnl.0000319648.65173.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garber AM, Phelps CE. Economic foundations of cost-effectiveness analysis. Journal of Health Economics. 1997;16(1):1–31. doi: 10.1016/s0167-6296(96)00506-1. [DOI] [PubMed] [Google Scholar]
- 57.Meltzer D. Accounting for future costs in medical cost-effectiveness analysis. Journal of Health Economics. 1997;16:33–64. doi: 10.1016/s0167-6296(96)00507-3. [DOI] [PubMed] [Google Scholar]
- 58.Meltzer D, Johannesson M. Inconsistencies in the "Societal Perspective" on Costs of the Panel on Cost-Effectiveness in Health and Medicine. Medical Decision Making. 1999;19:371–377. doi: 10.1177/0272989X9901900401. [DOI] [PubMed] [Google Scholar]
- 59.Weinstein MC, O'Brien BJ, Hornberger J. Principles of good practice of decision analytic modeling in health care evaluation: Report of the ISPOR Task Force on Good Research Practices-Modeling Studies. Value in Health. 2003;6:9–17. doi: 10.1046/j.1524-4733.2003.00234.x. [DOI] [PubMed] [Google Scholar]
- 60.Caro JJ, Briggs AH, Siebert U, Kuntz KM. Modeling good research practices--Overview: A report of the ISPOR-SMDM Modeling Good Research Practices Task Force-1. Value in Health. 2012;15:796–803. doi: 10.1016/j.jval.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 61.Smyth KA. Cost-effectiveness analyses of treatments for multiple sclerosis: are they clinically relevant? Neurology. 2011;77(4):317–318. doi: 10.1212/WNL.0b013e318227066d. [DOI] [PubMed] [Google Scholar]
- 62.Boult C, Wieland GD. Comprehensive primary care for older patients with multiple chronic conditions: "nobody rushes you through". JAMA. 2010;304:1936–1943. doi: 10.1001/jama.2010.1623. [DOI] [PubMed] [Google Scholar]
- 63.Meltzer D, Basu A, Conti R. The economics of comparative effectiveness studies: societal and private perspectives and their implications for prioritizing public investments in comparative effectiveness research. Pharmacoeconomics. 2010;28(10):843–853. doi: 10.2165/11539400-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chilcott J, McCabe C, Tappenden P, et al. Modelling the cost effectiveness of interferon beta and glatiramer acetate in the management of multiple sclerosis. Commentary: evaluating disease modifying treatments in multiple sclerosis. BMJ. 2003;326(7388):522–526. doi: 10.1136/bmj.326.7388.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cahill J, Learner N. Managed care pharmacy sees potential of comparative effectiveness research to improve patient care and lower costs. Pharmacoeconomics. 2010;28(10):931–934. doi: 10.2165/11535610-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 66.Raftery J. Multiple sclerosis risk sharing scheme: a costly failure. BMJ. 2010;340:1672. doi: 10.1136/bmj.c1672. [DOI] [PubMed] [Google Scholar]
- 67.Schafer JA, Gunderson BW, Gleason PP. Price increases and new drugs drive increased expenditures for multiple sclerosis. Journal of Managed Care Pharmacy. 2010;16:713–717. doi: 10.18553/jmcp.2010.16.9.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pickin M, Cooper CL, Chater T. The Multiple Sclerosis Risk Sharing Scheme Monitoring Study: early results and lessons for the future. BMC Neurology. 2009;9:1–8. doi: 10.1186/1471-2377-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sanchez-de la Rosa R, Sabater E, Casado MA. Budget impact analysis of the first-line treatment of relapsing remitting multiple sclerosis in Spain [in Spanish] Rev Neurol. 2011;53:129–138. [PubMed] [Google Scholar]
- 70.Coyle PK, Foley JF, Fox EJ, et al. Best practice recommendations for the selection and management of patients with multiple sclerosis receiving natalizumab therapy. Multiple Sclerosis. 2009;15:S26–S35. [Google Scholar]
- 71.Chambers JD, Neumann PJ, Buxton MJ. Does Medicare have an implicit cost-effectiveness threshold? Med Decis Making. 2010;30:E14–E27. doi: 10.1177/0272989X10371134. [DOI] [PubMed] [Google Scholar]
- 72.O'Neill P, Devlin NJ. An analysis of NICE's "restricted" (or "optimized") decisions. Pharmacoeconomics. 2010;28(11):987–993. doi: 10.2165/11536970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 73.Eckermann S, Karnon J, Willan AR. The value of value of information: best informing research design and prioritization using current methods. Pharmacoeconomics. 2010;28(9):699–709. doi: 10.2165/11537370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 74.Chalkidou K, Walley T. Using comparative effectiveness research to inform policy and practice in the UK NHS: past, present and future. Pharmacoeconomics. 2010;28(10):799–811. doi: 10.2165/11535260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 75.Jankovic SM, Kostic M, Radosavljevic M. Cost-effectiveness of four immunomodulatory therapies for relapsing-remitting multiple sclerosis: a Markov model based on data a Balkan country in socioeconomic transition. Vojnosanit Pregl. 2009;66(7):556–562. doi: 10.2298/vsp0907556j. [DOI] [PubMed] [Google Scholar]
- 76.Romero A, Arango C, Alvis N, et al. The cost of treatment in multiple sclerosis in Colombia. Value Health. 2011;14(Suppl. 1):S48–S50. doi: 10.1016/j.jval.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 77.Traboulsee AL, Li DK. The role of MRI in the diagnosis of multiple sclerosis. Adv Neurol. 2006;98:125–146. [PubMed] [Google Scholar]
- 78.Knies S, Evers SM, Candel MJ, et al. Utilities of the EQ-5D: Transferable or not? Pharmacoeconomics. 2009;27(9):767–779. doi: 10.2165/11314120-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 79.Norman R, Cronin P, Viney R, et al. International comparisons in valuing EQ-5D health states: a review and analysis. Value Health. 2009;12(8):1194–1200. doi: 10.1111/j.1524-4733.2009.00581.x. [DOI] [PubMed] [Google Scholar]
- 80.Parkin D, McNamee P, Jacoby A, et al. A cost-utility analysis of interferon beta for multiple sclerosis. Health Technol Assess. 1998;2(4):iii-54. [PubMed] [Google Scholar]
- 81.Kobelt G, Lindgren P, Parkin D, et al. Scandinavian Working Papers in Economics. Stockholm: The Economic Research Institute, Stockholm School of Economics; 2000. Costs and Quality of Life in Multiple Sclerosis: A Cross-Sectional Observational Study in the UK. [Google Scholar]
- 82.Prosser LA, Kuntz KM, Bar-Or A, et al. Patient and community preferences for treatments and health states in multiple sclerosis. Multiple Sclerosis. 2003;9(3):311–319. doi: 10.1191/1352458503ms903oa. [DOI] [PubMed] [Google Scholar]
- 83.Kobelt G, Texier-Richard B, Lindgren P. The long-term cost of multiple sclerosis in France and potential changes with disease-modifying interventions. Multiple Sclerosis. 2009;15(6):741–751. doi: 10.1177/1352458509102771. [DOI] [PubMed] [Google Scholar]
- 84.Otten N. Interferon Beta 1-B and Multiple Sclerosis. Issue 5.0, 1. Ottawa: Canadian Coordinating Office for Health Technology Assessment (CCOHTA); 1996. [Google Scholar]
- 85.Otten N. Comparison of Drug Treatments for Multiple Sclerosis. Ottawa, Canada: Canadian Coordinating Office for Health Technology Assessment (CCOHTA); 1998. [Google Scholar]
- 86.Levy AR, Mitton C, Johnston KM, et al. International comparison of comparative effectiveness research in five jurisdictions: insights for the US. Pharmacoeconomics. 2010;28(10):813–830. doi: 10.2165/11536150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 87.Garrison LP. Regulatory benefit-risk assessment and comparative effectiveness research: strangers, bedfellows or strange bedfellows? Pharmacoeconomics. 2010;28(10):855–865. doi: 10.2165/11538640-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 88.Brown MG, Murray TJ, Fisk JD, et al. A therapeutic and economic assessment of betaseron in multiple sclerosis. Ottawa: Canadian Coordinating Office for Health Technology Assessment (CCOHTA); 1996. Jul, [Google Scholar]
- 89.Forbes RB, Lees A, Waugh N, et al. Population based cost utility study of interferon beta-1b in secondary progressive multiple sclerosis. BMJ. 1999;319(7224):1529–1533. doi: 10.1136/bmj.319.7224.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kobelt G, Jonsson L, Henriksson F, et al. Cost-utility analysis of interferon beta-1b in secondary progressive multiple sclerosis. Int J Technol Assess Health Care. 2000;16(3):768–780. doi: 10.1017/s0266462300102041. [DOI] [PubMed] [Google Scholar]
- 91.Parkin D, Jacoby A, McNamee P, et al. Treatment of multiple sclerosis with interferon beta: an appraisal of cost-effectiveness and quality of life. J Neurol Neurosurg Psychiatry. 2000;68(2):144–149. doi: 10.1136/jnnp.68.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bose U, Ladkani D, Burrell A. Cost-effectiveness analysis of glatiramer acetate in the treatment of relapsing-remitting multiple sclerosis. Journal of Medical Economics. 2001;4:207–219. [Google Scholar]
- 93.Phillips C, Gilmour L, Gale R. A cost utility model of beta-interferon in the treatment of relapsing-remitting multiple sclerosis. Journal of Medical Economics. 2001;4:35–50. [Google Scholar]
- 94.Kobelt G, Jonsson L, Miltenburger C, et al. Cost-utility analysis of interferon beta-1B in secondary progressive multiple sclerosis using natural history disease data. Int J Technol Assess Health Care. 2002;18(1):127–138. [PubMed] [Google Scholar]
- 95.Lepen C, Coyle PK, Vollmer T, et al. Long-term cost-effectiveness of interferon-beta-1a in the treatment of relapsing-remitting multiple sclerosis. Clin Drug Invest. 2003;23(9):571–581. doi: 10.2165/00044011-200323090-00003. [DOI] [PubMed] [Google Scholar]
- 96.Touchette DR, Durgin TL, Wanke LA, et al. A cost-utility analysis of mitoxantrone hydrochloride and interferon beta-1b in the treatment of patients with secondary progressive or progressive relapsing multiple sclerosis. Clin Ther. 2003;25(2):611–634. doi: 10.1016/s0149-2918(03)80100-5. [DOI] [PubMed] [Google Scholar]
- 97.Iskedjian M, Walker JH, Gray T, et al. Economic evaluation of Avonex (interferon beta-Ia) in patients following a single demyelinating event. Mult Scler. 2005;11(5):542–551. doi: 10.1191/1352458505ms1211oa. [DOI] [PubMed] [Google Scholar]
- 98.Gani R, Giobannoni G, Bates D, et al. Cost-effectiveness analysis of natalizumab (Tysabri) compared with other disease-modifying therapies for people with highly active relapsing-remitting multiple sclerosis in the UK. Pharmacoeconomics. 2008;26(7):617–627. doi: 10.2165/00019053-200826070-00008. [DOI] [PubMed] [Google Scholar]
- 99.Chiao E, Meyer K. Cost effectiveness and budget impact of natalizumab in patients with relapsing multiple sclerosis. Current Medical Research and Opinion. 2009;25(6):1445–1454. doi: 10.1185/03007990902876040. [DOI] [PubMed] [Google Scholar]
- 100.Nuijten M, Mittendorf T. A health-economic evaluation of disease-modifying drugs for the treatment of relapsing-remitting multiple sclerosis from the German societal perspective. Clinical Therapeutics. 2010;32(4):717–727. doi: 10.1016/j.clinthera.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 101.Becker R, Dembeck C. Effects of cohort selection on the results of cost-effectiveness analysis of disease-modifying drugs for relapsing-remitting multiple sclerosis. Journal of Managed Care Pharmacy. 2011;17(5):377–387. doi: 10.18553/jmcp.2011.17.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.O'Day K, Meyer K, Miller RM, Agarwal S, Franklin M. Cost-effectiveness of natalizumab versus fingolimod for the treatment of relapsing multiple sclerosis. Journal of Medical Economics. 2011;14(5):617–627. doi: 10.3111/13696998.2011.602444. [DOI] [PubMed] [Google Scholar]