Abstract
Background
Spontaneous clearance of Hepatitis C virus (HCV) following liver transplantation (OLT) is a rare occurrence. Here we present detailed immunological analysis of an interferon naive OLT recipient receiving uninterrupted immunosuppression who cleared HCV spontaneously two years after transplantation.
Methods
Enzyme linked immunospot assay (ELISpot) analysis of peripheral T-cell IFNγ, IL-10, and IL-17 response to HCV core and non-structural antigen 4 (NS4) and enzyme linked immunosorbent assay (ELISAs) to collagen (Col) subtypes I, II, IV, and V were performed in the index patient at the time of viral clearance and compared to an OLT cohort with persistent viremia matched for time from OLT, immunosuppression, and histology. ELISpot and ELISA analysis were repeated on the patient 4 years after OLT. Transcription-mediated amplification assays were used to confirm viral clearance.
Results
Compared to a cohort of post-OLT and non-transplanted viremic HCV patients, the index patient with HCV clearance demonstrated higher IL-17, IL-10 and lower IFN-γ response to NS4 and core antigen and a higher titer of Abs to Col subtypes I, II, and V during clearance. On follow-up 2 years later, HCV specific IFN-γ was increased in the index patient, with a decline in IL-17 and IL-10 response as well as Col I, II, and V Ab titer.
Conclusions
Virus induced activation of Th-17 cells may contribute to HCV clearance post- OLT. Maintenance of viral suppression may be facilitated by restoration of Th1 (IFNγ) responses. Modulation of Th17 immunity deserves further attention as a therapeutic strategy in the treatment of HCV recurrence post-OLT.
INTRODUCTION
Spontaneous clearance of hepatitis C virus (HCV) infection after liver transplantation (OLT) is rare (1). The natural history of HCV is accelerated in liver transplant recipients (2). T-cell mediated immunity is critical in determining the outcome of HCV infection both pre- and post-transplantation (3). Recent reports have also demonstrated a potential role for T helper type 17 (Th-17) in multiple inflammatory conditions associated with liver fibrosis (4–6). Here we present immunological analysis of an interferon naïve OLT recipient receiving uninterrupted immunosuppression who cleared HCV spontaneously two years after transplantation. IL-17 response to HCV core and NS4 antigens measured by ELISpot in this patient was significantly higher during the time frame of presumed viral clearance when compared to groups of both transplanted and non-transplanted patients with persistent HCV viremia. This was followed by a decrease in IL-17 and increase in IFN-γ responses following clearance. The occurrence of Abs to extracellular matrix protein collagen (Col) was also significantly different in the patient during clearance when compared to follow-up 2 years later. We propose that differential initial activation of Th-17 followed by sustained activation of IFN-γ by HCV antigens plays a role in viral clearance post-transplantation.
CASE REPORT
A 48 year-old Caucasian male was transplanted for chronic HCV and hepatocellular carcinoma in 2006. He had come to medical attention one year prior (2005) with portal hypertension manifested by esophageal variceal bleeding, ascites and portosystemic encephalopathy. Workup as to the etiology of liver disease revealed HCV genotype 1 with a viral titer of 675,000 IU/ml. Medical history was otherwise unremarkable. Model for End-Stage Liver Disease (MELD) score was 14 and the patient was listed for transplantation.
Four months later he underwent successful OLT. Induction of immunosuppression included methylprednisolone 1000mg preoperatively with subsequent tapering to 20 mg daily, 5 days after transplantation. In addition, tacrolimus was given 0.05 mg/kg on day 1 after transplantation (adjusted for serum trough levels between 6–10 ng/mL) and mycophenolate mofetil 1 gram twice daily.
On day 28 after transplantation, elevation of liver enzymes was noted (Aspartate aminotransferase (AST) 234 IU/mL, ALT 465 IU/mL, alkaline phosphatase 535 IU/mL, direct bilirubin 8.2 mg/dL). Liver biopsy showed mild acute and chronic portal and lobular inflammation and portal fibrosis with focal hepatocyte dropout around the central vein. The bile ducts appeared unremarkable. There was no endothelialitis or evidence of rejection. Both cytomegalovirus immunohistological stain and in-situ hybridization for Epstein-Barr virus early ribonucleic acid (RNA) were negative. Portal fibrosis and the presence of inflammation were suggestive of early HCV recurrence. Endoscopic Retrograde Cholangiopancreatography (ERCP) was performed which revealed choledocholithiasis treated with stone extraction and stent placement. Serum enzymes normalized over the following week.
Eight months after transplantation the patient had elevated transaminases and a repeat ERCP revealed biliary anastomotic stricture that was treated with stent placement. Serum enzymes normalized over the following week. HCV RNA was noted to be 2,850,000 IU/ml. Over the following year the patient had normal liver chemistries and his immunosuppression was tailored with discontinuation of mycophenolate mofetil.
On Day 825 (2.25 years) after transplantation, there was an increase in transaminases (AST 79 IU/mL, alanine aminotransferase 88 IU/mL, and alkaline phosphatase 95 IU/mL, direct bilirubin 2.1 mg/dL). Cholangiogram showed no signs of obstruction and doppler ultrasound of the allograft was unremarkable. Biopsy revealed portal tract infiltrate with focal lymphoid aggregates and interface hepatitis as well as lobulitis consistent with recurrent HCV (grade 3 inflammation/mild portal fibrosis with minimal bridging features). Consideration was given to initiating antiviral therapy. HCV RNA PCR assay was performed to assess viral load prior to therapy; however, this was negative (<50 IU/mL). Repeat HCV RNA 4 months, 8 months, and 2 years later were persistently negative and confirmed using transcription mediated amplification assays (Quest Diagnostics, Chantilly, VA)(<5 IU/ml). Liver function tests gradually normalized approximately 4 months after first documented negative HCV RNA PCR and the patient is well with good allograft function.
RESULTS
Decreased IFNγ and Increased IL-10 and IL-17 During Clearance in the Index Patient Compared to Persistently Viremic Patients
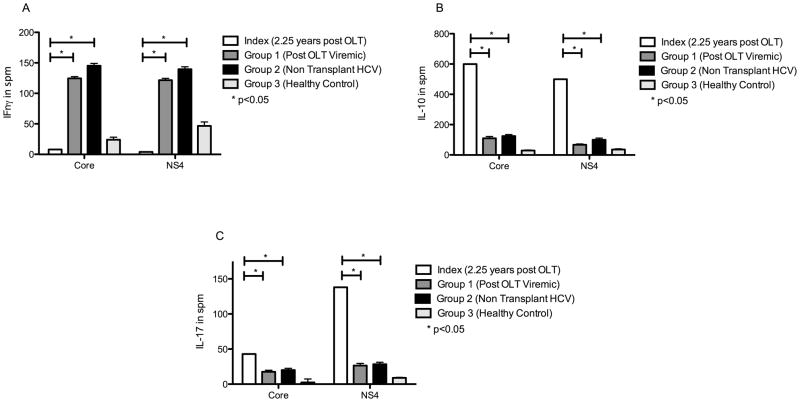
At the time of presumed viral clearance (2.25 years after transplantation), HCV (Core and NS4) specific CD4 T-Cell secretion of IFNγ, IL-10, and IL-17 was different compared to a cohort of post-OLT (Group 1) and non-transplanted (Group 2) viremic patients and healthy controls (Group 3) (Figure 1). IFNγ response to HCV core was lower compared to group 1, 2 and 3 subjects (index, post-OLT viremic, non-transplanted viremic and healthy control represented as mean standard error measurement (SEM) [95% CI] expressed in spots per million (spm): 8.0 vs. 124.8 (2.7) [117.2–132.4] vs. 145.2 (3.8) [132.1–159.4] vs. 24.1 (4.1) [13.1–35.7]. IFNγ response to HCV NS4 antigen was also lower in the index patient compared to all three groups (represented as mean (SEM), [95% CI] expressed in spm): 4 vs. 121.8 (2.9) [113.5–130.1] vs. 139.5 (4.1) [123.2 – 153.6] vs. 46.8 (6.5) [28.67–64.9].
Figure 1. At the time of presumed clearance (2.25 years post-OLT) index patient had decreased IFNγ, and increased IL-10 and IL-17 response to HCV antigens when compared to post-OLT patients with persistent viremia, non-transplanted HCV patients and healthy controls.
ELISpot comparing IFNγ (Fig 1A), IL-10 (Fig 1B) and IL-17 (Fig 1C) responses to HCV core and NS4 antigen among index patient at time of presumed viral clearence (2.25 years post-OLT - white bars), post-OLT persitent viremic patients (Group 1, n=5, dark grey bars), non-transplanted viremic HCV patients (Group 2, n=5, black bars), healthy controls (Group 3, n=5, light grey bars). Values represented as spm ± SEM. HIV Gp120 peptide and phytohemagglutinin were used as postive and negative controls respectively (not shown). * denotes p<0.05
IL-10 and IL-17 response to both HCV core and NS4 antigen were higher in the index patient compared to both post-OLT and non-transplanted patients with viremia and controls at the time of viral clearance (Figure 1). For IL-10 secretion upon core stimulation (index, Group 1, Group 2 and Group 3 represented as mean (SEM) [95% CI] expressed in spm): 600 vs. 110 (12) [76.7–143.4] vs. 125.4 (10) [97.2–156.5] vs. 30 (3.5) [20.2–39.8]. IL-10 secretion upon NS4 stimulation: 500 vs. 67 (5.6) [51.4–82.6] vs. 100.2 (11.4) [78.3–130.2] vs. 37.2 (4.6) [24.5–49.9]. IL-17 secretion upon core stimulation: 43 vs. 17.6 (.9) [15–20.2] vs. 20.1 (1.2) [17.3–22.1] vs. 2.4 (.5) [1–3.8]. IL-17 upon NS4 stimulation: 138 vs. 26.4 (1.3) [22.7–30.1] vs. 28.3 (1.2) [26.4–31.1] vs. 9 (.7) [7–11].
Increased Frequency Of Abs To Extracellular Matrix (ECM) Col During Spontaneous Clearance Compared To Patients With Persistent Viremia
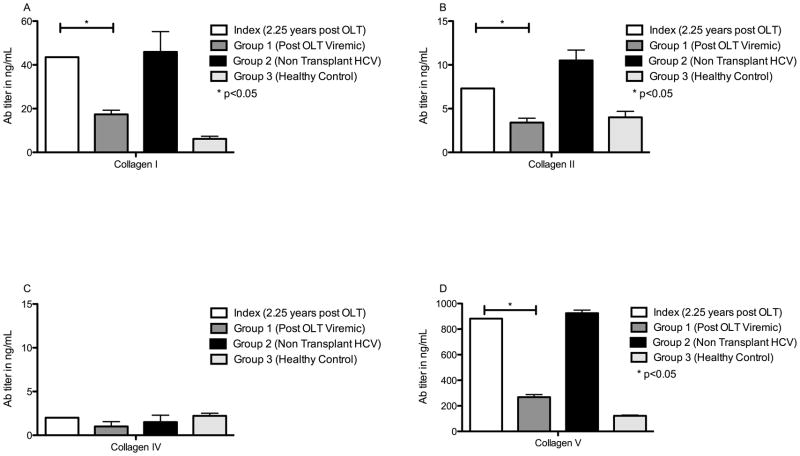
In comparison to the cohort with post-OLT viremic and healthy controls, the index patient demonstrated a higher level of Abs to ECM Col I, II, and V, but this was less than the non-OLT HCV subjects (Figure 2). Abs to Col I were (patient, post-OLT persistently viremic, non-OLT HCV subjects, healthy control expressed as mean (SEM) [95% CI] in ng/mL): 43.5 vs. 17.4 (1.9) [12–22.9] vs. 45.9 (9.3) [19.5–70.3] vs. 6.2 (1.2) [3–9.4]. Col II Ab: 7.3 vs. 3.4 (.5) [2–4.8] vs. 10.5 (1.2) [6.4–13.8] vs. 4 (.7) [2–6]. Col IV Ab: 2 vs. .56 (.07) [.37–.75] vs. 1.5 (0.8) [0.2–2.9] vs. 2.2 (.32) [1.3–3.1]. Col V Ab: 882 vs. 268.6 (10.1) [240.5–296.7] vs. 924.6 (20.3) [890.5–956.3] vs. 121.8 (5.7) [106–137.6].
Figure 2. Increased serum Abs to Col I, II and V in index patient at time of presumed viral clearance compared to post-OLT viremic patients.
Serum titer of Abs to Col I (Fig 2A), II (Fig 2B), IV (Fig 2C) and V (Fig 2D) were determined by ELISA. Index patient (white bars) had significantly higher titer of Abs to Col types I, II and V at time of viral clearance when compared to post-OLT patients with persistent viremia (Group 1 - dark grey bars) and healthy controls (Group 3 - light grey bars). These were not different from those in non-transplanted viremic patients (group 2 - black bars). Values expressed as mean Ab titer in ng/mL ± SEM. * denotes p<0.05
Increased IFNγ, Decreased IL-10, IL-17 Secretion and Decreased ECM Col Abs During Longitudinal Follow Up of the Index Patient
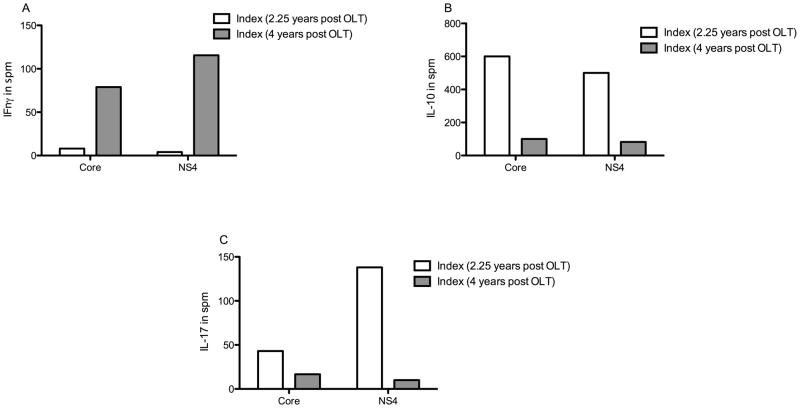
To assess for a longitudinal trend in the patient, we performed ELISpot on the patient at 4 years post-OLT (2 years from documented HCV clearance). In comparison to the timeframe of HCV clearance, the patient demonstrated increased secretion of IFNγ, diminished IL-10 and IL-17 upon stimulation with HCV core and NS4 antigens (Figure 3). Core antigen stimulation of IFNγ was higher 4 years post-OLT: 78.9 spm vs. during clearance: 8 spm. NS4 antigen stimulation of IFNγ 115.6 spm at 4 years compared with 4 spm during clearance. Both core and NS4 antigen stimulation of IL-10 were decreased at 4 years: IL-10 (core and NS4 at 4 yrs vs. 2.25 years (during clearance) in spm): 82.2 and 100.2 vs. 600 and 500. Core and NS4 antigen stimulation of IL-17 were also decreased at 4 years: IL-17 (core and NS4 at 4 yrs vs. core and NS4 during clearance in spm): 16.7 and 10 vs. 43 and 138.
Figure 3. Increase in IFNγ response and decrease in IL-10 and IL-17 response to HCVantigens in the index patient approximately 2 years after spontaneous clearance (4 years post-OLT) compared to at the time of viral clearance (2.25 years post OLT).
ELISpot comparing IFNγ (Fig 3A), IL-10 (Fig 3B) and IL-17 (Fig 3C) responses to HCV core and NS4 antigen among index patient at time of presumed viral clearance (2.25 years post OLT - white bars), and two years following that (4 years post-OLT, grey bars). Following the period of presumed viral clearance there was an increase in IFN-γ response to HCV antigens with a decrease in IL-17 and IL-10 responses. (Statistical comparisons not made due to insufficient data points)
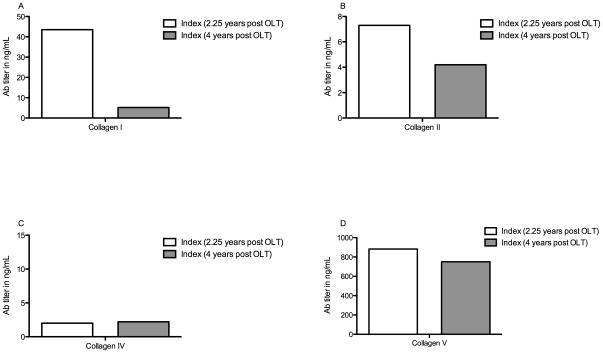
To evaluate the development of Abs to Col we performed ELISA on serum obtained 4 years post-OLT. The patient demonstrated decreased levels of Abs to Col I, II, and V at 4 years compared to the timeframe of viral clearance (Figure 4). Col I Ab (4 years vs. 2 years expressed in ng/mL): 5.2 vs. 43.5. Col II Ab: 4.2 vs. 7.3. Col V Ab: 750 vs. 882.
Figure 4. Decrease in serum Abs to Col I, II and V in index patient approximately 2 years after spontaneous clearance (4 years post-OLT) compared to at the time of viral clearance (2.25 years post OLT).
Serum titer of Abs to Col I (Fig 4A), II (Fig 4B), IV (Fig 4C) and V (Fig 4D) were determined by ELISA. Index patient two years post presumed viral clearance (4 years post-OLT, grey bars) had a decrease in titer of Abs in Col I, II, and V when compared to at the time of presumed viral clearance (2.25 years post-OLT, white bars). (Statistical comparisons not made due to insufficient data points)
DISCUSSION
HCV recurrence following OLT is a universal phenomenon (7). Approximately 20–30% of patients will develop allograft cirrhosis by five years (8). This rapid course is in contrast to the history of HCV infection in the native liver in which up to 15–20% of infected individuals spontaneously clear the virus (9). Viral clearance in both the pre- and post-transplant setting is associated with anti-HCV T-cell responses (10). The accelerated history of HCV in the post-transplant setting is thought largely due to chronic immunosuppression with blunting of T-cell mediated clearance mechanisms (11).
Recently, we reported that HCV recurrence in OLT recipients induces an inflammatory milieu characterized by increased Th2 cytokines and decreased IFN-γ which facilitates the induction of HCV specific CD4+ Th-17 cells (6). These cells are resistant to suppression by Tregs and may promote an inflammatory cascade leading to progression of cirrhosis. In addition, it was found that occurrence of Abs to Col proteins I, II, and V positively correlate with liver fibrosis in HCV positive OLT recipients (12). Spontaneous clearance of HCV following transplantation is extremely rare and thus provided a unique opportunity to evaluate HCV specific T-cell responses and potential immunologic mechanisms involved in viral clearance. This investigation reports a patient with biopsy proven HCV recurrence that cleared the virus approximately 2.25 years after OLT. Clearance was documented at 8 months after a decrease in his immunosuppression, and clearance has been sustained on follow-up over a 2 year period. During the timeframe of clearance, HCV specific (core and NS4 antigen) T-cell cytokine response were demonstrably different compared with a cohort of OLT patients matched for immunosuppression, allograft histology, and time from transplant as well as a cohort of interferon naïve non-transplanted HCV viremic patients. Specifically, the patient demonstrated lower HCV specific IFN-γ and higher IL-10 and IL-17 secreting cells when compared to the other HCV groups. Abs to Col subtypes I, II, and V were elevated in the index patient during clearance when compared to patients with persistent viremia. On follow-up 2 years later the immunologic profile had changed, with an increase in HCV specific IFN-γ response and diminished IL-10 and IL-17 responses along with a decrease in the titer of Abs to Col I, II, and V.
A handful of case reports exist in the literature describing spontaneous resolution of HCV infection after OLT (13–17). Samonakis et al reported spontaneous resolution of HCV in two patients after liver transplantation with a change in their immunosuppressive regimen to sirolimus secondary to renal insufficiency (13). Bhagat et al (14) reported two cases of HIV-HCV co-infection with Falconer et al (15) commenting that a lower chronic T cell activation along with higher degree of T cell function on highly active anti-retroviral therapy may contribute to this phenomena in the co-infected patient. Doughty et al presented a case of HCV clearance 41 months after liver transplantation following an episode of cholestatic hepatitis (16). The authors encountered a large number of quasispecies during decreased immune suppression postulating its association (as a surrogate for enhanced innate immunity) with HCV clearance. In the largest case series with spontaneous clearance of HCV after liver transplantation, Casanovas-Taltavull et al. examined IFN-γ and IL-10 production in five patients with spontaneous clearance and five sustained virological responders to treatment compared with normal controls (17). They reported that IFN-γ levels in transplanted patients with spontaneous clearance were similar to healthy controls and patients who achieved sustained virologic response (SVR) on antiviral therapy and were significantly higher compared to non-responders. IL-10 production was similar in cases of spontaneous clearance, SVR, and controls but was lower in pre-transplant patients and non-responders. Non responders demonstrated high basal IL-10 production with little increase after stimulation.
Our patient demonstrated a significantly different IFNγ response profile compared with the aforementioned study. Similar to previous reports, we cannot define the precise timing of viral clearance; however, given the documented HCV RNAs around and elevated transaminases at 2.25 yrs, we suspect it to have occurred then. We performed LUMINEX assay on banked serum at this time point which revealed demonstrable circulating peripheral IFNγ (224.8 pg/ml) levels implicating innate immune cells as a potential source. It is plausible that with clearance of infection, Th1 predominant immunity was restored, resulting in the increase in HCV specific IFNγ response at 2 years following viral clearance. We hypothesize that restoration of Th1 immunity facilitates sustained HCV clearance. In addition, during the time of clearance, Abs to ECM Col were elevated, suggesting a significant amount of ECM turnover probably due to inflammation; with resolution of infection these titers decreased.
To our knowledge, this is the first report to evaluate Th-17 responses in spontaneous HCV clearance following OLT. IL-17 secreting T (Th-17) cells have been associated with inflammation and pathogenesis in liver disorders (18). HCV-specific Th1 and Th-17 cells have been shown to be suppressed by NS4-induced production of the anti-inflammatory cytokines IL-10 and TGF-β (19). During the clearance phase the patient demonstrated both enhanced IL-10 and IL-17 following NS4 stimulation both in comparison to non-transplanted and post-OLT viremic patients. These responses declined with clearance of the virus. Thus, contrary to our previous report, wherein increased IL-17 responses were associated with increased fibrosis post-OLT (6), the findings in this single patient suggest that transient high IL-17 and IL-10 response may also result in viral clearance and subsequent reactivation of Th1 immunity, which in turn prevents recurrence. The requirement for Th1 responses to maintain viral clearance is supported by studies wherein augmented Th1 with decrease in Th2 responses in non-transplanted patients were associated with viral clearance after antiviral therapy (20).
Spontaneous clearance of HCV post-transplantation is a dynamic process in part due to viral specific T-cell mediated necroinflammation and activation of hepatic stellate cells under the influence of immunosuppression. However, the consequences of immunosuppression and the mechanisms of T-cell response and viral clearance following recurrence have not been fully delineated. Our evaluation of responses was performed only at two points in a single patient and as such is not sufficient to carefully dissect out temporal trends in T-cell responses. Studies to define HCV immune profile along with serial monitoring of viral titers are currently underway. Nevertheless, recurrent HCV infection in the allograft may engage the recipient’s immune system in multiple unique and non-exclusive pathways. Virus induced activation of Th-17 cells may contribute to clearance of the virus, and this may be facilitated by a decrease in immunosuppression. Further subsistence of viral suppression may be maintained by a Th1 (IFNγ) response. An attempt to treat HCV infection post liver transplantation is often warranted in order to abrogate progression of fibrosis and delay allograft loss, and modulation of Th-17 immunity may prove to be a therapeutic strategy.
METHODS AND MATERIALS
To investigate this rare case of spontaneous viral clearance after OLT we analyzed HCV specific IFNγ, IL-10, and IL-17 secreting cells during the timeframe of viral clearance (2.25 years from OLT). In this cross-sectional analysis, we compared the index patient’s HCV responses to three groups: 1) a cohort of post-OLT patients with persistent HCV viremia (n=5) matched for immunosuppression with tacrolimus, allograft histology (grade 3 inflammation/minimal fibrosis) and time from OLT (2 years [range: 1.8–2.2 yrs]); 2) interferon naïve non-transplanted chronic HCV subjects (n=5) matched for HCV genotype, liver fibrosis, age and race; and 3) non-HCV infected healthy controls (n=5). Non-transplanted and post-OLT cohorts with persistent viremia were selected from patients from ongoing lab studies (6, 12). All experiments were repeated on the index patient at 4 years post-OLT (2 years from documented viral clearance). Informed consent was obtained from all subjects after approval by the Human Research Protection Office (institutional review board).
Isolation of Serum and Peripheral Blood Mononuclear Cells (PBMC)
PBMC were isolated from blood samples by Ficoll-Hypaque gradient centrifugation. Cells were used for experiments immediately. Serum was obtained from whole blood and used immediately or frozen till future use at −70°C.
Antigens
Recombinant HCV core, and NS4 (Fitzgerald Industries, Acton MA), HIV Gp120 peptide (Biosynthesis Inc, TX), and phytohemagglutinin (PHA) (Sigma, St. Louis, MO) peptide antigens were obtained and tested endotoxin-free. PBMCs were stimulated with 5μg/mL of antigen overnight in 24 well plates (Corning Inc, NY) at 37°C in 5% CO2.
Enzyme Linked Immunospot assay (ELISpot)
ELISpot was carried out as described previously (6). Briefly, stimulated PBMCs were cultured in triplicate (3×105 cells /200μL) in immunospot plates with antigens (5μg/mL) for 48–72 in 5% CO2 at 37°C. IFNγ and IL-10 (BD Biosciences), IL-17 (eBioscience) ELISpots were performed per manufacturer’s instructions. Spots were analyzed in an ImmunoSpot Image Analyzer (Cellular Technology Ltd., Cleveland, OH). Cells cultured in medium (Cellular Technology, Cleveland) and irrelevant peptide (HIV Gp120) were used as negative and PHA as positive control. Spots in the experimental wells greater than two standard deviations of those in negative control wells were considered significantly positive and expressed as spm cells.
Enzyme Linked Immunosorbent Assay (ELISA)
We used ELISA to determine concentrations of Abs to Col proteins (12). Briefly, ninety-six well ELISA plates were coated with 100ng/100μL of human Col I (Cell Sciences, Canton, MA), 50ng/100μL of human Col II (Sigma), 2ng/100μL of human Col IV (Biodesign International, Saco, ME) and 200ng/100μL of human Col V (Sigma). Serum was tested for binding to Col and Abs detected by peroxidase-conjugated goat anti-human IgA, IgG and IgM (Jackson Immunoresearch, West Grove, PA). Reactions were developed using tetramethylbenzidine and read at 450nm (μ Quant, Bio-TEK instrument Inc, Winooski, VT). Ab concentrations were determined using the standard curve of a known concentration of anti-Col (Santa Cruz Biotechnology, CA). Positive cut off for Ab was set as +2 SD of mean obtained in normal healthy subjects. This was determined to be 4ng/mL for anti Col I, 2ng/mL for anti Col II, 2ng/mL for anti Col IV, and 12ng/mL for anti Col V.
Statistical Analysis
Analysis was performed using GraphPad Prism v5.0b (La Jolla, CA). Cohort group values were evaluated with SEM and 95% CIs where appropriate.
Acknowledgments
This publication was made possible by an award from the NIH DK065982 (TM), and from BJC Foundation (TM). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. AS and BB were supported by National Institutes of Health, National Research Service Award 5-T32-DK07301-35, from the National Institute of Digestive Diseases and Kidney. The authors would like to thank Ms. Billie Glasscock for assistance in the preparation of the manuscript.
Abbreviations
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- BSA
Bovine serum albumin
- CI
Confidence Interval
- Col
Collagen
- ECM
Extracellular matrix
- ELISA
Enzyme Linked Immunosorbent Assay
- ERCP
Endoscopic Retrograde Cholangiopancreatography
- HCV
Hepatitis C Virus
- NS4
Non-structural antigen 4
- OLT
Orthotopic Liver Transplantation
- PBMC
Peripheral Blood Mononuclear Cell
- PCR
Polymerase Chain Reaction
- PHA
Phytohemagglutinin
- RNA
Ribonucleic Acid
- SEM
Standard Error Measurement
- SPM
spots per million
- SVR
Sustained Virologic Response
- Th-17
T helper type 17
Footnotes
Conflict of Interest: The authors have no relevant conflicts of interests to disclose.
References
- 1.Haque M, Hashim A, Greanya ED, Steinbrecher UP, Erb SR, Yoshida EM. Spontaneous clearance of hepatitis C infection post-liver transplant: A rare but real phenomenon? A case report and review of the literature. Annals of hepatology: official journal of the Mexican Association of Hepatology. 2010;9 (2):202. [PubMed] [Google Scholar]
- 2.McCaughan GW, Shackel NA, Bertolino P, Bowen DG. Molecular and cellular aspects of hepatitis C virus reinfection after liver transplantation: how the early phase impacts on outcomes. Transplantation. 2009;87 (8):1105. doi: 10.1097/TP.0b013e31819dfa83. [DOI] [PubMed] [Google Scholar]
- 3.Suneetha PV, Mederacke I, Heim A, et al. Spontaneous clearance of chronic hepatitis C after liver transplantation: are hepatitis C virus-specific T cell responses the clue? Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2008;14 (8):1225. doi: 10.1002/lt.21559. [DOI] [PubMed] [Google Scholar]
- 4.Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clinical and experimental immunology. 2007;148 (1):32. doi: 10.1111/j.1365-2249.2007.03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008;43 (3):402. doi: 10.1016/j.cyto.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basha HI, Subramanian V, Seetharam A, et al. Characterization of HCV-Specific CD4+Th17 Immunity in Recurrent Hepatitis C-Induced Liver Allograft Fibrosis. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11 (4):775. doi: 10.1111/j.1600-6143.2011.03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gane EJ. The natural history of recurrent hepatitis C and what influences this. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2008;14 (Suppl 2):S36. doi: 10.1002/lt.21646. [DOI] [PubMed] [Google Scholar]
- 8.Burra P. Hepatitis C. Seminars in liver disease. 2009;29 (1):53. doi: 10.1055/s-0029-1192055. [DOI] [PubMed] [Google Scholar]
- 9.Sreenarasimhaiah J, Jaramillo A, Crippin J, Lisker-Melman M, Chapman WC, Mohanakumar T. Lack of optimal T-cell reactivity against the hepatitis C virus is associated with the development of fibrosis/cirrhosis during chronic hepatitis. Human immunology. 2003;64 (2):224. doi: 10.1016/s0198-8859(02)00781-4. [DOI] [PubMed] [Google Scholar]
- 10.Burke KP, Cox AL. Hepatitis C virus evasion of adaptive immune responses: a model for viral persistence. Immunologic research. 2010;47 (1–3):216. doi: 10.1007/s12026-009-8152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber NK, Trotter JF. Spontaneous clearance of hepatitis C virus after liver transplantation. Transplantation. 2009;87 (7):1102. doi: 10.1097/TP.0b013e31819d407c. [DOI] [PubMed] [Google Scholar]
- 12.Borg BB, Seetharam A, Subramanian V, et al. Immune response to extracellular matrix collagen in chronic hepatitis C induced liver fibrosis. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2011 doi: 10.1002/lt.22303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samonakis DN, Cholongitas E, Triantos CK, et al. Sustained, spontaneous disappearance of serum HCV-RNA under immunosuppression after liver transplantation for HCV cirrhosis. Journal of hepatology. 2005;43 (6):1091. doi: 10.1016/j.jhep.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Bhagat V, Foont JA, Schiff ER, Regev A. Spontaneous clearance of hepatitis C virus after liver transplantation in two patients coinfected with hepatitis C virus and human immunodeficiency virus. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2008;14 (1):92. doi: 10.1002/lt.21351. [DOI] [PubMed] [Google Scholar]
- 15.Falconer K, Gonzalez VD, Reichard O, Sandberg JK, Alaeus A. Spontaneous HCV clearance in HCV/HIV-1 coinfection associated with normalized CD4 counts, low level of chronic immune activation and high level of T cell function. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2008;41 (2):160. doi: 10.1016/j.jcv.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Doughty AL, Zekry A, Spencer JD, Turhan S, Painter D, McCaughan GW. Spontaneous clearance of hepatitis C virus infection post-liver transplantation is associated with rapidly changing quasispecies: a single case report. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2000;6 (5):648. doi: 10.1053/jlts.2000.9740. [DOI] [PubMed] [Google Scholar]
- 17.Casanovas-Taltavull T, Ercilla MG, Gonzalez CP, et al. Long-term immune response after liver transplantation in patients with spontaneous or post-treatment HCV-RNA clearance. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2004;10 (5):584. doi: 10.1002/lt.20105. [DOI] [PubMed] [Google Scholar]
- 18.Burrell BE, Bishop DK. Th17 cells and transplant acceptance. Transplantation. 2010;90 (9):945. doi: 10.1097/TP.0b013e3181f5c3de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowan AG, Fletcher JM, Ryan EJ, et al. Hepatitis C virus-specific Th17 cells are suppressed by virus-induced TGF-beta. Journal of immunology. 2008;181 (7):4485. doi: 10.4049/jimmunol.181.7.4485. [DOI] [PubMed] [Google Scholar]
- 20.Sreenarasimhaiah J, Jaramillo A, Crippin J, Lisker-Melman M, Chapman WC, Mohanakumar T. Concomitant augmentation of type 1 CD4+ and CD8+ T-cell responses during successful interferon-alpha and ribavirin treatment for chronic hepatitis C virus infection. Human immunology. 2003;64 (5):497. doi: 10.1016/s0198-8859(03)00041-7. [DOI] [PubMed] [Google Scholar]