Abstract
BACKGROUND
Sildenafil, a selective phosphodiesterase-type-5 (PDE-5) inhibitor, produces vasodilation that improves erectile dysfunction and pulmonary hypertension. Sildenafil could also cause baroreflex sympathetic activation that would enhance vascular tone and oppose direct vasodilation. We tested the hypothesis that sildenafil administration increases sympathetically mediated vascular tone in healthy middle-aged men.
METHODS
We randomized 9 healthy, middle-aged, male volunteers (mean age 45±2 years) in a double-blind, crossover fashion to receive a single oral dose of sildenafil 100mg or placebo on 2 separate study days. Hemodynamics and forearm blood flow responses were measured at baseline, at 30 and 45 minutes after study drug administration, and then during intra-arterial infusions of vasoactive drugs. After sildenafil and placebo administration, intrabrachial medications were infused to test forearm alpha receptor sensitivity (norepinephrine), cyclic-AMP–mediated vasodilation (isoproterenol), and sympathetically mediated vascular tone (phentolamine) (adenosine was a control vasodilator). Blood samples were taken before and 60 minutes after study drug administration and at the end of the intrabrachial infusions for measurement of plasma norepinephrine concentrations.
RESULTS
Forearm vascular responses to norepinephrine, isoproterenol, and adenosine were not different after placebo and sildenafil administration. Percentage reduction in forearm vascular resistance during phentolamine was significantly lower after sildenafil than placebo (−73% ± 3% vs −63% ± 3%; P = 0.0002). Sildenafil significantly increased plasma norepinephrine compared with placebo 60 minutes after study drug administration and at the end of the study session (P = 0.02).
CONCLUSIONS
Sildenafil increased sympathetically mediated vascular tone in middle-aged healthy men. Alpha-adrenergic–mediated vasoconstriction may offset vasodilation during PDE-5 inhibition and may explain the significant hypotension observed in patients taking alpha-blockers with sildenafil.
Keywords: blood pressure, hypertension, norepinephrine, sildenafil, sympathetic nervous system, vascular tone, vasodilation.
Sildenafil citrate (Viagra, Revatio) is a selective inhibitor of cyclic guanosine monophosphate-specific phosphodiesterase-type-5 (PDE-5) approved for the treatment of erectile dysfunction and pulmonary arterial hypertension.1–3 Inhibition of PDE-5 prolongs the action of cyclic guanosine monophosphate, resulting in enhanced smooth muscle relaxation and vasodilation in the corpus cavernosum, in vessels in the lungs, and in vessels elsewhere in the body.4
Numerous studies have found that PDE-5 inhibition improves endothelial function.5–10 In healthy subjects, local intra-arterial administration of sildenafil into the brachial artery produces a modest dose-dependent increase in forearm blood flow (FBF), suggesting that PDE-5 plays a role in tonic constriction of resistance vessels.11 Sildenafil also increases endothelium-dependent and -independent vasodilation in young smokers and nonsmokers.12 Oral sildenafil, however, does not appear to affect venodilation or flow-mediated dilatation of conduit vessels.13 Recent evidence demonstrates that sildenafil also enhances cyclic adenosine monophosphate (cAMP)–mediated vasodilation in healthy subjects, demonstrating ability to modulate action and effects of other PDE isozymes.14
The therapeutic vasodilatory potential of PDE-5 inhibitors is being widely investigated. In addition to use for the treatment of erectile dysfunction and pulmonary hypertension, PDE-5 inhibitors are being investigated for use with other urologic and cardiovascular indications.15 Studies have suggested that PDE-5 inhibitors may have a role in the treatment of hypertension and heart failure.16,17 However, PDE-5 inhibition has been shown in animal and human studies to increase sympathetic drive,18,19 which could limit the cardiovascular benefits. Our laboratory found that acute PDE-5 inhibition increased muscle sympathetic nerve activity and plasma norepinephrine (NE) in healthy volunteers.18 An animal study demonstrated that injection of sildenafil into the central nervous system (lateral cerebral ventricles) of rats significantly heightened sympathetic nerve activity that was not baroreflex mediated.19 In contrast, acute PDE-5 inhibition did not significantly increase NE spillover in patients with heart failure.20 It is possible that sympathetic activation after PDE-5 inhibition may cause vasoconstriction that attenuates vasodilatory actions. However, the consequences of sympathetic activation depend on the type of tissue innervated (e.g., muscle versus fat vs. skin) and the dominant receptor subtypes in those tissues. Specifically, it is not known if sildenafil-induced sympathetic activation increases alpha-adrenergic sympathetic vascular tone. Therefore, we tested the hypothesis that PDE-5 inhibition alters alpha-receptor sensitivity, beta-receptor sensitivity, and alpha-adrenergic sympathetic vascular tone in healthy, middle-aged men.
METHODS
Subjects
We studied healthy, middle-aged, male subjects. Subjects were randomized to receive a single oral dose of 100mg sildenafil or placebo in a double-blind fashion at 2 study visits separated by at least 4 days. Subjects were nonsmokers, normotensive, free of disease, and not taking any medications or vitamins. Fasting serum lipids and glucose were measured to rule out unrecognized hypercholesterolemia (total cholesterol < 240mg/dl) or diabetes (glucose < 110mg/dl). Informed written consent was obtained from each subject. The study was approved by the University of Iowa Institutional Review Board and complied with all institutional guidelines.
Measurements
Heart rate was measured continuously with a lead 2 electrocardiogram, and blood pressure was measured by an automatic sphygmomanometer (Lifestat 200; Physio-Control, Redmond, WA). Bilateral FBF was measured by venous occlusion plethysmography (EC4 Hokanson, Bellevue, WA) using indium/gallium-in-silastic strain gauges.21
Protocol and procedures
After a screening visit, all subjects were studied at similar times in the supine position after fasting for at least 12 hours. All studies were performed in a General Clinical Research Center Human Cardiovascular Physiology Laboratory, which was kept at a constant room temperature between 22 ºC and 24 ºC. Subjects were asked to refrain from caffeine for 12 hours, alcohol for 24 hours, and nonsteroidal anti-inflammatory drugs or antihistamines for 7 days before study sessions. Baseline FBF, blood pressure, and heart rate were recorded during 3 5-minute periods during a 30-minute baseline period before oral study drug administration. These variables were measured in an identical fashion 30 minutes, 45 minutes, and 60 minutes after study drug administration. FBF was not measured 60 minutes after study drug administration because intra-arterial infusions were being performed at that time. Immediately after study drug administration (and before measurement of variables 30 minutes after study drug), the brachial artery of the nondominant arm was cannulated with a 27 standard wire gauge steel cannula and then infused with normal saline.
Sympathetic vascular tone responses
Forearm vascular reactivity studies were performed to evaluate the effect of PDE-5 inhibition on alpha-receptor sensitivity, cAMP-mediated vasodilation, and sympathetically mediated vascular tone. Forty-five minutes after study drug administration, NE (48 and 480 pmol/min; Bedford Laboratories, Bedford, OH), adenosine (ADEN) (30 and 300 mcg/min; Astellas Pharma, Northbrook, IL), isoproterenol (ISO) (25 and 250ng/min; Hospira, Lake Forest, IL), and phentolamine (PHEN) (120 mcg/min; Bedford Laboratories, Bedford, OH) were infused intra-arterially. Each dose was given for 6 minutes, with the exception of PHEN (18 minutes). NE was infused to test alpha-receptor function; ADEN was a control vasodilator; ISO was infused to test the effect of sildenafil on cAMP vasodilation; and PHEN was used to test the effect of sildenafil on sympathetically mediated vascular tone. PHEN was given last because its maximal effect is not seen immediately. NE was always infused first to maximize washout time before PHEN administration. The order of ISO and ADEN was randomized and kept consistent for each subject at the placebo and sildenafil visits. After the PHEN infusion, NE (480 pmol/min) and PHEN (120 mcg/min) were coinfused to test the extent of alpha-receptor blockade. Normal saline was infused for up to 20 minutes between intra-arterial drugs to allow blood flow to return to baseline. FBF responses were measured bilaterally (i.e., infused and noninfused arm). Forearm vascular resistance (FVR) was calculated by dividing mean arterial pressure by blood flow to account for confounding variables (e.g., hemodynamic changes) on resting vascular tone.
Plasma NE was determined before study drug administration, at 60 minutes after study drug administration, and at the end of the study session. Blood samples for NE determinations were collected in prechilled heparinized tubes and placed on ice. Plasma was immediately separated at 2800rpm for 10 minutes and stored at −80 °C until the day of analysis.
Assays
Plasma catecholamines were determined using high-performance liquid chromatography with electrochemical detection in the General Clinical Research Center Analytical Laboratory.22 The inter- and intra-assay coefficients of variation were 3.4% and 3.1%, respectively, and the lower limit of detection was 25 pg/mL. Total cholesterol and serum glucose concentrations were measured using established methods by the University of Iowa Hospitals and Clinics Clinical Laboratory.
Analyses
Hemodynamics, electrocardiogram, and FBF were recorded simultaneously on a computerized data acquisition system (MacLab; AD Instruments, Colorado Springs, CO) in combination with a Macintosh Quadra 950 Computer (Apple Computer, Cupertino, CA). FBF was expressed as milliliters per minute per 100ml forearm volume. To account for any systemic effects, forearm vasodilatory responses were expressed as absolute values or percentage change from baseline in the ratio between the infused arm and noninfused arm.
Results are expressed as mean ± SEM. Baseline differences between treatment groups (sildenafil vs. placebo) were determined using Wilcoxon signed rank tests for hemodynamic variables. Differences between treatment groups were determined using mixed model analysis for repeated measures (SAS version 8.2; SAS Institute, Cary, NC) for hemodynamic variables (heart rate, mean arterial pressure), plasma catecholamines, and resistance vessel responses to NE, ADEN, and ISO. A 2-way analysis of variance was used to analyze differences in FBF and FVR during PHEN infusion. The key variable for mixed model analysis was the group-by-time or group-by-dose interaction. Statistical significance was defined as P < 0.05 using 2-tailed tests.
RESULTS
Subjects
We enrolled 9 middle-aged male subjects (mean age 45±2 years) who completed both study visits. Subjects had normal blood pressure, cholesterol, and serum glucose concentrations. Subject characteristics can be found in Table 1.
Table 1.
Subject characteristics
| Age, years | 45±2 |
| Body mass index, kg/m2 | 27±0.6 |
| Systolic blood pressure, mm Hg | 127±2 |
| Diastolic blood pressure, mm Hg | 77±2 |
| Total cholesterol, mg/dl | 186±13 |
| Triglycerides, mg/dl | 101±18 |
| HDL, mg/dl | 48±4 |
| LDL, mg/dl | 118±11 |
| Serum glucose, mg/dl | 94±2 |
Values are means ± SE.
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Hemodynamics and forearm vascular responses
Mean hemodynamic and forearm vascular response values before and 30, 45, and 60 minutes after study drug administration are found in Table 2. Mean arterial pressure at baseline on sildenafil and placebo study days was similar (P = 0.82). Mean arterial pressure increased slightly from baseline over the 60-minute study period after placebo administration (mean change = +4±1mm Hg; P = 0.01) and was slightly reduced after sildenafil administration (mean change = −4±1mm Hg; P = 0.006) (P = 0.001, sildenafil vs. placebo). Heart rate was similar at baseline and slightly but significantly increased after sildenafil administration compared with placebo administration (P = 0.03). FVR at baseline was similar before sildenafil and placebo administration, respectively, and remained relatively unchanged over the subsequent 45-minute study periods (P = 0.16 sildenafil vs. placebo). FBF was similar at baseline and did not change significantly during the 45 minutes after sildenafil and placebo administration (P = 0.19 sildenafil vs. placebo).
Table 2.
Hemodynamic and forearm vascular responses to sildenafil and placebo
| Sildenafil | Placebo | P valuea | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 30 min | 45 min | 60 min | Baseline | 30 min | 45 min | 60 min | ||
| MAP, mm Hg | 93±3 | 92±3 | 91±3 | 89±3 | 93±3 | 95±4 | 96±3 | 96±4 | 0.001 |
| HR, bpm | 53±3 | 57±4 | 55±4 | 55±4 | 55±3 | 53±3 | 55±4 | 52±3 | 0.03 |
| FVR, mm Hg • min • 100mL/mL | 36±4 | 35±5 | 37±6 | — | 31±3 | 39±6 | 38±5 | — | 0.16 |
| FBF, ml/min/100ml tissue | 2.8±0.3 | 2.9±0.3 | 2.7±0.3 | — | 3.2±0.3 | 2.8±0.3 | 2.8±0.3 | — | 0.19 |
Abbreviations: bpm, beats per minute; FBF, forearm blood flow; FVR, forearm vascular resistance; HR, heart rate; MAP, mean arterial pressure.
aAnalysis of variance: group × time interaction (group = sildenafil or placebo).
Forearm skeletal muscle vascular responses to NE, ADEN, ISO, and PHEN
Infusion of vasoactive NE, ADEN, ISO, and PHEN into the brachial artery did not change blood pressure, heart rate, or noninfused arm FBF (results not shown). NE produced a dose-dependent decrease in FBF (Table 3) and a dose-dependent increase in FVR, which were similar after sildenafil and placebo administration (Table 4). Both ADEN and ISO produced dose-dependent increases in FBF (Table 3) and decreases in FVR, which were similar after sildenafil and placebo administration (Table 4). FBF before PHEN was 2.7±0.3ml/min/100ml at the sildenafil and 2.7±0.2ml/min/100ml at the placebo visits. FBF increased to 8.8±1.2, 9.0±1.1, and 9.4±1.1ml/min/100ml during PHEN after sildenafil administration and increased to 7.1±0.7, 7.3±0.8, and 7.6±0.9ml/min/100ml during PHEN after placebo administration (P = 0.04 sildenafil vs. placebo). Percentage reduction in FVR (P = 0.0002) and percentage increase in FBF (P = 0.01) were also significantly greater (approximately 35% relative difference in percentage increase FBF) during PHEN after sildenafil administration compared with placebo administration (Figure 1). Coinfusion of PHEN and NE completely prevented vasoconstriction seen with NE alone and, in fact, produced vasodilation, indicating that alpha-receptor blockade was maximal (Figure 2).
Table 3.
Forearm blood flow responses to intra-arterial norepinephrine (NE), adenosine (ADEN), and isoproterenol (ISO)
| Sildenafil | Placebo | P valuea | |
|---|---|---|---|
| FBF (ml/min/100ml tissue) | FBF (ml/min/100ml tissue) | ||
| NE | |||
| Baseline | 2.7±0.3 | 2.7±0.2 | 0.65 |
| 48 pmol/min | 2.1±0.2 | 2.1±0.1 | |
| 480 pmol/min | 1.7±0.2 | 1.7±0.1 | |
| ADEN | |||
| Baseline | 2.7±0.3 | 2.7±0.2 | 0.90 |
| 30 mcg/min | 6.3±1 | 6.9±1.1 | |
| 300 mcg/min | 14.7±2.4 | 14.7±1.9 | |
| ISO | |||
| Baseline | 2.7±0.3 | 2.7±0.2 | 0.26 |
| 25 mcg/min | 6.7±1.2 | 5.4±0.6 | |
| 250 mcg/min | 14.6±2.4 | 11.5±1.5 | |
Abbreviation: FBF, forearm blood flow (ratio of infused arm to noninfused arm).
aAnalysis of variance: Group × dose interaction (group = sildenafil or placebo).
Table 4.
Forearm vascular resistance during norepinephrine (NE), adenosine (ADEN), and isoproterenol (ISO)
| Sildenafil | Placebo | P valuea | |
|---|---|---|---|
| % Change in FVR | % Change in FVR | ||
| NE | |||
| 48 pmol/min | +24±7 | +22±7 | 0.68 |
| 480 pmol/min | +64±15 | +56±14 | |
| ADEN | |||
| 30 mcg/min | −55±5 | −56±5 | 0.67 |
| 300 mcg/min | −80±2 | −78±2 | |
| ISO | |||
| 25ng/min | −52±5 | −50±4 | 0.47 |
| 250ng/min | −77±3 | −72±3 | |
Abbreviation: FVR, forearm vascular resistance.
aAnalysis of variance: Group × dose interaction (group = sildenafil or placebo).
Figure 1.
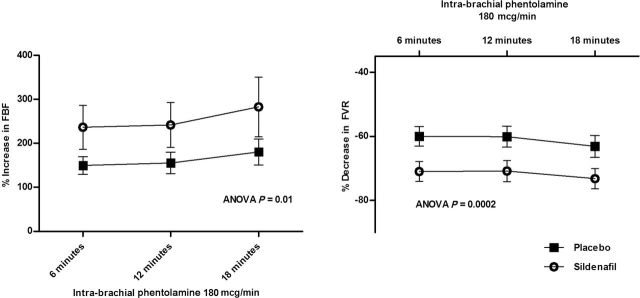
Percentage change in forearm blood flow (FBF) (left panel) and forearm vascular resistance (FVR) (right panel) during intra-arterial infusion of phentolamine (PHEN) for sildenafil (open circles) and placebo (filled squares). Percentage increase in FBF was significantly greater during infusion of PHEN after sildenafil administration compared with placebo administration (P = 0.001). Similarly, percentage reduction in FVR during PHEN was also significantly greater after sildenafil administration compared with placebo administration (P = 0.001). Abbreviation: ANOVA, analysis of variance.
Figure 2.
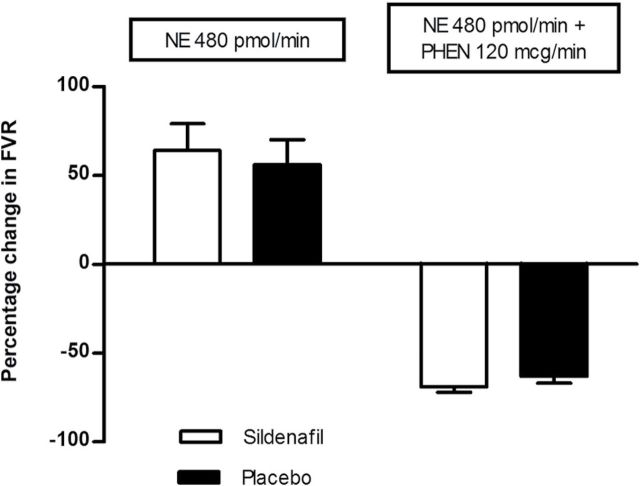
Similar forearm vasoconstriction to norepinephrine (NE) 480 pmol/min is depicted after sildenafil (open bar) and placebo (filled bar) administration (P = 0.62) (left side). Similar forearm vasodilation to NE 480 pmol/min coinfused with phentolamine (PHEN) 120 mcg/min is depicted after sildenafil (open bar) and placebo (filled bar) administration (P = 0.20) (right side).
Plasma NE
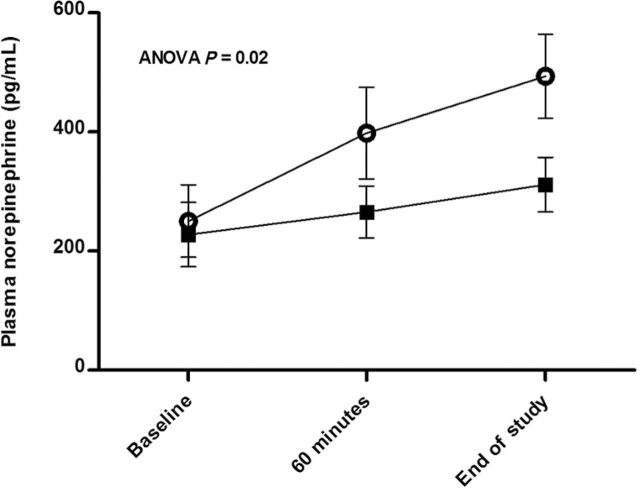
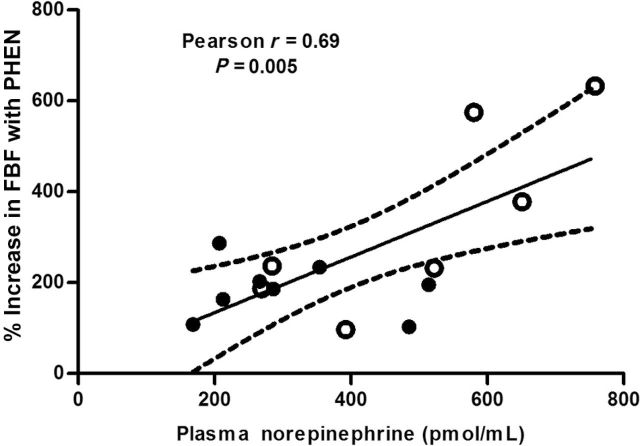
Plasma NE was similar at baseline before sildenafil and placebo administration (219±29 pg/ml and 225±28 pg/ml, respectively). Plasma NE significantly increased after sildenafil administration (P = 0.0006) but not placebo administration (P = 0.12) at 60 minutes and at the end of the study session (Figure 3). Compared with placebo, sildenafil increased NE over the study period (P = 0.02) (Figure 3). A significant positive correlation was found between percentage increase in forearm blood flow during PHEN and plasma NE concentrations at the end of the study visit (Pearson r = 0.69; P = 0.005) (Figure 4).
Figure 3.
Plasma norepinephrine concentrations (pg/ml) at baseline, 60 minutes after study drug administration, and at the end of the study session after sildenafil (open circles) and placebo (filled squares) administration. After sildenafil administration, plasma norepinephrine significantly increased compared with baseline and placebo and was significantly different from placebo at 60 minutes (P = 0.03) and at the end of the study session (P = 0.001) (P = 0.02 group × time interaction). Plasma norepinephrine did not significantly increase from baseline following placebo (P = 0.12). Abbreviation: ANOVA, analysis of variance.
Figure 4.
Correlation of plasma norepinephrine concentrations at the end of the study visit to increase in forearm blood flow (FBF) during phentolamine (PHEN) infusion. Change in FBF after 18 minutes was positively correlated with plasma norepinephrine concentrations (r = 0.69; P = 0.005). Filled dots represent values from placebo visit, and open dots are from sildenafil visit.
DISCUSSION
The novel finding of this study is that sildenafil significantly increases sympathetically mediated resistance vessel tone in human subjects. In addition, sildenafil significantly increases plasma NE concentrations, confirming our previous findings that demonstrated sildenafil-induced sympathetic activation.18 The positive correlation we observed between plasma NE concentrations and vasodilatation to PHEN infusion provides additional support for this idea and suggests that PDE-5–induced sympathetic activation is directed to the vasculature. In contrast with a previous study,14 we did not find that PDE-5 inhibition influenced beta-adrenergic–mediated vasodilation (via cyclic AMP). The increase in sympathetic vascular tone after PDE-5 inhibition cannot be explained by differences in alpha-receptor sensitivity because responses to intra-arterial NE were similar after sildenafil and placebo.
We have previously shown that sildenafil is associated with sympathetic activation in healthy male volunteers.18 In this study, circulating NE levels were significantly elevated after sildenafil administration, consistent with our previous findings. Subsequently, an animal study demonstrated that injection of sildenafil into the lateral cerebral ventricles in rats significantly increased lumbar sympathetic nerve activity and sympathetic activation assessed by heart rate and blood pressure variability.19 Sildenafil may be acting to increase SNA through the baroreflex, which seems likely given its vasodilator actions. Alternatively, central sympathetic activation induced by PDE-5 inhibition is possible because sildenafil crosses the blood–brain barrier23,24 and PDE-5 is found in the central nervous system.25 Sympathetic activation after PDE-5 inhibition may be particularly problematic in populations known to have elevated baseline sympathetic nervous system activity, like heart failure. However, studies indicate that sildenafil is well tolerated in patients with heart failure over the long term26 and that heart failure patients may not experience sympathetic activation with PDE-5 inhibitors. Whereas Piccorillo et al.27 reported increased sympathetic modulation of sinus node discharge (heart rate variability) after sildenafil administration in heart failure patients, Al-Hesayen et al. did not find evidence of increased systemic sympathetic activation in patients with heart failure.20 In fact, Al-Hesayen et al. reported that sildenafil reduced cardiac efferent sympathetic activity assessed by NE spillover.20 Thus, sympathetic responses after PDE-5 inhibition may differ in various patient populations. As a result, it will be important to further characterize the sympathetic effects of PDE-5 inhibition in both healthy patients and in patients with different cardiovascular disorders.
Sildenafil increased alpha-adrenergic sympathetic vascular tone compared with placebo in our study. A 35% increase in sympathetic vascular tone may be clinically relevant in patients with concomitant conditions and medications predisposing to hemodynamic instability. PDE-5 inhibitors have labeled interactions with alpha-adrenergic blockers and may produce significant hypotension when used concurrently.3,28 The effects of coadministration of PDE-5 inhibitors and alpha-blockers on blood pressure have been evaluated in a number of studies.3,28–30 PDE-5 inhibitors do not appear to significantly reduce blood pressure when used concomitantly with the uroselective alpha-blockers tamsulosin and alfuzosin.29,30 However, when used with nonuroselective alpha blockers (doxazosin, terazosin), PDE-5 inhibitors have produced significant blood pressure reductions in some patients.3,28 Increasing evidence suggests that erectile dysfunction and lower urinary tract symptoms are associated,31 and thus, patients may require simultaneous treatment with both alpha-blockers and PDE-5 inhibitors. The results of the current study support the premise that sympathetic activation maintains vascular tone after PDE-5 inhibition. Loss of adrenergic tone during alpha blockade may render certain patients more susceptible to significant hypotension. To our knowledge, this is the first study to document a mechanism for the pharmacodynamic interaction between alpha-blockers and PDE-5 inhibitors.
Similar to PDE-5 inhibitors, vasodilating agents also increase sympathetic activity that may cause significant, additive reductions in blood pressure with concomitant use. Direct vasodilators (hydralazine and nifedipine) increase sympathetic activity and produce additive reductions in blood pressure with alpha blockers.32–34 However, because patients treated with nifedipine and hydralazine most often have high blood pressure, clinicians are monitoring blood pressure and expect reductions from dual therapy. In contrast, patients may be taking alpha-blockers for prostate issues and may not expect significant reductions in blood pressure with addition of a PDE-5 inhibitor.
Although we have demonstrated sympathetic activation after acute administration of a PDE-5 inhibitor, it is unknown whether PDE-5 inhibition maintains sympathetic activation with chronic daily use. Understanding sympathetic effects of chronic PDE-5 inhibition is important because patients are increasingly taking PDE-5 inhibitors daily for treatment of erectile dysfunction and pulmonary arterial hypertension on a chronic basis. Although PDE-5 inhibitors effectively reduce pulmonary artery pressures, improve exercise ability, and delay clinical worsening over 12 weeks,35 PDE-5 inhibitor–induced sympathetic activation and elevated NE concentrations may have deleterious effects on pulmonary vessels over time. Pulmonary hypertension is characterized by remodeling of pulmonary arteries and arterioles and increased pulmonary vascular tone.36 Proliferation of vascular smooth muscle cells and fibroblasts appears to underlie a portion of the vascular remodeling in pulmonary hypertension. Stimulation of alpha-receptors by NE may promote proliferation, hypertrophy, and movement of vascular smooth muscle cells and adventitial fibroblasts both in cell culture and in vivo.37–40 Recently, Faber et al. demonstrated that catecholamine stimulation of alpha receptor subtypes 1B and 1D contribute to vascular remodeling in an animal model of pulmonary hypertension caused by hypoxia.33 A recent, long-term, open-label extension study followed subjects taking sildenafil for 3 years after an initial 12-week placebo-controlled clinical study.41 After 3 years, 60% of patients improved or maintained their functional status.41 Overall, these numbers are encouraging, but the data suggest that functional status declines in a subset of patients taking long-term sildenafil. Testing whether sympathetic activation is maintained during chronic PDE-5 inhibitor therapy will be important in elucidating the long-term benefits and possible deleterious effects of PDE-5 inhibitors in the treatment of pulmonary hypertension.
Unlike a previous Swiss study, we did not demonstrate differences in cAMP-mediated vasodilation after sildenafil and placebo administration.14 Absolute forearm blood flow (Table 3) responses to isoproterenol after sildenafil and placebo administration in our study are comparable with the values from the Swiss study. However, after accounting for possible systemic effects of intra-arterial infusions and environmental influences (by evaluating the ratio of bilateral forearm blood flow responses) and hemodynamic changes (by evaluating FVR) during the study, forearm responses to ISO were similar between sildenafil and placebo in our study. Differences between these findings and others may be due to study design, age of the subjects studied, and possible ethnic differences in PDE-5 distribution within the cardiovascular system. Our findings suggest that PDE-5 inhibition does not augment cAMP-mediated vasodilation in healthy, middle-aged, American men.
Our study had multiple important strengths. First, all participants were free of disease, medications, and vitamins and had normal lipids, eliminating any confounding effect on endothelial function. Second, subjects studied were within the age range of a substantial proportion of patients who are prescribed sildenafil. Third, we employed a randomized, double-blind study design. The order of placebo and sildenafil and the order of administration for intra-arterial ADEN and ISO were randomized. Fourth, hemodynamics and forearm blood flow responses to vasodilators were measured within the time window when sildenafil serum concentrations were maximal. Fifth, differences in alpha-receptor sensitivity and blockade were excluded as reasons for differences in PHEN responses by infusing NE alone and with PHEN. Last, forearm blood flow was measured in both arms, ruling out any possible confounding systemic effects of intra-arterially administered vasodilators or external influences.
There are limitations to our study. We measured responses to acute PDE-5 inhibition and not after chronic therapy. Future studies should evaluate sympathetic and vascular responses to PDE-5 inhibition after chronic therapy. Second, we measured vascular responses in forearm resistance vessels, which may not represent the responses in all vascular beds. Third, we only tested alpha-receptor sensitivity with NE, and not phenylephrine. Use of phenylephrine may avoid potential beta-adrenergic receptor-mediated vasodilation with NE. However, in our past studies, vasoconstriction with NE and phenylephrine was similar.42 In addition, because we demonstrated similar beta-adrenergic mediation vasodilation to isoproterenol, we do not believe that differential beta-mediated vasodilation affected responses to NE. Lastly, our study evaluated healthy, middle-aged men and therefore these results cannot be extrapolated to women or to patients with cardiovascular disease or diabetes.
In conclusion, our study shows that sildenafil increases sympathetically mediated vascular tone and plasma NE concentrations in middle-aged men. Sildenafil slightly reduced blood pressure and slightly increased heart rate but did not significantly change resting forearm vascular resistance compared with placebo. Sympathetic activation directed to vasculature maintains vascular tone after PDE-5 inhibition and may explain the pharmacodynamic interaction between alpha blockers and PDE-5 inhibitors.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
J.M.D. was a recipient of an American College of Clinical Pharmacy Research Institute/Merck and Company Cardiovascular Training Fellowship and completed this work when he was at the University of Iowa. W.G.H. is supported by National Insitutes of Health grant HL-14388. This research was supported through grant M01 RR00059 from the General Clinical Research Centers Program, National Center for Research Resources.
REFERENCES
- 1. Rendell MS, Rajfer J, Wicker PA, Smith MD. Sildenafil for treatment of erectile dysfunction in men with diabetes. A randomized controlled trial. JAMA 1999; 281:421–426 [DOI] [PubMed] [Google Scholar]
- 2. Herrmann HC, Chang G, Klugherz BD, Mahoney PD. Hemodynamic effects of sildenafil in men with severe coronary artery disease. N Engl J Med 2000; 342:1622–1626 [DOI] [PubMed] [Google Scholar]
- 3. Pfizer Sildenafil prescribing information. Pfizer:New York, 2010. [Google Scholar]
- 4. Boolell M, Allen MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM, Osterloh IH, Gingell C. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res 1996; 8:47–52 [PubMed] [Google Scholar]
- 5. Behr-Roussel D, Oudot A, Caisey S, Coz OL, Gorny D, Bernabé J, Wayman C, Alexandre L, Giuliano FA. Daily treatment with sildenafil reverses endothelial dysfunction and oxidative stress in an animal model of insulin resistance. Eur Urol 2008; 53:1272–1280 [DOI] [PubMed] [Google Scholar]
- 6. Rossi R, Nuzzo A, Lattanzi A, Coppi F, Modena MG. Sildenafil improves endothelial function in patients with pulmonary hypertension. Pulm Pharmacol Ther 2008; 21:172–177 [DOI] [PubMed] [Google Scholar]
- 7. Aversa A, Greco E, Bruzziches R, Pili M, Rosano G, Spera G. Relationship between chronic tadalafil administration and improvement of endothelial function in men with erectile dysfunction: a pilot study. Int J Impot Res 2007; 19:200–207 [DOI] [PubMed] [Google Scholar]
- 8. Gori T, Sicuro S, Dragoni S, Donati G, Forconi S, Parker JD. Sildenafil prevents endothelial dysfunction induced by ischemia and reperfusion via opening of adenosine triphosphate-sensitive potassium channels: a human in vivo study. Circulation 2005; 111:742–746 [DOI] [PubMed] [Google Scholar]
- 9. Halcox JP, Nour KR, Zalos G, Mincemoyer R, Waclawiw MA, Rivera CE, Willie G, Ellahham S, Quyyumi AA. The effect of sildenafil on human vascular function, platelet activation, and myocardial ischemia. J Am Coll Cardiol 2002; 40:1232–1240 [DOI] [PubMed] [Google Scholar]
- 10. Desouza C, Parulkar A, Lumpkin D, Akers D, Fonseca VA. Acute and prolonged effects of sildenafil on brachial artery flow-mediated dilatation in type 2 diabetes. Diabetes Care 2002; 25:1336–1339 [DOI] [PubMed] [Google Scholar]
- 11. Jackson G, Benjamin N, Jackson N, Allen MJ. Effects of sildenafil citrate on human hemodynamics. Am J Cardiol 1999; 83:13C–20C [DOI] [PubMed] [Google Scholar]
- 12. Kimura M, Yukihito H, Hara K, Noma K, Sasaki S, Nakagawa K, Goto C, Oshima T, Yoshizumi M, Chayama K. PDE5 inhibitor sildenafil citrate augments endothelium-dependent vasodilation in smokers. Hypertension 2003; 41:1106–1110 [DOI] [PubMed] [Google Scholar]
- 13. Dishy V, Sofowora G, Harris PA, Kandcer M, Zhan F, Wood AJ, Stein CM. The effect of sildenafil on nitric oxide-mediated vasodilation in healthy men. Clin Pharmacol Ther 2001; 70:270–279 [DOI] [PubMed] [Google Scholar]
- 14. Schalcher C, Schad K, Brunner-La Rocca P, Schindler R, Oechslin E, Scharf C, Suetsch G, Bertel O, Kiowski W. Interaction of sildenafil with camp-mediated vasodilation in vivo. Hypertension 2002; 40:763–767 [DOI] [PubMed] [Google Scholar]
- 15. Konstantinos G, Petros P. Phosphodiesterase-5 inhibitors: future perspectives. Curr Pharm Des 2009; 15:3540–3551 [DOI] [PubMed] [Google Scholar]
- 16. Oliver JJ, Melville VP, Webb DJ. Effect of regular phosphodiesterase type 5 inhibition in hypertension. Hypertension 2006; 48:622–627 [DOI] [PubMed] [Google Scholar]
- 17. Guazzi M, Samaja M, Arena R, Vicenzi M, Guazzi MD. Long-term use of sildenafil in the therapeutic management of heart failure. J Am Coll Cardiol 2007; 50:2136–2144 [DOI] [PubMed] [Google Scholar]
- 18. Phillips BG, Kato M, Pesek CA, Winnicki M, Narkiewicz K, Davison D, Somers VK. Sympathetic activation by sildenafil. Circulation 2000; 102:3068–3073 [DOI] [PubMed] [Google Scholar]
- 19. Fazan R, Jr, Huber DA, Silva CA, Dias da Silva VJ, Salgado MC, Salgado HC. Sildenafil acts on the central nervous system increasing sympathetic activity. J Appl Physiol 2008; 104:1683–1689 [DOI] [PubMed] [Google Scholar]
- 20. Al-Hesayen A, Floras JS, Parker JD. The effects of intravenous sildenafil on hemodynamics and cardiac sympathetic activity in chronic human heart failure. Eur J Heart Fail 2006; 8:864–868 [DOI] [PubMed] [Google Scholar]
- 21. Haynes WG, Stuchan FE, Webb DJ. Endothelin ETa and ETb receptors mediate vasoconstriction of human resistance and capacitance vessels in vivo. Circulation 1995; 92:357–363 [DOI] [PubMed] [Google Scholar]
- 22. Hoffman RP, Sinkey CA, Dopp JM, Phillips BG. Systemic and local adrenergic regulation of muscle glucose utilization during hypoglycemia in healthy subjects. Diabetes 2002; 51:734–742 [DOI] [PubMed] [Google Scholar]
- 23. Vobig MA, Klotz T, Staak M, Bartz-Schmidt KU, Engemann U, Walter P. Retinal side effects of sildenafil. Lancet 1999; 353:375 [DOI] [PubMed] [Google Scholar]
- 24. Milman HA, Arnold SB. Neurologic, psychological, and aggressive disturbances with sildenafil. Ann Pharmacother 2002; 36:1129–1134 [DOI] [PubMed] [Google Scholar]
- 25. Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev 1995; 75:725–748 [DOI] [PubMed] [Google Scholar]
- 26. Guazzi M, Vicenzi M, Arena R, Guazzi MD. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo-controlled study. Circ Heart Fail 2011; 4:8–17 [DOI] [PubMed] [Google Scholar]
- 27. Piccorillo G, Nocco M, Lionetti M, Moise A, Naso C, Marigliano V, Cacciafesta M. Effects of sildenafil citrate (Viagra) on cardiac repolarization and on autonomic control in subjects with chronic heart failure. Am Heart J 2002; 143:703–710 [DOI] [PubMed] [Google Scholar]
- 28. Kloner RA, Jackson G, Emmick JT, Mitchell MI, Bedding A, Warner MR, Pereira A. Interaction between the phosphodiesterase 5 inhibitor, tadalafil and 2 alpha-blockers, doxazosin and tamsulosin in healthy normotensive men. J Urol 2004; 172:1935–1940 [DOI] [PubMed] [Google Scholar]
- 29. Giuliano F, Kaplan SA, Cabanis MJ, Astruc B. Hemodynamic interaction study between the alpha1-blocker alfuzosin and the phosphodiesterase-5 inhibitor tadalafil in middle-aged healthy male subjects. Urology 2006; 67:1199–1204 [DOI] [PubMed] [Google Scholar]
- 30. Auerbach SM, Gittelman M, Mazzu A, Cihon F, Sundaresan P, White WB. Simultaneous administration of vardenafil and tamsulosin does not induce clinically significant hypotension does not induce clinically significant hypotension in patients with benign prostatic hyperplasia. Urology 2004; 64:998–1003 [DOI] [PubMed] [Google Scholar]
- 31. Rosen RC, Wei JT, Althof SE, Seftel AD, Miner M, Perelman MA. BPH Registry and Patient Survey Steering Committee. Association of sexual dysfunction with lower urinary tract symptoms of BPH and BPH medical therapies: results from the BPH registry. Urology 2009; 73:562–566 [DOI] [PubMed] [Google Scholar]
- 32. Oates HF, Stoker LM, Stokes GS. Interaction between prazosin, clonidine, and direct vasodilators in the anaesthetized rat. Clin Exp Pharm Physiol 1978; 5:85–89 [DOI] [PubMed] [Google Scholar]
- 33. Leenen FH, Burns RJ, Myers MG, Frankel D. Effects of nifedipine versus hydralazine on sympathetic activity and cardiac function in patients with hypertension persisting on diuretic plus beta-blocker therapy. Cardiovasc Drugs Ther 1990; 4:499–504 [DOI] [PubMed] [Google Scholar]
- 34. Donnelly R, Elliott HL, Meredith PA, Howie CA, Reid JL. Combination of nifedipine and doxazosin in essential hypertension. J Cardiovasc Pharmacol 1992; 19:479–486 [DOI] [PubMed] [Google Scholar]
- 35. Galie N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G. Sildenafil citrate for pulmonary arterial hypertension. N Engl J Med 2005; 353:2148–2157 [DOI] [PubMed] [Google Scholar]
- 36. Faber JE, Szymeczek CL, Cotecchia S, Thomas SA, Tanoue A, Tsujimoto G, Zhang H. α1-Adrenoreceptor-dependent vascular hypertrophy and remodeling in murine hypoxic pulmonary hypertension. Am J Physiol Heart Circ Physiol 2007; 292:H2316–H2323 [DOI] [PubMed] [Google Scholar]
- 37. Erami C, Zhang H, Ho JG, French DM, Faber JE. α1-Adrenoreceptor stimulation directly induces growth of the injured vascular wall in vivo. Am J Physiol Heart Circ Physiol 2002; 283:H1577–H1587 [DOI] [PubMed] [Google Scholar]
- 38. Zhang H, Facemire C, Banes AJ, Faber JE. Different alpha-adrenoreceptors mediate migration of vascular smooth muscle cells and adventitial fibroblasts in vitro. Am J Physiol Heart Circ Physiol 2002; 282:H2364–H2370 [DOI] [PubMed] [Google Scholar]
- 39. Vecchione C, Fratta L, Rizzoni D, Notte A, Poulet R, Porteri E, Frati G, Guelfi D, Trimarco V, Mulvany MJ, Agabiti-Rosei E, Trimarco B, Cotecchia S, Lembo G. Cardiovascular influences of alpha1b-adrenergic receptor defect in mice. Circulation 2002; 105:1700–1707 [DOI] [PubMed] [Google Scholar]
- 40. Faber JE, Yang N, Xin X. Expression of α-adrenoreceptor subtypes by smooth muscle cells and adventitial fibroblasts in rat aorta and in cell culture. J Pharmacol Exp Ther 2001; 298:441–452 [PubMed] [Google Scholar]
- 41. Rubin LJ, Badesch DB, Fleming TR, Galie N, Simonneau G, Ghofrani HA, Oakes M, Layton G, Serdarevic-Pehar M, McLaughlin VV, Barst RJ. Super-2 Study Group Long-term treatment with sildenafil citrate in pulmonary arterial hypertension. Chest 2011; 140:1274–1283 [DOI] [PubMed] [Google Scholar]
- 42. Agapitov AV, Correia ML, Sinkey CA, Haynes WG. Dissociation between sympathetic nerve traffic and sympathetically-mediated vascular tone in normotensive human obesity. Hypertension 2008; 52:687–695 [DOI] [PMC free article] [PubMed] [Google Scholar]