Abstract
Whereas, most cancer research data come from high-profile academic centers, little is known about the outcomes of cancer care in rural communities. We summarize the experience of building a multi-institution partnership to develop a cancer outcomes research infrastructure in Southwest Georgia (SWGA), a primarily rural 33-county area with over 700,000 residents. The partnership includes eight institutions: the Emory University in Atlanta, the Centers for Disease Control and Prevention (CDC), the Georgia Comprehensive Center Registry (the Registry), the Southwest Georgia Cancer Coalition (the Coalition), and the four community cancer centers located within the SWGA region. The practical application of the partnership model, its organizational structure, and lessons learned are presented using two specific examples: a study evaluating treatment decisions and quality of life among prostate cancer patients, and a study of treatment discontinuation among prostate, breast, lung, and colorectal cancer patients. Our partnership model allowed us to (1) use the Coalition as a link between Atlanta-based researchers and local community; (2) collaborate with the area cancer centers on day-to-day study activities; (3) involve the Registry personnel and resources to identify eligible cancer cases and to perform data collection; and (4) raise community awareness and sense of study ownership through media announcements organized by the Coalition. All of the above activities were performed in consultation with the funding institution (CDC) and its project directors who oversee several other studies addressing similar research questions throughout the country. Our partnership model may provide a useful framework for cancer outcomes research projects in rural communities.
Keywords: Cancer, Rural population, Outcomes research, Partnership
Introduction
Today, we know more about cancer prevention, detection, and treatment than ever before, yet not all segments of the US population have fully benefited from these advances [1]. For example, a recent study has shown that overall improvements in cancer survival have eluded populations with low levels of education [2]. Although nearly three-quarters of all cancer cases are treated in non-teaching community hospitals and clinics throughout the United States, [3] most clinical research findings and treatment recommendations are born out of experience accumulated in large academic institutions located in major metropolitan centers [4].
The optimal interpretation and application of research findings may be difficult if the study participants do not represent the population receiving cancer care [5]. Of particular concern are hard-to-reach groups that include individuals residing in rural areas. These rural residents tend to be poorer, less educated, and are more likely to be uninsured compared to their urban counterparts, and their outcomes following cancer treatment may be quite different than those reported in the literature [6].
Concerns about the applicability of cancer outcomes research findings in rural settings can only be addressed by conducting studies in these settings. However, access to data and adequate recruitment and retention of participants from rural areas is often complicated due to logistical, cultural, and economic barriers which may include: (1) financial and organizational problems associated with distances from major academic centers; (2) lack of culturally appropriate recruitment methodologies; (3) distrust of researchers and research institutions attributed, at least in part, to a legacy of past unethical practices exemplified by the Tuskegee Syphilis Experiment; and (4) lack of sense of study ownership in the community (e.g., due to perception of low relevance of the disease under investigation) [7].
Despite these barriers, progress can be made when research institutions partner with local healthcare providers and community leaders and all parties contribute to study design and study implementation [8, 9]. The following communication summarizes the experience of building a multi-institution partnership to develop a sustainable cancer outcomes research infrastructure in Southwest Georgia (SWGA), a largely rural area characterized by its relatively remote location, large geographic area, and sizeable medically underserved population. In this paper, we will identify the main components of the SWGA cancer outcomes research partnership (the Partnership), and present specific examples of the Partnership in action using case studies from two population-based projects.
Southwest Georgia
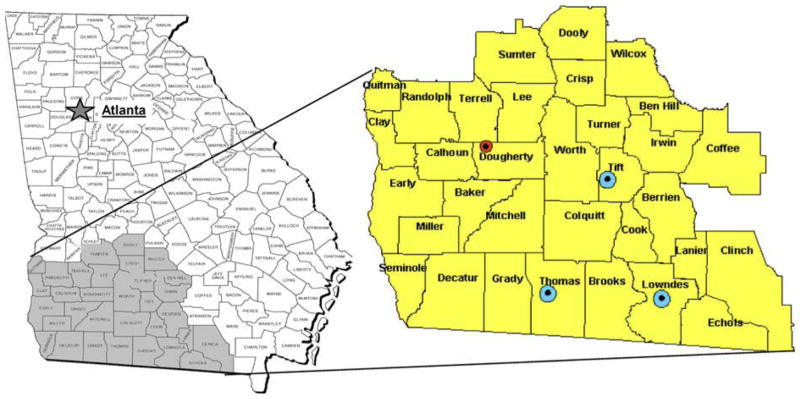
The Southwest Georgia (SWGA) region is a 33-county area with the most recent census-based population of 724,327––larger than at least four US states (Alaska, North Dakota, Vermont, and Wyoming) [10]. Figure 1 shows the location of SWGA within the state of Georgia; its territory is approximately the size of Massachusetts. Census data, summarized in Table 1, indicate that the population of SWGA differs substantially from the rest of the state and the nation as a whole in a number of respects including having a higher percentage of African-Americans, lower median household income, and lower levels of educational attainment. Only two of the 33 counties, representing 18% of the SWGA population, are classified as belonging to metropolitan statistical areas (MSA’s). For reference, 80% of the US population, and 69% of Georgia population overall, live in MSA’s [10].
Fig. 1.
The geographic location of Southwest Georgia and enlarged area showing locations of the four cancer centers and of the Southwest Georgia Cancer Coalition Headquarters. Small (red) dot indicates Southwest Georgia Cancer Coalition Headquarters and Phoebe Cancer Center in Albany; large (blue) dots indicate Tift Regional Oncology Center, Singletary Oncology Center, and Pearl-man Cancer Center in Tifton, Thomasville and Valdosta, respectively
Table 1.
Sociodemographic characteristics of the Southwest Georgia population
| Region | African-Americans % | College graduates % | Living below poverty % | Median household income |
|---|---|---|---|---|
| United States | 12.3 | 24.40 | 12.40 | $41,994 |
| Georgia | 29.10 | 24.30 | 13.00 | $42,433 |
| SWGA | 38.30 | 13.90 | 21.20 | $30,290 |
Source: U.S. Census Bureau: 2000 Census of population and housing, summary population and housing characteristics, PHC-1-12, Georgia. Washington, DC; 2002
According to the Georgia Comprehensive Cancer Registry data (Table 2), 3,196 cancers were diagnosed among residents of SWGA in 2003, which translates into an age-adjusted incidence rate of 459 cases per 100,000 persons. With respect to specific tumor sites, the incidence rates in SWGA relative to the rest of the state were higher for a number of cancers including, for example, cancers of oral cavity, esophagus, liver, lung, uterus, and prostate. By contrast, cancers of colon and rectum, pancreas, breast, and certain hematopoietic malignancies among others appeared to be less common in SWGA than in other parts of Georgia.
Table 2.
Cancer incidence and death rates in Southwest Georgia compared to the rest of the statea
| Cancer site | State of Georgia
|
Southwest Georgia
|
||||||
|---|---|---|---|---|---|---|---|---|
| Incidence rate | Cases | Death rate | Deaths | Incidence rate | Cases | Death rate | Deaths | |
| All cancers | 449.7 | 33,675 | 200 | 69,828 | 458.6 | 3,196 | 214 | 5,763 |
| Oral cavity and pharynx | 12.6 | 964 | 3 | 1,058 | 14.0 | 99 | 4.3 | 117 |
| Esophagus | 5.5 | 415 | 5 | 1,632 | 6.2 | 45 | 5.5 | 152 |
| Stomach | 6.8 | 490 | 4 | 1,433 | 6.6 | 46 | 4 | 110 |
| Colon and rectum | 50.0 | 3,631 | 19 | 6,598 | 48.5 | 337 | 20 | 539 |
| Liver and bile duct | 4.1 | 309 | 4 | 1,486 | 4.9 | 34 | 5 | 133 |
| Pancreas | 10.7 | 757 | 11 | 3,643 | 8.6 | 59 | 11.1 | 297 |
| Larynx | 4.7 | 365 | 1.5 | 550 | 4.9 | 35 | 2 | 54 |
| Lung and bronchus | 73.9 | 5,355 | 60 | 21,117 | 79.4 | 552 | 68 | 1,834 |
| Breast | 66.6 | 5,157 | 15 | 5,345 | 65.2 | 453 | 13.5 | 363 |
| Cervix uteri | 5.1 | 424 | 1.5 | 596 | 7.1 | 48 | 2.1 | 57 |
| Uterus | 10.2 | 779 | 2.2 | 770 | 13.0 | 92 | 2.7 | 73 |
| Ovary | 6.8 | 512 | 5 | 1,827 | 6.5 | 46 | 4.2 | 113 |
| Prostate | 64.7 | 4,789 | 12 | 3,687 | 67.2 | 469 | 14.2 | 377 |
| Urinary bladder | 9.3 | 652 | 4 | 1,328 | 8.4 | 58 | 3.6 | 94 |
| Kidney and renal pelvis | 13.2 | 995 | 4 | 1,424 | 13.5 | 93 | 4.3 | 117 |
| Hodgkin lymphoma | 1.9 | 165 | 0.4 | 158 | 2.8 | 21 | 0.3 | 9 |
| Non-Hodgkin lymphoma | 17.0 | 1,275 | 7 | 2,361 | 15.2 | 107 | 5.9 | 157 |
| Myeloma | 5.8 | 418 | 4 | 1,455 | 5.8 | 40 | 4.2 | 113 |
| Leukemia | 12.2 | 914 | 7 | 2,551 | 10.7 | 75 | 7.6 | 204 |
Incidence data from 2003; mortality data from 2000 to 2004. All incidence and mortality estimates are per 100,000 and age-adjusted to the 2000 US standard population
Source: Georgia Comprehensive Cancer Registry (2000–2004)
A comparison of 2000–2004 cancer mortality in SWGA to that in the entire state demonstrated higher frequency of deaths due to several cancers, which included among others, cancers of lung and bronchus, as well as cancers of the uterus, and prostate cancers. On the other hand, mortality due to certain cancers, such as breast cancer and non-Hodgkin lymphoma was lower in SWGA than in the rest of Georgia.
The Partnership
The Partnership includes eight institutions: Emory University (School of Medicine and Rollins School of Public Health) in Atlanta, the Centers for Disease Control and Prevention (CDC), the Georgia Comprehensive Center Registry, the Southwest Georgia Cancer Coalition, and the four community cancer centers located throughout the region (Phoebe Cancer Center at Phoebe Putney Memorial Hospital in Albany, Tift Regional Oncology Center at Tift Regional Medical Center in Tifton, Singletary Oncology Center at Archbold Memorial Hospital in Thomasville, and Pearlman Cancer Center at South Georgia Medical Center in Valdosta). Each of the partners contributes a unique set of strengths and of skills.
The Southwest Georgia Cancer Coalition is the key organizational force for cancer prevention, education, care and research in SWGA. It represents a diverse constituency of health care institutions and physicians; business, community and faith leaders; public health agencies; academic institutions; cancer survivors and others. Its Board of Directors includes the chief executive officers of the four SWGA cancer centers, leading cancer care providers and community representatives.
The majority of cancer cases in SWGA receive care at one (or more) of the four SWGA cancer centers accredited by the American College of Surgeons’ (ACoS) Commission on Cancer (Fig. 1). While all four facilities offer a full range of medical, surgical, and radiation oncology services, they differ substantially in cancer case load. According to data provided by the ACoS Commission on Cancer, one of these facilities (Phoebe Cancer Center in Albany) can be regarded as “high volume” by national standards; with an average of about 1,000–1,100 cancer cases per year, it ranks at about the 82nd percentile with respect to annual case load among the 1,431 approved programs across the US (personal communication with Andrew Stewart, Senior Manager of the National Cancer Data Base, June 2005). Two other SWGA facilities (Pearlman Cancer Center in Valdosta and Singletary Oncology Center in Thomasville), with annual case loads in the 400–600 range, are at about the 50th percentile; and the fourth SWGA program (Tift Regional Oncology Center in Tifton), with annual case loads of about 300–400, is at about the 30th percentile.
The Emory University Rollins School of Public Health (RSPH) includes six academic departments and hosts multiple interdisciplinary centers. More than 160 full-time, doctoral-level faculty members teach and conduct research in many areas of public health including nutrition and chronic disease, cancer causation and control, social determinants of health-risk behaviors, and cost of health care and allocation of health resources.
The Georgia Center for Cancer Statistics is based in the RSPH Department of Epidemiology. The Center was founded in 1975 as part of the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) Program. In 1995, the Georgia Department of Human Resources (GA DHR), established the state-wide Georgia Comprehensive Cancer Registry (GCCR) [11] with funding from the CDC National Program of Cancer Registries (http://www.cdc.gov/cancer/npcr). The Georgia Center for Cancer Statistics was authorized by the GA DHR to serve as its agent in conducting the day-to-day operation of the state cancer registry in addition to SEER-related activities. The GCCR operations are divided into five geographic regions, each staffed with a regional coordinator and a team of cancer registrars; the 33-county area of SWGA represents one of those regions.
Much of the financial support for cancer research projects in SWGA comes from supplemental funding to the Emory Prevention Research Centers (EPRC). The EPRC, one of 33 CDC-supported Prevention Research Centers in the country, aims to build long-term relationships for engaging communities as partners in research, to develop public health researchers’ skills for working with communities, and to create research networks for priority health issues, such as cancer prevention and control [12]. Funding for the two projects described here was secured through the Special Interest Projects (SIP) initiative, by which CDC funds collaborations between CDC scientists and academic researchers to conduct special studies. These ongoing SIP projects serve as case studies to illustrate our cancer outcomes research partnership model.
Study I: Treatment Decisions and Quality of Life Among Prostate Cancer Patients in Southwest Georgia
Research Objectives and Study Design
The purpose of this ongoing study is to determine factors that influence first course of treatment choices for men with localized prostate cancer residing in SWGA and to measure prostate cancer-specific and general quality of life (QOL) from the perspective of the patient, his family, and his health care providers. Although substantial amount of research on this topic is available, the data pertaining to underserved populations, such as rural and semi-rural men, are lacking [13].
The study design involves recruitment of 300 (target sample size) newly diagnosed localized prostate cancer patients who are <76 years of age and reside in one of the 33 SWGA counties. Men with previous history of prostate cancer and those with mental or cognitive problems are not considered eligible for the study. The main data collection procedures include three in-person patient interviews: at baseline (within 1 month after diagnosis), six-months after diagnosis, and 12 months after diagnosis. In addition to interviewing the patient, there is an interview at six-months with the primary caregiver (usually the spouse) and a written questionnaire sent 12 months after the diagnosis to the patient’s physician (usually a urologist or a radiation oncologist) responsible for the prostate cancer treatment. To verify questionnaire responses, and to learn about those patients who did not volunteer to participate, we supplemented the data collected for cancer registration purposes with information on additional variables such as the pre-diagnosis levels of prostate specific antigen (PSA), hormonal treatment, and Gleason score (a measure of cancer grade).
The Role of the Partnership
To maximize the use of the Partnership, we developed the following organizational study structure which takes advantage of strengths provided by each partner institution (Fig. 2). It is important to point out that the Partnership model for this study evolved as the study progressed and became fully developed in early 2007. In its final form the model worked as follows: newly diagnosed prostate cancer patients were identified via a rapid case ascertainment (RCA) system which was established in collaboration with GCCR specifically for this study. The RCA implementation relied on weekly transmission of pathology reports from the regional GCCR office to the Emory project staff. Community awareness of the study was raised through newspaper and broadcast media announcements organized by the SWGA Cancer Coalition. A Coalition/Emory team periodically visited local physicians to inform them of study progress and maintain support and participation. The participation of local physicians would be impossible without a local liaison. Our local liaison is a member of the Coalition Board of Directors, a clinical oncologist with long-term ties to both the patient and the health care communities in the area and a strong supporter of cancer research in SWGA.
Fig. 2.
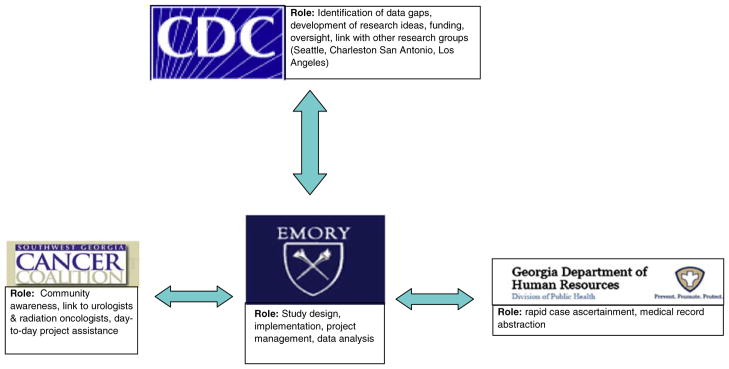
Use of the cancer research partnership to implement the study of prostate cancer treatment decisions and quality of life in Southwest Georgia
The actual enrollment involved coordinated efforts of the SWGA study personnel who sent letters to eligible patients and conducted interviews, professional Emory-based recruiters who contacted potential participants by phone, and local health care providers who distributed informational fliers and discussed the study with their patients. After the patients were enrolled, all interviews were conducted by local field investigators hired within the community and trained by the joint Emory-GCCR project team. Medical record abstraction was performed by local abstractors with oversight from the SWGA regional GCCR coordinator. All of the above activities were performed in collaboration with the CDC scientists who also acted as a link with other funded investigators conducting comparable studies in other parts of the country.
Progress to Date
The study recruitment is complete; however, interviews are still ongoing. To allow for loss to follow up we were able to enroll 330 newly diagnosed prostate cancer patients over 3 years. Of those, seven patients discontinued their participation prior to the first interview. To date, of the remaining 323 patients, 306 (95%) completed a baseline interview, 210 (69%) completed two interviews (a baseline and a 6-month) and 77 (24%) completed all three interviews. The remaining 6-and 12-month interviews will be completed in 2008.
Table 3 compares frequency of successful contact and enrollment in our study to those reported for the previously conducted multi-center Prostate Cancer Outcomes Study (PCOS), which included patients from the states of Connecticut, Utah, and New Mexico and from the metropolitan areas of Atlanta, GA, Los Angeles, CA, and Seattle, WA [14–17]. In our study, 72% of those contacted agreed to participate for an overall enrollment of 57%. This is similar to the percentage of eligible patients who enrolled at different PCOS sites (range 54% to 74%). A comparison of the pre-2007 enrollment percentages to those achieved in 2007 (after the Partnership became fully operational), demonstrated an increase from 53% to 62% (Table 3).
Table 3.
Enrollment status in the Southwest Georgia prostate cancer study compared to the NCI multicenter prostate cancer outcomes study (PCOS)
| STUDY | Eligible | Contacted
|
Recruited
|
|||
|---|---|---|---|---|---|---|
| N | N | % of eligible | N | % of contacted | % of eligible | |
| PCOS Atlanta site | 515 | 441 | 86 | 379 | 86 | 74 |
| PCOS (other sites)a | 5,157 | 4,295 | 83 | 3,154 | 73 | 61 |
| SW Georgia | 581 | 460 | 79 | 330 | 72 | 57 |
| Pre-2007 | 318 | 246 | 77 | 168 | 68 | 53 |
| 2007 | 263 | 214 | 81 | 162 | 76 | 62 |
Includes states of Connecticut, Utah, and New Mexico and the metropolitan areas of Los Angeles, CA, and Seattle, WA
Lessons Learned
The initial difficulties of the study implementation highlight the importance of partnerships in rural areas such as SWGA. The implementation of RCA involving the regional registry coordinator shortened the interval between diagnosis and recruitment from 2–3 months to just one or two weeks. Involvement of experienced Emory-based recruiters in addition to local project staff and a weekly exchange of data between the Emory and the SWGA teams ensured a systematic approach towards patient contact and enrollment. Periodic contacts with area radiation oncologists and urologists, mediated by the SWGA Cancer Coalition, also had a positive impact on recruitment as physicians and practices providing care to prostate cancer patients became aware of the study and had an opportunity to interact with researchers.
Study II: Treatment Discontinuation Among Prostate, Breast, Lung, and Colorectal Cancer Patients in Southwest Georgia
Research Objectives and Study Design
The primary aims of this study are to examine the frequency of failure to complete all cancer treatment prescribed within the first year among residents of SWGA and to investigate patient-, provider-, tumor-, and health system-related factors associated with premature treatment discontinuation in this population. This is a retrospective chart review of breast cancer, lung cancer, colorectal cancer, and prostate cancer cases diagnosed and treated between 2001 and 2003 in SWGA. Approximately 15% of cases (n = 631) that were known to receive treatment outside the 33 county study area were excluded from the study. All data were extracted from medical records by trained local abstractors and included patient demographics, co-morbidities, insurance status, treatment plan, whether the plan was followed, type of surgery, dates and doses of radiation therapy, dates, doses and drugs delivered for chemotherapy cycles, and hormonal therapy type and dates given. Treatment information was limited to the first 12 months post-diagnosis. Reasons for treatment discontinuation were determined from documentation in medical records.
The Role of the Partnership
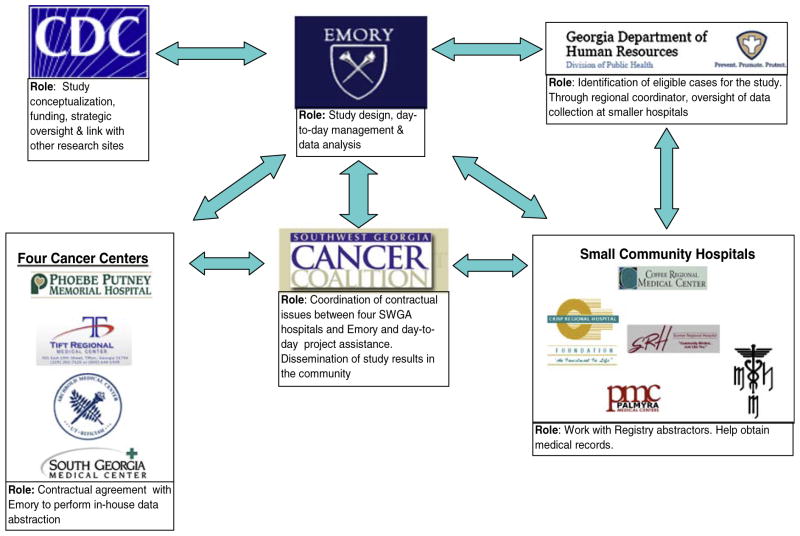
The Partnership model and the role of each partner in study implementation are shown in Fig. 3. Briefly, the Partnership model for this study worked as follows: cases were identified by the Registry and their medical records were obtained from the four SWGA Cancer Centers and from small area hospitals and medical practices. Once eligible cases were identified, the Registry/Emory project team developed a list of patients treated at each facility.
Fig. 3.
Use of the cancer research partnership to implement the study of treatment discontinuation in Southwest Georgia
The actual medical record abstraction was divided among five teams: one team assigned to each of the four SWGA cancer centers and the fifth team was charged with data abstraction from smaller hospitals and free-standing clinics. Each cancer center-based team was employed by the respective institution, but their project-related work was funded by the study. The SWGA Cancer Coalition acted as a liaison between Emory and the four Cancer Centers to help coordinate research efforts and contractual issues. The fifth study team was employed directly by the study and all work was performed under the supervision of the regional Registry coordinator using trained Registry abstractors. Thus, patient confidentiality was maintained at all times because medical record abstraction was performed either in-house or under the auspices of the cancer Registry which has established data collection and handling procedures that are mandated by law.
The above efforts were performed in collaboration with the funding institution (CDC) and its scientists, who conceptualized, sponsored and supervised several other studies addressing similar research questions. This ‘big-picture’ oversight by CDC allows future data pooling as well as inter-regional and urban-rural comparisons.
Progress to Date
Data abstraction is now complete for 3,898 medical records, which represents over 97% of all eligible cases. The summary of medical record abstraction is presented in Table 4. Not all records required abstraction as many localized prostate cancer and early-stage breast and colorectal cancer patients were treated with surgery only and thus were not at risk for treatment discontinuation. Records that did require abstraction were most commonly those of lung cancer patients (31.5%) followed by records of patients treated for prostate cancer (27.6%), breast cancer (26.8%) and colorectal cancer (14.1%).
Table 4.
Summary of medical records abstracted for the cancer treatment discontinuation study by age, race, and cancer care facility
| Patient characteristics | All cases (N = 3996) | Breast cancer (N = 1096) | Prostate cancer (N = 1103) | Lung cancer (N = 1259) | Colorectal cancer (N = 565) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | Number | % | ||
| Age | |||||||||
| <65 | 1,780 (44.5%) | 643 | 60.1 | 357 | 32.4 | 510 | 40.5 | 270 | 47.8 |
| ≥65 | 2,216 (55.5%) | 426 | 39.9 | 746 | 67.6 | 749 | 59.5 | 295 | 52.2 |
| Race | |||||||||
| White | 2,656 (66.5%) | 738 | 69.0 | 617 | 55.9 | 926 | 73.6 | 375 | 66.4 |
| Black | 1,319 (33.0%) | 322 | 30.1 | 478 | 43.3 | 329 | 26.1 | 190 | 33.6 |
| Other/Unknowna | 21 (0.5%) | 9 | 0.8 | 8 | 0.7 | 4 | 0.3 | 0 | 0.0 |
| Facility | |||||||||
| Phoebe cancer center | 1,582 (39.6%) | 433 | 40.5 | 436 | 39.5 | 526 | 41.8 | 187 | 33.1 |
| Singletary oncology center | 746 (18.7%) | 165 | 15.4 | 251 | 22.8 | 239 | 19.0 | 91 | 16.1 |
| Pearlman cancer center | 724 (18.1%) | 197 | 18.4 | 170 | 15.4 | 268 | 21.3 | 89 | 15.8 |
| Tift regional oncology center | 436 (10.9%) | 126 | 11.8 | 119 | 10.8 | 110 | 8.7 | 81 | 14.3 |
| All other SWGA facilities | 508 (12.7%) | 148 | 13.8 | 127 | 11.5 | 116 | 9.2 | 117 | 20.7 |
Includes Asians/Pacific Islanders and Hispanics, which are not reported due to small numbers
With the exception of breast cancer, most cases abstracted for the study were 65 years of age or older. African-Americans represented approximately one-third of all cases. The proportion of cases that were treated outside of the four major cancer centers ranged from 9% for lung cancer to 21% for colorectal cancer. Thus, the study presents an opportunity to examine cancer treatment outcomes in a previously understudied population that includes rural whites and blacks and an interesting subgroup of patients receiving care at small rural community hospitals.
Lessons Learned
During study implementation it became clear that cancer patients often receive care at more than one SWGA facility. For example, a patient diagnosed with early-stage lung cancer at Tift Regional Oncology Center may undergo surgery at Phoebe Cancer Center and then receive chemotherapy at a smaller hospital closer to home. As a result there may be a need to combine multiple abstracts pertaining to the same patient into a single record.
Although we were successful in obtaining detailed information about radiation, surgery, and chemotherapy, the data regarding hormonal therapy were less complete. Abstracting of all outpatient medical records related to hormone therapy was not feasible within the scope of this study; however, it may be possible to obtain hormonal therapy information by linking registry data to ancillary sources such as Medicare or Medicaid claims.
It also became evident that even very detailed data abstraction instruments fail to capture all possible scenarios leading to treatment discontinuation. To document information that cannot be readily coded, we instructed abstractors to use free-text fields which we then reviewed and coded during data cleanup.
Discussion
Our case studies demonstrate a workable model for engaging state and federal public health agencies, academia, local health care institutions, and the community in a long-term research partnership to study cancer outcomes in rural Georgia. In this model, each of the partners plays a unique role that allows addressing scientific, logistical, cultural, and economic obstacles that cannot be overcome by any single institution.
The state Cancer Registry allows rapid identification of new cases for prospective follow up and selection of existing cases for retrospective studies. Availability of regional coordinators and trained abstractors facilitates efficient data collection and transfer enhanced by the already-established relationships between the registry and the hospitals. The use of existing protocols and software minimizes the time required to develop data collection tools for new studies. Despite its strengths, the Cancer Registry does not have sufficient funds or personnel to conduct original hypothesis-driven research.
The expertise of the Emory faculty includes nearly all aspects of a successful scientific project including proposal writing, study design, day-to-day study management, data analysis, and reporting. Nevertheless, much of Emory’s population-based cancer outcomes research in Georgia has been conducted within metropolitan Atlanta, and without a local partner, such as the SWGA Cancer Coalition, obtaining cooperation of local physicians and successful recruitment and retention of participants in Southwest Georgia presents a challenge.
The Coalition’s active involvement in research projects greatly increases the likelihood of obtaining support and collaboration of the community leaders, including local politicians, health care providers, educators, and members of the faith community. Moreover, by serving as a link between Emory-based researchers, SWGA hospitals and local investigators (project managers, interviewers and abstractors), the Coalition ensures a more effective use of funding. The Coalition’s mission includes providing opportunities for residents of SWGA to participate in research studies; however, the Coalition lacks expertise to lead federally-funded studies. Access to such studies requires collaborations with Emory and other academic centers.
Both studies reported in this communication were conceptualized and funded by CDC as part of the strategic plan to involve communities as partners in research; and in both research projects, SWGA was only one of several funded sites. For the prostate cancer quality of life study (Study I) other funded sites included Seattle, WA, Los Angeles, CA, Charleston, SC, and San Antonio, TX; whereas, the counterparts for the treatment discontinuation study (Study II) included Birmingham AL, and Seattle, WA. Thus, the benefits of CDC involvement extend beyond funding. Perhaps as importantly, CDC leadership identifies gaps in research and allows addressing similar research questions in diverse geographic areas and in different populations.
Partnerships similar to ours have been described previously. For example, the National Cancer Institute initiated the development of the Special Populations Network (SPN) program, a nationwide initiative that included several independent yet coordinated projects [18]. At the present time the existing projects involve such diverse populations as New York City immigrants [19]; the Asian-American communities in Boston, New York, Houston, Seattle, California and Hawaii [20]; the Native American and Alaska Natives in Western and Midwestern states and Alaska [21]; and the underserved communities in Maryland [22, 23]. The one SPN project that involves a population, which is geographically and demographically most similar to ours, is the Deep South Network (DSN), which includes selected rural and urban areas in Mississippi and Alabama [24]. After five years of operation, the DSN has been reported to be effective in raising cancer awareness, improving education and outreach to the target population, and increasing the use of cancer screening services [25].
Although the SWGA Partnership and the Deep South Network have a number of similarities, the two projects are also different in several respects. First, unlike the DSN, the SWGA Partnership did not develop as an externally funded infrastructure-building project. Rather, it evolved out of necessity, driven by specific research needs and using already existing organizations united by common interests. Second, while the main focus of DSN is on African-American populations living in selected communities, the SWGA Partnership is focused on a large well-defined geographic area with a population comprised of about 60% whites and 40% blacks. Finally, the initial DSN activities involved education and outreach whereas, the SWGA Partnership developed in the context of clinical outcomes research.
The methodological differences notwithstanding, both the DSN and the SWGA Partnership serve the common cause of eliminating cancer disparities. Both the CDC and the NCI continue to identify the reduction of cancer-related health disparities and a better understanding of cancer outcomes in different populations, as priorities for the near future [26, 27]. These priorities require lasting partnerships that involve members of academia, the government, and local communities, and such partnerships must maintain a sense of mutual trust. Our experience and the experience of others provide a framework for future studies and public health interventions in underserved areas.
Acknowledgments
Funding was made possible by cooperative agreement # U48 DP 000043 for the Emory Prevention Research Center, from the Centers for Disease Control and Prevention (CDC). The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Contributor Information
Michael Goodman, Email: mgoodm2@sph.emory.edu, Department of Epidemiology, Emory University Rollins School of Public Health, 1518 Clifton Road, NE, Atlanta, GA 30322, USA.
Lyn Almon, Georgia Comprehensive Cancer Registry, 2 Peachtree Street, Atlanta, GA 30303-3142, USA.
Rana Bayakly, Georgia Comprehensive Cancer Registry, 2 Peachtree Street, Atlanta, GA 30303-3142, USA.
Susan Butler, Emory University Rollins School of Public Health, 1518 Clifton Road, NE, Atlanta, GA 30322, USA.
Carol Crosby, Georgia Comprehensive Cancer Registry, 2 Peachtree Street, Atlanta, GA 30303-3142, USA.
Colleen DiIorio, Emory University Rollins School of Public Health, 1518 Clifton Road, NE, Atlanta, GA 30322, USA.
Donatus Ekwueme, Division of Cancer Prevention and Control, National Center for Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, 4770 Buford Highway, MS K-55, Atlanta, GA 30341, USA.
Diane Fletcher, Southwest Georgia Cancer Coalition, Inc, 507 West Third Ave, Suite 6A, Albany, GA 31701, USA.
John Fowler, Georgia Comprehensive Cancer Registry, 2 Peachtree Street, Atlanta, GA 30303-3142, USA.
Theresa Gillespie, Emory University School of Medicine, 1365 Clifton Road, NE, Atlanta, GA 30322, USA.
Karen Glanz, Emory University Rollins School of Public Health, 1518 Clifton Road, NE, Atlanta, GA 30322, USA.
Ingrid Hall, Division of Cancer Prevention and Control, National Center for Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, 4770 Buford Highway, MS K-55, Atlanta, GA 30341, USA.
Judith Lee, Division of Cancer Prevention and Control, National Center for Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, 4770 Buford Highway, MS K-55, Atlanta, GA 30341, USA.
Jonathan Liff, Emory University Rollins School of Public Health, 1518 Clifton Road, NE, Atlanta, GA 30322, USA.
Joseph Lipscomb, Emory University Rollins School of Public Health, 1518 Clifton Road, NE, Atlanta, GA 30322, USA.
Lori A. Pollack, Division of Cancer Prevention and Control, National Center for Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, 4770 Buford Highway, MS K-55, Atlanta, GA 30341, USA
Lisa C. Richardson, Division of Cancer Prevention and Control, National Center for Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, 4770 Buford Highway, MS K-55, Atlanta, GA 30341, USA
Phillip Roberts, Southwest Georgia Cancer Coalition, Inc, 507 West Third Ave, Suite 6A, Albany, GA 31701, USA.
Kyle Steenland, Emory University Rollins School of Public Health, 1518 Clifton Road, NE, Atlanta, GA 30322, USA.
Kevin Ward, Georgia Comprehensive Cancer Registry, 2 Peachtree Street, Atlanta, GA 30303-3142, USA.
References
- 1.Institute of Medicine, Committee on Cancer Research among Minorities and the Medically Underserved. The unequal burden of cancer: An assessment of NIH research and programs for ethnic minorities and the medically underserved. Washington, DC: National Academy Press; 1999. [PubMed] [Google Scholar]
- 2.Kinsey T, Jemal A, Liff J, Ward E, Thun M. Trends in mortality from four major cancers by education, United States, 1993–2001. American Association for cancer research. First conference on the science of cancer health disparities; Atlanta, GA: 2007. [Google Scholar]
- 3.Menck HR, Cunningham MP, Jessup JM, Eyre HJ, Winchester DP, Scott-Conner CE, et al. The growth and maturation of the National Cancer Data Base. Cancer. 1997;80(12):2296–2304. doi: 10.1002/(sici)1097-0142(19971215)80:12<2296::aid-cncr11>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 4.Center for Health Policy Research and Ethics, C. Linking rural health services research with health policy. Pentagon City, VA: 2000. [Google Scholar]
- 5.Fayter D, McDaid C, Eastwood A. A systematic review highlights threats to validity in studies of barriers to cancer trial participation. Journal of Clinical Epidemiology. 2007;60(10):990–1001. doi: 10.1016/j.jclinepi.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Engelman KK, Perpich DL, Peterson SL, Hall MA, Ellerbeck EF, Stanton AL. Cancer information needs in rural areas. Journal of Health Communication. 2005;10(3):199–208. doi: 10.1080/10810730590934217. [DOI] [PubMed] [Google Scholar]
- 7.Paskett ED, Cooper MR, Stark N, Ricketts TC, Tropman S, Hatzell T, et al. Clinical trial enrollment of rural patients with cancer. Cancer Practice. 2002;10(1):28–35. doi: 10.1046/j.1523-5394.2002.101006.x. [DOI] [PubMed] [Google Scholar]
- 8.Couto R, Simpson N, Harris G. Sowing seeds in the mountains: Community based coalitions for cancer prevention and control. Rockville, MD: National Cancer Institute; 1994. NIH Publication No. 94-3779. [Google Scholar]
- 9.Lopez D, Chen M, Maddox Y, Boyce C, Burhansstipanov L, Clanton M, Freeman H, Gines V, Hiatt R, Howard G, et al. Making cancer health disparities history: Report to the trans-HHS cancer health disparities progress review group. Washington, DC: 2004. [Google Scholar]
- 10.U.S. Census Bureau. 2000 Census of population and housing, summary population and housing characteristics, PHC-1-12. Georgia; Washington, DC: 2002. [Google Scholar]
- 11.Georgia Department of Human Resources, and Health, Division of Public Health. Georgia comprehensive cancer registry policy and procedure manual for reporting facilities. Atlanta, GA: 2006. [Google Scholar]
- 12.Centers for Disease Control and Prevention. Facts about the PRC Program. 2007 Retrieved 12/28/2007, from http://www.cdc.gov/prc/about-prc-program/facts-about-prc-program.htm.
- 13.Ramsey S, Zeliadt S, Hall I, Ekwueme D, Penson D. On the importance of race, socioeconomic status and comorbidity when evaluating quality of life in men with prostate cancer. Journal of Urology. 2007;177(6):1992–1999. doi: 10.1016/j.juro.2007.01.138. [DOI] [PubMed] [Google Scholar]
- 14.Gilliland FD, Hoffman RM, Hamilton A, Albertsen P, Eley JW, Harlan L, et al. Predicting extracapsular extension of prostate cancer in men treated with radical prostatectomy: Results from the population based prostate cancer outcomes study. Journal of Urology. 1999;162(4):1341–1345. [PubMed] [Google Scholar]
- 15.Potosky AL, Harlan LC, Stanford JL, Gilliland FD, Hamilton AS, Albertsen PC, et al. Prostate cancer practice patterns and quality of life: The prostate cancer outcomes study. Journal of the National Cancer Institute. 1999;91(20):1719–1724. doi: 10.1093/jnci/91.20.1719. [DOI] [PubMed] [Google Scholar]
- 16.Potosky AL, Legler J, Albertsen PC, Stanford JL, Gilliland FD, Hamilton AS, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: Results from the prostate cancer outcomes study. Journal of the National Cancer Institute. 2000;92(19):1582–1592. doi: 10.1093/jnci/92.19.1582. [DOI] [PubMed] [Google Scholar]
- 17.Stanford JL, Feng Z, Hamilton AS, Gilliland FD, Stephenson RA, Eley JW, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: The prostate cancer outcomes study. Journal of the American Medical Association. 2000;283(3):354–360. doi: 10.1001/jama.283.3.354. [DOI] [PubMed] [Google Scholar]
- 18.Van Duyn MA, Reuben SH, Macario E. Special populations networks: Themes and lessons learned. Cancer. 2006;107(Suppl 8):1945–1954. doi: 10.1002/cncr.22165. [DOI] [PubMed] [Google Scholar]
- 19.Gany FM, Shah SM, Changrani J. New York city’s immigrant minorities. Reducing cancer health disparities. Cancer. 2006;107(Suppl 8):2071–2081. doi: 10.1002/cncr.22155. [DOI] [PubMed] [Google Scholar]
- 20.Chen MS, Shinagawa SM, Bal DG, Bastani R, Chow EA, Ho RC, et al. Asian American network for cancer awareness, research, and training’s legacy. The first 5 years. Cancer. 2006;107(Suppl 8):2006–2014. doi: 10.1002/cncr.22160. [DOI] [PubMed] [Google Scholar]
- 21.Kaur JS, Dignan M, Burhansstipanov L, Baukol P, Claus C. The “Spirit of Eagles” legacy. Cancer. 2006;107(Suppl 8):1987–1994. doi: 10.1002/cncr.22152. [DOI] [PubMed] [Google Scholar]
- 22.Baquet CR, Mack KM, Bramble J, DeShields M, Datcher D, Savoy M, et al. Maryland’s special populations cancer network: Cancer health disparities reduction model. Journal of Health Care for the Poor and Underserved. 2005;16(2):192–206. doi: 10.1353/hpu.2005.0022. [DOI] [PubMed] [Google Scholar]
- 23.Baquet CR, Mack KM, Mishra SI, Bramble J, Deshields M, Datcher D, et al. Maryland’s special populations network. A model for cancer disparities research, education, and training. Cancer. 2006;107(Suppl 8):2061–2070. doi: 10.1002/cncr.22158. [DOI] [PubMed] [Google Scholar]
- 24.Partridge EE, Fouad MN, Hinton AW, Hardy CM, Liscovicz N, White-Johnson F, et al. The Deep South network for cancer control: Eliminating cancer disparities through community-academic collaboration. Family and Community Health. 2005;28(1):6–19. doi: 10.1097/00003727-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Lisovicz N, Johnson RE, Higginbotham J, Downey JA, Hardy CM, Fouad MN, et al. The Deep South network for cancer control. Building a community infrastructure to reduce cancer health disparities. Cancer. 2006;107(Suppl 8):1971–1979. doi: 10.1002/cncr.22151. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, and Division of Cancer Prevention and Control. National comprehensive cancer control program 2006/2007. Atlanta, GA: 2006. [Google Scholar]
- 27.Niederhuber J. The nation’s investment in cancer research: A plan and budget proposal for fiscal year 2008 (NIH Publication No 06–6090) Rockville, MD: National Cancer Institute; 2007. [Google Scholar]