Abstract
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used; however, they are also nephrotoxic with both acute and chronic effects on kidney function. Here we determined NSAID prescribing before and after estimated GFR (eGFR) reporting and evaluate renal function in patients who used NSAIDs but stopped these after their first eGFR report. A population-based longitudinal analysis using a record-linkage database was conducted with the GFR estimated using the four-variable equation from the MDRD study and analyzed by trend test, paired t-test, and logistic regression modeling. Prescriptions for NSAIDs significantly decreased from 39,459 to 35,415 after implementation of eGFR reporting from the second quarter of 2005 compared with the first quarter of 2007. Reporting eGFR was associated with reduced NSAID prescriptions (adjusted odds ratio, 0.78). NSAID prescription rates in the 6 months before April 2006 were 18.8, 15.4, and 7.0% in patients with CKD stages 3, 4, and 5 and 15.5, 10.7, and 6.3%, respectively, after eGFR reporting commenced. In patients who stopped NSAID treatment, eGFR significantly increased from 45.9 to 46.9, 23.9 to 27.1, and 12.4 to 26.4 ml/min per 1.73 m2 in 1340 stage 3 patients, 162 stage 4 patients, and 9 stage 5 patients, respectively. Thus, NSAID prescribing decreased after the implementation of eGFR reporting, and there were significant improvements in estimated renal function in patients who stopped taking NSAIDs. Hence, eGFR reporting may result in safer prescribing.
Keywords: eGFR, NSAIDs, prescribing rate, renal function
Chronic kidney disease (CKD) is a worldwide public health problem with an increasing incidence and prevalence, particularly in elderly populations.1, 2, 3, 4 Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used in elderly populations. They are also nephrotoxic agents with both acute and chronic effects on kidney function.5 Previous studies have shown that NSAIDs are associated with a decrease in kidney function.6, 7, 8 Data from Scotland have shown a decrease in the use of NSAIDs over the period 2004–2008.9 In April 2006, the Scottish Renal Registry and the National Service Framework (NSF) recommended that reporting of creatinine measurements should be accompanied by an estimated glomerular filtration rate (eGFR). The aims of this study were to determine NSAID prescribing before and after the implementation of eGFR reporting and to evaluate renal function in patients who used NSAIDs but stopped these after eGFR reporting was implemented.
RESULTS
NSAID prescribing rates during the two time intervals
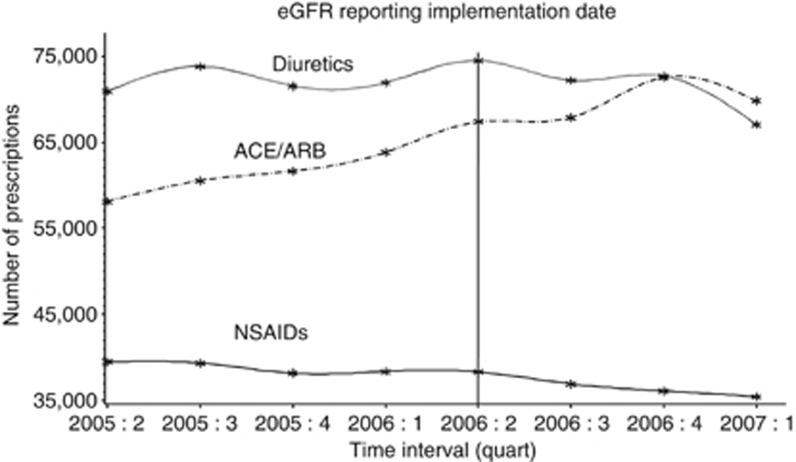
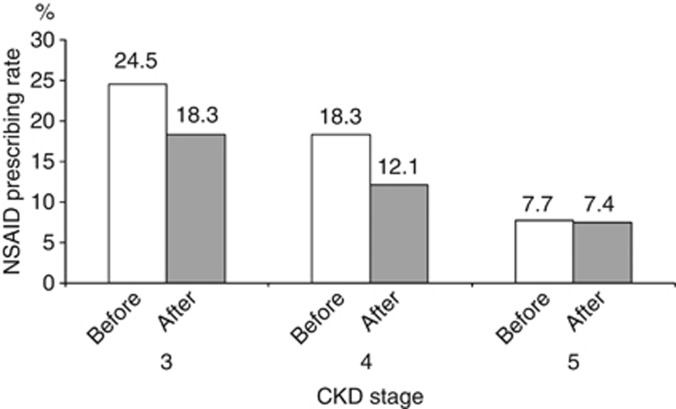
Prescriptions of NSAIDs decreased after the implementation of eGFR reporting (39,459 in the second quarter of 2005 vs. 35,415 in the first quarter of 2007, P<0.01; Figure 1). NSAID prescribing rates in patients with CKD stages 3, 4, and 5 were 24.5% (7746/31,600), 18.3% (257/1406), and 7.7% (20/259) in the year before April 2006 and 18.3% (5052/27,474), 12.1% (196/1625), and 7.4% (26/352) in the year after eGFR reporting commenced (Figure 2). The corresponding figures for NSAID prescribing 6 months on either side of 1 April 2006 were 18.8, 15.4, and 7.0% (before eGFR reporting) and 15.5, 10.7, and 6.3% (after eGFR reporting), respectively.
Figure 1.
Frequency of nonsteroidal anti-inflammatory drugs (NSAIDs), angiotensin-converting enzyme/angiotensin receptor blocker (ACE/ARB) inhibitors, and diuretics prescriptions in Tayside between 2005 and 2007. quart, quarter.
Figure 2.
Nonsteroidal anti-inflammatory drug (NSAID) prescribing rate in the 1 year on either side of the implementation date of 1 April 2006. CKD, chronic kidney disease.
Examining the changes in renal function in patients who used NSAIDs
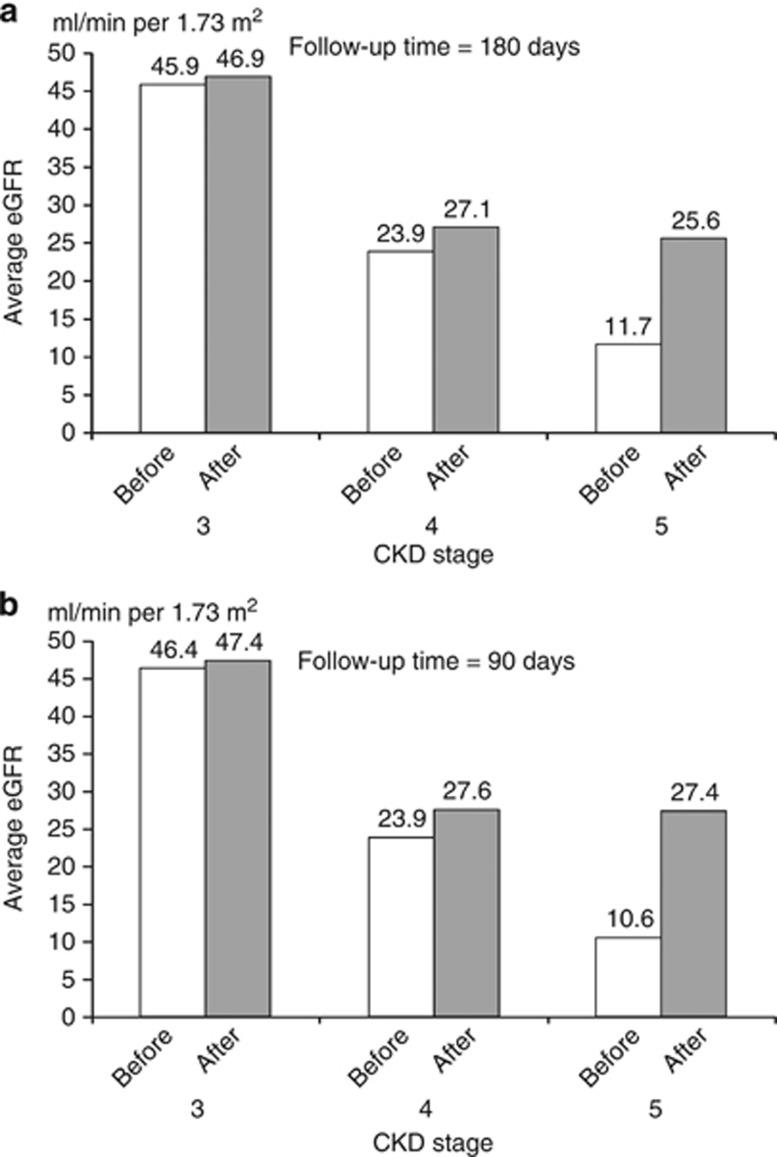
A total of 1522 patients had two reported eGFRs with a time interval of >180 days and had NSAID prescriptions recorded before eGFR reporting but stopped after the first reported eGFR measurement. They were all in stages 3, 4, and 5 (1340, 162, and 20 patients in stages 3, 4, and 5, respectively). Table 1 shows the characteristics of patients by CKD stage. Patients in stage 5 were significantly younger than patients in stages 3 and 4. There were no differences in gender, socioeconomic status, type of NSAIDs used, and diabetes history between the patients in the different CKD stages. Medical notes were reviewed for patients in the stage 5 group. Of the 20 patients studied, 11 were on dialysis and their results were excluded from the analysis in Figure 3a. The average eGFR in each stage (Figure 3a) was improved significantly in all three groups, with the largest improvement in stage 5 patients. eGFR increased from 45.9 to 46.9 ml/min per 1.73 m2 (n=1340, P<0.01), 23.9 to 27.1 ml/min per 1.73 m2 (n=162, P<0.01), and 12.4 to 26.4 ml/min per 1.73 m2 (n=9, P<0.01), respectively. The absolute differences were 1.0, 3.2, and 13.9 ml/min per 1.73 m2 for stages 3, 4, and 5, respectively. Figure 3b shows the results in patients with a 3-month follow-up time (1700, 181, and 24 patients for stages 3, 4, and 5, respectively). The absolute difference of eGFR was similar in stage 3 patients, and bigger in patients with stages 4 and 5 (3.7 vs. 3.2 ml/min per 1.73 m2, and 16.8 vs. 14.3 ml/min per 1.73 m2, respectively).
Table 1. Characteristics of patients by CKD stages.
|
CKD stage |
P-value | |||
|---|---|---|---|---|
| 3 | 4 | 5 | ||
| n=1340 | n=162 | n=20 | ||
| Age, mean (s.d.) | 74.3 (10.1) | 78.0 (9.0) | 65.4 (17.8) | <0.01 |
| Gender, n (%) | ||||
| Male | 461 (34.4) | 45 (27.8) | 8 (40.0) | 0.20 |
| Female | 879 (65.6) | 117 (72.2) | 12 (60.0) | |
| Socioeconomic status, n (%) | ||||
| 1 (most deprived) | 215 (16.2) | 30 (18.5) | 6 (30.0) | 0.40 |
| 2 | 222 (16.7) | 27 (16.7) | 5 (25.0) | |
| 3 | 249 (18.8) | 28 (17.3) | 1 (5.0) | |
| 4 | 413 (31.1) | 47 (29.0) | 3 (15.0) | |
| 5 (most affluent) | 228 (17.2) | 30 (24.7) | 5 (25.0) | |
| NSAID type, n (%) | ||||
| Diclofenac | 472 (35.2) | 52 (32.1) | 7 (35.0) | 0.18 |
| Ibuprofen | 387 (28.9) | 39 (24.1) | 3 (15.0) | |
| Others | 481 (35.9) | 71 (43.8) | 10(50.0) | |
| Diabetes history, n (%) | 333 (24.9) | 40 (24.7) | 6 (30.0) | 0.87 |
| Use of diuretics, n (%) | ||||
| Before interval | 842 (85.4) | 132 (13.4) | 12 (1.2) | <0.01 |
| After interval | 871 (85.8) | 133 (13.1) | 11 (1.1) | <0.01 |
| Use of ACE/ARB inhibitors, n (%) | ||||
| Before interval | 734 (86.4) | 104 (12.2) | 12 (1.4) | 0.07 |
| After interval | 850 (88.1) | 108 (11.2) | 7 (0.7) | 0.02 |
| Calculated eGFR before intervala | ||||
| Stage 2 | 107 (8.2) | 0 (0.0) | 1 (5.0) | <0.01 |
| Stage 3 | 1183 (90.0) | 53 (32.9) | 1 (5.0) | |
| Stage 4 | 24 (1.8) | 107 (66.5) | 7 (35.0) | |
| Stage 5 | 0 (0.0) | 1 (0.6) | 11 (55.0) | |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CKD, chronic kidney disease; eGFR; estimated glomerular filtration rate; NSAID, nonsteroidal anti-inflammatory drug.
A total of 1495 patients had creatinine results.
Figure 3.
The average of estimated glomerular filtration rate (eGFR) in nonsteroidal anti-inflammatory drug (NSAID) users who stopped NSAIDs after the first reported eGFR measurement. (a) Follow-up time=180 days. (b) Follow-up time=90 days.
A sensitivity analysis was performed by using the closest eGFR after the last NSAID was prescribed. In this case, more patients were included in the study (n=15,212). There were 10,669 patients in stage 3, 3163 patients in stage 4, and 1380 patients in stage 5. The absolute changes in eGFR were −0.3, 4.2, and 7.4 ml/min per 1.73 m2 for stages 3, 4, and 5, respectively.
Implementation of eGFR reporting was associated with reduced NSAID prescribing in patients who had creatinine and eGFR measurements (n=62,716) during the study period (adjusted odds ratio, 0.78 95% confidence interval 0.75–0.82).
DISCUSSION
We observed a decrease in NSAID prescribing in Tayside after eGFR reporting was introduced on 1 April 2006. eGFR reporting was not associated with decline in the use of other cardiovascular drugs, such as angiotensin-converting enzyme inhibitors or angiotensin receptor-blocking drugs. Our study provides evidence that eGFR reporting was associated with improved estimates of renal function in patients who stopped NSAID treatment. By providing additional information, in addition to serum creatinine, eGFR reporting may provide additional renal benefit and reduced prescribing of NSAIDs in primary care.
CKD and NSAID use in elderly people are major challenges to health-care systems with aging populations. CKD is a risk factor for multiple adverse outcomes such as kidney failure, cardiovascular disease, cognitive impairment, and death.10 However, despite the importance of recognizing CKD, it may be missed when serum creatinine alone is used as a marker of renal function, because of confounders, including muscle mass, age, sex, and ethnicity; eGFR reporting was introduced mainly in recognition of this. NSAIDS are known to affect renal function most likely by inhibiting renal prostaglandin production, an effect that is modified by factors such as sodium status.11, 12 NSAIDs, except aspirin, should generally be avoided in CKD patients if possible. However, in practice, patients with CKD are likely to be older and to have multiple comorbid conditions or symptoms that lead to increased use of NSAIDs. NSAIDs have been associated with acute kidney injury and disease progression in those with CKD, leading to sodium retention, edema, hypertension, and hyperkalemia.5, 13, 14 Therefore, it is important to monitor renal function in patients taking NSAIDs. Previous studies have shown that early recognition, intervention, and management of patients with CKD by physicians lead to slow progression of the disease and disease complications.15, 16, 17, 18 However, a recent American survey showed that among patients with CKD stage ⩾3, NSAIDs were prescribed in 5% of patients, and that awareness of the association between NSAIDs and kidney disease was not associated with reduced NSAID use (3.8% aware vs. 3.9% unaware; P=0.979).15 Our data suggest that awareness of CKD in patients prescribed NSAIDs increased after the introduction of eGFR reporting, and that the reduction in NSAID prescribing was associated with improvement in renal function in patients with CKD after they stopped taking NSAIDs. We observed a substantial improvement in eGFR in stage 5 patients. To clarify the clinical status of patients at the time of the estimates of GFR and to make sure the results were not affected by dialysis patients, we further reviewed the medical notes of stage 5 patients after the main analysis. The results of eGFR change in stage 5 patients after excluding the dialysis patients (n=11) remained the same. This suggested that our findings were valid. Furthermore, within stage 5 patients, we observed decreased eGFRs between the eGFR before the date of the last NSAID prescription (within 2 months) and the eGFR after the date of last NSAID prescription (within 2 months), with a mean change of −4 ml/min per 1.73 m2. This supports the evidence that NSAIDs are associated with a decreased renal function. Although only 9 patients were included in this group, similar results were also observed in a further sensitivity analysis that included 1380 patients in this group when using the closest eGFR after the last NSAID. eGFR reporting did not affect the community prescribing of diuretics, angiotensin-converting enzyme inhibitors, or angiotensin receptor blockers. This might be explained by the perceived antihypertensive and nephroprotective benefits of these drugs, resulting in clinicians being reluctant to discontinue them.
The main strength of this study is its population-based cohort design, with complete regional biochemistry data, which is unique. However, the study has some limitations. First, the study was confined to a single National Health Service (NHS) region, and it is possible that the association between NSAID prescribing and CKD reporting may be different in other regions. A further study such as a questionnaire survey of physician behavior would strengthen the study finding. Second, the study period was historical. We do not know whether the prescribing behaviors in recent years were sustained or accelerated or lapsed. A follow-up study would be able to answer this question. Third, the estimated eGFR did not correct for ethnicity because we did not have this information. However, 97% of Tayside population are Caucasians, and therefore this is unlikely to have been a major confounder. This is unlikely to be a major source of misclassification. Fourth, unmeasured risk factors and confounders may have biased the use of NSAIDs. However, we have previously shown little impact of unmeasured risk factors and confounders in the same population.19 Finally, we did not use a reference method for assessing glomerular function.
In conclusion, introduction of eGFR reporting may result in safer prescribing of NSAIDs in primary care.
MATERIALS AND METHODS
The study was conducted in Tayside, Scotland, using the MEMO (Medicines Monitoring Unit) record-linkage database held by the Health Informatics Centre in the University of Dundee.20 The MEMO database covers a geographically compact population and serves ∼400,000 patients in the NHS in Scotland, 97% of whom are white. In brief, this database contains several data sets including all dispensed community prescriptions, hospital discharge data, biochemistry data, and other data that are linked by a unique patient identifier, the community health index number. The data have been validated and made anonymous for the purposes of research as approved by the government-appointed guardians of patient confidentiality. The project was also approved by the Tayside committee on research medical ethics and the Tayside Caldicott Guardians.
Study population
Subjects resident in Tayside and registered with a general practitioner between January 2005 and December 2007 formed the study population.
Study subjects
Subjects were those who had at least one NSAID prescription or serum creatinine measurement between April 2005 and March 2007. There are two time intervals for this study period, 1 year on either side of the eGFR implementation date of 1 April 2006. For each patient, eGFR and creatinine were followed up for 6 months.
Estimation of eGFR
Glomerular filtration rate was estimated using the four-variable equation from the Modification of Diet in Renal Disease (MDRD) study21 (the four variables used in this equation are serum creatinine, age, sex, and ethnicity). An information sheet about eGFR reporting was circulated to all users of laboratory services in March 2006. For creatinine results reported before April 2006, GFR was estimated retrospectively. The creatinine assay was a rate-blanked compensated Jaffe method, measured on a Roche modular P analyzer (Roche Diagnostics, Lewes, UK). There was no change in method throughout the study period. External quality assurance of the creatinine assay was through the United Kingdom National External Quality Assessment Scheme (UK NEQAS). Bias was acceptable and consistent throughout.
Kidney disease was stratified using the system advocated by the American National Kidney Foundation Kidney Disease Outcomes Initiative (NKF KDOQI).22 Briefly, CKD is stratified into five stages, defined as follows: stage 1, eGFR ⩾90.0 ml/min per 1.73 m2; stage 2, eGFR 60.0–89.9 ml/min per 1.73 m2; stage 3, eGFR 30.0–59.9 ml/min per 1.73 m2; stage 4, eGFR 15.0–29.9 ml/min per 1.73 m2; and stage 5, eGFR<15.0 ml/min per 1.73 m2.
Outcome variables
The primary outcome variable was the change in NSAID prescribing rates during the two time intervals (1 year on either side of the eGFR implementation date). The secondary outcome was the change in renal function as estimated by the four-variable MDRD equation (eGFR) in patients who were on NSAID treatment but who stopped after the first eGFR report. Patients in the analysis of renal function change were those who were ⩾18 years old, as eGFR measured by MDRD is only valid in adults. Medical notes were retrospectively reviewed for stage 5 patients by a renal physician to establish whether the patients were dialysis patients after an additional ethical approval was obtained.
Statistical analysis
Data were summarized as mean (s.d.)/median (interquartile range) for continuous variables and number of subjects (percent) for categorical variables. The χ2, paired t-test, and analysis of variance tests were performed to determine significant differences. Rates of NSAID prescribing in CKD patients were compared between the users who did not get an eGFR report and who had an eGFR report (that is, before the implementation of eGFR reporting vs. after the implementation of eGFR reporting). Potential confounding by indication for eGFR reporting such as age, gender, socioeconomic status, comorbidity of diabetes, and use of diuretics, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers was adjusted for in a logistic regression model. All statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC).
Acknowledgments
This study was funded by the TENOVUS Scotland (T09/36). The funding body did not have any role in study design, data analysis, result interpretations, and report submission.
AUTHOR CONTRIBUTIONS
LW designed the study, did the statistical analysis, and wrote the manuscript. TMM and MJM contributed to the study design and result interpretation and reviewed/edited the manuscript. CJ reviewed medical records, contributed to result interpretation, and reviewed the manuscript. XS helped with data assembly and reviewed the manuscript. RWF contributed to result interpretation and reviewed the manuscript. LW is the guarantor.
TMM received consultancy fees, honoraria, and travel expenses in the past 3 years from Pfizer, Servier, Novartis, Wyeth, Kaiser Permanante, Takeda, Recordati, and NiCox. All the other authors declared no competing interests.
References
- Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- Renal Data System US. Excerpts from the USRDS 2004 Annual Report. Am J Kindney Dis. 2005;45:S1–280. [Google Scholar]
- Clase CM, Garg AX, Kiberd BA. Prevalence of low glomerular filtration rate in nondiabetic Americans: Third National Health and Nutrition Examination Survey (NHANES III) J Am Soc Nephrol. 2002;13:1338–1349. doi: 10.1097/01.asn.0000013291.78621.26. [DOI] [PubMed] [Google Scholar]
- Culleton BF, Larson MG, Evans JC, et al. Prevalence and correlates of elevated serum creatinine levels: the Framingham Heart Study. Arch Intern Med. 1999;159:1785–1790. doi: 10.1001/archinte.159.15.1785. [DOI] [PubMed] [Google Scholar]
- Gooch K, Culleton BF, Manns BJ, et al. NSAID use and progression of chronic kidney disease. Am J Med. 2007;120:280. e1–7. doi: 10.1016/j.amjmed.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Adams DH, Howie AJ, Michael J, et al. Non-steroidal anti-inflammatory drugs and renal failure. Lancet. 1986;327:57–60. doi: 10.1016/s0140-6736(86)90714-2. [DOI] [PubMed] [Google Scholar]
- Curhan GC, Knight EL, Rosner B, et al. Lifetime nonnarcotic analgesic use and decline in renal function in women. Arch Intern Med. 2004;164:1519–1524. doi: 10.1001/archinte.164.14.1519. [DOI] [PubMed] [Google Scholar]
- Rexrode KM, Buring JE, Glynn RJ, et al. Analgesic use and renal function in men. JAMA. 2001;286:315–321. doi: 10.1001/jama.286.3.315. [DOI] [PubMed] [Google Scholar]
- Prescribing statistics . Available at http://www.isdscotland.org/Health-Topics/Prescribing-and-Medicines/Community-Dispensing/Prescription-Cost-Analysis/ (accessed 27 February2013
- Stevens LA, Viswanathan G, Weiner DE. Chronic kidney disease and end-stage renal disease in the elderly population: current prevalence, future projections, and clinical significance. Adv Chronic Kidney Dis. 2010;17:293–301. doi: 10.1053/j.ackd.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SK, Rudy DW, Lasseter KC, et al. Effect of cyclooxygenase-2 inhibition on renal function in elderly persons receiving a low-salt diet. A randomized, controlled trial. Ann Intern Med. 2000;133:1–9. doi: 10.7326/0003-4819-133-1-200007040-00002. [DOI] [PubMed] [Google Scholar]
- Schwartz JI, Vandormael K, Malice MP, et al. Comparison of rofecoxib, celecoxib, and naproxen on renal function in elderly subjects receiving a normal-salt diet. Clin Pharmacol Ther. 2002;72:50–61. doi: 10.1067/mcp.2002.126182. [DOI] [PubMed] [Google Scholar]
- Schneider V, Lévesque LE, Zhang B, et al. Association of selective and conventional nonsteroidal antiinflammatory drugs with acute renal failure: a population-based, nested case-control analysis. Am J Epidemiol. 2006;164:881–889. doi: 10.1093/aje/kwj331. [DOI] [PubMed] [Google Scholar]
- Clive DM, Stoff JS. Renal syndromes associated with nonsteroidal antiinflammatory drugs. N Engl J Med. 1984;310:563–572. doi: 10.1056/NEJM198403013100905. [DOI] [PubMed] [Google Scholar]
- Plantinga L, Grubbs V, Sarkar U, CDC CKD Surveillance Team et al. Nonsteroidal anti-inflammatory drug use among persons with chronic kidney disease in the United States. Ann Fam Med. 2011;9:423–430. doi: 10.1370/afm.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartarolo JM, Thoelke M, Schafers SJ. Reporting of estimated glomerular filtration rate: effect on physician recognition of chronic kidney disease and prescribing practices for elderly hospitalized patients. J Hosp Med. 2007;2:74–78. doi: 10.1002/jhm.172. [DOI] [PubMed] [Google Scholar]
- Kinchen KS, Sadler J, Fink N, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med. 2002;137:479–486. doi: 10.7326/0003-4819-137-6-200209170-00007. [DOI] [PubMed] [Google Scholar]
- Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- Wei L, MacDonald TM, Walker BR. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med. 2004;141:764–770. doi: 10.7326/0003-4819-141-10-200411160-00007. [DOI] [PubMed] [Google Scholar]
- Wei L, Parkinson J, MacDonald TM.The Tayside Medicines Monitoring Unit (MEMO)In Strom BL, (ed)Pharmacoepidemiology4th ednJohn Wiley and Sons: Chichester; 2005323–336. [Google Scholar]
- Levey AS, Greene T, Kusek J.A simplified equation to predict glomerular filtration rate from serum creatinine J Am Soc Nephrol 200011155A[abstract]. [Google Scholar]
- National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39 (Suppl 1:S1–S266. [PubMed] [Google Scholar]