Abstract
Both sleep-disordered breathing (SDB) and cognitive impairment are common among older adults, yet few studies have examined their relationship within this population to determine whether the effect of SDB on cognition is of similar or greater magnitude as that observed in younger- and middle-aged adults. Here, we review the extant literature and report that studies are largely supportive of an association between SDB and cognitive impairment in older adults, particularly in the domains of attention/vigilance, executive function, and verbal delayed recall memory. Presence of the APOE4 allele may confer increased vulnerability to SDB-associated cognitive dysfunction among elderly individuals. Although findings are mixed, there is strong evidence to suggest that SDB-related intermittent hypoxemia is the primary mechanism through which SDB exerts its adverse effects on cognition. We propose a microvascular model in which chronic intermittent hypoxemia causes vasculopathy that ultimately is expressed as cognitive impairment in the older adult. However, it remains unclear if the effects of SDB on cognition are the same regardless of age, or if there is a synergistic interaction between age and SDB.
Keywords: sleep-disordered breathing, obstructive sleep apnea, sleep apnea, cognition, cognitive, neuropsychology, older adults, elderly
Introduction
Estimated prevalence rates of both sleep-disordered breathing (SDB) and cognitive impairment increase with age [1, 2]. Previous studies have examined relationships between SDB and cognition in younger- and middle-aged adults [3–5]. In recent years, however, a number of influential reports have examined these relationships exclusively among older adults. These endeavors are both timely and important as older adults occupy relatively increasing proportions of the general population [6] and research efforts intensify to identify remediable risk factors, such as SDB, for cognitive impairment, serious medical illness, and associated functional declines. The goal of the current article is to summarize findings from the literature on SDB and cognition in older adults and highlight several recent developments. Emerging convergent findings, possible mechanisms underlying SDB-related cognitive dysfunction, and considerations for future research are also discussed.
Sleep-disordered breathing is a broad term used to describe the presence of abnormal respiratory events that occur during sleep. The clinical diagnosis of sleep apnea syndrome is made when apneas (complete cessations of breathing) and hypopneas (partial cessations of breathing) are present in conjunction with excessive daytime somnolence. The etiology of apneas and hypopneas is frequently anatomical and involves a narrowing or collapse of the pharyngeal upper airway when an individual is lying in a supine position during sleep. Apneas and hypopneas may occur occasionally or up to several hundred times a night and are associated with intermittent hypoxemia (i.e., significant drops in blood oxygenation). In addition, each breathing event causes involuntary and usually unconscious arousals in order to resume respiration, ultimately leading to sleep fragmentation. There are three primary types of sleep apnea events. Obstructive sleep apnea (OSA) is defined as the presence of at least 5 or more apneas and hypopneas per hour of sleep associated with collapse of the upper airway, despite adequate respiratory muscle effort, that result in excessive nocturnal or daytime symptoms. Obstructive Sleep Apnea may also be diagnosed with a presentation of 15 or more apneas or hypopneas per hour of sleep in the absence of associated sequelae [7]. Central sleep apnea events are characterized by the presence of apneas and hypopneas in the context of a relatively patent upper airway and reduced respiratory muscle effort. Central events are most common among individuals with stroke, congestive heart failure, Cheyne-Stokes respiration, renal failure, or those sleeping at high altitudes [8]. Finally, mixed sleep apnea events may also occur, such as when the upper airway collapses at the end of a central event. These breathing events are commonly identified using an overnight polysomnography (PSG) procedure that can be used to generate an Apnea Hypopnea Index (AHI; the number of apneas and hypopneas per hour of sleep) and Respiratory Disturbance Index (RDI; the number of apneas, hypopneas, and arousals per hour of sleep). Although severity guidelines vary, in general, an AHI of 5–19 is termed mild, an AHI of 20–29 is moderate, and an AHI of 30 or greater is severe [9]. A common treatment is nightly use of a positive airway pressure (PAP) device, which functions as pneumatic splint that prevents collapse of upper airway through use of a nasal mask attached to an external air pump. PAP is effective at mitigating the primary causes and associated sequelae of OSA [10–12], yet adherence to nightly PAP use is generally poor [13].
Increased Prevalence of Sleep-Disordered Breathing in Older Adults
Low levels of SDB present in otherwise healthy individuals are often undetected or underreported due to minimal interference with activities of daily living. However, the clinically significant symptoms of OSA have estimated prevalence rates among middle-aged adults of 2% for women and 4% for men [14]. Of particular note to clinicians and researchers who work with older adults, however, are the critically higher prevalence rates of OSA and SDB among the elderly of up to 62% [1]. In 1981, Carskadon and Dement [15] reported an early account of relatively higher rates of respiratory events among older adults, noting that 37.5% of their sample of community dwelling volunteers met criteria for sleep apnea syndrome and stating, “… there is little doubt that this is an age related finding.” The increased prevalence of SDB among older adults has led some third-party payers to consider a different cutoff of at least ten abnormal breathing events per hour of sleep as clinically significant among this population. What is the reason for a disproportionately higher rate of SDB among the elderly? Competing mechanistic causes include age-related changes in pharyngeal and upper airway muscle size and function, increased sleep fragmentation, instability in ventilator control, and differential effects of hormones on upper airway function (for review, see [16]). International Classification of Sleep Disorders diagnostic criteria for OSA do not currently vary as a function of age [7], although age-associated alterations in diagnostic thresholds have been proposed [17].
Sleep-Disordered Breathing and Cognition in Older Adults
Sleep-disordered breathing and age have been shown to be independent risk factors for cognitive dysfunction and dementia [1, 18, 19] and cognitive dysfunction is a key contributor to quality of life and independent living among older adults. In this review, we excluded studies that focused on prevalent dementia samples because our goal was to examine the effect of SDB on cognition in healthy elderly adults rather than the effect of pathological neurodegeneration on sleep function. It should be noted, however, that there is evidence [20–23] that older adults with dementia experience a disproportionate amount of day and nighttime sleep disturbances that suggest shared biological underpinnings. Publications for this review were identified using the National Institutes of Health National Library of Medicine PubMed literature search system. Additional relevant references included within identified articles were also evaluated for inclusion in the review. Search terms included “sleep-disordered breathing”, “obstructive sleep apnea”, “sleep apnea”, “cognition”, “cognitive”, “neuropsychology”, “older adults”, and “elderly.” Inclusion criteria required that original research articles: 1) were written in English, 2) examined an older adult population aged ≥ 50 years, 3) included at least one measure of SDB, and 4) included at least one measure of cognitive function.
Table 1 depicts the general characteristics of 18 studies examining SDB and cognition in older adults. Sample setting tended to systematically influence other study characteristics, such as sample size, SDB severity, and comprehensiveness of both cognitive and SDB measures. Therefore, major findings are broadly discussed on the basis of the origin of their sample setting, i.e., sleep clinic or community-dwelling. Table 2 provides specific examples of neuropsychological tests that generally fall within the domains that have been found to be associated with measures of SDB in older adults; examples are drawn from both the papers reviewed herein as well as those that are commonly used in the clinical neuropsychological assessment of older adults.
Table 1.
Characteristics of Studies on Sleep-Disordered Breathing and Cognition among Older Adults
| Authors, Year Published |
Sample Setting, design |
Subjects | Cognitive Domains | Major Findings | |||
|---|---|---|---|---|---|---|---|
| N | Age | Gender | SDB Severity | ||||
| Yaffe et al., 2011[44] | Community, longitudinal | 105 SDB 193 NC |
83(3) SDB 82(3) NC |
Women only | AHI N=298: median=10; interquartile range=5–20 | Global, Attention, Executive Function, Memory |
|
| Kim et al., 2011[29] | Clinic, cross-sectional | 30 MCI 30 NC |
68(4) MCI 67(4) NC |
42 M 18 W |
AHI: 13(12) MCI; 15(14) NC | Executive Function, Language, Memory, Visuospatial Construction |
|
| Blackwell et al., 2011[45] | Community, cross-sectional | 2909 | 76(6) | Men only | 43% had AHI≥ 15 | Global, Vigilance, Executive Function |
|
| Sforza et al., 2010[35] | Community, cross-sectional | 827 | 68(2) | 343 M 484 W |
AHI: 20(15); 53% had AHI≥15 | Global, Attention, Executive Function, Memory, Language |
|
| Spira et al., 2008[39] | Community, cross-sectional | 448 | 83(3) | Women only | AHI: 16(15); 13% had AHI≥30 | Global, Executive Function |
|
| Alchanatis et al., 2008[28] | Sleep Clinic, cross-sectional | 58 OSAS 41 NC |
Range=32–65 OSAS; Range=33–63 NC | N/A | AHI: range 31–137 OSAS; range 1–7 NC | Reaction time |
|
| Mathieu et al., 2008[27] | Sleep Clinic, cross-sectional | 28 OSAS 30 NC |
38(2) OSAS younger; 62(2) OSAS older | 26 M 2 W |
AHI: 51(4) OSAS younger; 43(4) OSAS older | Attention, Executive Function, Memory |
|
| O’Hara et al., 2005[38] | Community, cross-sectional | 36 | 70(6) APOE4+ 72(10) APOE4− |
12 M 24 W |
AHI: 9(8) APOE4+; 6(7) APOE4− |
Global, Attention, Executive Function, Memory |
|
| Kim et al., 2007[40] | Community, cross-sectional | 611 | Range=35–74 | 346 M 265 W |
AHI: median =2.8; range=0–121 | Vigilance |
|
| Aloia et al., 2003[26] | Clinic, cross-sectional | 12 | 65(6) OSAS compliant; 65(3) noncompliant | N/A | RDI: 51(20) OSAS compliant; 46(22) OSAS noncompliant | Attention, Executive Function, Construction, Motor Speed, Memory, Language |
|
| Foley et al., 2003[34] | Community, cross-sectional | 718 | Range=79–97 | Men only | 71% had AHI≥5; 19% had AHI≥30 | Global, Attention, Executive Function, Construction, Memory, Language |
|
| Boland et al., 2002[33] | Community, cross-sectional | 1700 | 62; range=52–75 | 837 M 923 W |
RDI median range: 0.4–23 | Attention, Executive Function, Memory |
|
| Cohen-Zion et al., 2001[43] | Community, longitudinal | 46 | 80(3) | 7 M 39 W |
RDI: 10(9) | Global |
|
| Dealberto et al., 1996[42] | Community, cross-sectional | 1389 | 65(3) | 574 M 815 W |
Questionnaire: breathing stoppage and snoring | Global, Attention, Executive Function, Memory |
|
| Hayward et al., 1992[41] | Community, cross-sectional | 96 | 78(4) | 21 M 75 W |
RDI: 6(6) | Attention, Executive Function, Memory, Language, Motor |
|
| Phillips et al., 1992[32] | Community, cross-sectional | 92 | 64(9) | 44 M 48 W |
AHI:3(4) | Global, IQ, Attention, Executive Function, Memory, Language, Motor |
|
| Berry et al., 1990[25] | Sleep Clinic, cross-sectional | 8 OSAS 12 HC |
67(5) OSAS 68(2) HC |
Men only | AHI: 28(12) OSAS; 3(3) NC | Global, IQ, Memory |
|
| Yesavage et al., 1985[24] | Sleep Clinic, cross-sectional | 41 | 70(6) | Men only | RDI: 26(30); 73% had RDI>5 | Attention, Executive Function, Memory |
|
Age and Symptom Severity presented in mean(sd) unless otherwise specified; M=Men; W=Women; AHI=Apnea Hypopnea Index; RDI=Respiratory Disturbance Index; EDS=Excessive Daytime Sleepiness; MCI=Mild Cognitive Impairment; NC=Normal Control; OSAS=Obstructive Sleep Apnea Syndrome; APOE4=apolipoprotein E ε4 allele; IQ=Intelligence Quotient
Table 2.
Examples of Neuropsychological Tests in Domains Associated with SDB among Older Adults
| Domain | Neuropsychological Test |
|---|---|
| Attention/Vigilance | |
| Psychomotor Vigilance Test [57] | |
| Digit Vigilance Test [58] | |
| Trail Making Test Part A [59] | |
| Digit Symbol Substitution/Coding Subtest [60] | |
| Digit Span Subtest [60] | |
| Connors’ Continuous Performance Test [61] | |
| Executive Function | |
| Trail Making Test Part B [59] | |
| Stroop Color and Word Test (Golden version) [62] | |
| Raven’s Progressive Matrices [63] | |
| Controlled Oral Word Association Test [64] | |
| Memory | |
| Hopkins Verbal Learning Test – Revised [65] | |
| Rey Auditory Verbal Learning Test [66] | |
| Brief Visuospatial Memory Test – Revised [67] | |
| California Verbal Learning Test [68] | |
| Logical Memory I and II Subtests [69] | |
Sleep clinic studies of SDB and cognition in older adults
Of 18 studies reviewed, six (33%) utilized data drawn from individuals referred from a sleep or general health clinic. Although clinic-based samples tended to be relatively smaller in size, they also generally included attended overnight PSG, comprehensive cognitive batteries, and participants with the most severe AHI or RDI. In addition, all of the clinic-based studies found significant relationships between indices of SDB and performance on at least one measure of cognitive function. A 1985 study by Yesavage and colleagues [24] is notable for its extensive neuropsychological battery that assessed a wide-range of cognitive domains as well as inclusion of participants with moderate to severe SDB symptoms. After controlling for age, education, depression, and sleepiness, they found that RDI was associated with poorer performance on almost half of the cognitive tests administered, including psychomotor speed, language, and several tests of executive function. This was an important study because it highlighted critical questions that continue to be investigated today; that is, 1) whether the observed cognitive impairments were due to daytime sleepiness and/or hypoxemia associated with SDB and 2) postulated that SDB and age-related neurodegenerative disease “… may overlap and interact in the elderly population.” The first study on this topic that included a matched healthy control comparison group was published by Berry and colleagues in 1990 [25]. Although the sample size was small (n=8), patients performed more poorly on tests of nonverbal intelligence and nonverbal delayed recall memory. In the first study to examine the effect of PAP use on cognition among older adults with sleep apnea, Aloia and colleagues [26] reported that prior to PAP initiation, RDI was associated with poorer verbal delayed recall memory performance while hypoxemia was associated with verbal delayed recall memory and constructional abilities. Again, the sample size was small, but following three months of PAP use, older adults who were compliant with treatment (average use 8.5 hours/night) demonstrated greater improvements on tests of attention, psychomotor speed, executive function, and nonverbal delayed recall memory that those who were not compliant. In 2008, two separate clinic-based studies compared older and younger OSA patients to older and younger matched controls. Both studies used 50 years of age as a cut-off for age group comparisons. These studies addressed the question of whether age and SDB interact or whether the association between SDB and cognition is invariant across age groups. The first [27] found that older participants performed more poorly across a range of different tests of cognition than younger participants and OSA patients performed more poorly across tests of cognition than healthy controls. However, there was no age by group interaction, suggesting that older OSA patients did not demonstrate relatively greater impairments than younger OSA patients. In fact, younger OSA patients demonstrated a greater number of different relationships with daytime sleepiness and hypoxemia variables and tests of attention than older OSA patients, leading the authors to speculate that younger OSA patients may be more vulnerable to the deleterious effects of hypoxemia than older OSA patients, who may habituate to hypoxemia or possibly demonstrate “survivor effects.” The second of these studies [28] used a similar study design and a neuropsychological battery focused on the domains of attention and reaction time in patients with severe OSA. They also found differences on attention tests between younger and older OSA patients. However, younger OSA patients performed similarly to their matched controls, while older OSA patients demonstrated poorer performance compared to their controls, suggesting, contrary to the report from Mathieu and colleagues, that it is older OSA patients who are more vulnerable to the effects of sleep fragmentation and hypoxemia on cognition while younger OSA patients may be able to compensate for any negative effects. Finally, a recent study examined older adults recruited from a general health clinic with mild cognitive impairment (MCI)[29]. Individuals with MCI demonstrate clinically significant cognitive impairments in the absence of associated declines in activities of daily living and are at high risk for the development of dementia [30, 31]. No differences between the MCI and control groups on PSG measures were found. However, higher AHI was associated with poorer performance on a language test among individuals with MCI. The authors concluded that this finding suggests that older patients with MCI are more vulnerable to the negative effects of SDB. This study was distinct for its specific examination of older adults with MCI. However, a large number of comparisons were performed without statistical adjustment and future studies with larger sample sizes and addition of an older adult control group without MCI or SDB will be necessary to determine whether SDB potentiates the risk of developing dementia among individuals with MCI.
Community-based studies of SDB and cognition in older adults
Community-based studies have the advantage of examining large samples with relatively heterogeneous yet healthy presentations that facilitate generalizations to the broader population. One of the earliest community-based studies of SDB and cognition in older adults [32] examined 92 men and women aged 50–80 years with mild AHI (3/hr on average) using an extensive neuropsychological battery. They found no relationships between measures of SDB and a range of different measures of cognition after adjustment for multiple comparisons. The lack of a relationship remained even after they dichotomized their sample on the basis of AHI≥5 in order to examine effects of SDB severity. Several other community-based studies reported a similar absence of relationships between SDB and cognition among older adults [33–35]. Two studies specifically examined the effect of the apolipoprotein E ε4 (APOE4) allele, the strongest known genetic risk factor for late-onset Alzheimer’s disease, on cognitive function among older adults with SDB. APOE4 has pertinent relevance as it is a risk factor for both cognitive decline in older adults [36] and SDB [37]. The first study [38] found that AHI was associated with verbal delayed recall memory difficulties in older adults with APOE4, but not those without the ε4 allele, and that hypoxemia was not related to cognition in either group of older adults. The second of these studies [39] reported that AHI and hypoxemia were associated with a decline in a global measure of cognition in a sample of older adults with mild SDB, and that presence of the ε4 allele was associated with a five-fold increased risk of global cognitive impairment. Several other community-based studies found relationships between AHI and poorer performance on tests of vigilance [40], RDI and attention and executive function [41], and a questionnaire-based measure of breathing cessations and performance on a test of attention [42]. The average participant in each of these studies had mild SDB. Only two studies examined longitudinal cognitive data to identify cognitive changes associated with the presence of SDB among older adults. Cohen-Zion and colleagues [43] examined 46 older adults with mild-moderate SDB and low levels of oxygen desaturations with an approximate 3-year interval of cognitive follow-up data. They found that excessive daytime sleepiness, but not hypoxemia or RDI, was associated with a gross measure of global cognitive decline. Although not significant in full models, their data suggested that RDI mediated the relationship between daytime sleepiness and cognitive decline. A seminal 2011 longitudinal study by Yaffe and colleagues [44] examined 298 older community-dwelling women with an approximate 5-year follow-up interval that provided ascertainment of cognitive status as either normal, MCI, or demented. They found that women with SDB had a nearly two-fold risk of developing either MCI or dementia and that within women with SDB, measures of hypoxemia, but not measures of sleep fragmentation and sleep duration, were associated with MCI or dementia risk. This study is distinguished by its large sample size, length of follow-up interval, well-controlled models, and robust assessment of both SDB variables of interest and cognitive status outcomes. The findings strongly suggest that intermittent hypoxemia is the likely mediator of the relationship between SDB and cognition in older adults. Finally, a 2011 community-based study of older men from the Osteoporotic Fractures in Men Sleep Study [45] found that 43% of their sample had AHI≥15 and severe hypoxemia (Sa02<80%) was associated with declines in vigilance. There were no relationships between cognitive performance and mild hypoxemia, AHI, or arousal index, suggesting that there may be a threshold effect at which hypoxemia exerts a negative impact on cognition in community-dwelling older adults.
Proposed Mechanisms for SDB-related Cognitive Dysfunction in Older Adults
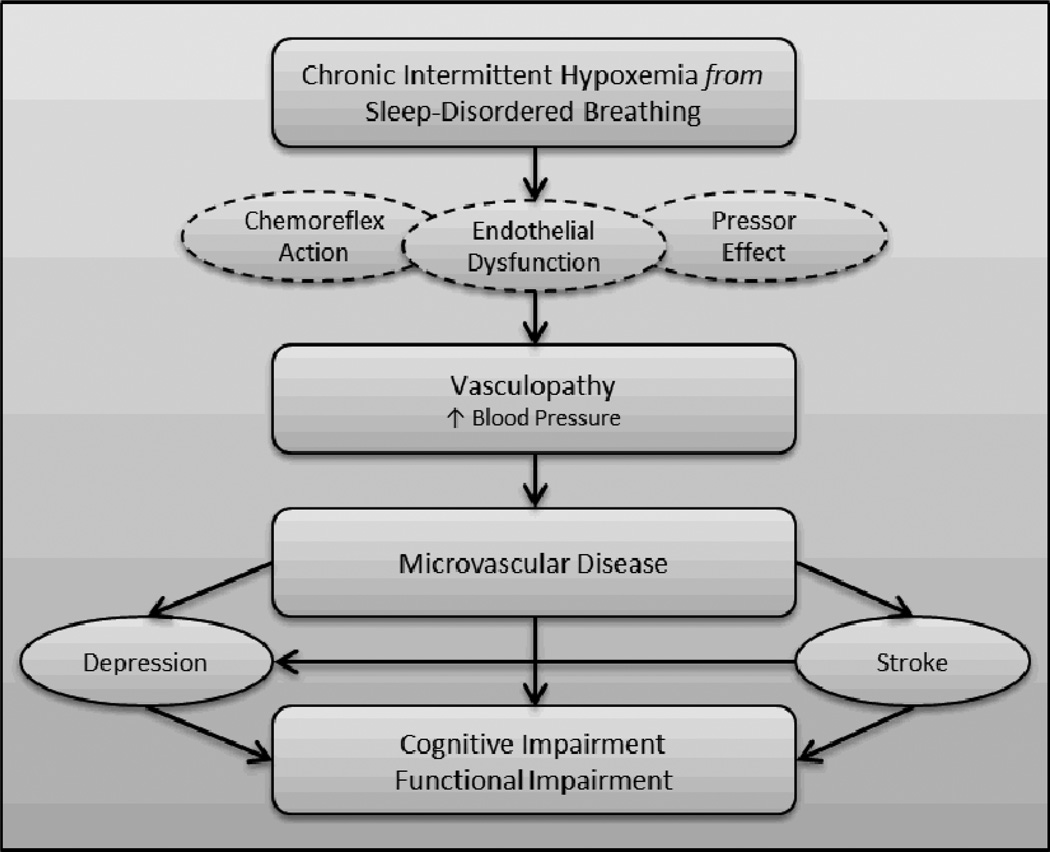
The existing literature largely supports the hypothesis that SDB is associated with cognitive impairment in older adults, particularly in the domains of attention/vigilance, executive function, and memory. Although findings are still mixed, there is evidence to suggest that intermittent hypoxemia triggered by untreated SDB is likely the cause of associated cognitive dysfunction [44]. What is the mechanism through which hypoxemia exerts adverse effects? Based on the predominant pattern of cognitive impairments as well as the work of Lanfranchi and Somers [46], we propose a microvascular model whereby chronic intermittent hypoxemia causes vasculopathy that ultimately is expressed as cognitive impairment and functional decline in the older adult (see Figure). Hypoxemia may contribute to vasculopathy in several ways; here we highlight three potential mechanisms. First, hypoxemia is known to cause a chemoreflex activation that affects sympathetic vasoconstriction, nocturnal blood pressure, and circulating catecholamines. A second possible mechanism is the pressor effect of endothelin. Hypoxemia also causes increased production of the vasoconstrictor endothelin, which has sustained hypertensive effects that may result in persistent nocturnal and diurnal blood pressure elevations. Third, the structural integrity of the endothelial cells themselves may be directly compromised by hypoxemia. Endothelial cells that line the wall of the blood vessel produce nitric oxide in response to hypoxemia exposure. Nitric oxide is a vasodilator that protects the blood vessel walls from constriction. Studies have shown that individuals with sleep apnea have a lowered nitric oxide response to hypoxemia compared to healthy controls, again putting them at higher risk for vasoconstriction and elevated nocturnal blood pressure [47]. These mechanisms may act independently or in concert to produce vasculopathy, negatively impacting the integrity of the blood vessel. The prevalence of hypertension in SDB is 50% and approximately 83% of individuals with drug-resistant hypertension have SDB [48, 49], lending support to the plausibility of this relationship. Such vascular dysfunction is likely to lead to ischemic damage in the brain, affecting primarily small vessels. Ongoing and significant microvascular disease may ultimately give rise to cognitive impairment in the older adult, especially in the domains of attention and executive function with secondary effects on memory abilities. Importantly, subtle microvascular abnormalities may be “silent” in the older adult, meaning there may be no overt clinical manifestations that are directly observable. However, accumulation of subclinical microvascular disease in apparently healthy older adults may confer significant risk for the development of future vascular disease. Indeed, several studies have reported that individuals with SDB have an increased risk for stroke [50, 51]. It is also important to note that additional models have been proposed for SDB-related sequelae [9] and our model is not necessarily mutually exclusive of others that have been described. However, the types of cognitive deficits often observed in older adults with SDB suggest that microvascular disease may be the most likely contributor in populations of advanced age.
Figure.
Proposed Mechanism linking Sleep-Disordered Breathing Hypoxemia to Cognitive Dysfunction.
Considerations for Future Research on SDB-related Cognitive Dysfunction in Older Adults
Here, we enumerate several areas germane to the study of SDB and cognition in elderly populations that require additional systematic exploration.
Double Insult Hypothesis. Case-controlled prospective studies should examine the “double insult” hypothesis [52] of SDB-associated cognitive dysfunction in older adults. Alchanatis and colleagues [28] and Mathieu and colleagues [27] reported somewhat contradictory findings using optimal study designs to address the question of whether the cognitive deficits observed in older adults with SDB are of similar or greater magnitude than those observed in middle and younger aged adults with SDB and normal control subjects. However, both studies used age 50 as a cut point for “older” adult and employed somewhat restricted cognitive assessments. Future studies using this design should include older adults aged > 65 and comprehensive cognitive batteries.
Exposure Period of Untreated SDB. Although difficult to validly quantify, examination of onset of SDB and length of untreated SDB may be equally critical as the severity of SDB, particularly in the older adult. Longitudinal observational studies with repeated assessments may be necessary to fully address this issue, but development of retrospective bed partner questionnaires or a series of questions targeting this issue would be a useful first step toward understanding the impact of SDB chronicity on cognitive outcomes in adults across the lifespan.
Cognitive Response to PAP Treatment. Many studies have shown that consistent, optimal adherent PAP use confers benefits on a multitude of associated nocturnal and diurnal sequelae among younger and middle-aged adults [53–55], but few studies have examined the effect of PAP use on cognitive performance in older adults. This is an essential understudied topic as the effect of PAP as well as barriers to PAP adherence may not be the same in the elderly individual as they are in the younger or middle-aged adult.
Hypoxemia and Sleep Fragmentation. Although recent studies have suggested that intermittent hypoxemia is the primary mechanism through which SDB affects cognition[44, 45], several other studies have found a central role for sleep fragmentation and/or associated daytime sleepiness [38, 43] and not all studies reviewed specifically examined the role of each on cognitive function. In addition, findings that optimal PAP adherence results in improvements in cognitive performance among adults with existing deficits brings to question the primacy of the contribution of hypoxemia as it would seem easier to reverse the effects of sleep fragmentation than the presumably downstream microvascular effects of intermittent hypoxemia. Future studies are necessary to continue to carefully consider the unique or synergistic contributions of intermittent hypoxemia and sleep fragmentation to observed cognitive dysfunction.
Role of APOE4 allele. APOE4 has unique relevance to the study of SDB and cognition among older adults as the APOE4 allele is widely considered a “vulnerability” factor that has been shown to be influential in the development of SDB, cognitive impairment, and dementia. The two studies that have examined APOE4 as a modifier of the relationship between SDB and cognition in the elderly [38, 39] have found significant effects on memory and global cognitive impairment, supporting the future investigation of this as well as other vulnerability or moderating genes in the expression of SDB-associated cognitive function.
Longitudinal Studies. Prospective longitudinal studies are necessary to study both the natural history of untreated SDB as well as the development of cognitive dysfunction associated with the presence of SDB in the older adult. Association studies are useful for hypothesis generation, but longitudinal studies should be used to determine causal relationships. This need is particularly salient in studies of aging as older adults may experience cognitive difficulties in the course of normal aging. Carefully controlled longitudinal designs are critical agents toward efforts to understand the complex relationship among SDB, cognition, and aging and development of prevention and intervention strategies.
Conclusions
Findings from the reviewed studies indicate that SDB is widely prevalent in an elderly population and that it adversely impacts cognition and the development of cognitive impairment in older adults, particularly in the domains of attention, executive function, and memory. Although cognitive batteries varied widely in their breadth and focus, it is notable that nearly all studies found associations between cognition and SDB whether the samples under study were clinic- or community-based and whether SDB was subclinical or clinical. Presence of the APOE4 allele and previously existing cognitive impairment may confer greater vulnerability to SDB-associated cognitive dysfunction in the elderly. Hypoxemia associated with SDB may be the most likely mechanism supporting the development of cognitive impairment, although further study is needed on the effects of sleep fragmentation and daytime sleepiness and whether these potential mediators vary as a function of SDB severity. The pattern of SDB-related cognitive deficits is consistent with that observed in younger and middle-aged adults [4, 5]. At the present time, it is unclear if the effects of SDB on cognition are the same regardless of age, or if there is a synergistic interaction with age whereby the older adult with SDB may be either more or less susceptible to its effects [27, 28]. It is possible that the older adult with lifelong subclinical untreated SDB has developed a compensatory tolerance for its effects, or “pre-conditioning” and therefore does not exhibit associated cognitive disturbance[56]. It is also possible that the older adult with lifelong subclinical untreated SDB has reached a threshold whereby normal age-associated neurodegenerative processes render them no longer able to withstand the impact of SDB on cognition, resulting in significant cognitive impairment and decline in ability to care for self [9, 52]. Neither of these hypothetical scenarios considers the impact of new onset SDB in the older adult, which the epidemiological literature suggests is highly widespread [1]. The urgent need for additional study of these proposed models is underscored by the fact that SDB is a remediable risk factor not only for cognitive impairment, but also cardiovascular disease, cerebrovascular disease and other serious medical comorbidities that significantly interfere with the quality of life of elderly adults.
References
Papers of particular interest published since 2009 have been highlighted as:
* of importance
** of outstanding importance
- 1.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14(6):486–495. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salthouse TA. Selective review of cognitive aging. J Int Neuropsychol Soc. 2010;16(5):754–760. doi: 10.1017/S1355617710000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson ML, Stough C, Howard ME, Spong J, Downey LA, Thompson B. The contribution of fatigue and sleepiness to depression in patients attending the sleep laboratory for evaluation of obstructive sleep apnea. Sleep & breathing = Schlaf & Atmung. 2011;15(3):439–445. doi: 10.1007/s11325-010-0355-2. [DOI] [PubMed] [Google Scholar]
- 4.Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26(3):298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- 5.Aloia MS, Arnedt JT, Davis JD, Riggs RL, Byrd D. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10(5):772–785. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- 6.He W, Sengupta M, Velkoff VA, DeBarros KA. US census bureau current population reports. Washington, D.C.: U.S. Government Printing Office; 2005. 65+ in the united states; pp. 23–209. [Google Scholar]
- 7.Medicine AAoS. International classification of sleep disorders, edition 2: diagnostic and coding manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 8.De Backer WA. Central sleep apnoea, pathogenesis and treatment: an overview and perspective. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 1995;8(8):1372–1383. doi: 10.1183/09031936.95.08081372. [DOI] [PubMed] [Google Scholar]
- 9.Norman D, Loredo JS. Obstructive sleep apnea in older adults. Clinics in geriatric medicine. 2008;24(1):151–165. doi: 10.1016/j.cger.2007.08.006. ix. [DOI] [PubMed] [Google Scholar]
- 10.McMahon JP, Foresman BH, Chisholm RC. The influence of CPAP on the neurobehavioral performance of patients with obstructive sleep apnea hypopnea syndrome: a systematic review. WMJ : official publication of the State Medical Society of Wisconsin. 2003;102(1):36–43. [PubMed] [Google Scholar]
- 11.Mazza S, Pepin JL, Naegele B, Rauch E, Deschaux C, Ficheux P, Levy P. Driving ability in sleep apnoea patients before and after CPAP treatment: evaluation on a road safety platform. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2006;28(5):1020–1028. doi: 10.1183/09031936.06.00112905. [DOI] [PubMed] [Google Scholar]
- 12.Zimmerman ME, Arnedt JT, Stanchina M, Millman RP, Aloia MS. Normalization of memory performance and positive airway pressure adherence in memory-impaired patients with obstructive sleep apnea. Chest. 2006;130(6):1772–1778. doi: 10.1378/chest.130.6.1772. [DOI] [PubMed] [Google Scholar]
- 13.Aloia MS, Arnedt JT, Stepnowsky C, Hecht J, Borrelli B. Predicting treatment adherence in obstructive sleep apnea using principles of behavior change. J Clin Sleep Med. 2005;1(4):346–353. [PubMed] [Google Scholar]
- 14.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 15.Carskadon MA, Dement WC. Respiration during sleep in the aged human. Journal of gerontology. 1981;36(4):420–423. doi: 10.1093/geronj/36.4.420. [DOI] [PubMed] [Google Scholar]
- 16.Launois SH, Pepin JL, Levy P. Sleep apnea in the elderly: a specific entity? Sleep Med Rev. 2007;11(2):87–97. doi: 10.1016/j.smrv.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Berry DT, Webb WB, Block AJ. Sleep apnea syndrome. A critical review of the apnea index as a diagnostic criterion. Chest. 1984;86(4):529–531. doi: 10.1378/chest.86.4.529. [DOI] [PubMed] [Google Scholar]
- 18.Ancoli-Israel S, Palmer BW, Cooke JR, Corey-Bloom J, Fiorentino L, Natarajan L, Liu L, Ayalon L, He F, Loredo JS. Cognitive effects of treating obstructive sleep apnea in Alzheimer's disease: a randomized controlled study. Journal of the American Geriatrics Society. 2008;56(11):2076–2081. doi: 10.1111/j.1532-5415.2008.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shochat T, Pillar G. Sleep apnoea in the older adult : pathophysiology, epidemiology, consequences and management. Drugs & aging. 2003;20(8):551–560. doi: 10.2165/00002512-200320080-00001. [DOI] [PubMed] [Google Scholar]
- 20.Beaulieu-Bonneau S, Hudon C. Sleep disturbances in older adults with mild cognitive impairment. Int Psychogeriatr. 2009;21(4):654–666. doi: 10.1017/S1041610209009120. [DOI] [PubMed] [Google Scholar]
- 21.Naismith SL, Rogers NL, Hickie IB, Mackenzie J, Norrie LM, Lewis SJ. Sleep well, think well: sleep-wake disturbance in mild cognitive impairment. J Geriatr Psychiatry Neurol. 2010;23(2):123–130. doi: 10.1177/0891988710363710. [DOI] [PubMed] [Google Scholar]
- 22.McCurry SM, Ancoli-Israel S. Sleep Dysfunction in Alzheimer's Disease and Other Dementias. Curr Treat Options Neurol. 2003;5(3):261–272. doi: 10.1007/s11940-003-0017-9. [DOI] [PubMed] [Google Scholar]
- 23.Neikrug AB, Ancoli-Israel S. Sleep disorders in the older adult - a mini-review. Gerontology. 2010;56(2):181–189. doi: 10.1159/000236900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yesavage J, Bliwise D, Guilleminault C, Carskadon M, Dement W. Preliminary communication: intellectual deficit and sleep-related respiratory disturbance in the elderly. Sleep. 1985;8(1):30–33. doi: 10.1093/sleep/8.1.30. [DOI] [PubMed] [Google Scholar]
- 25.Berry DT, Phillips BA, Cook YR, Schmitt FA, Honeycutt NA, Arita AA, Allen RS. Geriatric sleep apnea syndrome: a preliminary description. Journal of gerontology. 1990;45(5):M169–M174. doi: 10.1093/geronj/45.5.m169. [DOI] [PubMed] [Google Scholar]
- 26.Aloia MS, Ilniczky N, Di Dio P, Perlis ML, Greenblatt DW, Giles DE. Neuropsychological changes and treatment compliance in older adults with sleep apnea. Journal of psychosomatic research. 2003;54(1):71–76. doi: 10.1016/s0022-3999(02)00548-2. [DOI] [PubMed] [Google Scholar]
- 27.Mathieu A, Mazza S, Decary A, Massicotte-Marquez J, Petit D, Gosselin N, Malo J, Montplaisir J. Effects of obstructive sleep apnea on cognitive function: a comparison between younger and older OSAS patients. Sleep medicine. 2008;9(2):112–120. doi: 10.1016/j.sleep.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Alchanatis M, Zias N, Deligiorgis N, Liappas I, Chroneou A, Soldatos C, Roussos C. Comparison of cognitive performance among different age groups in patients with obstructive sleep apnea. Sleep & breathing = Schlaf & Atmung. 2008;12(1):17–24. doi: 10.1007/s11325-007-0133-y. [DOI] [PubMed] [Google Scholar]
- 29. Kim SJ, Lee JH, Lee DY, Jhoo JH, Woo JI. Neurocognitive dysfunction associated with sleep quality and sleep apnea in patients with mild cognitive impairment. Am J Geriatr Psychiatry. 2011;19(4):374–381. doi: 10.1097/JGP.0b013e3181e9b976. This study is notable because it specifically examined SDB in older adults with MCI. Individuals with MCI are at high risk of developing dementia. Although no differences were found between MCI and control groups on measures of AHI and hypoxemia, higher AHI was associated with poorer performance on a language test among individuals with MCI.
- 30.Katz MJ, Lipton RB, Hall CB, Zimmerman ME, Sanders AE, Verghese J, Dickson DW, Derby CA. Age-specific and Sex-specific Prevalence and Incidence of Mild Cognitive Impairment, Dementia, and Alzheimer Dementia in Blacks and Whites: A Report From the Einstein Aging Study. Alzheimer disease and associated disorders. 2011 doi: 10.1097/WAD.0b013e31823dbcfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phillips BA, Berry DT, Schmitt FA, Magan LK, Gerhardstein DC, Cook YR. Sleep-disordered breathing in the healthy elderly. Clinically significant? Chest. 1992;101(2):345–349. doi: 10.1378/chest.101.2.345. [DOI] [PubMed] [Google Scholar]
- 33.Boland LL, Shahar E, Iber C, Knopman DS, Kuo TF, Nieto FJ. Measures of cognitive function in persons with varying degrees of sleep-disordered breathing: the Sleep Heart Health Study. J Sleep Res. 2002;11(3):265–272. doi: 10.1046/j.1365-2869.2002.00308.x. [DOI] [PubMed] [Google Scholar]
- 34.Foley DJ, Masaki K, White L, Larkin EK, Monjan A, Redline S. Sleep-disordered breathing and cognitive impairment in elderly Japanese-American men. Sleep. 2003;26(5):596–599. doi: 10.1093/sleep/26.5.596. [DOI] [PubMed] [Google Scholar]
- 35.Sforza E, Roche F, Thomas-Anterion C, Kerleroux J, Beauchet O, Celle S, Maudoux D, Pichot V, Laurent B, Barthelemy JC. Cognitive function and sleep related breathing disorders in a healthy elderly population: the SYNAPSE study. Sleep. 2010;33(4):515–521. doi: 10.1093/sleep/33.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 37.Kadotani H, Kadotani T, Young T, Peppard PE, Finn L, Colrain IM, Murphy GM, Jr, Mignot E. Association between apolipoprotein E epsilon4 and sleep-disordered breathing in adults. JAMA. 2001;285(22):2888–2890. doi: 10.1001/jama.285.22.2888. [DOI] [PubMed] [Google Scholar]
- 38.O'Hara R, Schroder CM, Kraemer HC, Kryla N, Cao C, Miller E, Schatzberg AF, Yesavage JA, Murphy GM., Jr Nocturnal sleep apnea/hypopnea is associated with lower memory performance in APOE epsilon4 carriers. Neurology. 2005;65(4):642–644. doi: 10.1212/01.wnl.0000173055.75950.bf. [DOI] [PubMed] [Google Scholar]
- 39.Spira AP, Blackwell T, Stone KL, Redline S, Cauley JA, Ancoli-Israel S, Yaffe K. Sleep-disordered breathing and cognition in older women. Journal of the American Geriatrics Society. 2008;56(1):45–50. doi: 10.1111/j.1532-5415.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 40.Kim H, Dinges DF, Young T. Sleep-disordered breathing and psychomotor vigilance in a community-based sample. Sleep. 2007;30(10):1309–1316. doi: 10.1093/sleep/30.10.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayward L, Mant A, Eyland A, Hewitt H, Purcell C, Turner J, Goode E, Le Count A, Pond D, Saunders N. Sleep disordered breathing and cognitive function in a retirement village population. Age and ageing. 1992;21(2):121–128. doi: 10.1093/ageing/21.2.121. [DOI] [PubMed] [Google Scholar]
- 42.Dealberto MJ, Pajot N, Courbon D, Alperovitch A. Breathing disorders during sleep and cognitive performance in an older community sample: the EVA Study. Journal of the American Geriatrics Society. 1996;44(11):1287–1294. doi: 10.1111/j.1532-5415.1996.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 43.Cohen-Zion M, Stepnowsky C, Marler, Shochat T, Kripke DF, Ancoli-Israel S. Changes in cognitive function associated with sleep disordered breathing in older people. Journal of the American Geriatrics Society. 2001;49(12):1622–1627. doi: 10.1046/j.1532-5415.2001.t01-1-49270.x. [DOI] [PubMed] [Google Scholar]
- 44. Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Ancoli-Israel S, Stone KL. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613–619. doi: 10.1001/jama.2011.1115. This longitudinal study of 298 older women drawn from the community found that women with SDB had a nearly two-fold increased risk of developing either mild cognitive impairment or dementia over a five -year follow-up period. Furthermore, within women with SDB, measures of hypoxemia, but not sleep fragmentation or sleep duration, were associated with mild cognitive impairment or dementia risk.
- 45. Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Ensrud KE, Stefanick ML, Laffan A, Stone KL. Associations between sleep architecture and sleep-disordered breathing and cognition in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. Journal of the American Geriatrics Society. 2011;59(12):2217–2225. doi: 10.1111/j.1532-5415.2011.03731.x. This community-based study of 2909 older men found relationships between severe hypoxemia and performance on a vigilance test. No relationships were found between cognitive performance and mild indicies of SDB, suggesting that there may be a threshold effect at which hypoxemia exerts a negative impact on cognition.
- 46.Lanfranchi P, Somers VK. Obstructive sleep apnea and vascular disease. Respiratory research. 2001;2(6):315–319. doi: 10.1186/rr79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kato M, Roberts-Thomson P, Phillips BG, Haynes WG, Winnicki M, Accurso V, Somers VK. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102(21):2607–2610. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- 48.Silverberg DS, Oksenberg A, Iaina A. Sleep-related breathing disorders as a major cause of essential hypertension: fact or fiction? Current opinion in nephrology and hypertension. 1998;7(4):353–357. doi: 10.1097/00041552-199807000-00001. [DOI] [PubMed] [Google Scholar]
- 49.Logan AG, Perlikowski SM, Mente A, Tisler A, Tkacova R, Niroumand M, Leung RS, Bradley TD. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. Journal of hypertension. 2001;19(12):2271–2277. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 50.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, O'Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. American journal of respiratory and critical care medicine. 2001;163(1):19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 51.Monahan K, Redline S. Role of obstructive sleep apnea in cardiovascular disease. Current opinion in cardiology. 2011;26(6):541–547. doi: 10.1097/HCO.0b013e32834b806a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ayalon L, Ancoli-Israel S, Drummond SP. Obstructive sleep apnea and age: a double insult to brain function? American journal of respiratory and critical care medicine. 2010;182(3):413–419. doi: 10.1164/rccm.200912-1805OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tomfohr LM, Ancoli-Israel S, Loredo JS, Dimsdale JE. Effects of continuous positive airway pressure on fatigue and sleepiness in patients with obstructive sleep apnea: data from a randomized controlled trial. Sleep. 2011;34(1):121–126. doi: 10.1093/sleep/34.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tregear S, Reston J, Schoelles K, Phillips B. Continuous positive airway pressure reduces risk of motor vehicle crash among drivers with obstructive sleep apnea: systematic review and meta-analysis. Sleep. 2010;33(10):1373–1380. doi: 10.1093/sleep/33.10.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barbe F, Duran-Cantolla J, Capote F, de la Pena M, Chiner E, Masa JF, Gonzalez M, Marin JM, Garcia-Rio F, de Atauri JD, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. American journal of respiratory and critical care medicine. 2010;181(7):718–726. doi: 10.1164/rccm.200901-0050OC. [DOI] [PubMed] [Google Scholar]
- 56.Lavie L, Lavie P. Ischemic preconditioning as a possible explanation for the age decline relative mortality in sleep apnea. Medical hypotheses. 2006;66(6):1069–1073. doi: 10.1016/j.mehy.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 57.Dinges DI, Powell JW. Microcomputer analysis of performance on a portable, simple visual RT task sustained operations. Behavior Research Methods, Instruments, & Computers. 1985;17:652–655. [Google Scholar]
- 58.Lewis RF, Rennick PM. Manual for the repeatable cognitive-perceptual-motor batter. Gross Point Park, MI: Axon Publishing; 1979. [Google Scholar]
- 59.Army Individual Test Battery. Manual of directions and scoring. Washington, D.C.: War Department, Adjutant General's Office; 1944. [Google Scholar]
- 60.Wechsler D. Wechsler Adult Intelligence Scale - Fourth Edition administration and scoring manual. San Antonio, TX: Psychological Corporation; 2008. [Google Scholar]
- 61.Connors K. CPT: Connors' Continuous Performance Test. Toronto, Ontario: Multi-Health Systems; 1995. [Google Scholar]
- 62.Golden CJ. Stroop Color and Word Test: a manual for clinical and experimental uses. Chicago, IL: Stoelting, Co; 1978. [Google Scholar]
- 63.Raven JC. Progressive matrices: a perceptual test of intelligence. Oxford: Oxford Psychologists Press Ltd; 1938. Individual form. [Google Scholar]
- 64.Spreen O, Benton AL. Neurosensory Center Comprehensive Examination for Aphasia (NCCEA) (Revised Edition) Victoria, CA: University of Victoria Neuropsychology Labratory; 1977. [Google Scholar]
- 65.Benedict RHB, Schretlen D, Brandt J. Hopkins Verbal Learning Test - Revised: instructions for administration and scoring. Odessa, FL: Psychological Assessment Resources; 1997. [Google Scholar]
- 66.Rey A. L'examine clinique en psychologie. Paris: Press Universitaire de France; 1958. [Google Scholar]
- 67.Benedict RHB. Brief Visuospatial Memory Test - Revised. Odessa, FL: Psychological Assessment Resources; 1998. [Google Scholar]
- 68.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test - Second Edition, Adult Version. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 69.Wechsler D. Wechsler Memory Scale - Fourth Edition administration and scoring manual. San Antonio, TX: Psychological Corporation; 2009. [Google Scholar]