Methods and software tools for optimal design in nonlinear mixed effect models, based on the Fisher information matrix, have been developed for a decade.1,2 Academic groups regularly proposed new versions.3–5 Present tools do not incorporate adaptive designs for these models although prior information is needed and adaptive designs are increasingly used in drug development.6 We conducted a study among drug companies of the Drug and Disease Model Resources consortium7 to identify current practices and expectations.
Study on the Use of Optimal Design in Pharmacometrics
In 2011, the Drug and Disease Model Resources (DDMoRe) European consortium was approved as one of the Innovative Medicines Initiative projects of the European Union with the objective of developing a drug–disease model library and an open-source interoperability framework.7 This project associates 9 academic groups, 6 small and medium sized enterprises, and 10 pharmaceutical companies that are members of the European Federation of Pharmaceutical Industries and Associations. One of the work packages within DDMoRe is responsible for the development and integration of new tools, among others also for adaptive optimal designs in pharmacokinetics/pharmacodynamics using nonlinear mixed effect models (NLMEM).
The working group members and authors of this article designed a questionnaire for this study. It was sent to each European Federation of Pharmaceutical Industries and Associations representative within DDMoRe in October 2011. Each representative was then charged with asking one to three scientists within the company to respond to the questionnaire, mostly to those indeed involved in designing pharmacokinetic/pharmacodynamic studies. The detailed questionnaire is available as Supplementary Figure S1 online. Responders first stated how clinical trials were generally designed, by simulations, heuristic approaches, and/or optimal design. The main body of the survey was composed of two parts, part 1: state of the art on the use of optimal design methods in industry and part 2: requests for future developments using adaptive optimal design.
Results
Results were obtained in November 2011 from all the 10 member companies of the DDMoRe consortium (100% response rate): AstraZeneca, GSK, Lilly, Merck Serono, Novartis, Novo Nordisk, Pfizer, Roche, Servier, and UCB Pharma.
Current situation
Part 1 of the study investigated the current situation. The first question showed that optimal design software tools in NLMEM are being used by nearly all companies (9 of 10), mostly during phase I and II for pharmacokinetics/pharmacodynamics, sometimes for biopharmaceutical studies later in development in special populations: pediatric patients, patients with renal or hepatic impairment, and the elderly. All currently available software tools were used among the respondents. Here, a list of the programs and their frequency of usage are given: PFIM (INSERM, University Paris Diderot, 6 of 9), POPED (University of Uppsala, 3 of 9), POPDES (University of Manchester, 3 of 9), and POPT (University of Otago, 3 of 9).
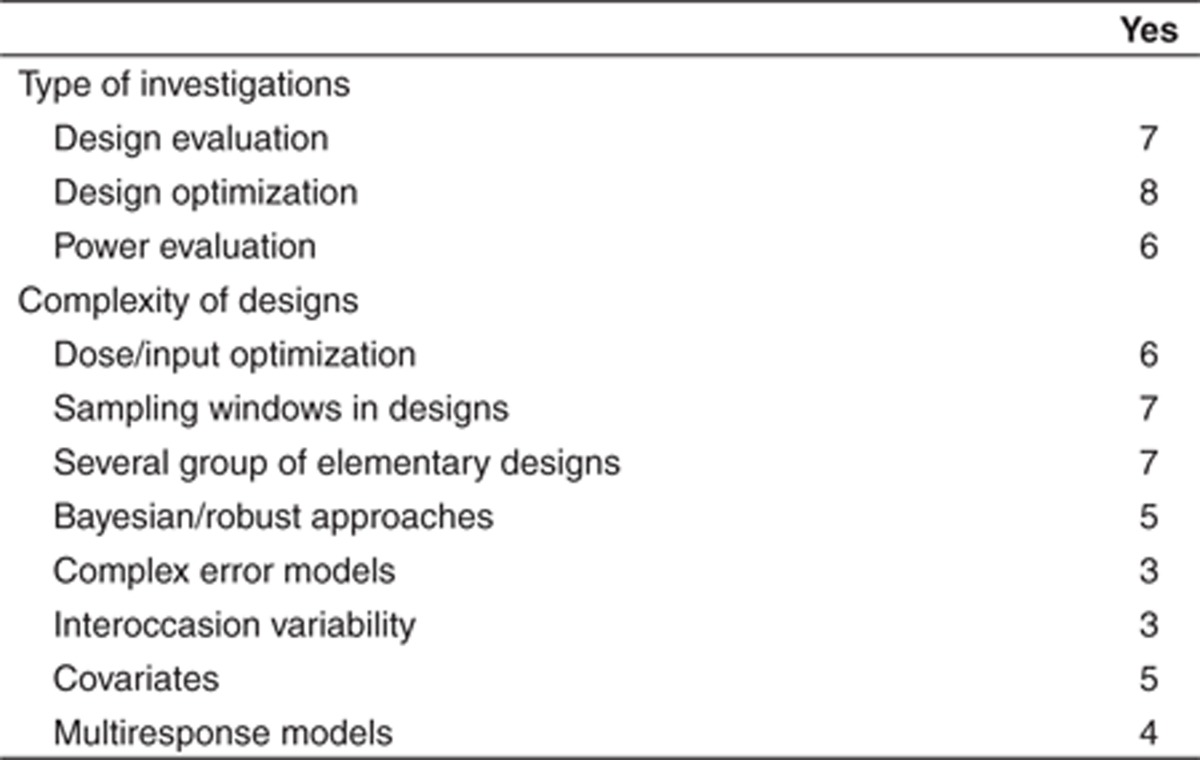
Optimal design approaches are used for a variety of investigations and design complexity (Table 1). Of note, optimal design is often used in early clinical phases (I and II), and less in phase III; one responder suggested that there is a lack of models able to handle complex end points encountered in later phases. Current limitations were expressed in free text that is available in Supplementary Table S1 online. The most common limitation was the need to change software when moving from estimation to design, showing a strong need for more integrative/global approaches/tools. Several companies were concerned about the limited models currently implemented in most optimal design software tools and suggested adding more flexibility. Overall, the perceived impact of optimal design was generally considered quite important, with potentially wider applications suggested in the industry.
Table 1. Current use of optimal design software tools for the n = 9 European Federation of Pharmaceutical Industries and Associations companies, out of the 10 of DDMoRe, presently using this approach.
Adaptive designs and further developments
Adaptive design in NLMEM is of high priority for most companies with a median of 4, on a scale of 0–5, with 4 companies quoting a 5 (very useful). The answers to specific needs were: (i) start from prior information (8 of 9), (ii) design optimization after each new cohort (8 of 9), and (iii) use stopping rules (6 of 9). One company highlighted that adaptive design is not possible in therapeutic areas where end points are attained slowly while recruitment is fast.
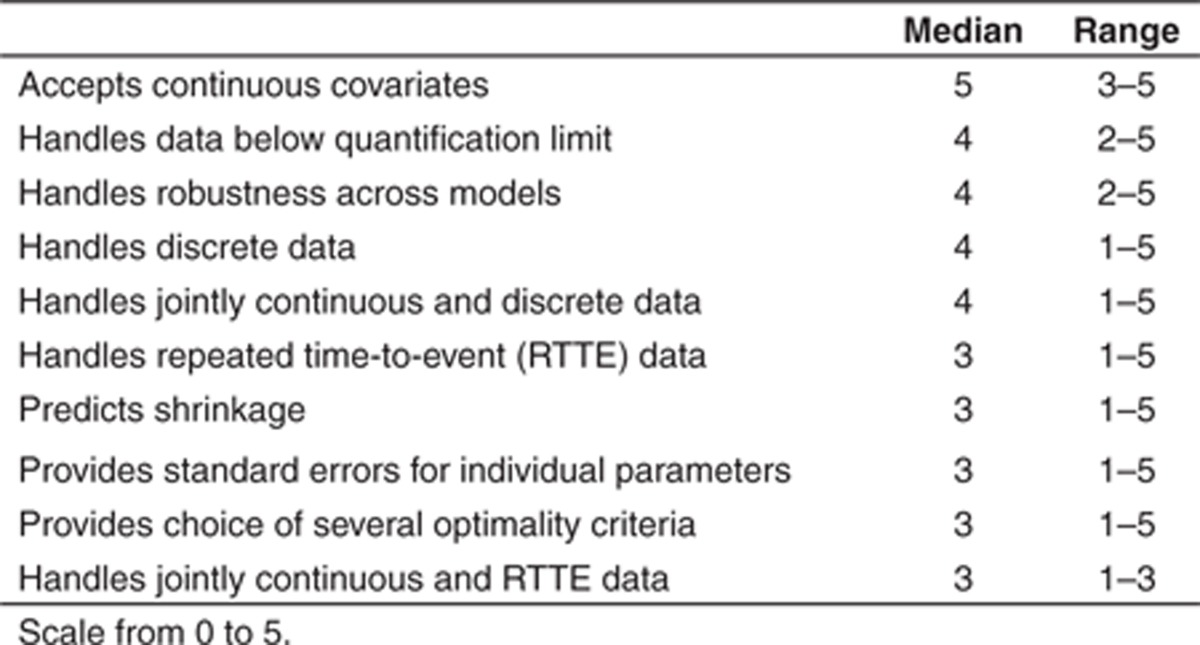
The importance of new developments in design tools was graded on a scale of 0–5. Results are given in Table 2 and show that the priorities are: (i) handling of continuous covariates, (ii) dealing with data below quantification limit, (iii) robustness across models, and (iv) design for discrete outcome data also in combination with continuous data. Additional expectations were expressed in text (Supplementary Table S1 online).
Table 2. Expectations of n = 10 European Federation of Pharmaceutical Industries and Associations companies of DDMoRe regarding the capabilities of a new optimal design software.
Conclusion
This study illustrates that optimal design methodology has been quickly adopted within the industry, especially in early phases where pharmacokinetics/pharmacodynamics is more important. This study further highlights the expected improvements in interoperability between optimal design and estimation software and in statistical capabilities of the optimal design methodology. A smooth workflow among estimation, model evaluation, and design will be facilitated on the DDMoRe platform. It should be noted that we did not perform a comprehensive systematic review of the use of optimal design software tool in NLMEM in drug companies outside of DDMoRe; therefore, the presented results may be biased.
Another outcome of the study is that the high priority was given to further development of adaptive optimal design in NLMEM with optimization not only of sampling times but of other design variables, e.g., doses (Table 1). Initial work on adaptive optimal design in population pharmacokinetics demonstrated its feasibility,8 but the approach has not yet been fully studied nor implemented in any available software. Those developments will only address some of the issues of the complexity of adaptive design in drug development. For instance, adaptive dose-ranging studies analyzed by nonlinear models without random effects are already being optimized.6 Moreover, as pharmacometrics has increased its scope beyond population pharmacokinetics, design tools for more complex models and for other types of data, especially discrete data, are now needed. Academic groups are actively working on those topics and are sharing their results to translate progress into new software tools, but collaborations with statisticians involved in other aspects of the complex issues in adaptive designs are also increasingly needed. Optimal design enriches clinical trial simulation, and both will work in concert to improve model-based drug development in the pharmaceutical industry.
Conflict of interest
As an Associate Editor for CPT:PSP, F.M. was not involved in the review or decision process of this article. The authors declared no conflict of interest.
Acknowledgments
The research leading to these results has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115156, resources of which are composed of financial contributions from the European Union's Seventh Framework Programme (FP7/2007-2013) and European Federation of Pharmaceutical Industries and Associations' companies in kind contribution. The DDMoRe project is also financially supported by contributions from Academic and small and medium enterprise partners.
Supplementary Material
References
- Mentré F, et al. Software for optimal design in population pharmacokinetics and pharmacodynamics: a comparison Annual Meeting of the Population Approach Group in EuropePAGE 16 (2007) Abstract 1179 < < http://www.page-meeting.org/?abstract=1179 >.
- Retout S., Duffull S., Mentré F. Development and implementation of the population Fisher information matrix for the evaluation of population pharmacokinetic designs. Comput. Methods Programs Biomed. 2001;65:141–151. doi: 10.1016/s0169-2607(00)00117-6. [DOI] [PubMed] [Google Scholar]
- Gueorguieva I., Ogungbenro K., Graham G., Glatt S., Aarons L. A program for individual and population optimal design for univariate and multivariate response pharmacokinetic-pharmacodynamic models. Comput. Methods Programs Biomed. 2007;86:51–61. doi: 10.1016/j.cmpb.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Bazzoli C., Retout S., Mentré F. Design evaluation and optimisation in multiple response nonlinear mixed effect models: PFIM 3.0. Comput. Methods Programs Biomed. 2010;98:55–65. doi: 10.1016/j.cmpb.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Nyberg J., Ueckert S., Strömberg E.A., Hennig S., Karlsson M.O., Hooker A.C. PopED: an extended, parallelized, nonlinear mixed effects models optimal design tool. Comput. Methods Programs Biomed. 2012;108:789–805. doi: 10.1016/j.cmpb.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Bornkamp B., et al. Innovative approaches for designing and analyzing adaptive dose-ranging trials. J. Biopharm. Stat. 2007;17:965–995. doi: 10.1080/10543400701643848. [DOI] [PubMed] [Google Scholar]
- Harnisch L., Karlsson M.O.Presentation of DDMoRe Annual Meeting of the Population Approach Group in EuropePAGE 20 (2011) Abstr 2294 < < http://www.page-meeting.org/?abstract=2294 >.
- Foo L.K., Duffull S. Adaptive optimal design for bridging studies with an application to population pharmacokinetic studies. Pharm. Res. 2012;29:1530–1543. doi: 10.1007/s11095-011-0659-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


