Abstract
Seizures are a common phenotype in many neurological disorders including Alzheimer’s disease, Down syndrome and fragile X syndrome (FXS). Mouse models of these disorders over-express amyloid β-protein precursor (AβPP) and amyloid beta (Aβ) and are highly susceptible to audiogenic-induced seizures (AGS). We observed decreased AGS in these mice fed a casein-based, purified diet (D07030301) as opposed to a standard soy protein-containing, non-purified diet (Purina 5015). Our objective in this manuscript was to determine if soy protein, and in particular soy isoflavones, in the Purina 5015 were contributing to the seizure phenotype. Wild running, AGS and death rates were assessed in juvenile mice fed Purina 5015, D07030301, D07030301 containing soy protein, or D07030301 supplemented with individual isoflavones (750 mg/kg daidzein or genistein). A short treatment (3 days) with Purina 5015 induced wild running and AGS in Alzheimer’s disease mice. A 3-day treatment with daidzein-supplemented diet, but not genistein, induced wild running in wild type mice. To understand the mechanism underlying daidzein activity, we assessed dendritic AβPP expression in primary, cultured, wild type neurons treated with daidzein or genistein. In vitro, daidzein significantly increased dendritic AβPP. Thus, the soy isoflavone daidzein recapitulated seizure induction in vivo and altered AβPP expression in vitro. These results have important implications for individuals on soy-based diets as well as for rodent model research.
Keywords: soy, daidzein, seizure, amyloid β-protein precursor, amyloid beta, isoflavone
Introduction
As many as 1 out of 10 Americans will experience a seizure at some point in their lifetime. Brain injury, infections, tumors, stroke and high fevers are all causes of epilepsy; however, in the majority of cases, the etiology is unknown. Furthermore, the underlying molecular mechanism(s) that initiate and propagate seizures are not well understood. An increased susceptibility to seizures is observed in both patients with fragile X syndrome (FXS) and the mouse model of the disorder, Fmr1KO mice [1-3]. Seizure susceptibility is manifested in the Fmr1KO mice as a high susceptibility to audiogenic-induced seizures (AGS) at a young age [4], which correlates well with juvenile susceptibility to seizures in the human disorder [5]. We have found that Fmr1KO mice, which lack expression of fragile X mental retardation protein (FMRP), over-express amyloid β-protein precursor (AβPP) and amyloid beta (Aβ) [6]. We hypothesized that excess AβPP and Aβ contributed to the seizure phenotype. In support of that hypothesis, mouse models used to study Alzheimer’s disease (Tg2576) and Down syndrome (Ts65Dn), and thus overexpress AβPP and Aβ, exhibited a robust AGS phenotype [7]. In addition, Tg2576 had a very low threshold to pentylenetetrazol-induced seizures [8], and AGS were attenuated by genetic knockout of one App allele in Fmr1KO mice [9], treatment with metabotropic glutamate receptor 5 (mGluR5) antagonists or passive immunization with anti-AβPP/Aβ [7]. These data strongly suggest that AβPP and Aβ are involved in seizure propensity through an mGluR5/FMRP-dependent pathway and that pharmacological interventions that reduce Aβ or block mGluR5 signaling may be therapeutic for seizure disorders.
During chronic dosing experiments with an mGluR5 antagonist as a feed supplement to reduce Aβ, we discovered that seizure-prone mice fed a purified diet with a casein-based protein source (D07030301) were more resistant to AGS than mice fed a soy protein-based, non-purified diet (Purina 5015). Mouse colonies are typically maintained on Purina diets, which provide the necessary nutrients for growth and breeding of the mice; however, these diets are grain-based with their protein content derived from soybeans, and there can be huge variations in phytoestrogen content due to crop variations in the soybeans [10]. Isoflavones are a type of phytoestrogen that are prevalent in soybeans and can be transferred to offspring through the placenta as well as maternal milk. The steady-state concentration of isoflavones found in serum (2,340 μg/L) of Purina-fed mice is 30,000-fold greater than endogenous estrogen and has immense potential to affect the results of animal research [11]. Thus, we hypothesized that soy isoflavones in the Purina 5015 were exacerbating AGS in mice. We explored this by supplementing the D07030301 diet with individual isoflavones and determining the subsequent effects on seizure frequency and intensity. We demonstrate herein that the soy isoflavone daidzein contributes to seizure induction possibly through increased AβPP levels.
Materials and Methods
Mouse husbandry
The Fmr1KO [12], wild type, Tg2576 [13], FRAXAD [8], Fmr1KO/AppKO double knockout [9] and Ts65Dn [14] mice were obtained from colonies maintained by our laboratory. These strains were originally received from Dr. Bill Greenough (Fmr1KO and wild type, University of IL-Urbana), Dr. Jeff Johnson (Tg2576, University of Wisconsin-Madison) and Jackson Laboratories (Ts65Dn females and B6EiC3SnF1/J males, Bar Harbor, ME), or generated by our laboratory as previously described (FRAXAD [8] and Fmr1KO/APPKO double KO [9]). Fmr1KO, wild type, Tg2576, FRAXAD and Fmr1KO/APPKO offspring were in a C57BL/6 background while the Ts65Dn [B6EiC3Sn a/A-Ts(1716)65Dn] females generated trisomic and nontrisomic littermates in a mixed background. Mating pairs were housed in microisolator cages on a 12 hr (0600-1800 hr) light cycle with ad libitum access to water and food (specific diets are described below). All husbandry and euthanasia procedures were performed in accordance with NIH and an approved University of Wisconsin Madison animal care protocol administered through the University of Wisconsin Research Animal Resources Center. Genotypes were determined by PCR analysis of DNA extracted from tail biopsies taken at postnatal day 21 (P21) after AGS testing.
Lab diets
Purina 5015 is a high-energy, complete life-cycle, non-purified diet designed to support the reproduction, growth and maintenance of rodents. This grain-based diet contains not less than 17% protein and 11% fat and not more than 3% fiber and 6.5% ash. The main ingredients are ground wheat, dehulled soybean meal and ground corn. AIN-76A is a purified ingredient, rodent diet formulated by the American Institute of Nutrition with the main ingredients of granular sugar, lactic casein and cornstarch. A modified AIN-76A diet (product #D07030301) was synthesized, which matched Purina 5015 for the percentage of fat, protein and carbohydrate (Research Diets, Inc., New Brunswick, NJ) (Supplementary Table 1). The Purina 5015 and D07030301 contain low sucrose and similar macronutrients. Non-purified diets such as Purina 5015 contain soybean products including isoflavones and other plant-derived compounds that are not found in purified ingredient diets including D07030301. The casein protein source in the D07030301 was swapped gram for gram with soy protein SUPRO 661 to generate soy-supplemented diet (product #D07030304) (Research Diets, Inc., New Brunswick, NJ) (Supplementary Table 1). D07030301 was supplemented with 750 mg/kg genistein (product #D09080401) or daidzein (product #D09080402) to prepare isoflavone-supplemented diets (Research Diets, Inc., New Brunswick, NJ) (Supplementary Table 1).
Seizure testing
AGS were induced with a 118 dB personal body alarm as previously described [7]. AGS begin with wild running, which progresses to loss of the righting reflex, tonic hind limb extension and often death. Mice that do not die from the seizures regain the righting reflex during the post-ictal period and appear normal by the end of the 3-minute observation period. The number of mice exhibiting wild running, tonic seizures and death were scored, and statistical significance was assessed by Barnard’s exact test. Mice were tested at P21, the peak of AGS sensitivity in C57BL/6 [4].
Dosing
For the 3-day dosing strategy (Figure 1), mice were generated from breeding pairs that were bred and maintained on Purina 5015. When the pups were age P18, they were transferred to D07030301 for 3 days and tested at P21. For chronic restriction of soy constituents (Figures 2 & 3), mating pair were bred and litters maintained on D07030301 until testing at P21. To test the effect of soy, daidzein and genistein on seizures (Figure 2 & 3), pups bred and maintained on D07030301 until P18 at which time they were transferred to one of the supplemented diets (soy, daidzein or genistein) for 3 days until testing at P21.
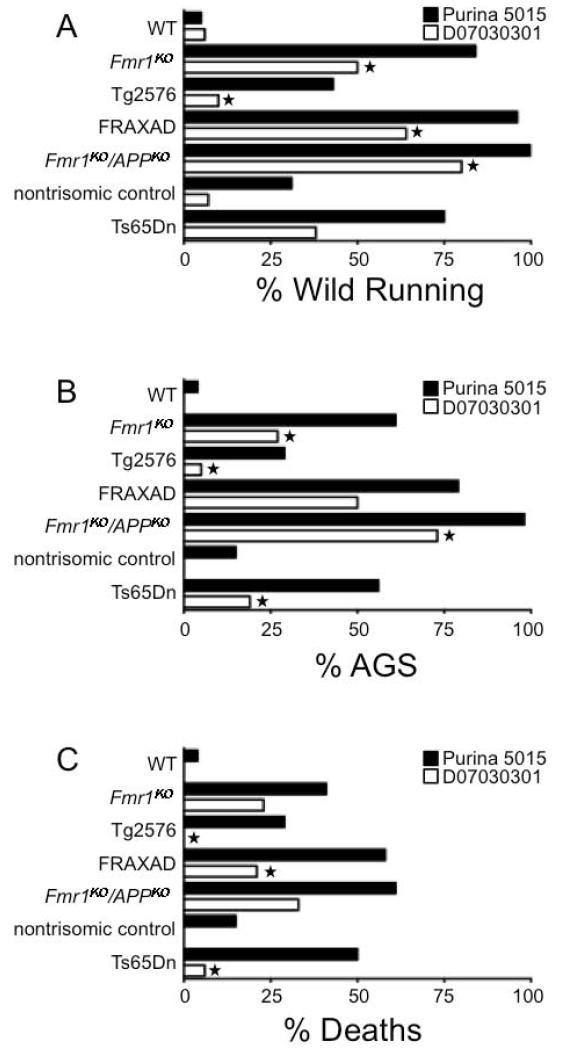
Figure 1.
Soy-free diet reduces seizure propensity in several strains of mice with altered AβPP and Aβ expression. Wild type, Fmr1KO (FXS mouse model that lacks FMRP expression), Tg2576 (Alzheimer disease mouse model that overexpresses the human AβPP695 gene with the Swedish familial mutation), FRAXAD (cross of Fmr1KO and Tg2576 mice that overexpresses both mouse and human AβPP), Fmr1KO/APPKO (double knockout mice that lack expression of AβPP and FMRP), and Ts65Dn (Down syndrome mouse model that is trisomic for chromosome 16 carrying the App gene) and nontrisomic littermate controls were conceived and maintained on Purina 5015. At age P18, mice were left on Purina 5015 (black bars) or transferred to purified diet D07030301 (white bars) for 3 days prior to seizure testing. Each cohort contained a minimum of 13 mice. Statistical significance between mice of the same genotype but fed different diets was determined by Barnard’s exact test (2-tail) and is denoted by a star (P ≤ 0.05).
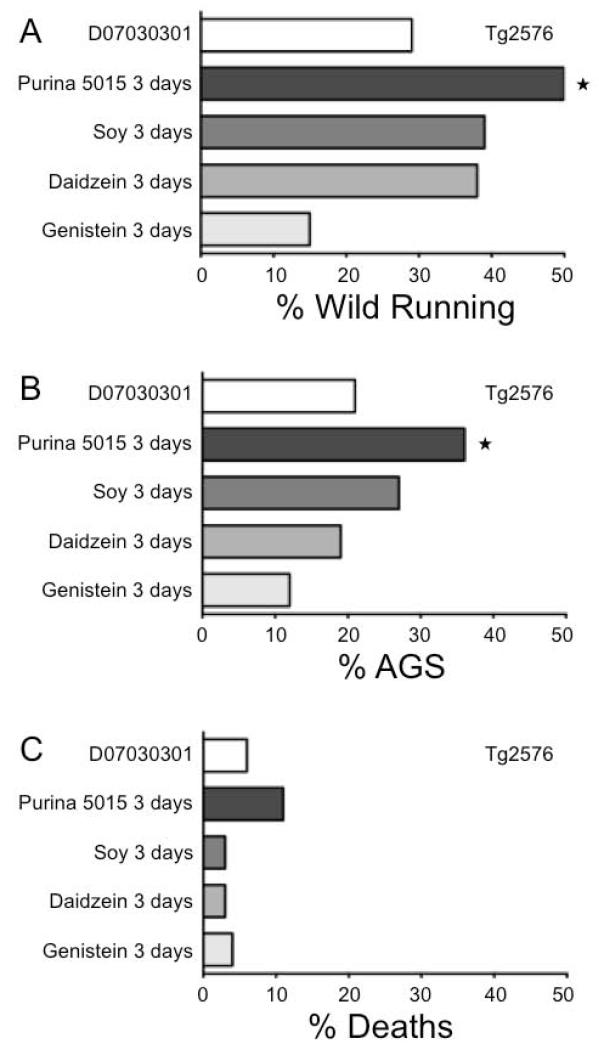
Figure 2.
Purina 5015 soy-containing diet restores seizure phenotype in Tg2576. Wild running, AGS and death rates were assessed in Tg2576 mice in response to diet. Mice were conceived and maintained on D07030301 (white bars) until P18 and 3 days prior to seizure testing transferred to Purina 5015 (dark gray bars), soy-supplemented (medium gray bars), daidzein-supplemented (light gray bars) or genistein-supplemented (pale gray bars) diets. Each cohort contained a minimum of 26 mice. Statistical significance was determined by Barnard’s exact test and is denoted by a star (P ≤ 0.05).
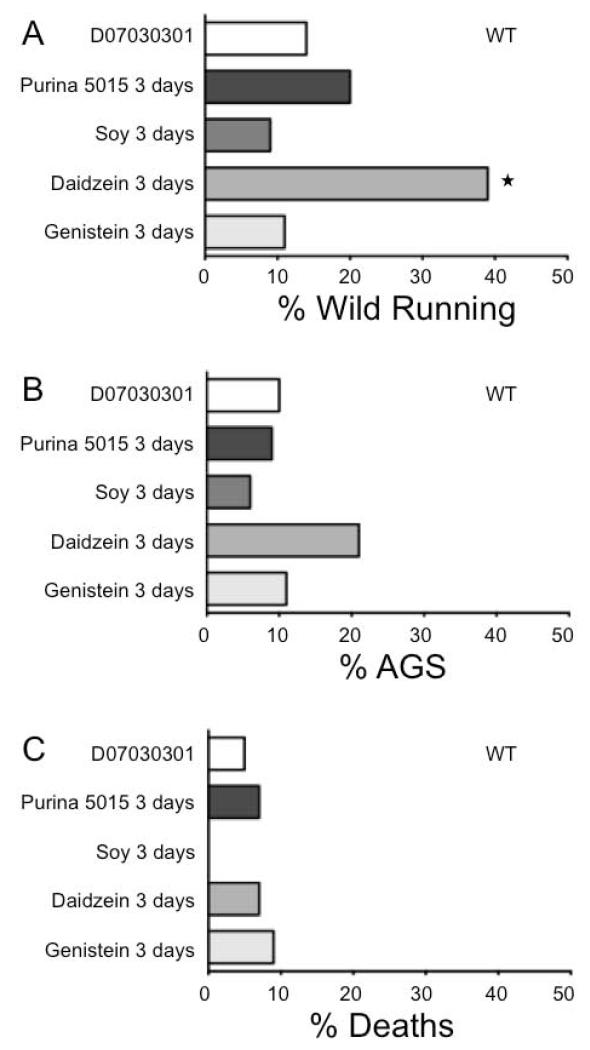
Figure 3.
Daidzein-supplemented diet induces wild running in wild type mice. Wild running, AGS and death rates were assessed in wild type mice in response to diet. Mice were conceived and maintained on D07030301 (white bars) until P18 and 3 days prior to seizure testing transferred to Purina 5015 (dark gray bars), soy-supplemented (medium gray bars), daidzein-supplemented (light gray bars) or genistein-supplemented (pale gray bars) diets. Each cohort contained a minimum of 28 mice. Statistical significance was determined by Barnard’s exact test and is denoted by a star (P ≤ 0.05).
Preparation, treatment and staining of primary neurons
Primary mouse neurons were prepared from embryonic cortex from timed-pregnant wild type mice as previously described [6]. Cells were cultured for 15 days on poly(D)-lysine coated glass coverslips inside of 12-well tissue culture dishes prior to treatment with daidzein or genistein at the indicated concentrations and times. For the analyses of dendritic AβPP levels, the cells were washed with DPBS, fixed with 4% paraformaldehyde, permeabilized with cold MeOH (−20°C) for 15 min, and stained with anti-22C11 against the amino-terminus of AβPP (catalog #MAB348, 1:2000) (Chemicon, Billerica, MA) followed by visualization with goat anti-mouse rhodamine-conjugated secondary antibody (1:500 for 30 min in the dark) (Invitrogen, Carlsbad, CA). Images were acquired with a Nikon C1 laser-scanning confocal microscope using a 60× objective and EZ-C1 v2.20 software. AβPP in the dendritic puncta was quantified with ImageJ software (Rashband, W.S., URL: http://rsb.info.nih.gov/ij) using the Analyze Particles function (minimum of 616 puncta analyzed per treatment). Values in the figure are means ± SEM.
Statistical analyses
Barnard’s exact test was used to quantitate statistical significance (P ≤ 0.05) for the wild running, AGS and death rates. One-way ANOVA was performed using GraphPad Prism version 5.0d for Mac OS X (GraphPad Software, San Diego, CA) to compare the means of three or more unmatched groups for the immunofluoresence data and the student t-test (P ≤ 0.05) was used to quantitate statistical significance.
Results
Non-soy diet attenuates AGS
Juvenile mice that over-express AβPP and Aβ (Fmr1KO, Tg2576, FRAXAD and Ts65Dn) are highly prone to AGS [2,3,7] whereas wild type C57BL/6 mice are resistant [15]. AGS can be reduced in these mouse models, which are used to study FXS, AD and DS, with mGluR5 antagonists [7,9]. During chronic dosing studies with the mGluR5 antagonist fenobam, which was incorporated into a purified diet (D07030301, Research Diets, Inc.) matched to Purina 5015 for protein, fat and carbohydrate content, AGS were reduced regardless of the presence of fenobam. The fenobam was incorporated into a purified diet because non-purified diets are grain-based and nutrients vary between lots [10]. Specifically, wild type, Fmr1KO, Tg2576, FRAXAD, Fmr1KO/APPKO and Ts65Dn mice were conceived and maintained on Purina 5015. At age P18, pups were transferred to D07030301 for 3 days prior to seizure testing at age P21. The D07030301 reduced wild running, seizure and/or death rates in Fmr1KO, Tg2576, FRAXAD, Fmr1KO/APPKO and Ts65Dn (Figure 1) compared with mice constantly maintained on the Purina 5015. Baseline data for mice chronically fed Purina 5015 were previously reported [7,9].
Wild running, seizure and death rates in the Fmr1KO, FRAXAD and Fmr1KO/APPKO mice on the D07030301 for 3 days were higher than those for the Tg2576 and Ts65Dn suggesting that a longer treatment period might further reduce seizure susceptibility in the Fmr1KO-based strains. Thus, we assessed seizures at age P21 in Fmr1KO mice conceived and maintained on D07030301 and found that wild running (56%) and AGS (33%) rates were significantly decreased, and the longer treatment decreased the death rates to attain statistical significance (11%, Barnard’s exact test, P ≤ 0.02).
To determine if the AGS phenotype resurfaced in mice conceived on D07030301 and later exposed to Purina 5015, wild type and Tg2576 mice born to parents maintained on the D07030301 were weaned onto Purina 5015 at P18 and tested for wild running, AGS and death rates at P21. The Tg2576 were chosen for these analyses as they over-express a single transgene as opposed to having the Fmr1KO gene inactivated, which alters the expression of numerous synaptic proteins. There was a statistically significant increase in wild running and AGS in the Tg2576 fed Purina 5015 for 3 days (Figure 2); however, the short treatment period was not long enough to fully recapitulate the death phenotype. Thus, a constituent of Purina 5015 lowers seizure threshold in Alzheimer’s disease mice. As expected, wild type littermates were unaffected by 3-days feeding with Purina 5015 (Figure 3).
The major difference between the D07030301 and Purina 5015 is the protein source. Soy protein is rich in phytoestrogens including the two most prevalent isoflavones, genistein and daidzein. The isoflavone concentration in soy products is lot dependent with published reports ranging from 490-1,500 mg/kg [16,17]. Thus, we tested the effects of total soy protein on seizures by preparing D07030301 in which the casein protein source was replaced with soy protein. We swapped the protein sources as opposed to removing isoflavones from the Purina 5015 because the standard process for removing isoflavones from feed is an alcohol wash, which can alter other components such as sterols, saponins, small peptides or the soy protein itself. Wild type and Tg2576 littermates were bred on the D07030301 and at P18 weaned onto soy-substituted D07030301 for 3 days prior to AGS testing at P21. Substitution of soy for casein protein in the D07030301 did not significantly increase wild running, AGS or death rates in Tg2576 (Figure 2) or wild type (Figure 3) mice. However, there was a strong trend for increased wild running (45%) and AGS (35%) (Barnard’s exact tests, P ≤ 0.20) selectively in the Tg2576 females (data not shown).
Daidzein exacerbates wild running
We hypothesized that soy isoflavones were the seizure-promoting constituent in the Purina 5015, and since the isoflavone content of soy products is highly variable [16,17], the lot of SUPRO 661 used to generate the soy-supplemented diet may have had a lower isoflavone content resulting in decreased AGS rates. Hence, we tested the effects of individual isoflavones on seizures by supplementing the D07030301 with genistein or daidzein at 750 mg/kg feed, which is within the concentration range of isoflavones naturally found in soy products. Wild type and Tg2576 littermates were bred on the D07030301 and at P18 weaned onto daidzein- or genistein-substituted D07030301 for 3-days prior to AGS testing at P21. Daidzein significantly increased wild running in wild type mice (39%, P ≤ 0.005), although 3 days feeding was not sufficient to induce a statistically significant increase in AGS (21%, P ≤ 0.1) (Figure 3). A 3-day treatment with daidzein did not significantly increase wild running in Tg2576 (Figure 2); however, there was a strong trend for increased wild running (54%, P ≤ 0.10) selectively in the Tg2576 females (data not shown). The genistein diet (3-days dosing) did not alter wild running, AGS or death rates in either wild type or Tg2576 mice (Figures 2 & 3) nor did diet containing both daidzein and genistein (data not shown).
Soy isoflavones increase AβPP levels
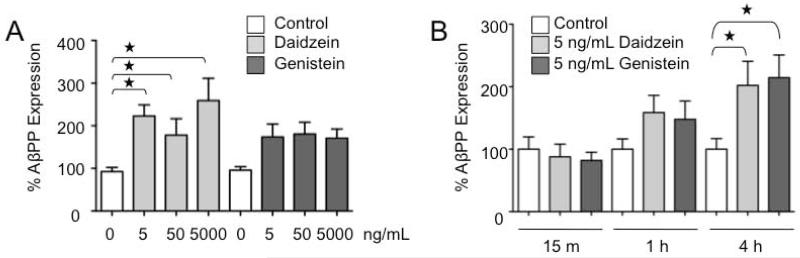
In vitro studies in primary cultured neurons indicate that treatment with daidzein and genistein increased dendritic AβPP, the parent molecule that is cleaved to form Aβ. Treatment with daidzein (5-5,000 ng/mL) resulted in a two-fold increase in dendritic AβPP after a 1-day treatment (one-way ANOVA, P ≤ 0.002) (Figure 4A). The ANOVA test failed to show a significant difference between control and genistein-treated samples after 1-day treatment (P ≤ 0.06). Shorter treatment periods (15 min, 1 hr and 4 hr) indicated that daidzein and genistein (5 ng/mL) significantly increased dendritic AβPP by 2-fold at 4 hr (one-way ANOVA, P ≤ 0.05), but there were no statistically significant differences at shorter time points (Figure 4B). Thus, isoflavones elicited time-dependent and concentration-independent effects on AβPP levels. The increase in AβPP in response to genistein was more transient in nature compared with daidzein treatment as statistical significance with genistein was lost at the 24 hr time points. The steady-state concentration of total isoflavones in serum of Purina-fed mice is 2,340 μg/L. Therefore, these data indicated that isoflavones at concentrations 500-fold lower than those in the serum of Purina-fed mice increase the production of AβPP.
Figure 4.
Daidzein increases AβPP expression in primary cultured neurons. (A) Primary wild type neurons were treated with 0, 5, 50 or 5,000 ng/mL daidzein or genistein for 1 day prior to fixation and staining for AβPP. A minimum of 616 puncta were analyzed per treatment. The percent of AβPP expression compared to untreated controls (white bars) was plotted against treatments (gray bars). Statistical significance was determined by one-way ANOVA (daidzein: P ≤ 0.002, F = 5; genistein: P = 0.06, F = 2.4) and by student t-test analyses (all daidzein-treated samples were statistically different from untreated controls, denoted by stars, P ≤ 0.01). (B) Primary wild type neurons were treated with 5 ng/mL daidzein or genistein for 15 min, 1 hr or 4 hr prior to fixation and staining for AβPP. A minimum of 746 puncta were analyzed per treatment. Statistical significance was determined by one-way ANOVA (4 h: P ≤ 0.05, F = 3.2) and student t-test (control versus daidzein 4 h: P ≤ 0.05; control versus genistein: P ≤ 0.01). Shorter time points were not statistically significant.
Discussion
Epileptic seizures are a common phenotype in many neurological disorders including FXS [18], autism [19], Alzheimer’s disease [20] and Down syndrome [21] as well as in the military population due to their high incidence of traumatic brain injury [22,23]. Anti-epileptic drugs are the first-line therapy for seizures. More than 20 medications are available, but seizures are not controlled in nearly a third of the patients and there are many side effects including cognitive impairment and neurobehavioral problems [24]. Due to the heterogeneity of seizures, the underlying cellular and molecular mechanisms that induce and propagate these abnormal electrical discharges in the brain remain poorly understood. It is imperative to understand these mechanisms in order to design better therapeutic interventions.
Accumulating evidence points to a role for AβPP, or a catabolite of AβPP such as Aβ, in seizure propensity [25]. We have shown that over- or under-expression of AβPP and Aβ in mice exacerbates the AGS phenotype [7,9]. Herein, we demonstrate reduced susceptibility to audiogenic-induced wild running, seizures and/or death in several strains of mice fed a purified, non-soy-based diet for 3-days prior to testing. The Tg2576 strain exhibited statistically significant reductions in all seizure phenotypes (wild running, seizure and death). These mice over-express a transgene carrying the human AβPP695 gene with the Swedish familial Alzheimer’s mutation and exhibit elevated Aβ production and deposition [13]. A longer period of soy restriction was required to attenuate deaths in Fmr1KO mice, a rodent model for the study of FXS and autism that lacks expression of FMRP and exhibits elevated levels of AβPP and Aβ. Genetic knockout of one App allele in Fmr1KO mice attenuated AGS by 54% suggesting that overexpression of AβPP and Aβ in an FMRP null background is an important contributing factor to seizure propensity [9]. The FRAXAD mice, which are a cross between the Tg2576 and Fmr1KO and thus overexpress both human and mouse AβPP, exhibit an additive effect on seizure propensity [7], which is reduced by soy restriction. However, Fmr1KO/APPKO mice, which lack expression of both AβPP and FMRP, exhibited the highest rates of wild running, seizures and death of all of the strains tested. Even though soy restriction reduced these rates, they were still higher than any of the other strains fed D07030301 for 3-days. Soluble AβPPα, a catabolite of AβPP, has neuroprotective effects and is absent in Fmr1KO/APPKO mice suggesting that both over- and under-expression of AβPP is detrimental. Ts65Dn mice are trisomic for mouse chromosome 16 that carries the App gene [14]. Soy restriction for 3-days was more effective at reducing seizures in the Ts65Dn and Tg2576 mice than in any of the Fmr1KO null strains (Fmr1KO, FRAXAD, Fmr1KO/APPKO). Thus, soy restriction was effective at reducing seizures in multiple mouse lines and expression levels of AβPP and Aβ appear to be a contributing factor.
The major difference between the diets was the protein source with Purina 5015 based on soy protein and D07030301 based on casein protein. We assessed seizure propensity in Tg2576 fed soy-supplemented diet for 3-days and found a strong trend for increased wild running in the females, but the results did not reach statistical significance. The isoflavone content in soy protein varies by crop. It is likely that a more isoflavone-rich lot of soy protein was required to recapitulate the seizure phenotype. Thus, we tested supplementation of D07030301 with the two most prevalent isoflavones, genistein and daidzein at concentrations typical of soy-based chows such as Purina 5015. Daidzein elicited a strong wild running phenotype in wild type mice after only 3-days feeding demonstrating that a short treatment with this soy isoflavone causes seizure induction. Daidzein did not induce AGS and death phenotypes suggesting that other unidentified constituent(s) of the Purina 501 contribute to seizure progression. FMR1KO Drosophila reared on food containing increased concentrations of glutamate die during development consistent with the theory that the loss of FMRP results in excessive glutamate signaling [26]; however, the D07030301 and Purina 5015 diets contained similar concentrations of glutamate. Dietary components other than soy isoflavones, such as zinc and iron, differ between the diets, but it is unlikely that the higher concentrations of these elements in the Purina 5015 contributed to the reduced seizure threshold. Branched-chain amino acids increase seizure threshold [27] whereas low levels increase seizure propensity [28]; however, all diets utilized herein contained comparable and nutritionally adequate quantities of leucine, isoleucine and valine. At this point, we have identified daidzein as a component of soy protein that elicits a strong wild running phenotype in wild type mice, but we do not know the identity of any other dietary factors that may cooperate with daidzein to mediate seizure progression. Daidzein did not increase AGS in the Tg2576, although similar to the soy, there was a strong trend for increased wild running in the females. The selective effect of daidzein-, and not genistein-supplemented diet, on seizure propensity could be due to differential metabolism of the isoflavones. Daidzein is metabolized by intestinal bacteria to produce S-equol, which binds to estrogen receptors with greater affinity than daidzein suggesting that it is a more potent estrogenic compound[29]. Daidzein, genistein and equol enter the brain and remain stable within the brain similar to steroids [30].
We utilized the AGS model in these studies to assess the effects of prevalent isoflavones on seizure propensity. Susceptibility to juvenile AGS in Black Swiss mice is inherited as an autosomal recessive trait localized to a critical region syntenic with human chromosome 19p13.3, which is implicated in juvenile febrile convulsions [31]. Although we were working with transgenic mice in the C57BL/6 background in which multiple loci are involved in AGS (chromosomes 4, 7 and 12) [32], the connection between AGS sensitivity in rodents and febrile seizures in humans is interesting. Work in progress indicates that autistic children fed soy-based infant formula have a higher incidence of febrile seizures (unpublished data, Westmark, 2012). In total, these data indicate that soy isoflavones are associated with decreased seizure threshold in both rodents and humans.
To begin to understand the mechanism underlying daidzein-induced seizure activity in mice that overexpress AβPP, we treated primary, cultured, wild type neurons with daidzein and genistein and assessed AβPP expression. We observed that both isoflavones increased dendritic AβPP levels in a time-dependent manner; however, the effect with genistein was more transient in nature compared to daidzein. These data in conjunction with seizure data demonstrating that diet supplemented with both daidzein and genistein did not induced wild running activity (data not shown) suggest that genistein may counteract the seizure-promoting effect(s) of daidzein and that the ratio of the isoflavones in soy batches may determine seizure propensity.
There are numerous health benefits for adults associated with the consumption of soy products in terms of prevention of age-related cardiovascular disease, osteoporosis and peri- and postmenopausal symptoms. However, the effects of isoflavones on individuals susceptible to seizures have not been studied. Seizures are comorbid with Alzheimer’s disease (10-22%) [33], FXS (18-23%) [18,34], Down syndrome (8%) [21], autism (21-38%) [19,35] and traumatic brain injury (TBI) (53%) [22,23]. Thus, the use of soy supplements or soy-based gastric tube feeding for these individuals may provoke seizures.
The effects of isoflavones on fetal and early childhood development are also not well understood. Soy formulas are the major source of nutrition for approximately 25% of infants. Studies in pregnant rats demonstrate that the placenta acts as a sink for isoflavones, and that while transport across the placenta is inefficient, low concentrations of isoflavones are found in the fetus at levels sufficient for activation of estrogen receptor β [36]. Twenty-five percent of infant formulas are based on soy protein [37] and have high isoflavone concentrations ranging from 32 to 47 mg/L compared to 5.6±4.4 μg/L in human breast milk resulting in circulating concentrations of isoflavones that are 13,000-22,000-fold higher than plasma estradiol concentrations [38,39]. Considering body weight and bioavailability, infants fed soy formula, as opposed to cow’s milk formula or breast milk, could be receiving the equivalent of five oral contraceptives per day [40]. The sex steroids bathe the rapidly developing, fetal brain during mid-pregnancy with males exposed to high testosterone and females to high estrogen concentrations. High exposure to estrogenic compounds during fetal and early childhood development through soy-based food products could disrupt the function of the natural steroid hormones and contribute to the high incidence of seizures associated with many childhood, neurological disorders including autism and FXS. Understanding the role of soy constituents, such as daidzein, on AβPP synthesis and metabolism and modulation of intake during pregnancy and infancy could reduce seizure incidence and prevent neurological damage.
In conclusion, seizures are a serious medical problem that is comorbid with many neurological disorders. Several mouse models of neurological disease are highly susceptible to AGS when maintained on soy-based rodent diets, which are rich in isoflavones that can increase AβPP expression. Accumulating evidence suggests that synaptic dysregulation of AβPP production and cleavage determines the level of Aβ in the brain interstitial fluid [6,41,42]. Thus, a clearer understanding of the environmental factors, such as soy, that modulate synaptic AβPP levels may provide a dietary intervention to reduce Aβ levels and seizures.
Supplementary Material
Acknowledgments
We thank Drs. Matt Ricci and Mike Pellizzon (Research Diets, Inc.) and Dr. Denise Ney (Department of Nutritional Sciences, University of WI-Madison) for helpful discussions regarding diet formulations. We thank Sara Abozeid and Brian Ray (Waisman Center, University of WI-Madison) for technical assistance. This project was supported by the Clinical and Translational Science Award (CTSA) program, previously through the National Center for Research Resources (NCRR) grant 1UL1RR025011, and now by the National Center for Advancing Translational Sciences (NCATS) grant 9U54TR000021 (CW), FRAXA Research Foundation (CW), and National Institutes of Health Grant P30 HD03352 (Waisman Center).
References
- [1].Berry-Kravis E, Raspa M, Loggin-Hester L, Bishop E, Holiday D, Bailey DB. Seizures in fragile X syndrome: characteristics and comorbid diagnoses. Am J Intellect Dev Disabil. 2010;115:461–472. doi: 10.1352/1944-7558-115.6.461. [DOI] [PubMed] [Google Scholar]
- [2].Musumeci SA, Bosco P, Calabrese G, Bakker C, De Sarro GB, Elia M, Ferri R, Oostra BA. Audiogenic seizures susceptibility in transgenic mice with fragile X syndrome. Epilepsia. 2000;41:19–23. doi: 10.1111/j.1528-1157.2000.tb01499.x. [DOI] [PubMed] [Google Scholar]
- [3].Chen L, Toth M. Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience. 2001;103:1043–1050. doi: 10.1016/s0306-4522(01)00036-7. [DOI] [PubMed] [Google Scholar]
- [4].Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- [5].Berry-Kravis E. Epilepsy in fragile X syndrome. Dev Med Child Neurol. 2002;44:724–728. doi: 10.1017/s0012162201002833. [DOI] [PubMed] [Google Scholar]
- [6].Westmark CJ, Malter JS. FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol. 2007;5:e52. doi: 10.1371/journal.pbio.0050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Westmark CJ, Westmark PR, Malter JS. Alzheimer’s Disease and Down Syndrome Rodent Models Exhibit Audiogenic Seizures. J Alzheimers Dis. 2010;20:1009–1013. doi: 10.3233/JAD-2010-100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Westmark CJ, Westmark P, Beard A, Hildebrandt S, Malter JS. Seizure susceptibility and mortality in mice that over-express amyloid precursor protein. Int J Clin Exp Pathol. 2008;1:157-157–168. [PMC free article] [PubMed] [Google Scholar]
- [9].Westmark CJ, Westmark PR, O’Riordan KJ, Ray BC, Hervey CM, Salamat MS, Abozeid SH, Stein KM, Stodola LA, Tranfaglia M, Burger C, Berry-Kravis EM, Malter JS. Reversal of Fragile X Phenotypes by Manipulation of AβPP/Aβ Levels in Fmr1 Mice. PLoS One. 2011;6:e26549. doi: 10.1371/journal.pone.0026549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ricci MR, Ulman EA. Laboratory animal diets: a critical part of your research. Animal Lab News. 2005;4:1. [Google Scholar]
- [11].Brown NM, Setchell KD. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab Invest. 2001;81:735–747. doi: 10.1038/labinvest.3780282. [DOI] [PubMed] [Google Scholar]
- [12].The Dutch-Belgian Fragile X Consortium Fmr1 knockout mice: a model to study fragile X mental retardation. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- [13].Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- [14].Davisson MT, Schmidt C, Akeson EC. Segmental trisomy of murine chromosome 16: a new model system for studying Down syndrome. Prog Clin Biol Res. 1990;360:263–280. [PubMed] [Google Scholar]
- [15].Schlesinger K, Griek BJ. 1970. The genetics and biochemistry of audiogenic seizures; pp. 219–257. [Google Scholar]
- [16].Thigpen JE, Li LA, Richter CB, Lebetkin EH, Jameson CW. The mouse bioassay for the detection of estrogenic activity in rodent diets: II. Comparative estrogenic activity of purified, certified and standard open and closed formula rodent diets. Lab Anim Sci. 1987;37:602–605. [PubMed] [Google Scholar]
- [17].Santell RC, Kieu N, Helferich WG. Genistein inhibits growth of estrogen-independent human breast cancer cells in culture but not in athymic mice. J Nutr. 2000;130:1665–1669. doi: 10.1093/jn/130.7.1665. [DOI] [PubMed] [Google Scholar]
- [18].Wisniewski KE, Segan SM, Miezejeski CM, Sersen EA, Rudelli RD. The Fra(X) syndrome: neurological, electrophysiological, and neuropathological abnormalities. Am J Med Genet. 1991;38:476–480. doi: 10.1002/ajmg.1320380267. [DOI] [PubMed] [Google Scholar]
- [19].Volkmar FR, Nelson DS. Seizure disorders in autism. J Am Acad Child Adolesc Psychiatry. 1990;29:127–129. doi: 10.1097/00004583-199001000-00020. [DOI] [PubMed] [Google Scholar]
- [20].Hauser WA, Morris ML, Heston LL, Anderson VE. Seizures and myoclonus in patients with Alzheimer’s disease. Neurology. 1986;36:1226–1230. doi: 10.1212/wnl.36.9.1226. [DOI] [PubMed] [Google Scholar]
- [21].Pueschel SM, Louis S, McKnight P. Seizure disorders in Down syndrome. Arch Neurol. 1991;48:318–320. doi: 10.1001/archneur.1991.00530150088024. [DOI] [PubMed] [Google Scholar]
- [22].Chen JW, Ruff RL, Eavey R, Wasterlain CG. Posttraumatic epilepsy and treatment. J Rehabil Res Dev. 2009;46:685–696. doi: 10.1682/jrrd.2008.09.0130. [DOI] [PubMed] [Google Scholar]
- [23].Weiss GH, Salazar AM, Vance SC, Grafman JH, Jabbari B. Predicting posttraumatic epilepsy in penetrating head injury. Arch Neurol. 1986;43:771–773. doi: 10.1001/archneur.1986.00520080019013. [DOI] [PubMed] [Google Scholar]
- [24].Ikonomidou C, Turski L. Antiepileptic drugs and brain development. Epilepsy Res. 2010;88:11–22. doi: 10.1016/j.eplepsyres.2009.09.019. [DOI] [PubMed] [Google Scholar]
- [25].Noebels J. A perfect storm: Converging paths of epilepsy and Alzheimer’s dementia intersect in the hippocampal formation. Epilepsia. 2011;52(Suppl 1):39–46. doi: 10.1111/j.1528-1167.2010.02909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chang S, Bray SM, Li Z, Zarnescu DC, He C, Jin P, Warren ST. Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila. Nat Chem Biol. 2008;4:256–263. doi: 10.1038/nchembio.78. [DOI] [PubMed] [Google Scholar]
- [27].Skeie B, Petersen AJ, Manner T, Askanazi J, Jellum E, Steen PA. Branched-chain amino acids increase the seizure threshold to picrotoxin in rats. Pharmacol Biochem Behav. 1992;43:669–671. doi: 10.1016/0091-3057(92)90393-t. [DOI] [PubMed] [Google Scholar]
- [28].Harris RA, Joshi M, Jeoung NH, Obayashi M. Overview of the molecular and biochemical basis of branched-chain amino acid catabolism. J Nutr. 2005;135:1527S–30S. doi: 10.1093/jn/135.6.1527S. [DOI] [PubMed] [Google Scholar]
- [29].Atkinson C, Frankenfeld CL, Lampe JW. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp Biol Med (Maywood) 2005;230:155–170. doi: 10.1177/153537020523000302. [DOI] [PubMed] [Google Scholar]
- [30].Lephart ED, Adlercreutz H, Lund TD. Dietary soy phytoestrogen effects on brain structure and aromatase in Long-Evans rats. Neuroreport. 2001;12:3451–3455. doi: 10.1097/00001756-200111160-00015. [DOI] [PubMed] [Google Scholar]
- [31].Misawa H, Sherr EH, Lee DJ, Chetkovich DM, Tan A, Schreiner CE, Bredt DS. Identification of a monogenic locus (jams1) causing juvenile audiogenic seizures in mice. J Neurosci. 2002;22:10088–10093. doi: 10.1523/JNEUROSCI.22-23-10088.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Neumann PE, Collins RL. Genetic dissection of susceptibility to audiogenic seizures in inbred mice. Proc Natl Acad Sci U S A. 1991;88:5408–5412. doi: 10.1073/pnas.88.12.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mendez M, Lim G. Seizures in elderly patients with dementia: epidemiology and management. Drugs Aging. 2003;20:791–803. doi: 10.2165/00002512-200320110-00001. [DOI] [PubMed] [Google Scholar]
- [34].Musumeci SA, Hagerman RJ, Ferri R, Bosco P, Dalla Bernardina B, Tassinari CA, De Sarro GB, Elia M. Epilepsy and EEG findings in males with fragile X syndrome. Epilepsia. 1999;40:1092–1099. doi: 10.1111/j.1528-1157.1999.tb00824.x. [DOI] [PubMed] [Google Scholar]
- [35].Giovanardi Rossi P, Posar A, Parmeggiani A. Epilepsy in adolescents and young adults with autistic disorder. Brain Dev. 2000;22:102–106. doi: 10.1016/s0387-7604(99)00124-2. [DOI] [PubMed] [Google Scholar]
- [36].Soucy NV, Parkinson HD, Sochaski MA, Borghoff SJ. Kinetics of genistein and its conjugated metabolites in pregnant Sprague-Dawley rats following single and repeated genistein administration. Toxicol Sci. 2006;90:230–240. doi: 10.1093/toxsci/kfj077. [DOI] [PubMed] [Google Scholar]
- [37].American Academy of Pediatrics. Committee on Nutrition American Academy of Pediatrics. Committee on Nutrition. Soy protein-based formulas: recommendations for use in infant feeding. Pediatrics. 1998;101:148–153. [PubMed] [Google Scholar]
- [38].Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet. 1997;350:23–27. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- [39].Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. Am J Clin Nutr. 1998;68:1453S–1461S. doi: 10.1093/ajcn/68.6.1453S. [DOI] [PubMed] [Google Scholar]
- [40].Chen A, Rogan WJ. Isoflavones in soy infant formula: a review of evidence for endocrine and other activity in infants. Annu Rev Nutr. 2004;24:33–54. doi: 10.1146/annurev.nutr.24.101603.064950. [DOI] [PubMed] [Google Scholar]
- [41].Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- [42].Westmark CJ. What’s hAPPening at synapses? The role of amyloid β-protein precursor and β-amyloid in neurological disorders. Molecular Psychiatry. 2012 doi: 10.1038/mp.2012.122. Accepted. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.