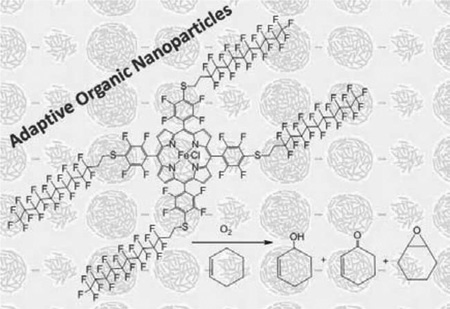
Abstract
Self-organized organic nanoparticles (ONP) are adaptive to the environmental reaction conditions. ONP of fluorous alkyl iron(III) porphyrin catalytically oxidize cyclohexene to the allylic oxidation products. In contrast, the solvated metalloporphyrin yields both allylic oxidation and epoxidation products. The ONP system facilitates a greener reaction because about 89% reaction medium is water, molecular oxygen is used in place of synthetic oxidants, and the ambient reaction conditions used require less energy. The enhanced catalytic activity of these ONP is unexpected because the metalloporphyrins in the nanoaggregates are in the close proximity and the TON should diminish by self-oxidative degradation. The fluorous alkyl chain stabilizes the ONP toward self-oxidative degradation.
Keywords: catalyst, dynamic light scattering, metalloporphyrin, organic nanoparticles, oxidation, green chemistry
1. Introduction
Allylic oxidation of olefins is a key organic transformation in a variety of synthetic strategies such as for steroids, and is often carried out using toxic metal oxidants as reagents or as catalysts. A variety of transition metal-based catalysts such as Cr, [1] Rh, [2] Mn, [3] Ru, [4] and Se [5] are reported but have different problems such as cost, scope, complete removal of the metal, and synthesis. From an environmental and cost-effective viewpoint, catalytic oxidation processes employing molecular oxygen, in aqueous media, and under ambient conditions are extremely valuable. Recently, Zhao and Yeung [6] reported the use of PhI(OAc)2 and t BuOOH reagents to generate the t BuO• radical to yield α, β-unsaturated enones in good yield and regioselectivity. Ammonium hypoiodite catalysis is another example of metal-free oxidation. [7]
Metalloporphyrins are an important class of organic molecules because of their tunable optical, photophysical, and chemical properties that enable a wide range of applications such as photonics, therapeutics, and catalysts. Various metalloporphyrins with Fe, Mn, Co, and other metal ions have been studied and used as catalysts in laboratory-scale reactions using a variety of oxygen sources. [8] These reactions can model the activity of cytochrome P450 monooxygenation reactions. [9] The catalytic activity of metalloporphyrins depends on the structure of the porphyrin, the central metal atom, the source of oxidant, solvent, the nature of axial ligand, temperature, pressure, and the structure of the substrate.[8]
Self-oxidative degradation during the catalytic process is a key issue that limits use of metalloporphyrin catalysts.[8a] To overcome this, electron withdrawing groups such as Cl and F, or bulky substituents are often appended to the porphyrin macrocycle.[10] Supramolecular porphyrin catalysts are designed to prevent self-oxidative degradation and to enhance substrate selectivity and/or regioselectivity[11] A variety of oxygen donors such as peracids, iodosylbenzene, peroxides, monopersulfates, and molecular oxygen are used for these reactions.[8a] In many ways, activation of molecular oxygen in air is preferable because it is cheap, readily available, and a greener reagent in that there is no energy used to manufacture and store it.
The use of inorganic nanomaterials composed of metal atoms, metal oxides, and composites have been well explored for a range of application such as catalysts.[12] However, the formation and activity of organic nanomaterials are more recent, and porphyrinoids are at forefront of this research because of their remarkable photochemical and catalytic properties, stability, and ease of preparation.[13] This work builds upon the principles of supramolecular chemistry as a versatile avenue for advanced functional materials. Hupp and co-workers,[11a, 14] for example, reported a self–assembled (discrete) metalloporphyrin catalyst formed from different porphyrinoids can be robust and have enhanced catalytic activity over the corresponding metalloporphyrin in solution.[11a, 14]
Supramolecular organic nanoparticles (ONP) of porphyrinoids are self-organized (non-discrete) systems formed via noncovalent interactions.[13b, 15] Because ONP are dynamic so can adapt to environmental changes, ONP are fundamentally different from inorganic nanomaterials. The formation of ONP of porphyrins was discussed previously and is generally accomplished by adding a concentrated solution of the porphyrin to a miscible nonsolvent with a few percent of a triethyleneglycol monomethylether glycol (PEG164) while vigorously mixing.[13a] Stabilization of the suspension of ONP to precipitation is effected by the small quantities of the PEG in the solvent system. We previously reported the effects of various conditions on the formation, size, and stability of porphyrinic ONP. For example, in addition to the specific molecular structure of the porphyrin, the molecular weight and quanitity of PEG stabilizer, the ratio of host to guest solvents, temperature, and type of mixing all factor in the size, stability and morphology of ONP.[13b] Though the specific preparative methods depend on the molecular structure (such as free base, metal ion, and exocyclic moieties), the conditions can be varied to afford ONP of different sizes.[13b, 16] For 5,10,15,20-tetrakis-[4-(1′H,1′H,2′H,2′H-heptadecafluorodecane -1-thiol)-2,3,5,6-tetrafluorophenyl]porphyrinato iron(III) chloride,[Fe(III)TPPF84]Cl, the particle size can be tuned by increasing in the volume of the PEG164, but without stabilizer no ONP formation was observed by dynamic light scattering (DLS) and UV–visible spectroscopy and the metalloporphyrin rapidly and quantitatively precipitates. The size and internal structure of porphyrinic nanoparticles depend on: (a) the nature and magnitude of intermolecular forces between the various components, for example, the porphyrin, host and guest solvents, and the stabilizer; (b) the ratio of the host to guest solvent, (c) the type of mixing, and (d) the nature of peripheral substituents on the porphyrin and chelated metal ion.[13c] These ONP are stable for many weeks at 4 °C, and ONP of catalytically active metalloporphyrins are generally more efficient catalysts than the component molecule in solution.[13c]
Previously, we reported that 10 nm diameter ONP of Fe(III)TPPF20 (Scheme 1) have about 10-fold enhanced catalytic activity for the allylic oxidation of cyclohexene to yield the α, β-unsaturated ketone and alcohol (Scheme 2) under ambient conditions, and using molecular oxygen as oxidant.[13c] Conversely, the epoxide (Scheme 2) is the only product from the completely solvated metalloporphyrin in organic solvents, and synthetic oxygen sources such as H2O2 are required. During both catalytic processes, the porphyrin decomposes completely. The objective of the present study is to evaluate the catalytic activity of ONP of a metalloporphyrin bearing fluorous alkanes designed to increasing the stability toward self-oxidative degradation.
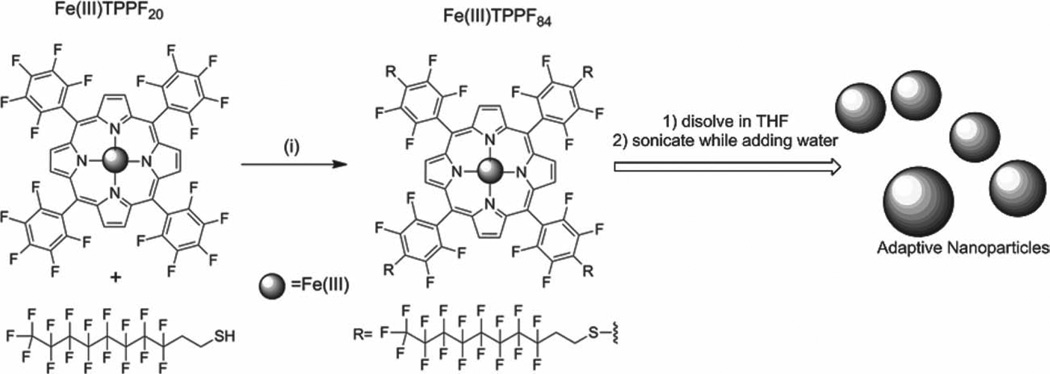
Scheme 1.
Preparation of Fe(III)TPPF84 from Fe(III)TPPF20: (i) DMF/DIPEA under N2 at rt. for 2 h. Addition of water with 2% triethyleneglycol monomethylether to a THF solution of the catalytic porphyrin forms the ONP.
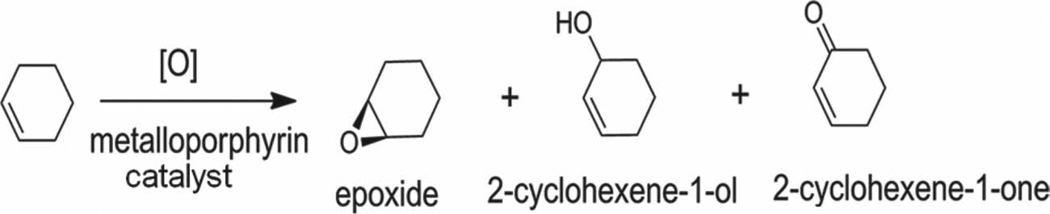
Scheme 2.
Oxidation of cyclohexene using metalloporphyrin catalysts.
Our hypothesis was that appending fluorous alkanes to the macrocycle would increase metalloporphyrin stability by inhibiting cofacial interactions. Indeed,[Fe(III)TPPF84]Cl (Scheme 1) is found to be more stable toward self-oxidative degradation, but the overall yields of the α, β-unsaturated ketone and alcohol are less than ONP of[Fe(III)TPPF20]Cl.
2. Experimental Section
The Fe(III)TPPF84 catalyst was prepared according to our previous report,[17] wherein 3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-heptadecafluoro-1-decanethiol was reacted with the perfluorophenylporphyrin, [Fe(III)TPPF20]Cl, in DMF with N,N-diisopropylethylamine for 15 min under a N2 atmosphere (Scheme 1, see Supporting Information). All spectral data are consistent with the structure (Figure S1 and S2, Supporting Information). The nanoparticles of Fe(III)TPPF84 were prepared similar to our previous report on the formation of ONP of the other porphyrinoids. 0.4 mL of a 1 × 10−3 m stock solution of Fe(III)TPPF84 in THF was mixed with 0.2 mL of PEG164 in a 10.0 mL vial and 5.0 mL nanopure water was then added over 60 s while sonicating and then the solution was further sonicated for another 2–3 min.[13a–c] The ONP were characterized by UV–visible spectroscopy and DLS. The average diameter of the ONP of Fe(III)TPPF84 is 15–20 nm (Figure S3 and S4, Supporting Information). The aggregation of the metalloporphyrins in the nanoscaled particles was also confirmed by the broadening of the Soret and Q bands as discussed previously.[13b, c] The broadening is due to edge-to-edge (J) and face-to-face (H) aggregation of the dyes in the ONP.
2.1. Catalytic Oxidation of Cyclohexene
The catalytic activity for the oxidation of cyclohexene by Fe(III) TPPF84 in solution and as supramolecular ONP was explored here. We used the ≈21% O2 in air, pure O2, and 30% H2O2, as oxidants. The metalloporphyrin was the limiting reagent and the reactions run exhaustively in all the reactions to assure accurate comparisons in turnovers in systems that have different activities (rates). Reactions were run for 24 h and then were quenched in an ice bath. Twenty microliters of toluene were used as internal standard and then 2 µL of the diluted solution was then injected into an Agilant 5975 series GC-MS for analysis of the oxidation products of cyclohexene (see Supporting Information). All reactions were stirred using a magnetic stirring bar and were run a minimum of four times and the reported data represents the average of these reactions.
3. Results and Discussion
The preparation and use of metalloporphyrin ONP catalysts has several advantages: ease of preparation, use of water as an eco-friendly solvent, avoids hazardous chemicals such as halogenated solvents or toxic catalysts, and reduces the energy requirement because the reactions runs at ambient temperature and pressure.
Cyclohexene is widely used as a standard substrate to study the catalytic activity of various metalloporphyrins using a variety of oxidizing agents and conditions.[18] The formation of different products or product ratios depends on the catalytic system. The possible products for cyclohexene oxidation are cyclohexenoxide, and the allylic substitution products: 2-cyclohexene-1-ol and 2-cyclohexene-1-one (Scheme 2).
The catalytic activity of ONP of Fe(III)TPPF84 using pure oxygen, H2O2, and ≈21% O2 in air as oxidizing agents is discussed. The highly fluorinated alkyl groups on the para-positions of the meso tetrafluorophenyl groups in Fe(III)TPPF84 increase the stability of the metalloporphyrin toward self-oxidative degradation, but render the system less catalytically active under these conditions.
3.1. Solution Phase Catalysis by Fe(III)TPPF84
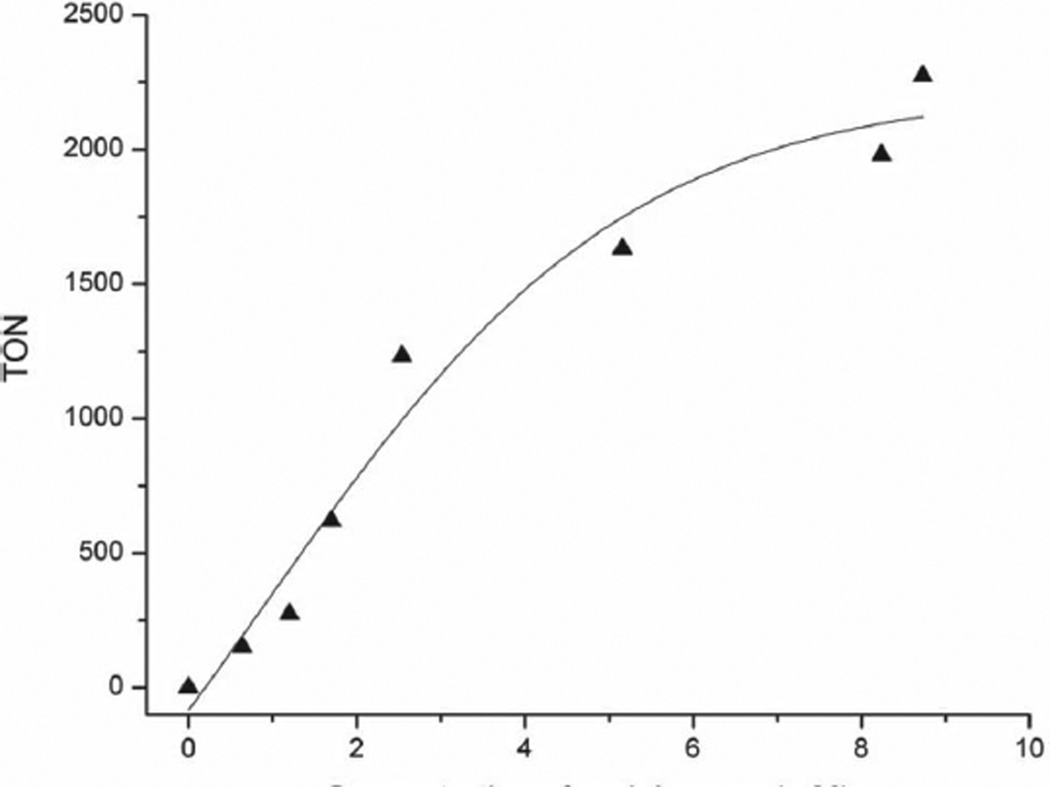
In solution, the Fe(III)TPPF84 catalyst activates O2 for the oxidation of cyclohexene in CH3OH: CH3CN (1:3) to yield all three products, epoxide:enol: enone = 1:1.5:3 (Scheme 2, Table 1). This activity is in contrast to Fe(III)TPPF20, which requires H2O2, does not activate O2, and yields only the epoxidation product.[19] The rate of the former reaction is slow compared with the latter. For the Fe(III)TPPF84 solution phase reaction, both solubilized metalloporphyrin and a small amount of large aggregates (≈1100 nm diameter) were observed by DLS but are not obvious with UV–visible spectroscopy. The formation of the allylic oxidation products along with the epoxide reflects this observation. The TON is defined as the total amount of products formed per porphyrin molecule. UV–visible analysis of the reaction mixture after the reaction was run exhaustively shows ≈36% porphyrin remains (Figure S5, Supporting Information). Increased concentration of cyclohexene in these reactions increases the TON (Figure 1). The highly fluorous alkyl chains moieties dramatically increase the stability toward self-oxidation compared with[Fe(III)TPPF20]Cl, which is more stable than the nonfluorinated compound.[19] When H2O2 is used as the oxidizing agent, even halogenated metalloporphyrins undergo complete decomposition in a few minutes during the olefinic oxidation catalysis.[19]
Table 1.
Fe(III)TPPF 84 catalysis of cyclohexene oxidation.
| Solution a) or ONP b) |
Conditions | % epoxide | % enol | % enone | TON | Comments |
|---|---|---|---|---|---|---|
| Solution | 125 mL O2 | 16 | 29 | 55 | 158 | 36% porphyrin left |
| Solution | 125 mL O2 | 20 | 27 | 53 | 147 | 72 h, no porphyrin left |
| Solution | 125 mL O2, 1.73 atm | 13 | 32 | 55 | 129 | No porphyrin left |
| Solution | 125 mL O2, 40 °C | 8 | 35 | 57 | 140 | No porphyrin left |
| Solution | 125 mL O2, 50 °C | 6 | 38 | 56 | 123 | No porphyrin left |
| ONP | 125 mL O2 | <1 | 29 | 70 | 670 | 26% porphyrin left |
| ONP | 125 mL O2 | <1 | 32 | 67 | 350 | 72 h, no porphyrin left |
| ONP | 125 mL O2, 1.73 atm | <1 | 31 | 68 | 305 | No porphyrin left |
| ONP | 125 mL O2, 40 °C or 50 °C |
X | X | X | X | No porphyrin left |
Reactions were run at room temperature and 1 atm for 24 h unless noted;
Solution reactions: 0.4 mL (1 × 10−3 m, 4 × 10−7 mol of porphyrin) of catalyst was mixed with 2.5 mL of methanol: acetonitrile (1:3) and 0.2 mL of cyclohexene to a final concentration of 0.13 × 10−3 m. 125 mL of O2 , porphyrin: cyclohexene: O2 = 1:4800:13000 was used;
ONP reactions with O2: 15–20 nm diameter ONP suspension (2.5 mL, 70 × 10−6 M, 1.75 × 10−7 mol of porphyrin) mixed with cyclohexene (0.2 mL) and O2 (125 mL); porphyrin: cyclohexene: O2 = 1:11300: 29000. TON = molproducts/molporphyrin has an error of ±5%. Products were extracted into CH2Cl2 and analyzed by using an Agilent 5975 series GC – MS. Control reactions: neither H2O2 nor O2 react directly with cyclohexene under these conditions (Table S1, Supporting Information).
Figure 1.
Catalysis of cyclohexene oxidation by Fe(III)TPPF84 ONP verus TON.
The reduced activity and enhanced stability may arise from the long fluorinated alkyl chains folding back over the metalloporphyrin faces, driven by hydrophobic effects and intermolecular interactions among the fluorous groups. The shielding of the reactive face may also decrease access of the hydrocarbon substrate. The increased solubility of O2 in fluorous phases may also contribute to the observed activity of Fe(III)TPPF84. Reactions run for up to 72 h have the same ratio of products and TON, but the porphyrin is completely decomposed. Recharging the reaction after 24 h by providing more oxygen is no more efficacious than the 72 h reaction. Reactions run under elevated temperature and pressure also result in complete decomposition of the porphyrin, gives similar oxidation product ratios, but with a reduced TON (Table 1). The increased rate of catalyst decomposition and decreased TON may be because the elevated T increases the dynamics of the fluorinated alkyl chains to expose the macrocycle to approach of another Fe(III)TPPF84 (Figure S6, Supporting Information). Changing the reaction solvent to ethanol/toluene (1:3) quenches the reaction using O2. Addition of anthracene to the reaction mixture can increase the catalytic activity of metalloporphyrins,[14a] but did not effect the Fe(III)TPPF84 reaction. No cyclohexene oxidation products were observed when the reactions used H2O2, but the catalyst decomposes in ≈15 min. The ≈21% O2 in air is not effective in solution phase reaction. Note that the nonfluorinated analogue, the dodecanethiol adduct (R =C12H25S-), is catalytically inactive and no porphyrin decomposition was observed.
The initial metalloporphyrin oxidation products, speculated to be the porphyrin radical cation formed from an intramolecular metal ion macrocycle electron transfer,[20] are more readily oxidized such that once the macrocycle opens, and is trapped in the ONP, it further oxidizes to as yet unidentified products. The UV–visible analysis of the reaction mixture before and after the reaction does not detect porphyrin oxidation intermediates such as chlorin, isobacteriochlorin, bacteriochlorin, bilirubin, or dipyrromethane. All of these compounds are known and have specific peaks in the UV–visible spectrum. GC–MS data do not show significant amounts of compounds >50 amu.
3.2. Catalysis by Nanoparticles of Fe(III)TPPF84
The 15–20 nm diameter ONP of Fe(III)TPPF84 are more catalytically active than the solvated metalloporphyrin. These ONP oxidize cyclohexene using O2 to yield only the allylic oxidation products, 2-cyclohexene-1-one and 2-cyclohexene-1-ol with a trace amount of epoxide (Scheme 2) at about a fourfold greater TON (Table 1).
UV–visible spectra of reaction mixtures after a 24 h reaction show that ≈26% of the porphyrin remains, but eventually Fe(III)TPPF84 decomposes after longer reaction times (Figure S7, Supporting Information). Intermediate oxidation products for the metalloporphyrin decomposition, such as chlorin, isobacteriochlorin, bacteriochlorin, bilirubin, or dipyrromethane were not observed by UV– visible analysis of the reaction mixture during the course of the reaction. Though the allylic oxidation products for ONP of Fe(III)TPPF84 is consistent with our previous report on Fe(III)TPPF20 ONP,[13c] and the catalytic activity is less, but they are more robust toward self-oxidative degradation. Fe(III)TPPF84 ONP are inactive with other oxidants such as the ≈21% O2 in air and H2O2. ONP of the hydrocarbon analogue are inactive under the same conditions. Kinetic assays indicate that the nanoparticles have very low or almost no activity during the first few hours of the reaction. GC–MS analysis of the reactions mixture for different time intervals for first few hours shows no oxidation product for cyclohexene. Also, UV–visible spectra indicate no degradation of ONP for the first few hours of the reactions. After 8–10 h of reaction time, we see a noticeable conversion of cyclohexene and then this saturates after about 20 h (Figure S8, Supporting Information).
3.3. Internal Organization of the ONP
The detailed structural organization of the porphyrins inside the ONP remains unknown because the intermolecular forces, which hold these molecules into nanoaggregates are weak, nonspecific, and reversible.[13b, c] The organization of the metalloporphyrins in the ONP strongly depends on the structure of component molecule, nature and ratio of host–guest solvent, stabilizer used, the mode of preparation, and temperature. We[13b,c] and others[21] found that the ONP may contain a number of subdomains of porphyrins and the solvent–stabilizer filled voids or channels of unknown size and distribution.
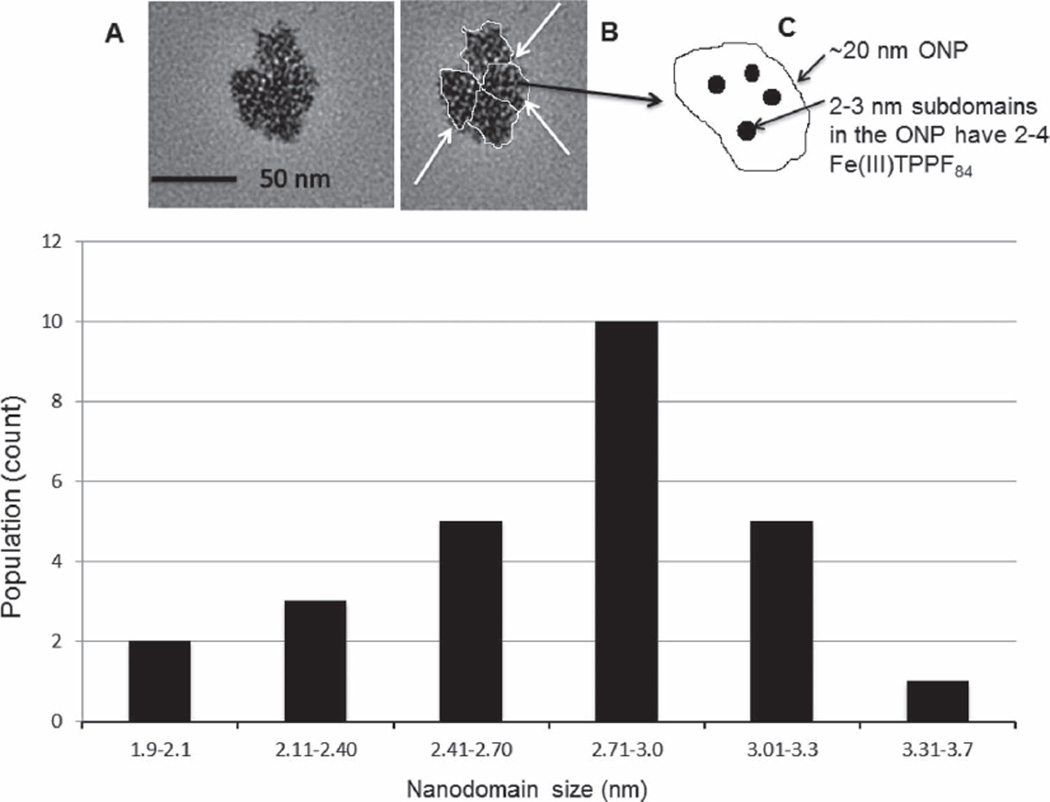
Because of the supramolecular interactions, the organization of the ONP is also adaptive in that it responds to the presence of substrates. Our group previously examined a number of ONP of porphyrins and metalloporphyrins using UV–visible and DLS in solution, and compared this to atomic force microscopy (AFM) studies of the ONP cast on the surfaces. AFM studies of nanoparticles of porphyrins cast on surfaces do not always show same size as in solution because of the surface energetics and the adaptive properties of the ONP.[13a,b,22] There is no a priori correlation between particle size and structure in solution with the morphology on surfaces. On hydrophobic surfaces, ONP of some metalloporphyrins disaggregate to reveal the constituent subdomains.[23] TEM characterization of ONP of Fe(III)TPPF84 shows a range of particle sizes. The 25–30 nm cluster in Figure 2 consists of 3–4 ONP from the cast solution. The DLS give a hydrodynamic diameter of 15–20 nm (Figure S4, Supporting Information). The observed agrregation of 3–4 of the 15–20 nm diameter ONP on the grid as the solvent evaporates is typical for these types of systems. The TEM also shows that the 15–20 nm diameter ONP are composed of smaller particles corresponding to the subdomains. The histogram in Figure 2 shows the size distribution for the subdomains on the grid and is consistent with the presence of dimers or trimers of the metalloporphyrins.
Figure 2.
(A) TEM image of a cluster of four ONP of Fe(III)TPPF84 on a carbon-coated copper grid, (B) where the 15–20 nm diameter ONP are outlined and indicated by arrows. (C) The scheme shows a cartoon of the hierarchical organization of one ONP and a few of the many 2–3 nm subdomains, each consisting of 2–4 metalloporphyrins. The histogram at the bottom shows the size distribution of the small subdomains contained within the ONP.
3.4. Mechanism
The mechanism for metalloporphyrin-catalyzed olefinic oxidation reactions have been well reviewed and depends on the specific compounds used. One main mechanism is H-atom abstraction.[19, 24] The aggregation of metalloporphyrins in the ONP may facilitate oxo-bridge complex formation, which is known to effect catalytic activity.[8a] Formation of allylic oxidation products by the ONPcatalyzed reaction suggest that the reaction goes through a different mechanistic pathway than when the epoxide is formed. The oxidation by the ONP may proceed through a radical mechanism.[13c] One plausible explanation for the slow reactivity and increased TON for these ONP catalytic reactions may be that only the outer layer of the metalloporphyrins in the nanoaggregates is available for the initial oxidative process. When the porphyrins in the outer layer eventually decompose, they fall off to expose the next active layer. The onion type was recently discussed,[13c] and is consistent with the DLS data that show that the size of the ONP decreases as the reaction progresses (Figure S12, Supporting Information). The higher ratio of ketone than alcohol for these ONP catalytic reactions may be because the initially formed alcohol gets further oxidized to ketone before it escapes the ONP cage. As cyclohexene is hydrophobic and the reaction solvent is mostly water, the substrate rapidly partitions into the ONP as indicated by the change in color of ONP and shift in the UV–visible spectral peaks. The organization of the metalloporphyrins within the ONP can change with the binding of the substrate.[13f] Thus, the mechanism has three convolved components in terms of the ONP. (1) The fundamental chemical reactivity in terms of the metalloporphyrin, substrate, solvent, temperature, and axial ligands; as extensively studied previously. (2) The role of the aggregation of the metalloporphyrins in the ONP in facilitating oxo-bridge formation, slowing down the reaction, and taking up the substrate from the mostly aqueous solution. (3) The nanoarchitecture of the aggregates may limit the approach of the substrate to the metal ion active site.
4. Conclusion
Appending fluorous alkanes onto a core Fe(III)TPPF20 platform using simple, high-yield chemistry affords a robust catalyst that can activate O2 for allylic oxidations. Although these adaptive ONP of Fe(III)TPPF84 exhibit reduced activity compared with the ONP of the Fe(III)TPPF20 platform, this strategy to make porphyrin derivatives provides a testbed to examine a plethora of other porphyrin derivatives and substrates for diverse applications.[16, 25] The porphyrin ONP catalysis reported herein represents a model for the green chemistry because ≈89% of the reaction solvent is water, halogenated solvents are avoided, and O2 is used as the oxidant. Allylic oxidation reactions are widely used in many areas of organic synthesis ranging from agricultural products to pharmaceuticals, and these ONP systems avoid use of reagents such as SeO2 and other metal oxides.[26] New high-yield synthetic methods may make simple metalloporphyrins attractive use of porphyrin catalysts on larger scales,[25a, 27] for example, in solvent-free reactions. [28] These studies suggests that all catalytic reactions using compounds such as metalloporphyrinoids should be examined for the presence of nanoparticles or aggregates because ONP can be much more active than the compounds in solution, thereby dominating the observed activity.
Supplementary Material
Acknowledgements
Supported by the National Science Foundation (NSF) CHE-0847997 to C.M.D. Hunter College Chemistry infrastructure is supported by the NSF, National Institutes of Health including the RCMI program (G12-RR-03037 and 8 G12 MD007599), and the City University of New York.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
References
- 1.Muzart J. Chem. Rev. 1992;92:113. [Google Scholar]
- 2.a) Choi H, Doyle MP. Org. Lett. 2007;9:5349. doi: 10.1021/ol7025284. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Doyle MP. J. Org. Chem. 2006;71:9253. doi: 10.1021/jo061411m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shing TKM, Yeung Y-Y, Su PL. Org. Lett. 2006;8:3149. doi: 10.1021/ol0612298. [DOI] [PubMed] [Google Scholar]
- 4.Harre M, Haufe R, Nickisch K, Weinig P, Weinmann H, Kinney WA, Zhang X. Org. Proc. Res. Dev. 1998;2:100. [Google Scholar]
- 5.Crich D, Zou Y. Org. Lett. 2004;6:775. doi: 10.1021/ol036501h. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Y, Yeung Y-Y. Org. Lett. 2010;12:2128. doi: 10.1021/ol100603q. [DOI] [PubMed] [Google Scholar]
- 7.Uyanik M, Okamoto H, Yasui T, Ishihara K. Science. 2010;328:1376. doi: 10.1126/science.1188217. [DOI] [PubMed] [Google Scholar]
- 8.a) Meunier B. Chem. Rev. 1992;92:1411. [Google Scholar]; b) Costas M. Coord. Chem. Rev. 2011;255:2912. [Google Scholar]
- 9.a) Silaghi-Dumitrescu R. J. Biol. Inorg. Chem. 2004;9:471. doi: 10.1007/s00775-004-0543-2. [DOI] [PubMed] [Google Scholar]; b) Meunier B, de Visser SP, Shaik S. Chem. Rev. 2004;104:3947. doi: 10.1021/cr020443g. [DOI] [PubMed] [Google Scholar]; c) Newcomb M, Hollenberg PF, Coon MJ. Arch. Biochem. Biophys. 2003;409:72. doi: 10.1016/s0003-9861(02)00445-9. [DOI] [PubMed] [Google Scholar]; d) Chandrasena REP, Vatsis KP, Coon MJ, Hollenberg PF, Newcomb M. J. Am. Chem. Soc. 2004;126:115. doi: 10.1021/ja038237t. [DOI] [PubMed] [Google Scholar]
- 10.a) Poltowicz J, Pamin K, Haber J. J. Mol. Catal. A: Chem. 2006;257:154. [Google Scholar]; b) Arasasingham RD, He GX, Bruice TC. J. Am. Chem. Soc. 1993;115:7985. [Google Scholar]; c) Ellis PE, Jr, Lyons JE. Coord. Chem. Rev. 1990;105:181. [Google Scholar]
- 11.a) Lee JY, Farha OK, Roberts J, Scheidt KA, Nguyen ST, Hupp JT. Chem. Soc. Rev. 2009;38:1450. doi: 10.1039/b807080f. [DOI] [PubMed] [Google Scholar]; b) Shultz AM, Farha OK, Hupp JT, Nguyen ST. J. Am. Chem. Soc. 2009;131:4204. doi: 10.1021/ja900203f. [DOI] [PubMed] [Google Scholar]
- 12.a) Bao J, Chen W, Liu T, Zhu Y, Jin P, Wang L, Liu J, Wei Y, Li Y. ACS Nano. 2007;1:293. doi: 10.1021/nn700189h. [DOI] [PubMed] [Google Scholar]; b) Huang Y, Ma W, Li J, Cheng M, Zhao J, Wan L, Yu JC. J. Phys. Chem. B. 2003;107:9409. [Google Scholar]
- 13.a) Gong X, Milic T, Xu C, Batteas JD, Drain CM. J. Am. Chem. Soc. 2002;124:14290. doi: 10.1021/ja027405z. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Drain CM, Smeureanu G, Patel S, Gong X, Garno J, Arijeloye J. New J. Chem. 2006;30:1834. [Google Scholar]; c) Smeureanu G, Aggarwal A, Soll CE, Arijeloye J, Malave E, Drain CM. Chem. Eur. J. 2009;15:12133. doi: 10.1002/chem.200901086. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Cho K, Kerber WD, Lee SR, Wan A, Batteas JD, Goldberg DP. Inorg. Chem. 2010;49:8465. doi: 10.1021/ic101035q. [DOI] [PubMed] [Google Scholar]; e) Qiu Y, Chen P, Liu M. J. Am. Chem. Soc. 2010;132:9644. doi: 10.1021/ja1001967. [DOI] [PubMed] [Google Scholar]; f) Aggarwal A, Qureshy M, Johnson J, Batteas JD, Drain CM, Samaroo D. J. Porphyrin Phthalocyanine. 2011;15:338. [Google Scholar]
- 14.a) Merlau ML, Cho S-H, Sun S-S, Nguyen ST, Hupp JT. Inorg. Chem. 2005;44:5523. doi: 10.1021/ic0505596. [DOI] [PubMed] [Google Scholar]; b) Lee SJ, Hupp JT. Coord. Chem. Rev. 2006;250:1710. [Google Scholar]; c) Merlau ML, Mejia Md P, Nguyen ST, Hupp JT. Angew. Chem. Int. Ed. 2001;40:4239. doi: 10.1002/1521-3773(20011119)40:22<4239::AID-ANIE4239>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 15.a) Wang Z, Medforth CJ, Shelnutt JA. J. Am. Chem. Soc. 2004;126:16720. doi: 10.1021/ja044148k. [DOI] [PubMed] [Google Scholar]; b) Wang Z, Medforth CJ, Shelnutt JA. J. Am. Chem. Soc. 2004;126:15954. doi: 10.1021/ja045068j. [DOI] [PubMed] [Google Scholar]
- 16.Drain CM, Bazzan G, Milic T, Vinodu M, Goeltz JC. Isr. J. Chem. 2005;45:255. [Google Scholar]
- 17.Varotto A, Todaro L, Vinodu M, Koehne J, Liu G-y, Drain CM. Chem. Commun. 2008:4921. doi: 10.1039/b806795c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.a) Kameyama H, Narumi F, Hattori T, Kameyama H. J. Mol. Catal. A: Chem. 2006;258:172. [Google Scholar]; b) Zhou X-T, Tang Q-H, Ji H-B. Tetrahedron Lett. 2009;50:6601. [Google Scholar]; c) Gharnati L, Doring M, Arnold U. Curr. Org. Syn. 2009;6:342. [Google Scholar]; d) Zhou X, Ji H. Chem. Eng. J. 2010;156:411. [Google Scholar]
- 19.Stephenson NA, Bell AT. Inorg. Chem. 2007;46:2278. doi: 10.1021/ic060757c. [DOI] [PubMed] [Google Scholar]
- 20.Bhuyan J, Sarkar S. Chem. Eur. J. 2010;16:10649. doi: 10.1002/chem.201001073. [DOI] [PubMed] [Google Scholar]
- 21.Kashani-Motlagh MM, Rahimi R, Kachousangi MJ. Molecules. 2010;15:280. doi: 10.3390/molecules15010280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu C. Synthesis and Application of Organic Thin Film Modified Surfaces, in Chemistry. New York: The City University of New York; 2005. p. Ph.D./. [Google Scholar]
- 23.a) Milic T, Garno JC, Batteas JD, Smeureanu G, Drain CM. Langmuir. 2004;20:3974. doi: 10.1021/la0359023. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Milic TN, Chi N, Yablon DG, Flynn GW, Batteas JD, Drain CM. Angew. Chem. Int. Ed. 2002;41:2117. [PubMed] [Google Scholar]
- 24.a) Arunkumar C, Lee Y-M, Lee JY, Fukuzumi S, Nam W. Chem. Eur. J. 2009;15:11482. doi: 10.1002/chem.200901362. [DOI] [PubMed] [Google Scholar]; b) Haber J, Matachowski L, Pamin K, Poltowicz J. J. Mol. Catal. A: Chem. 2003;198:215. [Google Scholar]; c) Lyons JE, Ellis PE, Myers HK. J. Catal. 1995;155:59. [Google Scholar]
- 25.a) Drain CM, Singh S. In: Combinatorial Libraries of Porphyrins: Chemistry and Applications, in The Handbook of Porphyrin Science with Applications to Chemistry, Physics, Materials Science, Engineering, Biology and Medicine. Kadish K, Smith KM, Guilard R, editors. Singapore: World Scientific Publisher; 2010. p. 485. [Google Scholar]; b) Drain CM, Batteas JD, Flynn GW, Milic T, Chi N, Yablon DG, Sommers H. Proc. Natl. Acad. Sci., USA. 2002;99:6498. doi: 10.1073/pnas.012521899. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Drain CM, Nifiatis F, Vasenko A, Batteas JD. Angew. Chem. Int. Ed. 1998;37:2344. doi: 10.1002/(SICI)1521-3773(19980918)37:17<2344::AID-ANIE2344>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]; d) Drain CM, Varotto A, Radivojevic I. Chem. Rev. 2009;109:1630. doi: 10.1021/cr8002483. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Jurow M, Schuckman AE, Batteas JD, Drain CM. Coord. Chem. Rev. 2010;254:2297. doi: 10.1016/j.ccr.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Radivojevic I, Varotto A, Farley C, Drain CM. Energy Environ. Sci. 2010;3:1897. [Google Scholar]
- 26.a) Andrus MB, Lashley JC. Tetrahedron. 2002;58:845. [Google Scholar]; b) Crich D, Zou Y. J. Org. Chem. 2005;70:3309. doi: 10.1021/jo0500333. [DOI] [PubMed] [Google Scholar]
- 27.a) Drain CM, Gong X. Chem. Commun. 1997:2117. [Google Scholar]; b) Cavaleiro JAS, Tomé AC, Neves MGPMS. In: “meso-Tetraarylporphyrin Derivatives: New Synthetic Methodologies”, in The Handbook of Porphyrin Science with Applications to Chemistry, Physics, Materials Science, Engineering, Biology and Medicine. Kadish K, Smith KM, Guilard R, editors. Singapore: World Scientific Publisher; 2010. p. 193. [Google Scholar]
- 28.Nia S, Gong X, Drain CM, Jurow M, Rizvi W, Qureshy M. J. Porphyrins Phthalocyanines. 2010;14:621. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.