Abstract
An increasing prevalence of morbid obesity has led to dramatic increases in the number of bariatric surgeries performed. Altered gastrointestinal physiology following surgery can be associated with modified oral drug bioavailability (Foral). In the absence of clinical data, an indication of changes to Foral via systems pharmacology models would be of value in adjusting dose levels after surgery. A previously developed virtual “post-bariatric surgery” population was evaluated through mimicking clinical investigations on cyclosporine and atorvastatin after bariatric surgery. Cyclosporine simulations displayed a reduced fraction absorbed through gut wall (fa) and Foral after surgery, consistent with reported observations. Simulated atorvastatin Foral postsurgery was broadly reflective of observed data with indications of counteracting interplay between reduced fa and an increased fraction escaping gut wall metabolism (FG). Inability to fully recover observed atorvastatin exposure after biliopancreatic diversion with duodenal switch highlights the current gap regarding the knowledge of associated biological changes.
The prevalence of obesity has increased dramatically in the USA and Europe over the past decade.1,2 Bariatric surgery has proven to be successful in treating morbid obesity with over 220,000 surgeries performed in the USA and Canada in 2008.3 Several bariatric surgical methods coexist in healthcare, where Roux-en-Y gastric bypass (RYGB) is considered the gold standard.4 RYGB results in a reduced gastric volume, complete bypass of the pylorus, partial bypass of the duodenum and proximal jejunum, and a delay in bile inflow to the distal jejunum. The more invasive biliopancreatic diversion with duodenal switch (BPD-DS) results in a partial resection of the stomach with the pylorus retained, bypass of jejunum and proximal ileum, and an approximately 250 cm delay of the bile inlet. Jejunoileal bypass (JIB) is considered to be the most invasive procedure, retaining only the stomach (with pylorus) and distal ileum.5,6
As a consequence of bariatric surgery, a number of physiological parameters influencing oral drug bioavailability (Foral) are altered, including: a reduced gastric capacity and emptying time, altered gastrointestinal (GI) pH, reduced absorption area, altered bile flow and small intestinal transit (SIT), altered substrate exposure to drug-metabolizing enzymes, and active efflux transporters.5,7,8 Patients undergoing bariatric surgery continue to receive various therapeutic drugs without dose adjustments for altered bioavailability, which can potentially lead to no therapeutic effect or higher than required systemic exposure. There are a very limited number of studies that have investigated oral drug exposure post-bariatric surgery.5,9
The direction and magnitude of impact on Foral following surgery may depend on the characteristics and invasiveness of the surgical procedure,10,11 where the extent of the small intestinal bypass may influence the fraction of dose absorbed through the gut wall (fa). Bypassing regions highly abundant in drug-metabolizing enzymes can affect the fraction of absorbed drug FG.6 FH, the fraction that escapes hepatic first-pass metabolism, may be assumed to remain unaltered (Eq. 1).5,7,8
 |
A level of uncertainty remains regarding the SIT postsurgery, where a reduction in motility has been observed, albeit well-powered clinical studies of SIT in man are lacking.6,12,13
The postsurgical physiology is further complicated by the possibility of small intestinal trauma or adaptation, where an enhanced permeability due to impairment of the mucosa or long-term villi elongation has been observed in rat BPD-DS models.14,15 Alterations in the levels of gastric hormones (including: peptide YY, ghrelin, and Glucagon-Like Peptide 1) may lead to redistribution of intestinal blood flow to the submucosa, as observed in dog models. Postsurgical hormonal levels have been observed to vary depending on the bariatric surgical procedure.13,16,17,18
Due to these multifactorial changes, physiologically based pharmacokinetic (PBPK) modeling enables one to predict, in silico, the effects of various bariatric surgeries in a morbidly obese population.6 The Advanced Dissolution Absorption and Metabolism (ADAM) model describes the variability in Foral through a physiologically based seven-segment model of the small intestine, including: duodenum, jejunum I and II, and ileum I–IV. The model describes drug release from formulation, dissolution, precipitation, degradation, absorption, active transport, and metabolism as the drug transits through the small intestine, allowing the incorporation of saturation effects and population variability. The ADAM model has been well described and used in the previous literature.8,19
In our previous work, a virtual “post-bariatric surgery” population was created using the ADAM model within a PBPK system containing characteristics of a morbidly obese population. Developed models for RYGB, BPD-DS, and JIB included specific anatomical and physiological parameters that are altered following surgery, namely: gastric capacity and fluid dynamics, gastric emptying time, small intestinal bypass, GI pH, bile flow, and alterations to regional abundance of drug-metabolizing enzymes (e.g. CYP3A) and efflux transporter P-glycoprotein. The model further incorporated whole body physiological changes, such as postsurgical recovery of renal function as a function of weight loss.6
Simulations predicted change in oral bioavailability of various drugs pre- to post-bariatric surgery, revealing the magnitude and direction of the effect to be surgery dependent, due to altered GI system parameters, and influenced by a complex interplay between drug characteristics, including: solubility, permeability, dissolution, gut wall metabolism, and dose level. However, no comparison so far has been made between the results of these simulations and existing clinical data.6 In the current study, we report on the evaluation of previously developed post-bariatric surgery models by comparing the observed vs. predicted impact of bariatric surgery on oral exposure of cyclosporine and atorvastatin acid using virtual simulations.
Results
Changes in oral drug bioavailability after bariatric surgery were demonstrated for cyclosporine and atorvastatin acid following RYGB, BPD-DS, and JIB.10,11,20,21 Sex-, age-, height-, and weight-matched simulations were carried out based on the corresponding clinical studies using post-bariatric surgery models coupled to a full PBPK distribution model into the Simcyp Simulator (Simcyp Limited (a Certara Company), Sheffield, UK). The results from the comparison of observed vs. simulation studies are as follows:
Cyclosporine
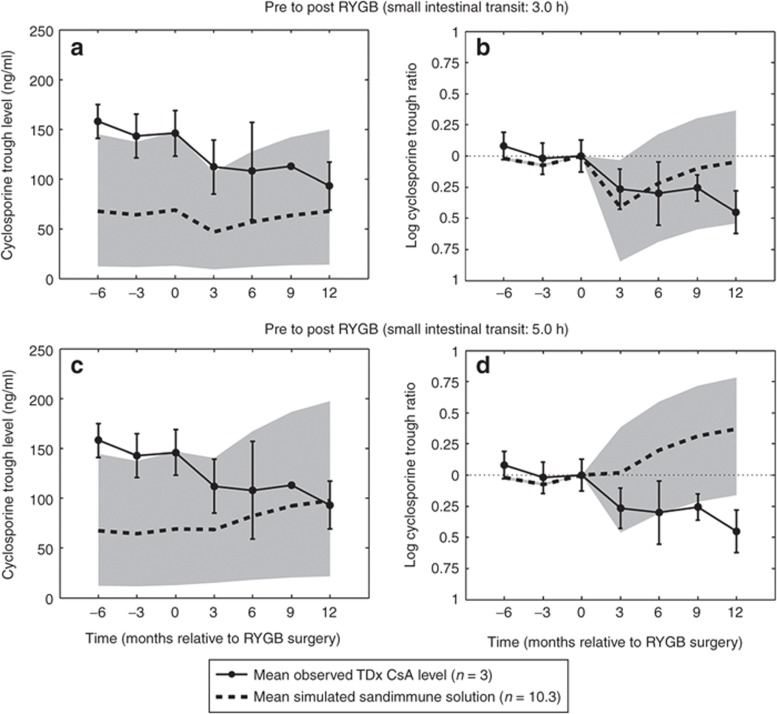
Roux-en-Y gastric bypass. Following RYGB, Marterre and coworkers reported a 194% increase in the daily dose per kg body weight in kidney transplant patients (n = 3) of cyclosporin A (CsA) Sandimmune solution. CsA trough blood levels were monitored from 6 months before RYGB up to 12 months postoperatively, using an immunoassay technique (TDx, Abbott Laboratories). The observed reduction in exposure prompted an increase in the oral dose from 1.8 (±0.5) to 3.5 (±1.1) mg/kg/day in order to maintain pre-RYGB CsA trough levels (20) (Figure 1a–d). Owing to the reported overprediction of the TDx immunoassay, observed and simulated data were normalized for trough levels immediately before RYGB surgery, indicated by the time of 0 months (Figure 1b,c).22
Figure 1.
Mean and SD of observed cyclosporin A (CsA) TDx trough levels at steady state pre to post-Roux-en-Y gastric bypass (RYGB) at −6, −3, 0, 3, 6, 9, and 12 months relative to RYGB surgical event (0 months). Time points from −6 to 12 months correspond to dose levels of 1.7, 1.7, 1.8, 2.4, 2.8, 3.2, 3.5 mg/kg/day, respectively (n = 3) administered twice daily. Observed data are compared with simulated 50, 95, and 5% prediction interval, indicated by gray area, of CsA Sandimmune trough levels (n = 10 × 3). (a) Simulated post-RYGB at a small intestinal transit time (SIT) of 3.0 h, (b) log-normalized simulated CsA trough ratio as compared with 0 months (RYGB SIT = 3.0 h), (c) simulated CsA trough levels at RYGB SIT of 5.0 h, (d) log-normalized simulated CsA trough ratio as compared with 0 months (RYGB SIT = 5.0 h).20
In an age-, sex-, and weight-matched virtual population, oral drug exposure of Sandimmune CsA solution was simulated in 10 randomized trials consisting of 3 individuals in each trial (n = 10·3). Cyclosporine displayed a reduction in fa from 0.40 (5–95% confidence interval (CI95): 0.24–0.59) to 0.19 (0.10–0.32) following RYGB, at a simulated SIT time of 3.0 h, whereas FG remained unaltered at 0.86 (0.74–0.87). A 194% increase in dose level, resulted in a trough level ratio of 0.96 (0.58–1.44) as compared with the preoperative exposure at 0 months. Assuming a postsurgical SIT time of 5.0 h, cyclosporine displayed reduction in fa to 0.26 (0.14–0.41), and FG remained unaltered. A 194% increase in the dose level resulted in an overprediction in post-RYGB CsA trough levels, with a simulated post/presurgical ratio of 1.45 (0.85–2.19) as compared with preoperative exposure at 0 months (Figure 1a–d).
Simulations of the theoretical impact of RYGB on the solubilization enhanced cyclosporine Neoral microemulsion, at a postsurgical SIT of 3.0 h, produced an area under the concentration–time curve (AUC) ratio of 1.84 (1.26–2.37) at 12 months postsurgery as compared with the levels at 0 months relative to RYGB surgery. The observed increase in oral exposure of Neoral was due to a 194% dose increase, where fa displayed an increase from 0.72 (0.47–0.92) to 0.82 (0.57–0.94), whereas FG increased from 0.90 (0.81–0.96) to 0.94 (0.90–0.98) (Supplementary Data online).
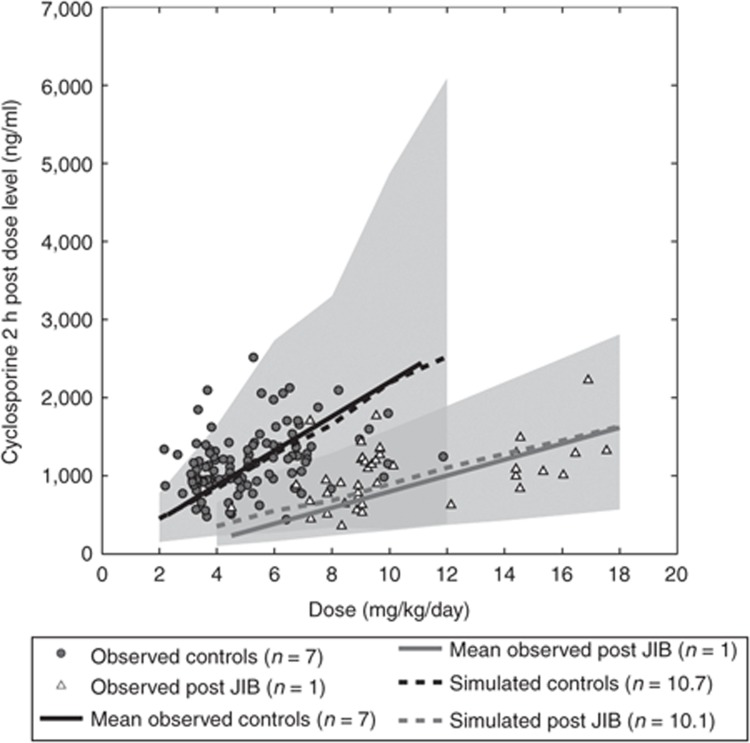
Jejunoileal bypass. In a case study by Chenhsu et al.,21 CsA blood levels at 2 h after administration at steady state (C2), administered as Neoral microemulsion in controls (n = 7) and post-JIB (n = 1), displayed a reduced exposure when comparing the mean C2 concentration over the administered dose range, reporting a reduction in C2 levels of ~59%.
Simulating demographically matched morbidly obese controls (n = 10·7) and post-JIB patients (SIT = 0.4 h; n = 10·1), cyclosporine displayed a reduction in C2 levels from 452 (153–776) to 357 (103–650) ng/ml at a dose level of 4 mg/kg/day and from 2,528 (388–6089) to 1,632 (571–2,811) ng/ml at a dose of 12 mg/kg/day. Simulated levels corresponded well with the linear regression of observed data as compared with the 5–95% prediction interval. The simulated reduction in oral drug exposure in the post-JIB population as compared with the controls was due to a reduction in fa from 0.61 (0.35–0.86) to 0.12 (0.04–0.19), whereas FG displayed a minor reduction from 0.90 (0.79–97) to 0.88 (0.75–0.96) at a therapeutic dose of 2 mg/kg/day. At a dose of 12 mg/kg/day, a major reduction in fa was observed from 0.34 (0.16–0.53) to 0.08 (0.01–0.18), whereas FG was reduced from 0.91 (0.81–0.93) to 0.90 (0.80–0.97) (Figure 2).
Figure 2.
Observed mean blood concentration of cyclosporine microemulsion (Sandimmune Neoral; Novartis) at steady state 2 h postdosing in controls (n = 10 × 7) and one patient (n = 10 × 1) post-jejunoileal bypass (JIB) as compared with simulated sex- and age-matched controls (n = 300) and post-JIB (n = 800) at a small intestinal transit time of 0.4 h over dose range of 300–1,000 mg, where 5, 50, and 95% prediction intervals are indicated by gray areas.21
Assuming a SIT of 0.7 h after JIB, cyclosporine displayed a less apparent reduction in C2 levels due to a less apparent reduction in fa to 0.19 (0.05–0.30) and 0.13 (0.02–0.26) at corresponding dose levels of 2 and 12 mg/kg/day, respectively. After JIB (SIT = 0.7 h), FG was altered to 0.89 (0.79–0.96) and 0.92 (0.84–0.98) at a therapeutic dose level of 2 and 12 mg/kg/day, respectively (Supplementary Data online).
Atorvastatin
Roux-en-Y gastric bypass. In a clinical trial carried out on 12 morbidly obese patients, atorvastatin was administered as an immediate release tablet of 20–80 mg in fasted state where patients were allowed to eat 2 h after administration. Plasma concentration profiles were obtained from 0–8 h after drug administration before and 3–6 weeks after RYGB. The pre to postsurgical trend in oral exposure displayed a high variability where the overall reported trend displayed a median post/pre surgery AUC ratio of 1.12 (range: 0.34–2.33), albeit being statistically insignificant.10
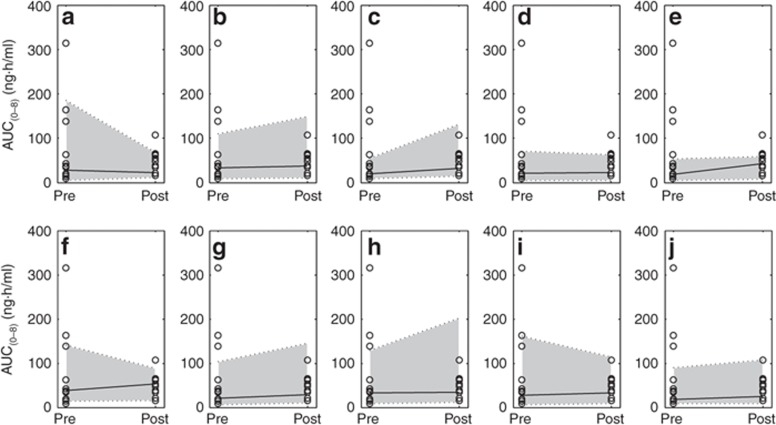
Virtual simulations for oral drug exposure of atorvastatin acid pre- to post-RYGB were conducted in 10 randomized trials, each consisting of 12 age-, sex-, and BMI-matched individuals (n = 10 × 12). Assuming a reduction in SIT as function of the small intestinal bypass (SIT = 3.0 h) resulted in an overall increase in AUC with a simulated median post/presurgical AUC ratio of 1.13, capturing 100% of observed data within the simulated 95% prediction interval of 0.27–3.80 (Figure 3a–j). Alternatively, with an increase in SIT time post-RYGB (SIT = 5.0 h), atorvastatin acid displayed a median post/presurgical AUC ratio of 1.42 (0.34–4.91) (Supplementary Data online).
Figure 3.
Simulated 50, 95, and 5% prediction interval (indicated by gray areas) of oral drug exposure of atorvastatin acid in randomized trials of age-, sex-, dose-, and BMI-matched patients pre- to post-Roux-en-Y gastric bypass surgery (small intestinal transit = 3.0 h) as compared with observed data. (a–j) Ten randomized simulated trials consisting of 12 individuals in each trial (n = 10 × 12), as compared with observed (n = 12; open circles).10 AUC, area under the concentration–time curve.
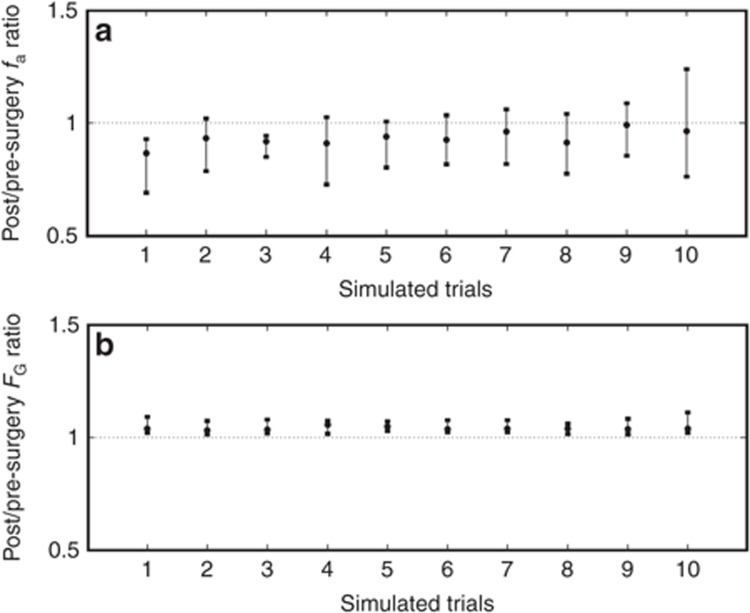
Simulated increase in exposure of atorvastatin following RYGB (SIT = 3.0 h) was due to a reduction in fa from 0.58 (0.33–0.77) to 0.54 (0.29–0.74) counteracted by an increase in FG from 0.69 (0.48–0.86) to 0.73 (0.53–0.88) (Figure 4a,b). Assuming a reduced small intestinal motility (RYGB SIT = 5.0 h), the more apparent increase in AUC was the result of an extensive increase in fa.
Figure 4.
Simulated 50, 95, and 5% prediction intervals of the ratio of (a) fraction of dose absorbed in the intestine (fa), and (b) fraction escaping gut wall metabolism (FG) of atorvastatin acid pre- to post-Roux-en-Y gastric bypass surgery (small intestinal transit = 3.0 h) in 10 individually simulated randomized trials (1–10) consisting of 10 individuals in each pre- and postsurgery (n = 10 × 10).
Biliopancreatic diversion with duodenal switch. Atorvastatin immediate release tablet 20–80 mg administered in the fasted state, allowing feeding at 2 h, displayed a significant increase in oral drug exposure following BPD-DS in 10 morbidly obese patients, with an observed mean AUC ratio of 2.0 (±1.0) and observed increase in the Cmax from 20.0 ng/ml (±24.9) to 28.0 (±22.5), whereas tmax increased from 1.2h (±0.8) to 2.3h (±1.0) post-BPD-DS.11
Predicted plasma concentration–time profiles of atorvastatin acid were consistent with observed data before surgery in a morbidly obese population (Supplementary Data online).
However, simulated plasma concentration–time profiles following BPD-DS (SIT = 1.2 h) were unable to capture the observed increase in oral drug exposure. The predicted AUC ratio of 0.90 (0.16–2.24) was lower than expected due to a reduction in fa from 0.61 (0.34–0.79) to 0.39 (0.06–0.72), which was counteracted by an increase in FG from 0.69 (0.59–0.79) to 0.72 (0.63–0.81). Cmax displayed a minor increase, with a post/pre-BPD-DS Cmax ratio of 1.32 (0.31–2.38), whereas tmax displayed a post/pre-BPD-DS ratio of 0.62 (0.31–1.00). The simulated pre- to post-BPD-DS alterations in AUC, Cmax, and tmax correspond to the central points of Figure 5a–c, respectively.
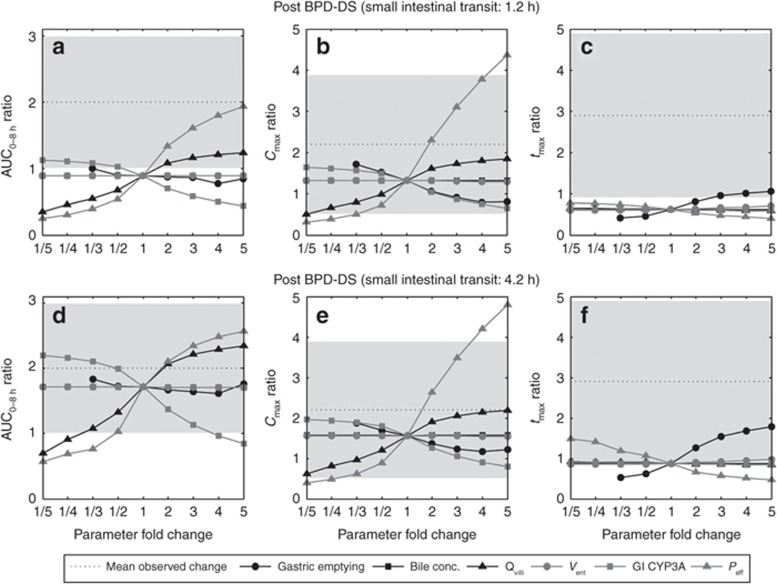
Figure 5.
Spider plot of sensitivity analysis of simulated post/pre-biliopancreatic diversion with duodenal switch (BPD-DS) at a small intestinal transit time (SIT) of 1.2 h; (a) AUC0–8 h, (b) Cmax, and (c) tmax ratio, BPD-DS (SIT = 4.2 h); (d) AUC0–8 h, (e) Cmax, and (f) tmax ratio, examining the impact of the fold change in physiological parameters: gastric emptying, ileal bile concentration (bile conc.), villous blood flow (Qvilli), enterocytic volume (Vent) in the remaining postsurgical small intestine, post-BPD-DS gastrointestinal CYP3A content, small intestinal permeability (Peff), as compared with mean observed ratio pre- to post-BPD-DS.11 AUC, area under the concentration–time curve; Cmax, maximum plasma drug concentration; tmax, time of maximum plasma drug concentration.
Sensitivity analysis was performed to assess the impact of potential physiological alterations postsurgery on the plasma exposure of atorvastatin acid, including the impact of: SIT, gastric emptying time, bile concentration in the terminal ileum, small intestinal enterocyte volume (Vent), GI CYP3A content, and small intestinal permeability (Peff). The AUC was insensitive to changes in the postsurgical bile concentration and Vent. Following BPD-DS (SIT = 1.2 h), a fivefold increase in Peff led to a post/presurgical AUC ratio of 1.93 (0.60–5.53), whereas Cmax displayed a post/presurgical ratio of 4.36 (1.23–9.68). A fivefold increase in Qvilli resulted in a minor increase in AUC with a simulated post/pre-BPD-DS AUC ratio of 1.24 (0.22–3.24) and a Cmax ratio of 1.86 (0.47–4.19). A reduction in GI CYP3A by fivefold gave a post/pre-BPD-DS AUC ratio of 1.13 (0.19–3.06) (Figure 5).
Pre to post-BPD-DS (SIT = 4.2 h) displayed an AUC ratio of 1.71 (0.74–5.79), Cmax ratio of 1.56 (0.69–3.39), and a tmax ratio of 0.87 (0.51–1.31). In the sensitivity analysis, a twofold increase in Qvilli resulted in a post/presurgical AUC ratio of 2.07 (0.86–6.96) and a Cmax ratio of 1.91 (0.84–4.22), whereas tmax remained unaltered. A twofold increase in Peff displayed a post/pre-BPD-DS AUC ratio of 2.11 (0.90–6.91), a Cmax ratio of 2.64 (1.17–5.66), and a minor reduction in tmax. A twofold reduction in GI CYP3A gave a post/presurgical AUC ratio of 1.99 (0.83–6.76), whereas Cmax increased to a minor extent, displaying an AUC ratio of 1.80 (0.79–4.00). Gastric emptying had the most apparent impact on tmax, where a fivefold increase resulted in a post/pre-BPD-DS ratio of 1.78 (1.11–2.58) (Figure 5).
Discussion
Cyclosporine
The immunosuppressant cyclosporine (molecular weight: 1202.61 g/mol) displays poor aqueous solubility and a relatively low permeability due to its lipophilic and bulky character making the compound dependent on bile-mediated solubility to facilitate absorption.23,24 The drug is mainly metabolized by CYP3A4 in the intestine and liver and is subject to P-glycoprotein efflux.25,26 The oral bioavailability displays a high interindividual variability, ranging from 5 to 89% for Sandimmune formulation, whereas Neoral displays improved absorption properties and an oral bioavailability of 21–73%.27
The simulated underprediction of Sandimmune TDx trough levels before RYGB surgery was expected and is most likely due to the unspecificity of the immunoassay displaying a high cross-reactivity between the parent compound and metabolites resulting in a significantly higher variability in the trough levels as compared with peak concentrations.20,22
Normalizing simulated cyclosporine trough levels to pre-RYGB levels produced a reduction in exposure comparable to observed data. Furthermore, a simulated 194% increase in Sandimmune dose levels recovered presurgical trough levels as stated in the publication.20
In the simulation study of cyclosporine Neoral, microemulsion pre to post-JIB observed data of the control population was well described within the 95% prediction interval of the simulated data, whereas the observed exposure post-JIB was well described within the 95% prediction interval of simulated data assuming a reduction in SIT time equivalent to the bypass (SIT = 0.4 h). An alternative “what-if” scenario simulating a longer transit time (SIT = 0.7 h) over predicted the observed mean blood concentration post-JIB as reported by Chenhsu et al. (Figure 2). The overprediction could suggest small intestinal motility to remain unaltered following JIB or be a result of a small postsurgical study population (n = 1), displaying a low study power, albeit the simulated magnitude of reduction in C2 levels post-JIB (SIT = 0.4 h) closely matched the estimated reduction in cyclosporine exposure following JIB in another case study where a patient was subject to a surgical reversal of the procedure. The reversed JIB produced an observed 2.78-fold increase in exposure in the case study.21,28
The simulated discrepancy in oral drug exposure of Sandimmune and Neoral pre to post-RYGB, where bariatric surgery had the highest effect on the oral bioavailability of Sandimmune, highlights the importance of formulation characteristics and its impact on oral drug bioavailability pre- to post-bariatric surgery. The choice of a solubilized biopharmaceutical formulation such as a self-microemulsifying drug delivery system, solution, or dispersible tablet may be considered as a first-hand choice for patients undergoing bariatric surgery and are treated with limited solubility drugs with a narrow therapeutic index. This suggestion supports earlier observations in clinical practice, where alterations in pharmacotherapy post-bariatric surgery aim at switching to formulations displaying improved dissolution properties.5
Atorvastatin
The HMG-CoA reductase inhibitor atorvastatin, which is administered in the acid form, displays a high solubility and permeability. The compound is extensively metabolized in the small intestine and liver, namely by CYP3A4 but also via the UGT1A1 and UGT1A3 route. Atorvastatin acid and the lactone metabolite are also subject to interconversion by the UGTs and another minor chemical pathway. Atorvastatin acid is a substrate of P-glycoprotein efflux and OATP1B1-mediated active hepatic uptake.29
Trends in simulated oral drug exposure of atorvastatin pre to post-RYGB were consistent with observed data, where simulated trends in fa and FG suggested an interplay between reduced absorption area and bypass of regions highly abundant in CYP3A to be of high importance when considering the overall effect on oral bioavailability. Simulated RYGB (SIT = 3.0 h) produced the closest agreement with observed data of atorvastatin exposure following RYGB, again suggesting a reduction in SIT time to be the most likely consequence of surgery.10
The inability to recover the observed twofold increase in atorvastatin AUC pre to post-BPD-DS at a simulated SIT of 1.2 h may suggest additional postsurgical physiological parameters to be governing the trend in oral drug bioavailability post-BPD-DS. The exploratory sensitivity analysis identified a number of potential parameters leading to a comparable increase in oral drug exposure pre to post-BPD-DS. An increase in SIT following BPD-DS, corresponding to the linear regression relationship between mouth to cecum transit time and plasma levels of peptide YY, resulted in an AUC ratio of 1.71 (0.74–5.79). Furthermore, a simulated twofold increase in Peff and Qvilli, or a twofold reduction in the GI content of CYP3A post-BPD-DS (SIT = 4.2 h) recovered the observed AUC of atorvastatin. The reoccurring underprediction of tmax following surgery may be explained by an altered postprandial response causing a delayed absorption.
These findings suggest that additional physiological parameters, such as impairment in permeability or redistribution of intestinal blood flow, may play an important role in governing trends in oral drug exposure pre to immediately post-BPD-DS. The results highlight the surgery-specific trends observed post-bariatric surgery due to the intricate interplay between fluid dynamics, absorption area, transporters, metabolism, and GI physiology.
Current limitations in simulating the impact of oral drug bioavailability following bariatric surgery include the lack of clinical and postsurgical physiological data. Nonetheless, the models integrate all available knowledge on changes known to occur in the GI tract following bariatric surgery and can assist with dosage recommendation when there is an absence of clinical observations.
Conclusions
In this work, we demonstrated the potential of a PBPK and simulation to predict oral drug bioavailability post-bariatric surgery by evaluating earlier developed models using observed data for cyclosporine and atorvastatin. Trends in oral drug exposure of atorvastatin and cyclosporine were predicted well within the 95% prediction interval following RYGB using the previously developed model at a SIT time of 3.0 h. The results suggest a reduction in SIT time to be the most likely scenario following RYGB. The observed increase in atorvastatin exposure following BPD-DS could not be captured using the developed BPD-DS model incorporating all known physiological alterations.
A mechanistic PBPK modeling approach has the potential to serve as a tool in examining the impact of physiological alterations on oral drug bioavailability in the absence of clinical data. The demonstrated approach may allow a framework for optimization of oral drug therapy post-bariatric surgery.
Methods
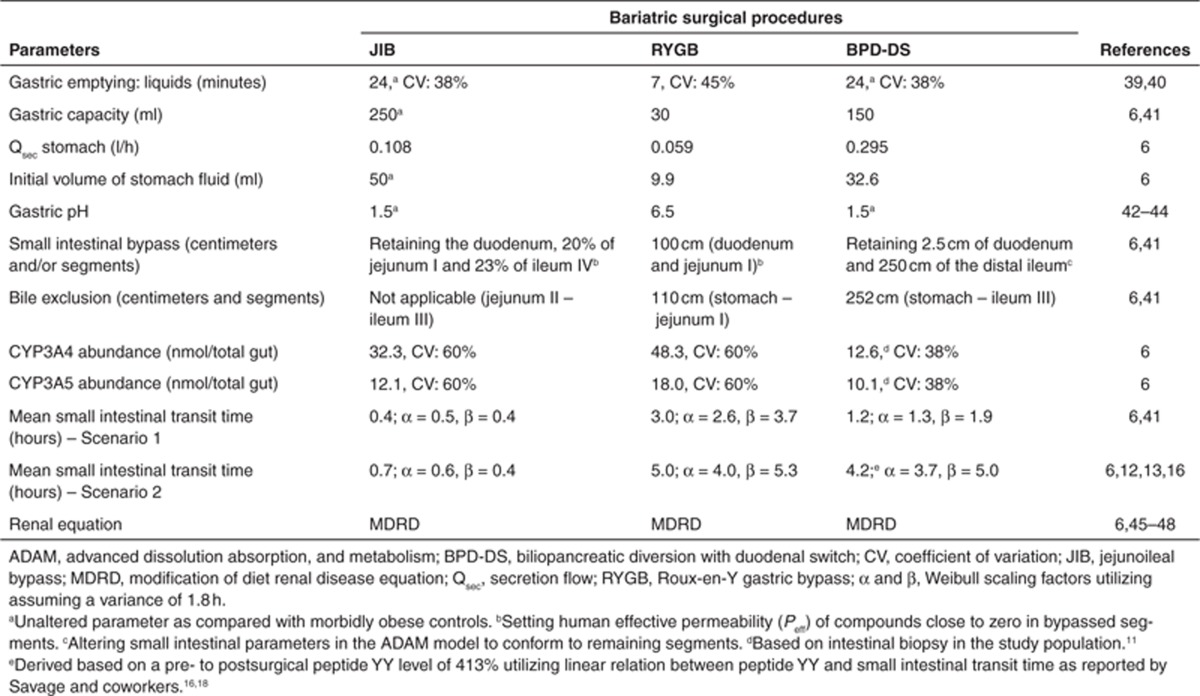
Bariatric surgery model. Sex-, age-, height-, and weight-matched simulations were carried out corresponding to identified clinical studies10,11,20,21 using the ADAM model coupled to a full PBPK distribution model, incorporated into the Simcyp Simulator (Simcyp, Sheffield, UK) (Eqs. 2–4).8,30 Detailed specification for the demographics and physiological parameters of the morbidly obese population have been published previously.7 Similarly, surgical changes to the anatomy and physiology of GI tract following bariatric surgeries, including: RYGB, BPD-DS, and JIB are defined in an earlier publication.6 Study population–specific surgical alterations are summarized in Table 1.
Table 1. Summary of alterations to population template in order to mimic and simulate postsurgical condition as per Darwich et al.6.
 |
 |
 |
Cyclosporine. The cyclosporine compound file, available in the Simcyp Simulator compound library, was adapted to account for formulation properties corresponding to Sandimmune solution and Sandimmune Neoral microemulsion (Novartis, East Hanover, NJ), using an aqueous solubility of 0.01 mg/ml and particle sizes of 3.73 and 0.03 μm, respectively, further allowing Neoral to supersaturate freely without precipitation assuming a linear dose–concentration relationship.31 A full PBPK model was used to describe the cyclosporine distribution, where tissue to plasma partition coefficients (Kps) were obtained from in vivo tissue to plasma concentrations at steady state following intravenous infusion in rat.32
Atorvastatin acid. Physicochemical parameters for atorvastatin acid were taken from the publication by Lennernäs.29 Metabolic data were obtained from the in vitro study reported by Jacobsen et al.;33 CYP3A4 was found to be the main enzyme involved in the formation of the two primary metabolites ortho- and para-hydroxyatorvastatin acid with a minor contribution from CYP2C8. Intersystem extrapolation factors, which correct for differences in intrinsic activity per unit CYP enzyme relative to its native environment, were applied to the kinetic data for recombinantly expressed CYP3A4 and CYP2C8, respectively. Acyl glucuronidation of atorvastatin acid to the lactone and UDPGA-dependent metabolism of atorvastatin acid mainly via UGT1A1 to a minor ether glucuronide was also considered.34,35 The resultant intrinsic clearance data were scaled to whole-organ values according to Eqs. 5 and 6.36 The uptake of atorvastatin acid has been demonstrated in an OATP1B1-transfected cell system (HEK293 cells).37,38 Although these in vitro data support the involvement of OATP1B1 in the hepatic uptake of atorvastatin acid, it was found that when used in combination with the metabolic data, the clearance was significantly underpredicted. To recover the plasma concentration time profile of atorvastatin acid before BPD-DS, Jmax,OATP1B1 and Kpmuscle were reestimated to 532.4 pmol/min/million cells and 4.0, using weighted least-square Nelder–Mead minimization method in the parameter estimation toolbox within the Simcyp Simulator.11
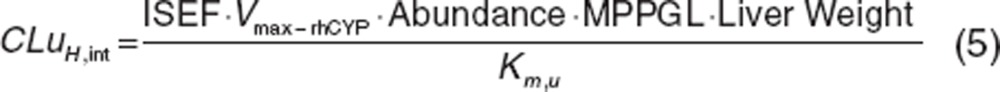 |
 |
Sensitivity analysis was carried out with regard to potential GI physiological parameters subject to potential alterations following surgery due to hormonal alterations, including: SIT time, villous blood flow (Qvilli), gastric emptying, small intestinal bile concentration, the enterocyte volume in the remaining small intestine (Vent), postsurgical abundance of GI CYP3A (GI CYP3A), and small intestinal effective permeability (Peff).
Analysis. Simulated data were visually inspected against observed data. In addition, the potential mechanism of changes to oral drug absorption was examined through the simulations in terms of assessing the effects on plasma drug concentration–time profile, maximum plasma drug concentration (Cmax), time of maximum plasma drug concentration (tmax), fa, and FG.
Author contributions
A.R.-H., A.S.D., D.P., K.R.-Y., M.J., A.Å., H.C., and D.M.A. wrote the manuscript. A.R.-H., A.S.D., D.P., and D.M.A. designed research. A.R.-H., A.S.D., D.P., and D.M.A. performed research. A.S.D. analyzed data.
Conflict of interest
D.P., K.R.-Y., and M.J. are employees in Simcyp Limited (a Certara Company). A.R.-H. is an employee of the University of Manchester and part-time secondee to Simcyp Limited (a Certara Company). The other authors declared no conflict of interest.
Study Highlights

Acknowledgments
The authors thank Basil Ammori, Salford Royal Hospital NHS Foundation Trust and David Turner, Simcyp (a Certara company) for discussions leading up to this work and James Kay for his assistance with the manuscript. Associate editor, A.R.-H. was not involved in the review or decision process for this paper. Simcyp's research is funded by a consortium of pharma companies.
Supplementary Material
References
- Flegal K.M., Carroll M.D., Ogden C.L., Curtin L.R. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Organisation for Economics Co-Operation and Development (OECD) Obesity and the Economics of Prevention: Fit not Fat - United Kingdom (England) Key Facts . < http://www.oecd.org/document/58/0,3746,en_2649_33929 _46039034_1_1_1_1,00.html#Further_Reading > ( 2011Accessed December 2012 [Google Scholar]
- Buchwald H., Oien D.M. Metabolic/bariatric surgery Worldwide 2008. Obes. Surg. 2009;19:1605–1611. doi: 10.1007/s11695-009-0014-5. [DOI] [PubMed] [Google Scholar]
- Picot J., et al. The clinical effectiveness and costeffectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol. Assess. 2009;13:215–357. doi: 10.3310/hta13410. [DOI] [PubMed] [Google Scholar]
- Darwich A.S., et al. Trends in oral drug bioavailability following bariatric surgery: examining the variable extent of impact on exposure of different drug classes. Br. J. Clin. Pharmacol. 2012;74:774–787. doi: 10.1111/j.1365-2125.2012.04284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwich A.S., Pade D., Ammori B.J., Jamei M., Ashcroft D.M., Rostami-Hodjegan A. A mechanistic pharmacokinetic model to assess modified oral drug bioavailability post bariatric surgery in morbidly obese patients: interplay between CYP3A gut wall metabolism, permeability and dissolution. J. Pharm. Pharmacol. 2012;64:1008–1024. doi: 10.1111/j.2042-7158.2012.01538.x. [DOI] [PubMed] [Google Scholar]
- Ghobadi C., et al. Application of a systems approach to the bottom-up assessment of pharmacokinetics in obese patients: expected variations in clearance. Clin. Pharmacokinet. 2011;50:809–822. doi: 10.2165/11594420-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Jamei M., et al. Population-based mechanistic prediction of oral drug absorption. AAPS J. 2009;11:225–237. doi: 10.1208/s12248-009-9099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone M., Alger-Mayer S.A. Medication use patterns after gastric bypass surgery for weight management. Ann. Pharmacother. 2005;39:637–642. doi: 10.1345/aph.1E393. [DOI] [PubMed] [Google Scholar]
- Skottheim I.B., et al. Significantly altered systemic exposure to atorvastatin acid following gastric bypass surgery in morbidly obese patients. Clin. Pharmacol. Ther. 2009;86:311–318. doi: 10.1038/clpt.2009.82. [DOI] [PubMed] [Google Scholar]
- Skottheim I.B., et al. Significant increase in systemic exposure of atorvastatin after biliopancreatic diversion with duodenal switch. Clin. Pharmacol. Ther. 2010;87:699–705. doi: 10.1038/clpt.2010.32. [DOI] [PubMed] [Google Scholar]
- Pellegrini C.A., Deveney C.W., Patti M.G., Lewin M., Way L.W. Intestinal transit of food after total gastrectomy and Roux-Y esophagojejunostomy. Am. J. Surg. 1986;151:117–125. doi: 10.1016/0002-9610(86)90021-8. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Ramos E.J., Goncalves C.G., Chen C., Meguid M.M. Changes in GI hormones and their effect on gastric emptying and transit times after Roux-en-Y gastric bypass in rat model. Surgery. 2005;138:283–290. doi: 10.1016/j.surg.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Gaggiotti G., et al. Modifications of intestinal permeability test induced by biliopancreatic diversion: preliminary results. Obes. Surg. 1995;5:424–426. doi: 10.1381/096089295765557539. [DOI] [PubMed] [Google Scholar]
- Mendieta-Zerón H., et al. Biliopancreatic diversion induces villi elongation and cholecystokinin and ghrelin increase. Diabetes Metab. Syndr. 2011;5:66–70. doi: 10.1016/j.dsx.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Savage A.P., Adrian T.E., Carolan G., Chatterjee V.K., Bloom S.R. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut. 1987;28:166–170. doi: 10.1136/gut.28.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell M.G., Harding R.K. Effects of peptide YY on intestinal blood flow distribution and motility in the dog. Regul. Pept. 1989;24:195–208. doi: 10.1016/0167-0115(89)90238-3. [DOI] [PubMed] [Google Scholar]
- Garcia-Fuentes E., et al. Different effect of laparoscopic Roux-en-Y gastric bypass and open biliopancreatic diversion of Scopinaro on serum PYY and ghrelin levels. Obes. Surg. 2008;18:1424–1429. doi: 10.1007/s11695-008-9560-5. [DOI] [PubMed] [Google Scholar]
- Darwich A.S., Neuhoff S., Jamei M., Rostami-Hodjegan A. Interplay of metabolism and transport in determining oral drug absorption and gut wall metabolism: a simulation assessment using the “Advanced Dissolution, Absorption, Metabolism (ADAM)” model. Curr. Drug Metab. 2010;11:716–729. doi: 10.2174/138920010794328913. [DOI] [PubMed] [Google Scholar]
- Marterre W.F., Hariharan S., First M.R., Alexander J.W. Gastric bypass in morbidly obese kidney transplant recipients. Clin. Transplant. 1996;10:414–419. [PubMed] [Google Scholar]
- Chenhsu R.Y., Wu Y., Katz D., Rayhill S. Dose-adjusted cyclosporine c2 in a patient with jejunoileal bypass as compared to seven other liver transplant recipients. Ther. Drug Monit. 2003;25:665–670. doi: 10.1097/00007691-200312000-00004. [DOI] [PubMed] [Google Scholar]
- Steimer W. Performance and specificity of monoclonal immunoassays for cyclosporine monitoring: how specific is specific. Clin. Chem. 1999;45:371–381. [PubMed] [Google Scholar]
- Venkataramanan R., et al. Biliary excretion of cyclosporine in liver transplant patients. Transplant. Proc. 1985;17:286–289. [PMC free article] [PubMed] [Google Scholar]
- Mehta M.U., et al. Effect of bile on cyclosporin absorption in liver transplant patients. Br. J. Clin. Pharmacol. 1988;25:579–584. doi: 10.1111/j.1365-2125.1988.tb03348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wójcikowski J., Pichard-Garcia L., Maurel P., Daniel W.A. Contribution of human cytochrome p-450 isoforms to the metabolism of the simplest phenothiazine neuroleptic promazine. Br. J. Pharmacol. 2003;138:1465–1474. doi: 10.1038/sj.bjp.0705195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lown K.S., et al. Role of intestinal P-glycoprotein (mdr1) in interpatient variation in the oral bioavailability of cyclosporine. Clin. Pharmacol. Ther. 1997;62:248–260. doi: 10.1016/S0009-9236(97)90027-8. [DOI] [PubMed] [Google Scholar]
- Paine M.F., Davis C.L., Shen D.D., Marsh C.L., Raisys V.A., Thummel K.E. Can oral midazolam predict oral cyclosporine disposition. Eur. J. Pharm. Sci. 2000;12:51–62. doi: 10.1016/s0928-0987(00)00139-1. [DOI] [PubMed] [Google Scholar]
- Knight G.C., Macris M.P., Peric M., Duncan J.M., Frazier O.H., Cooley D.A. Cyclosporine A pharmacokinetics in a cardiac allograft recipient with a jejuno-ileal bypass. Transplant. Proc. 1988;20:351–355. [PubMed] [Google Scholar]
- Lennernäs H. Clinical pharmacokinetics of atorvastatin. Clin. Pharmacokinet. 2003;42:1141–1160. doi: 10.2165/00003088-200342130-00005. [DOI] [PubMed] [Google Scholar]
- Jamei M., Marciniak S., Feng K., Barnett A., Tucker G., Rostami-Hodjegan A. The Simcyp population-based ADME simulator. Expert Opin. Drug Metab. Toxicol. 2009;5:211–223. doi: 10.1517/17425250802691074. [DOI] [PubMed] [Google Scholar]
- Mueller E.A., Kovarik J.M., van Bree J.B., Tetzloff W., Grevel J., Kutz K. Improved dose linearity of cyclosporine pharmacokinetics from a microemulsion formulation. Pharm. Res. 1994;11:301–304. doi: 10.1023/a:1018923912135. [DOI] [PubMed] [Google Scholar]
- Bernareggi A., Rowland M. Physiologic modeling of cyclosporin kinetics in rat and man. J. Pharmacokinet. Biopharm. 1991;19:21–50. doi: 10.1007/BF01062191. [DOI] [PubMed] [Google Scholar]
- Jacobsen W., et al. Lactonization is the critical first step in the disposition of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor atorvastatin. Drug Metab. Dispos. 2000;28:1369–1378. [PubMed] [Google Scholar]
- Goosen T.C., et al. Atorvastatin glucuronidation is minimally and nonselectively inhibited by the fibrates gemfibrozil, fenofibrate, and fenofibric acid. Drug Metab. Dispos. 2007;35:1315–1324. doi: 10.1124/dmd.107.015230. [DOI] [PubMed] [Google Scholar]
- Prueksaritanont T., Tang C., Qiu Y., Mu L., Subramanian R., Lin J.H. Effects of fibrates on metabolism of statins in human hepatocytes. Drug Metab. Dispos. 2002;30:1280–1287. doi: 10.1124/dmd.30.11.1280. [DOI] [PubMed] [Google Scholar]
- Howgate E.M., Rowland Yeo K., Proctor N.J., Tucker G.T., Rostami-Hodjegan A. Prediction of in vivo drug clearance from in vitro data. I: impact of inter-individual variability. Xenobiotica. 2006;36:473–497. doi: 10.1080/00498250600683197. [DOI] [PubMed] [Google Scholar]
- Lau Y.Y., Huang Y., Frassetto L., Benet L.Z. effect of OATP1B transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin. Pharmacol. Ther. 2007;81:194–204. doi: 10.1038/sj.clpt.6100038. [DOI] [PubMed] [Google Scholar]
- Amundsen R., Christensen H., Zabihyan B., Asberg A. Cyclosporine A, but not tacrolimus, shows relevant inhibition of organic anion-transporting protein 1B1-mediated transport of atorvastatin. Drug Metab. Dispos. 2010;38:1499–1504. doi: 10.1124/dmd.110.032268. [DOI] [PubMed] [Google Scholar]
- Hedberg J., Hedenström H., Karlsson F.A., Edén-Engström B., Sundbom M. Gastric emptying and postprandial PYY response after biliopancreatic diversion with duodenal switch. Obes. Surg. 2011;21:609–615. doi: 10.1007/s11695-010-0288-7. [DOI] [PubMed] [Google Scholar]
- Horowitz M., et al. Measurement of gastric emptying after gastric bypass surgery using radionuclides. Br. J. Surg. 1982;69:655–657. doi: 10.1002/bjs.1800691108. [DOI] [PubMed] [Google Scholar]
- Wittgrove A.C., Clark G.W. Laparoscopic gastric bypass, Roux-en-Y- 500 patients: technique and results, with 3-60 month follow-up. Obes. Surg. 2000;10:233–239. doi: 10.1381/096089200321643511. [DOI] [PubMed] [Google Scholar]
- Smith C.D., Herkes S.B., Behrns K.E., Fairbanks V.F., Kelly K.A., Sarr M.G. Gastric acid secretion and vitamin B12 absorption after vertical Roux-en-Y gastric bypass for morbid obesity. Ann. Surg. 1993;218:91–96. doi: 10.1097/00000658-199307000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrns K.E., Smith C.D., Sarr M.G. Prospective evaluation of gastric acid secretion and cobalamin absorption following gastric bypass for clinically severe obesity. Dig. Dis. Sci. 1994;39:315–320. doi: 10.1007/BF02090203. [DOI] [PubMed] [Google Scholar]
- Hedberg J., Hedenstrom H., Sundbom M. Wireless pH-metry at the gastrojejunostomy after Roux-en-Y gastric bypass: a novel use of the BRAVO system. Surg. Endosc. 2005;25:2302–2307. doi: 10.1007/s00464-010-1553-5. [DOI] [PubMed] [Google Scholar]
- Saliba J., et al. Roux-en-Y gastric bypass reverses renal glomerular but not tubular abnormalities in excessively obese diabetics. Surgery. 2010;147:282–287. doi: 10.1016/j.surg.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnac A., Weinstein T., Herman M., Hirsh J., Gafter U., Ori Y. The effects of weight loss on renal function in patients with severe obesity. J. Am. Soc. Nephrol. 2003;14:1480–1486. doi: 10.1097/01.asn.0000068462.38661.89. [DOI] [PubMed] [Google Scholar]
- Navarro-Díaz M., et al. Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. J. Am. Soc. Nephrol. 2006;17:S213–S217. doi: 10.1681/ASN.2006080917. [DOI] [PubMed] [Google Scholar]
- Serra A., et al. The effect of bariatric surgery on adipocytokines, renal parameters and other cardiovascular risk factors in severe and very severe obesity: 1-year follow-up. Clin. Nutr. 2006;25:400–408. doi: 10.1016/j.clnu.2005.11.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.