Abstract
BACKGROUND
This study examined the vasoprotective role of circulating angiotensin II (ANG II) levels in the cerebral circulation of high salt (HS)–fed (SS.BN-(D13hmgc41-13hmgc23)/Mcwi) (Ren1-BN) congenic rats, which carry a normally functioning renin allele from the Brown Norway (BN) rat on the Dahl salt-sensitive genetic background.
METHODS
Ren1-BN rats were placed on an HS (4.0% NaCl) diet for 3 days. The vasodilator response to acetylcholine (ACh; 10−10 – 10−6 mol/L) was assessed in isolated middle cerebral arteries (MCAs), and Western blots were performed to assess the expression of the antioxidant enzymes copper (Cu)/zinc (Zn) superoxide dismutase (SOD) and manganese (Mn) SOD in cerebral resistance vessels. A separate group of HS-fed animals were infused with either a subpressor dose of ANG II (100ng/kg/min) or saline vehicle via osmotic minipump for 3 days.
RESULTS
HS diet eliminated acetylcholine (ACh)-induced dilation in the MCAs of the congenic rats. Western blot analysis of antioxidant enzymes showed that Cu/Zn SOD and Mn SOD expression were significantly reduced in the cerebral resistance arteries of the HS-fed rats compared with control animals fed a normal salt diet. Infusion of ANG II restored the vasodilator response to ACh in the MCAs and increased Cu/Zn SOD (but not Mn SOD) expression compared with saline-infused animals.
CONCLUSIONS
These results indicate that prevention of salt-induced ANG II suppression prevents vascular dysfunction in the cerebral circulation by preventing the downregulation of Cu/Zn SOD and vascular oxidant stress that normally occurs with HS diet.
Keywords: angiotensin II, antioxidant enzymes, blood pressure, cerebral circulation, endothelial dysfunction, high salt, hypertension.
Endothelial dysfunction in salt-sensitive (SS) hypertensive patients is well characterized. However, recent studies have shown that short-term increases in dietary salt intake can also reduce vascular function in humans independent of changes in blood pressure.1,2 Consistent with this observation, multiple studies in rats3–10 and mice11 have shown blood pressure–independent impairment of vascular relaxation with increased dietary salt intake in multiple vascular beds, including the cerebral circulation.3,7,12 The loss of nitric oxide–mediated vasodilation in animals fed a high salt (HS) diet appears to be due to the suppression of the renin–angiotensin system because chronic intravenous infusion of a subpressor dose of angiotensin II (ANG II) to prevent salt-induced ANG II suppression13–15 restores normal vascular relaxation mechanisms7,12,16 and returns vascular superoxide levels to normal salt values in mesenteric resistance arteries.4
The goal of this study was to determine the effects of a short-term increase in dietary salt intake on endothelium-dependent vasodilation in response to acetylcholine (ACh) in middle cerebral arteries (MCAs) of the narrowed Ren1-BN congenic rat strain (SS.BN-(D13hmgc41-D13hmgc23)/Mcwi). Previous studies in this congenic rat strain have shown that these animals have genetically rescued vasodilator responses to ACh and higher expression of copper (Cu)/zinc (Zn) superoxide dismutase (SOD) in the cerebral vasculature compared with the Dahl SS parental strain because of the presence of the Brown Norway (BN) renin allele in the Dahl SS genetic background.17 The use of this congenic rat strain provides a unique model to study the specific role of the renin–angiotensin system on vascular function because, aside from a 2.0 Mbp region of chromosome 13 that contains the BN renin gene, the rats are genetically identical to the Dahl SS strain. In this study, we hypothesized that salt-induced ANG II suppression would eliminate endothelium-dependent dilation in MCAs of the congenic rats due to downregulation of Cu/Zn SOD and manganese (Mn) SOD expression in the cerebral vasculature. We also hypothesized that restoring ANG II to physiological levels during HS intake would restore endothelium-dependent vascular relaxation by increasing SOD expression in the cerebral vasculature.
MATERIALS AND METHODS
General procedures
All experiments were performed in age-matched (10–14 weeks old) male Ren1-BN congenic rats (SS.BN-(D13hmgc41-13hmgc23)/Mcwi) that were derived from backcrossing SS 13BN consomic rats to the parental Dahl SS rat strain, as previously described.18 All animals were maintained on a normal salt (0.4% NaCl) diet (Dyets, Bethlehem, PA) from weaning, with water to drink ad libitum. Additional experimental groups (n = 5–9) were switched to an HS diet (4.0% NaCl) for 3–5 days. In a separate group of Ren1-BN congenic rats, osmotic minipumps (ALZET, Cupertino, CA) containing either a subpressor dose of ANG II (100ng/kg/min) or saline vehicle were implanted subcutaneously on the day that the animals were switched to an HS diet. All procedures were approved by the Medical College of Wisconsin’s Institutional Animal Care and Use Committee.
Isolated vessel preparation and vasodilator stimuli
On the day of the experiment, animals were anesthetized with an intraperitoneal injection of a low dose of pentobarbital sodium (30mg/kg; Ovation Pharmaceuticals, Lake Forest, IL) because of the enhanced sensitivity of SS rats to barbiturate anesthesia. Mean arterial pressure of the anesthetized animals was measured by carotid artery cannulation.
MCAs were isolated from the ventral surface of the brain, cannulated with tapered glass micropipettes, and maintained at 37 ºC in a heated chamber for 1 hour while they were perfused and superfused with physiological salt solution bubbled with a 21% oxygen, 5% carbon dioxide, 74% nitrogen gas mixture, as previously described.19 The vessels were pressurized to 80mm Hg to simulate in vivo conditions, and internal diameters were measured by television microscopy. The response of the arteries to the endothelium-dependent vasodilator ACh (10−10 – 10−6 mol/L) were assessed by measuring vessel diameters during cumulative addition of the agonists to the tissue bath.
Western blot analysis of endothelial nitric oxide synthase (eNOS), phosphorylated e-NOS (p-eNOS) (S1177), Mn-SOD and Cu/Zn SOD enzyme expression
Protein expression of the antioxidant enzymes Cu/Zn SOD and Mn SOD, total expression of eNOS, and relative phosphorylation of eNOS at the S1177 activator site were assessed by performing Western blots using cerebral resistance arteries dissected from the ventral surface of the brain after the MCAs were removed for study, as previously described.17 Protein bands were quantified using the UNSCAN-IT software (Silk Scientific, Orem, UT), and final expression was normalized to β-actin. Relative phosphorylation of eNOS was normalized to total eNOS expression.
Statistical analysis
All data are summarized as mean value ± SEM. Because of limited availability of Ren1-BN rats, vessel responses to ACh in HS-fed control rats were compared with previously reported responses determined in congenic rats fed normal salt diet17 using 2-way analysis of variance with a Holm–Sidak test post hoc, and responses to ACh from control diameter in HS-fed Ren1-BN rats were also evaluated using a paired t test with a Bonferroni correction for multiple comparisons. Differences in enzyme expression between 2 groups were assessed using an unpaired Student t test. A probability value of P < 0.05 was considered to be statistically significant.
RESULTS
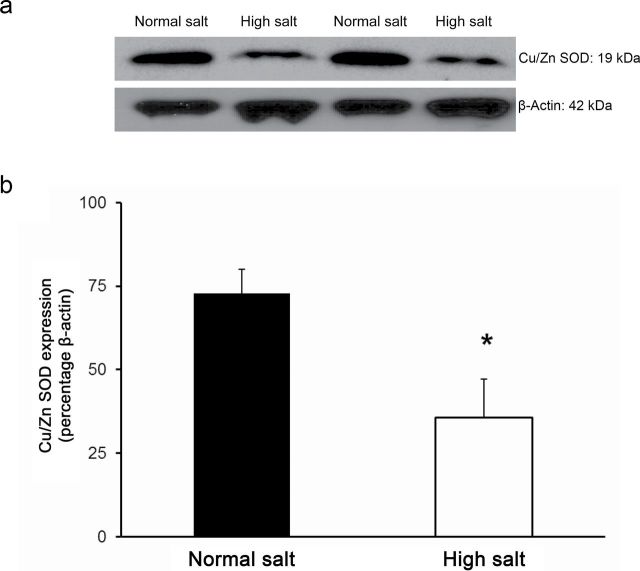
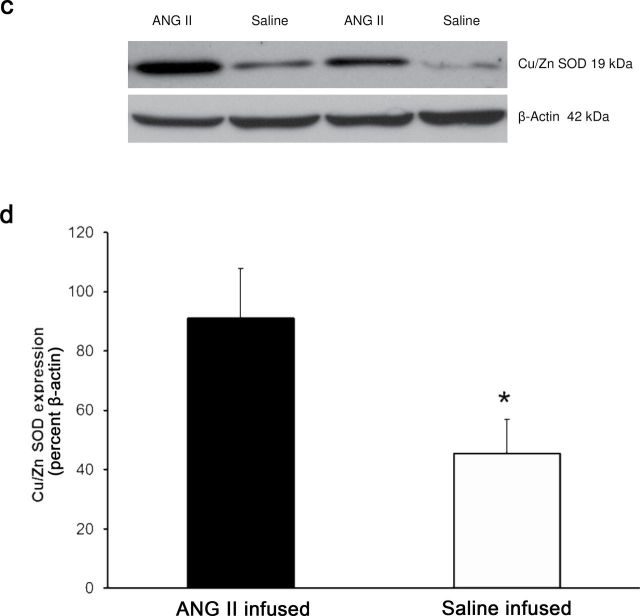
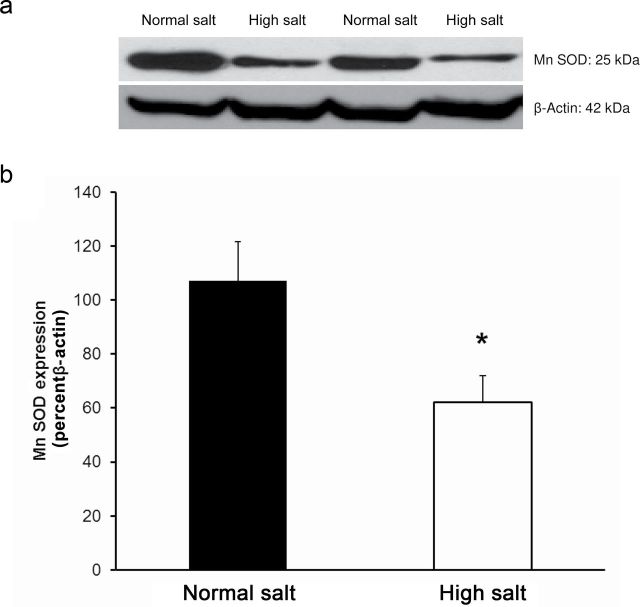
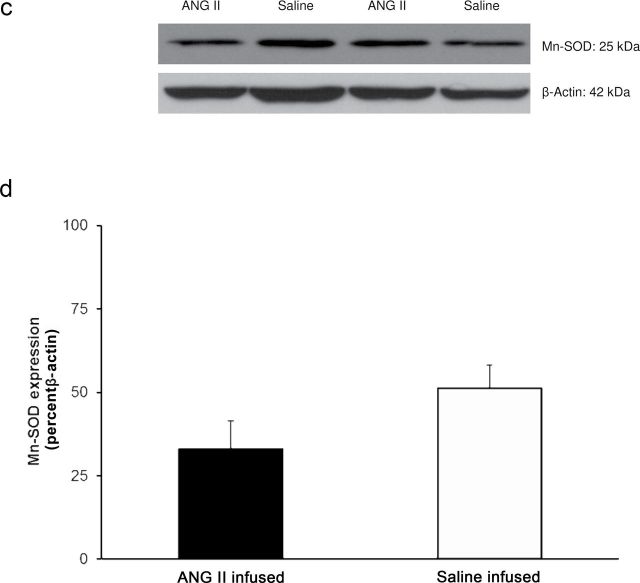
Placing Ren1-BN congenic rats on an HS diet for 3–5 days eliminated the vasodilator response to acetylcholine (ACh) in isolated MCAs (Figure 1a). Chronic infusion of a low dose of ANG II (100ng/kg/min) for 3 days ameliorated endothelial dysfunction in cerebral arteries of HS-fed Ren1-BN congenic rats (Figure 1b), as evidenced by the restoration of ACh-induced vasodilation in arteries of the ANG II-infused rats vs. saline-infused controls. ANG II infusion had no effect on mean arterial pressure in Ren1-BN rats fed an HS diet (113±5.3mm Hg in ANG II-infused rats (n = 8) vs. 111±5.5mm Hg in vehicle-infused controls (n = 7)), and vessel responses to the endothelium-independent vasodilator sodium nitroprusside were unaffected by ANG II infusion in HS-fed rats (data not shown). eNOS expression and phosphorylation of eNOS at S1177 in the cerebral vasculature were unaffected by increased dietary salt intake (Figure 2, a–c). In a similar fashion, ANG II infusion had no effect on eNOS expression or phosphorylation at S1177 in the cerebral arteries of congenic rats fed an HS diet (Figure 2, d–f). However, an HS diet caused a significant reduction in the expression of Cu/Zn SOD (Figure 3a,b) and Mn SOD (Figure 4a,b) in cerebral arteries compared with normal salt–fed controls. ANG II infusion caused a significant increase in Cu/Zn SOD expression in arteries of Ren1-BN rats fed an HS diet. (Figure 3c,d), whereas Mn-SOD expression was unaffected by ANG II infusion (Figure 4c,d).
Figure 1.
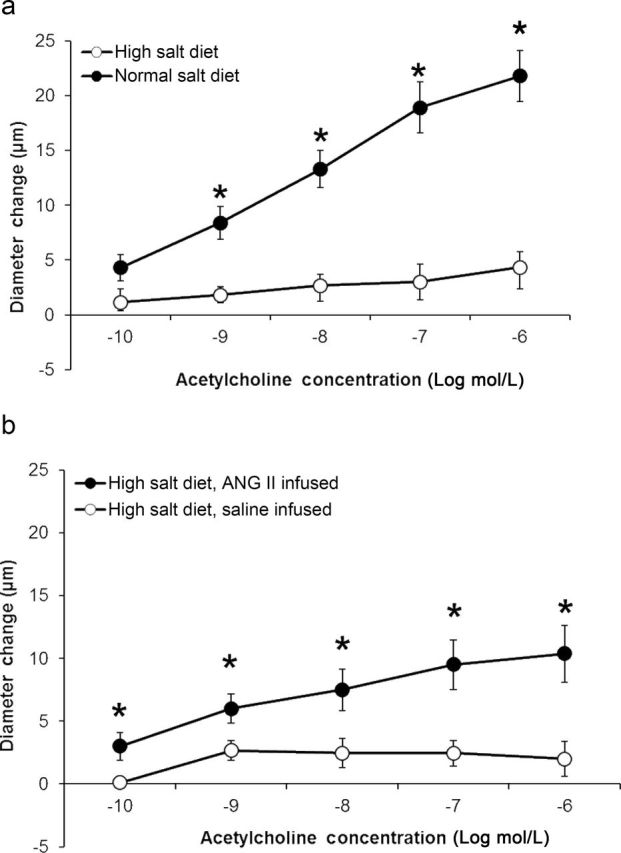
Evaluation of endothelium-dependent dilation to acetylcholine in cerebral arteries of Ren1-BN congenic rats. (a) Response to acetylcholine (10−10 – 10−6 mol/L) in isolated middle cerebral arteries from Ren1-BN congenic rats fed either a normal salt (n = 10) or high salt (n = 6) diet. Because of the limited availability of Ren1-BN rats, the normal salt response is replotted from Durand et al.17 (b) Response to acetylcholine (10−10 – 10−6 mol/L) in isolated middle cerebral arteries from high salt–fed Ren1-BN congenic rats that received an infusion of either angiotensin II (100ng/kg/min; n = 8) or saline vehicle (n = 9) by subcutaneously implanted osmotic minipump. Data are expressed as mean change from baseline diameter (µm). *Significant difference (P < 0.05) in high salt–fed rats vs. normal salt–fed controls (a) or in saline-infused rats vs. angiotensin II–infused rats fed a high salt diet (b). Acetylcholine had no significant effect on vessel diameter in high salt–fed Ren1-BN congenic rats.
Figure 2.
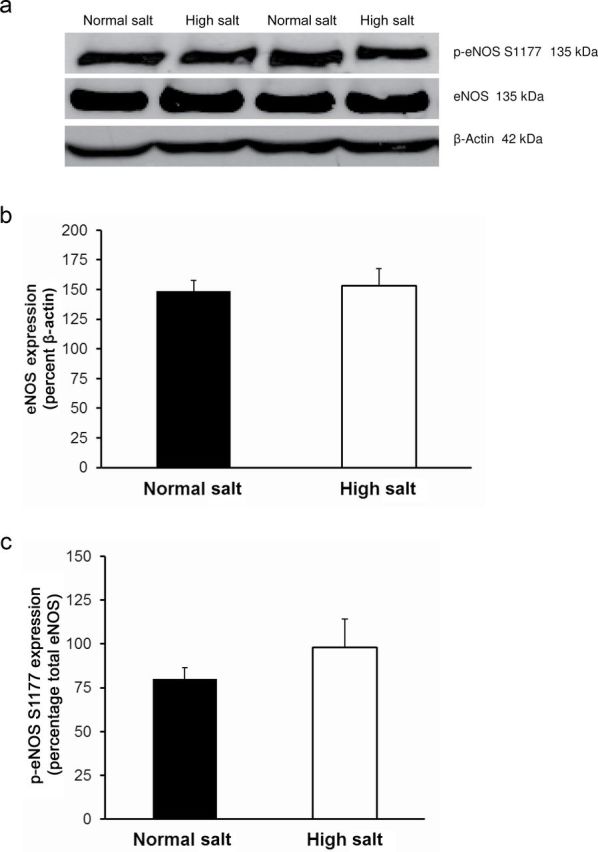
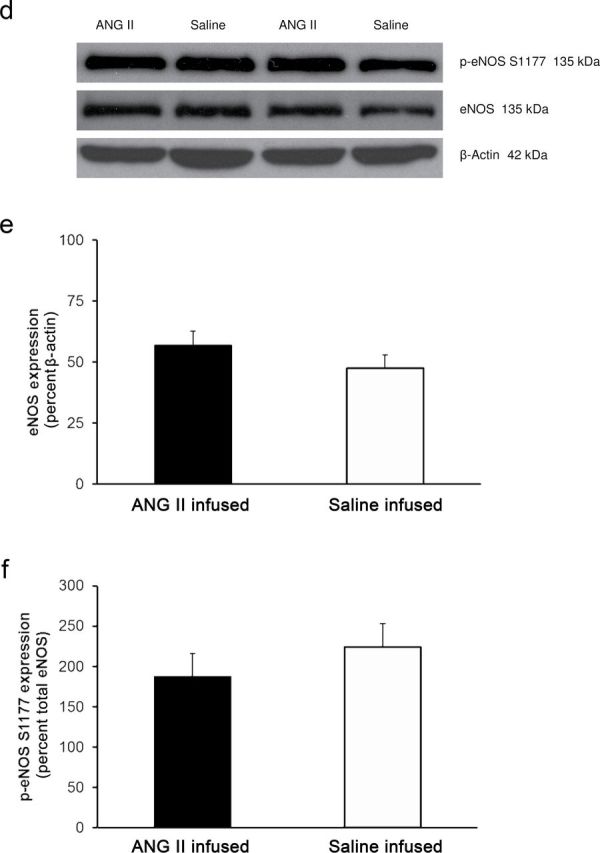
Endothelial nitric oxide synthase expression and phosphorylation at the S1177 activator site in cerebral arteries from normal salt–fed (n = 8) and high salt–fed (n = 6) Ren1-BN congenic rats (a–c) and high salt–fed Ren1-BN congenic rats receiving an infusion of either angiotensin II (ANG II; 100ng/kg/min; n = 4) or isotonic saline vehicle (n = 5) by subcutaneously implanted osmotic minipump (d–f). Panels a and d show representative Western blots of eNOS and phospho-eNOS (S1177). Panels b and e show total eNOS expression normalized to β-actin. Panels c and f show phosphorylation of eNOS at S1177, normalized to total eNOS. All values are summarized as mean ± SEM.
Figure 3.
Copper (Cu)/zinc (Zn) superoxide dismutase (SOD) expression in cerebral arteries from Ren1-BN congenic rats fed a normal salt (n = 4) or high salt (n = 5) diet (a,b) and Ren1-BN congenic rats fed a high salt diet and receiving infusion of either angiotensin II (ANG II; 100ng/kg/min; n = 4) or isotonic saline vehicle (n = 6) by subcutaneously implanted osmotic minipump (c,d). Cu/Zn SOD expression was normalized to β-actin, and all values are summarized as mean ± SEM. *Significant difference (P < 0.05) in high salt–fed rats vs. normal salt–fed rats or in high salt–fed + angiotensin II–infused rats vs. high salt–fed + saline-infused rats.
Figure 4.
Manganese (Mn) superoxide dismutase (SOD) expression in cerebral arteries from Ren1-BN congenic rats fed a normal salt (n = 8) or high salt (n = 7) diet (a,b) and Ren1-BN congenic rats fed a high salt diet and receiving infusion of either angiotensin II (ANG II; 100ng/kg/min; n = 4) or saline vehicle (n = 6) by subcutaneously implanted osmotic minipump (c,d). Mn SOD expression was normalized to β-actin, and all values are summarized as mean ± SEM. *Significant difference (P < 0.05) in high salt–fed rats vs. normal salt–fed rats.
DISCUSSION
The results of this study show that a short-term increase in dietary salt intake reduces the expression of the crucial antioxidant enzymes Cu/Zn SOD and Mn SOD in the cerebral circulation of congenic rats carrying the BN renin allele, concomitant with the development of endothelial dysfunction. Infusion of a subpressor dose of ANG II during the period of HS intake restored the vasodilator response to ACh in isolated MCAs and prevented the reduction of Cu/Zn SOD expression (but not Mn SOD expression) that occurred in HS-fed control animals.
A recent study by Tzemos and colleagues showed that nitric oxide–dependent vasodilation to ACh was severely impaired in young, normotensive humans placed on a short-term HS diet.20 In another study, Dickinson et al.21 reported that reducing sodium intake in obese, normotensive individuals dramatically improved flow-mediated vasodilation in the brachial artery. The exact mechanism by which increased dietary salt intake causes vascular dysfunction in humans remains to be elucidated. However, studies in normotensive rodents have shown that the vascular dysfunction induced by feeding the animals an HS diet is due to salt-induced suppression of the renin–angiotensin system because restoring plasma ANG II to normal physiological levels restores vascular reactivity in multiple vascular beds.16,17,22–24
It is well known that supraphysiological levels of ANG II cause vascular dysfunction and increase superoxide levels in the vasculature.25,26 However, there is increasing evidence that similar pathological conditions occur when circulating ANG II levels are suppressed in normotensive rodents either pharmacologically17,27,28 or because of an increased dietary salt intake.3,6,9–11,29–31 Studies in Sprague–Dawley rats fed an HS diet have shown that reduced expression or activity of the crucial antioxidant enzyme Cu/Zn SOD leads to vascular dysfunction and increased levels of superoxide in the vasculature.8,12 Consistent with these studies, chronic pharmacological inhibition of the renin–angiotensin system with angiotensin converting enzyme inhibitors or angiotensin type 1 receptor antagonists reduces nitric oxide–mediated vasodilation in different vascular beds of normotensive animals,16,17,27,28,32 providing further support for the hypothesis that normal physiological levels of ANG II are necessary to maintain vascular homeostasis.
Previous studies have shown that endothelium-dependent dilation is intact in isolated MCAs of Ren1-BN rats but absent in Dahl SS rats and in narrowed congenic strains carrying closely adjacent segments of BN chromosome 13 on either side of the renin locus (but still retaining the Dahl SS renin allele).17 The most novel and important observation of the present study is that an HS diet downregulates the expression of Cu/Zn SOD and Mn SOD concomitant with the development of endothelial dysfunction in the cerebral circulation in the Ren1-BN congenic strain.
The observation that Cu/Zn SOD expression, but not Mn SOD expression, was higher in the cerebral vasculature of HS-fed congenic rats receiving a low-dose ANG II infusion is interesting because it suggests that ANG II may regulate expression of the cytosolic isoform of SOD (Cu/Zn SOD) but not the mitochondrial form (Mn SOD). This hypothesis is supported by the observation that Ren1-BN congenic rats (which have a normally functioning renin allele) have significantly elevated Cu/Zn SOD expression but equal expression of Mn SOD in the cerebral vasculature compared with Dahl SS rats under normal salt diet conditions.33
The complex nature of the regulation of vascular SOD isoform expression and activity is highlighted in a recent study34 that showed that ANG II infusion increases Cu/Zn SOD expression in mesenteric arteries of wild-type mice but not in extracellular superoxide dismutase (ecSOD) knockout mice. By contrast, ANG II infusion caused a significant decrease in Mn SOD expression in mesenteric arteries of ecSOD knockout mice but not in wild-type mice. A particularly intriguing observation in that study was that ANG II infusion caused a significant increase in Cu/Zn SOD activity in the aorta (but not mesenteric arteries) of ecSOD knockout mice by upregulating copper chaperone protein for SOD and did so in the absence of a change in Cu/Zn SOD expression.
We can only speculate as to underlying mechanisms behind the differential effects of ANG II infusion on Cu/Zn SOD vs. Mn SOD expression in our study (and others as well).34 For example, regulation of Cu/Zn SOD and Mn SOD may involve different transcription factors that have different sensitivities to plasma ANG II levels. In addition, recent studies by Reed et al.35 showed that the ultimate effect of ANG II on specific signal transduction pathways and on coronary collateral development after transient ischemic episodes depends on existing levels of oxidant stress in the tissue, which may be affected by the plasma ANG II levels reached under different experimental conditions. Regardless of these considerations, it is important to note that because rats have a point mutation in the heparin-binding domain of ecSOD36 that is thought to render it dysfunctional at the endothelial–vascular smooth muscle boundary, Cu/Zn SOD contributes to the vast majority of total SOD activity in the vasculature of this species.37 This is further evidenced by multiple studies showing that acute inhibition of Cu/Zn SOD with diethyldithiocarbamate (DETC) increases superoxide levels and causes vascular dysfunction in multiple vascular beds.17,38,39
There are several limitations to this study. First, ANG II infusion did not completely restore the vasodilator response in MCAs of HS-fed rats. One possible explanation for this observation is that consumption of an HS diet may affect muscarinic receptor signaling, similar to altered prostacyclin receptor signaling observed in HS-fed Sprague–Dawley rats24 but in an ANG II-independent manner. Another possible reason for the partial restoration of Ach-induced dilation by ANG II infusion in HS-fed Ren1-BN rats is that the final plasma ANG II levels obtained with subcutaneous infusion may be lower than those obtained with intravenous infusion in other studies13–15 and could, therefore, affect different signal transduction pathways relevant to vascular oxidant stress.35
Another limitation of this study is that we were unable to directly evaluate vascular and mitochondrial superoxide levels in the cerebral vasculature because of limited availability of tissues. However, previous studies from our laboratory4,5,14 and others8–11 have shown that short-term dietary salt intake increases superoxide levels in multiple vascular beds, including the cerebral circulation, independent of an increase in blood pressure. Our laboratory has also shown that infusing HS-fed animals with a subpressor dose of ANG II restores superoxide levels to normal salt control values.4
The direct contributions of specific pro-oxidant enzymes to superoxide levels were also not directly evaluated in this study. However, a reduced capacity to scavenge vascular superoxide will inevitably lead to oxidative stress and reduced endothelial function, regardless of whether pro-oxidant enzyme expression and/or activity are increased.9–10
Despite these limitations, the results of this study provide additional support for the hypothesis that physiological levels of ANG II are necessary to maintain the expression of the crucial antioxidant enzyme Cu/Zn SOD in the cerebral circulation and emphasize the emerging role of physiological levels of ANG II in maintaining antioxidant defenses in the vasculature. However, several important questions remain that are clearly worthy of additional investigation. First, what is the relative role of mitochondrial superoxide levels in contributing to vascular dysfunction in the Dahl SS rat vs. other experimental models that show normal regulation of the renin–angiotensin system? Second, do similar mechanisms regulate Mn SOD expression in the Dahl SS genetic model of SS hypertension and in experimental models that regulate plasma renin activity normally? Finally, and most important, does the apparent absence of a role for plasma ANG II in regulating Mn SOD expression in HS-fed Ren1-BN congenic rats apply to other experimental models and pathophysiological conditions, such as low renin SS hypertension in humans, which is characterized by low levels of circulating ANG II? These intriguing questions are directly relevant to a recent editorial commentary by Wolin,40 emphasizing that the localized activities of different SOD isoforms in the arterial wall are crucial determinants of vascular function in experimental models of vascular disease and that differences in the influence of ANG II on the expression and activity of various forms of SOD in the vessel wall underscores the incomplete understanding that we have regarding the control of these crucial enzymes in health and disease.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by grants from the National Heart, Lung, and Blood Institute (HL-65289 and HL-92026) and a predoctoral fellowship from the American Heart Association Midwest Affiliate (0815532G). This work was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health (8UL1TR000055). The article’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1. Dickinson KM, Clifton PM, Keogh JB. Endothelial function is impaired after a high-salt meal in healthy subjects. Am J Clin Nutr 2011; 93:500–505 [DOI] [PubMed] [Google Scholar]
- 2. Liu FQ, Mu JJ, Liu ZQ, Shi DC, Huang Q, Yuan ZY, Lian QF, Zheng SH. Endothelial dysfunction in normotensive salt-sensitive subjects. J Hum Hypertens 2011; 26:247–252 [DOI] [PubMed] [Google Scholar]
- 3. Lombard JH, Sylvester FA, Phillips SA, Frisbee JC. High-salt diet impairs vascular relaxation mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol 2003; 284:H1124–H1133 [DOI] [PubMed] [Google Scholar]
- 4. Zhu J, Huang T, Lombard JH. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries. J Vasc Res 2007; 44:382–390 [DOI] [PubMed] [Google Scholar]
- 5. Zhu J, Mori T, Huang T, Lombard JH. Effect of high-salt diet on NO release and superoxide production in rat aorta. Am J Physiol Heart Circ Physiol 2004; 286:H575–H583 [DOI] [PubMed] [Google Scholar]
- 6. Weber DS, Lombard JH. Elevated salt intake impairs dilation of rat skeletal muscle resistance arteries via ANG II suppression. Am J Physiol Heart Circ Physiol 2000; 278:H500–H506 [DOI] [PubMed] [Google Scholar]
- 7. Drenjancevic-Peric I, Lombard JH. Reduced angiotensin II and oxidative stress contribute to impaired vasodilation in Dahl salt-sensitive rats on low-salt diet. Hypertension 2005; 45:687–691 [DOI] [PubMed] [Google Scholar]
- 8. Lenda DM, Boegehold MA. Effect of a high salt diet on microvascular antioxidant enzymes. J Vasc Res 2002; 39:41–50 [DOI] [PubMed] [Google Scholar]
- 9. Lenda DM, Boegehold MA. Effect of a high-salt diet on oxidant enzyme activity in skeletal muscle microcirculation. Am J Physiol Heart Circ Physiol 2002; 282:H395–H402 [DOI] [PubMed] [Google Scholar]
- 10. Lenda DM, Sauls BA, Boegehold MA. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. Am J Physiol Heart Circ Physiol 2000; 279:H7–H14 [DOI] [PubMed] [Google Scholar]
- 11. Nurkiewicz TR, Boegehold MA. High salt intake reduces endothelium-dependent dilation of mouse arterioles via superoxide anion generated from nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 2007; 292:R1550–R1556 [DOI] [PubMed] [Google Scholar]
- 12. McEwen ST, Schmidt JR, Somberg L, Cruz LL, Lombard JH. Time-course and mechanisms of restored vascular relaxation by reduced salt intake and angiotensin II infusion in rats fed a high-salt diet. Microcirculation 2009; 16:220–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petersen MC, Munzenmaier DH, Greene AS. Angiotensin II infusion restores stimulated angiogenesis in the skeletal muscle of rats on a high-salt diet. Am J Physiol Heart Circ Physiol 2006; 291:H114–H120 [DOI] [PubMed] [Google Scholar]
- 14. Zhu J, Drenjancevic-Peric I, McEwen S, Friesema J, Schulta D, Yu M, Roman RJ, Lombard JH. Role of superoxide and angiotensin II suppression in salt-induced changes in endothelial Ca2+ signaling and NO production in rat aorta. Am J Physiol Heart Circ Physiol 2006; 291:H929–H938 [DOI] [PubMed] [Google Scholar]
- 15. Gross V, Kurth TM, Skelton MM, Mattson DL, Cowley AW., Jr Effects of daily sodium intake and ANG II on cortical and medullary renal blood flow in conscious rats. Am J Physiol 1998; 274:R1317–R1323 [DOI] [PubMed] [Google Scholar]
- 16. Weber DS, Lombard JH. Angiotensin II AT1 receptors preserve vasodilator reactivity in skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol 2001; 280:H2196–H2202 [DOI] [PubMed] [Google Scholar]
- 17. Durand MJ, Moreno C, Greene AS, Lombard JH. Impaired relaxation of cerebral arteries in the absence of elevated salt intake in normotensive congenic rats carrying the Dahl salt-sensitive renin gene. Am J Physiol Heart Circ Physiol 2010; 299:H1865–H1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cowley AW, Jr, Roman RJ, Jacob HJ. Application of chromosomal substitution techniques in gene-function discovery. J Physiol 2004; 554:46–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drenjancevic-Peric I, Lombard JH. Introgression of chromosome 13 in Dahl salt-sensitive genetic background restores cerebral vascular relaxation. Am J Physiol Heart Circ Physiol 2004; 287:H957–H962 [DOI] [PubMed] [Google Scholar]
- 20. Tzemos N, Lim PO, Wong S, Struthers AD, MacDonald TM. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension 2008; 51:1525–1530 [DOI] [PubMed] [Google Scholar]
- 21. Dickinson KM, Keogh JB, Clifton PM. Effects of a low-salt diet on flow-mediated dilatation in humans. Am J Clin Nutr 2009; 89:485–490 [DOI] [PubMed] [Google Scholar]
- 22. Durand MJ, Raffai G, Weinberg BD, Lombard JH. Angiotensin 1–7 and low dose angiotensin II infusion reverse salt-induced endothelial dysfunction via different mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol 2010; 299:H1024–H1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McEwen ST, Balus SF, Durand MJ, Lombard JH. Angiotensin II maintains cerebral vascular relaxation via EGF receptor transactivation and ERK1/2. Am J Physiol Heart Circ Physiol 2009; 297:H1296–H1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu J, Yu M, Friesema J, Huang T, Roman RJ, Lombard JH. Salt-induced ANG II suppression impairs the response of cerebral artery smooth muscle cells to prostacyclin. Am J Physiol Heart Circ Physiol 2005; 288:H908–H913 [DOI] [PubMed] [Google Scholar]
- 25. Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 1994; 74:1141–1148 [DOI] [PubMed] [Google Scholar]
- 26. Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, Munzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res 2002; 90:E58–E65 [DOI] [PubMed] [Google Scholar]
- 27. Frisbee JC, Lombard JH. Short-term angiotensin converting enzyme inhibition reduces basal tone and dilator reactivity in skeletal muscle arterioles. Am J Hypertens 2000; 13:389–395 [DOI] [PubMed] [Google Scholar]
- 28. Frisbee JC, Weber DS, Lombard JH. Chronic captopril administration decreases vasodilator responses in skeletal muscle arterioles. Am J Hypertens 1999; 12:705–715 [DOI] [PubMed] [Google Scholar]
- 29. Liu Y, Rusch NJ, Lombard JH. Loss of endothelium and receptor-mediated dilation in pial arterioles of rats fed a short-term high salt diet. Hypertension 1999; 33:686–688 [DOI] [PubMed] [Google Scholar]
- 30. Nurkiewicz TR, Boegehold MA. High dietary salt alters arteriolar myogenic responsiveness in normotensive and hypertensive rats. Am J Physiol 1998; 275:H2095–H2104 [DOI] [PubMed] [Google Scholar]
- 31. Boegehold MA. Flow-dependent arteriolar dilation in normotensive rats fed low- or high-salt diets. Am J Physiol 1995; 269:H1407–H1414 [DOI] [PubMed] [Google Scholar]
- 32. Phillips SA, Lombard JH. Chronic AT1 receptor blockade alters the mechanisms mediating hypoxic dilation in middle cerebral arteries. J Cardiovasc Pharmacol 2005; 46:706–712 [DOI] [PubMed] [Google Scholar]
- 33. Durand MJ, Lombard JH. Introgression of the Brown Norway renin allele onto the Dahl salt-sensitive genetic background increases Cu/Zn SOD expression in cerebral arteries. Am J Hypertens 2011; 24:563–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gongora MC, Qin Z, Laude K, Kim HW, McCann L, Folz JR, Dikalov S, Fukai T, Harrison DG. Role of extracellular superoxide dismutase in hypertension. Hypertension 2006; 48:473–481 [DOI] [PubMed] [Google Scholar]
- 35. Reed R, Kolz C, Potter B, Rocic P. The mechanistic basis for the disparate effects of angiotensin II on coronary collateral growth. Arterioscler Thromb Vasc Biol 2008; 28:61–67 [DOI] [PubMed] [Google Scholar]
- 36. Marklund SL. Extracellular superoxide dismutase. Methods Enzymol 2002; 349:74–80 [DOI] [PubMed] [Google Scholar]
- 37. Stralin P, Karlsson K, Johansson BO, Marklund SL. The interstitium of the human arterial wall contains very large amounts of extracellular superoxide dismutase. Arterioscler Thromb Vasc Biol 1995; 15:2032–2036 [DOI] [PubMed] [Google Scholar]
- 38. Karasu C. Time course of changes in endothelium-dependent and -independent relaxation of chronically diabetic aorta: role of reactive oxygen species. Eur J Pharmacol 2000; 392:163–173 [DOI] [PubMed] [Google Scholar]
- 39. Paravicini TM, Chrissobolis S, Drummond GR, Sobey CG. Increased NADPH-oxidase activity and Nox4 expression during chronic hypertension is associated with enhanced cerebral vasodilatation to NADPH in vivo. Stroke 2004; 35:584–589 [DOI] [PubMed] [Google Scholar]
- 40. Wolin MS. Extracellular superoxide dismutase depletion in hypertension unmasks a new role for angiotensin II in regulating Cu,Zn-superoxide dismutase activity. Hypertension 2006; 48:368–369 [DOI] [PubMed] [Google Scholar]