Abstract
Chromatographic separation of the roots of a Kenyan medicinal plant, Clerodendrum eriophyllum, led to the isolation of ten abietane diterpenoids (1-10), one of which (1) was isolated for the first time from a natural source. Using spectroscopic data, the structure of 1 was determined to be 12-hydroxy-8,12-abietadiene-3,11,14-trione. Circular dichroism (CD) spectra showed that the stereochemistry of compounds 1, 3, and 6-8 belongs to the normal series of abietane diterpenes, which confirmed the absolute stereochemistry of the isolated compounds. Compounds 2-10 were evaluated for their in vitro antiplasmodial, antileishmanial, antifungal and antibacterial activities. Compounds 3 and 7 exhibited potent antifungal activity (IC50/MIC 0.58/1.25 and 0.96/2.5 µg/mL, respectively) against C. neoformans, whereas 3, 6 and 7 showed strong antibacterial activity against Staphylococcus aureus and methicillin-resistant S. aureus with IC50/MIC values between 1.33–1.75/2.5–5 and 0.96–1.56/2.5 µg/mL, respectively. In addition, compounds 3 and 9 exhibited potent antileishmanial activity (IC50 0.08 and 0.20 µg/mL, respectively) against L. donovani, while 3 and 7 displayed weak antimalarial activity against Plasmodium falciparum, but 9 was inactive.
Keywords: Clerodendrum eriophyllum, Verbenaceae, abietane diterpenoids, antimicrobial, antileishmanial, antimalarial
Clerodendrum eriophyllum Gürke (Verbenaceae), a small tree 0.5 – 2 m high, is scattered in the dry bushlands of Eastern Kenya where the plant is used by local communities for the treatment of malaria [1]. The plant has no record of previous phytochemical analysis. However, the methanol extract of C. eriophyllum root bark previously showed weak in vitro activity against Plasmodium falciparum D6 and W2 clones (IC50 9.51–10.56 µg/mL); while its methanol and aqueous extracts exhibited significant in vivo chemosuppression (i.e., 90.1% and 61.5%, respectively) against P. berghei infected mice treated intraperitoneally at a dose of 100 mg/kg body weight [2]. The genus Clerodendrum is known to contain iridoids [3, 4], abietane diterpenoids [5–7] and steroids [8]. In the quest for antiplasmodial compounds from Kenyan plants, we have investigated the roots of C. eriophyllum collected from Eastern Kenya. In this paper we report the isolation and structure elucidation of a new abietane diterpenoid, 12-hydroxy-8,12-abietadiene-3,11,14-trione (1), obtained alongside nine other known abietane diterpenoids (2-10).
We also report the antiplasmodial, antileishmanial, antibacterial, and antifungal activities of the isolated compounds.
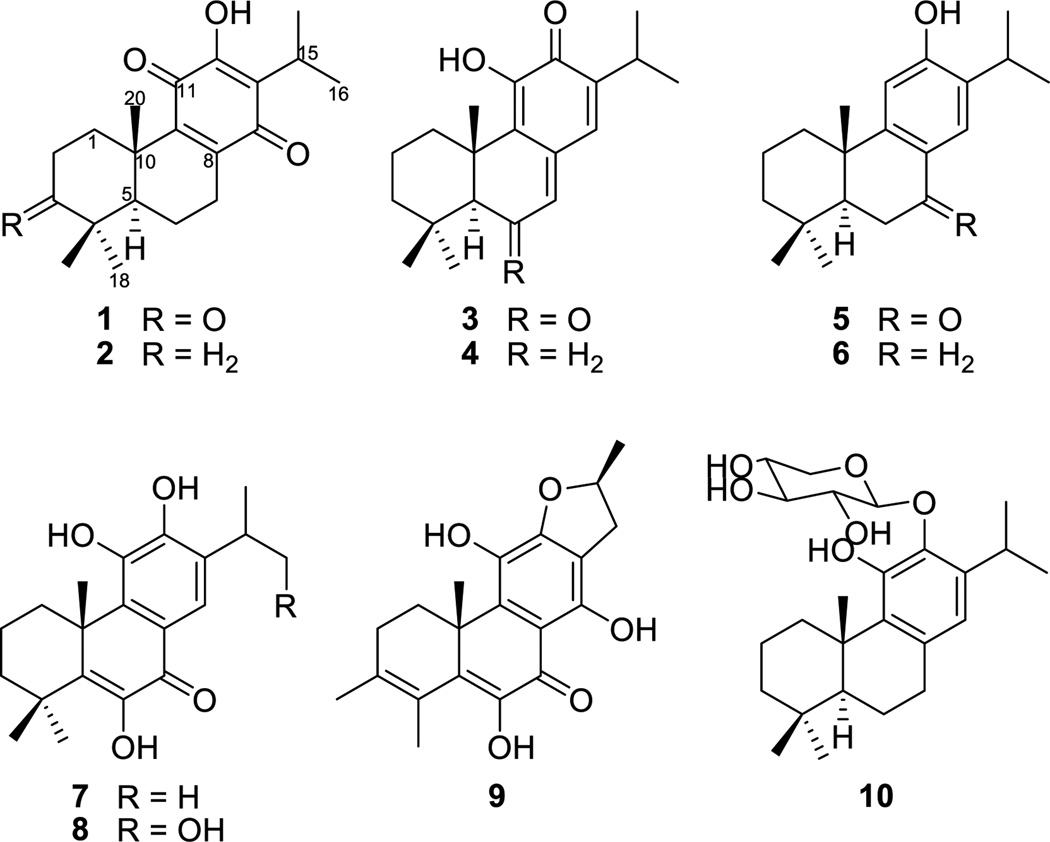
The 1:1 MeOH/CH2Cl2 extract of roots of C. eriophyllum showed moderate antiplasmodial activity with IC50 values of 8.8 µg/mL against chloroquine-sensitive (D6) and -resistant (W2) strains of P. falciparum. Repeated chromatographic purification of this extract gave 12-hydroxy-8,12-abietadiene-3,11,14-trione (1), as well as nine known abietane diterpenoids, namely royleanone (2) [9], taxodione (3) [10], 11- hydroxy-7,9(11),13-abietatrien-12-one (4) [11], sugiol (5) [12], ferruginol (6) [13], 6-hydroxysalvinolone (7) [14], 6,11,12,16-tetrahydroxy-5,8,11,13-abietatetra-en-7-one (8) [15], uncinatone (9) [16], and 11-hydroxy-8,11,13-abietatriene-12-O-β-xylopyranoside (10) [15].
The molecular formula of compound 1 was established as C20H26O4 (m/z 331.1910 [M+H+; calculated for m/z 331.1909) by HRESIMS. The UV absorption maxima at λmax 273 and 378 nm closely matched those of the p-quinone chromophore of royleanone 2 [9]. The IR spectrum indicated the presence of hydroxyl group(s) (νmax 3080–3430 cm−1), unconjugated carbonyl (νmax 1704 cm−1), together with olefinic and conjugated carbonyl absorptions of the p-quinone moiety (νmax 1609, 1633 and 1650 cm−1). The 13C NMR spectrum showed 20 signals, with the sp2 region displaying four olefinic quaternary carbons (δC 124.3, 144.1, 145. 8, 150.6) and two conjugated carbonyls (δC 183.2, 186.9) assignable to the p-quinone moiety. The 1H NMR spectrum did not show signals in either the olefinic or aromatic regions, but did show signals for three methyl singlets at δH 1.09, 1.13 and 1.24, assignable to C-18, C-19 and C-20, respectively, of an abietane skeleton, and an isopropyl group, with two methyl doublets at δH 1.19 and 1.20 (each 3H, J = 7.0 Hz) and a methine septet at δH-15 3.15. The position of the isopropyl group was deduced from HMBC correlations (Table 1) between δH-15 3.15 and δC-12 150.6, δC-13 124.3, and δC-14 186.9. The unconjugated carbonyl at δC 216.7 was established to be at C-3 from HMBC correlations between δH-18 1.13, δH-19 1.09 and the carbonyl carbon signal. Furthermore, a comparison of the 13C NMR spectral data of 1 with those of the known compound royleanone 2 showed close similarities of the carbon signals, except for the differences associated with C-2 – C-5 due to the presence of a carbonyl group at the C-3 position (δC 33.8, 216.7, 46.9 and 50.8 vs. 18.5, 41.2, 33.8 and 44.4 for 2, respectively). A complete set of 2D NMR experiments [1H-1H COSY, 1H-13C HMQC, 1H-13C HMBC (Table 1), 1H-1H NOESY] allowed the unambiguous establishment of the structure of 1 as 12-hydroxy-8,12-abietadiene-3,11,14- trione.
Table 1.
1H and 13C NMR spectroscopic data (J values in Hz, in parenthesis), and 1H-13C HMBC correlations of compound 1.
| H/C | δH | δC | HMBC |
|---|---|---|---|
| 1 | 1.76, m; 2.81, m | 34.5, t | C-2, C-3, C-5, C-10, C-20 |
| 2 | 2.59, ddd (15.6, 9.2, 5.4); 2.51, dt (15.6, 7.2) | 33.8, t | C-1, C-3, C-4, C-10 |
| 3 | - | 216.7, s | |
| 4 | - | 46.9, s | |
| 5 | 1.76, m | 50.8, d | C-1, C-3, C-4, C-6, C-7, C-10, C-18, C-19, C-20 |
| 6 | 1.46, ddd (21.8, 12.0, 4.5); 1.76,m | 18.6, t | C-4, C-5, C-7, C-8, C-10 |
| 7 | 2.31, ddd (19.6, 12.0, 6.0); 2.84, br dd (19.6, 5.4) | 26.0, t | C-5, C-6, C-8, C-9. C-14 |
| 8 | - | 145.8, s | |
| 9 | - | 144.1, s | |
| 10 | - | 37.3 ,s | |
| 11 | - | 183.2 ,s | |
| 12 | - | 150.6 ,s | |
| 13 | - | 124.3, s | |
| 14 | - | 186.9, s | |
| 15 | 3.15, sept (7.0) | 24.1, d | C-12, C-13, C-14, C-16, C-17 |
| 16 | 1.20, d (7.0) | 19.9, q | C-13, C-15, C-17 |
| 17 | 1.19, d (7.0) | 19.8, q | C-13, C-15, C-16 |
| 18 | 1.13, s | 27.7, q | C-3, C-4, C-5, C-19 |
| 19 | 1.09, s | 20.0, q | C-3, C-4, C-5, C-18 |
| 20 | 1.24, s | 20.6, q | C-1, C-5, C-9, C-10 |
| 12-OH | 7.21, s | - | C-11, C-12, C-13 |
Circular dichroism (CD) spectra showed that the stereochemistry of compounds 1, 3, and 6-8 belong to the normal series (A/B trans) of diterpenes. The positive Cotton effect at 275 and 284 nm for compound 1 supports the β-orientation of the methyl group at C-10, i.e. (10S)-Me configuration, according to the rule for π-π* transition of an α,β-unsaturated ketone [17] (Figure 2). The CD spectra of compounds 3 and 6 are in agreement with those of the known taxodione analog [18] and ferruginol [19], respectively, confirming their absolute stereochemistry. CD spectra of 7 and 8, not previously reported, are similar to the related abietane diterpene cyrtophyllone A, whose absolute stereochemistry was determined by X-ray crystallography [17]. Only recently, the absolute configuration of 6-hydroxysalvinolone (7) was determined as (10R)-Me by enhanced X-ray crystallography [20], thus, supporting its stereochemistry deduced from CD spectra.
The antiplasmodial, antileishmanial, antifungal, antibacterial and cytotoxic activities are summarized in Tables 2-4. Compounds 3 and 9 demonstrated potent antileishmanial activities with IC50 values of 0.08 and 0.20 µg/mL, respectively, against L. donovani, compared with those observed for the standard drug amphotericin B (IC50 0.13 µg/mL).
Table 2.
Antiplasmodial, antileishmanial and cytotoxic activity of compounds 2-10.
| Compound/extract | P. falciparum | VERO | L. donovani | ||
|---|---|---|---|---|---|
| D6a | W2b | TC50 µg/mL |
IC50 µg/mL |
IC90 μg/mL |
|
| IC50, µg/mL | |||||
| C.eriophyllum extract | 8.8 | 8.8 | NC | NT | NT |
| 2 | - | - | NC | NT | NT |
| 3 | 1.2 | 1.2 | NC | 0.08 | 0.21 |
| 6 | - | - | NC | 4 | 13 |
| 7 | 1.8 | 2.5 | 4.5 | 3.2 | 6.5 |
| 8 | 3.0 | 4.8 | NC | 12 | 22 |
| 9 | - | - | NC | 0.2 | 0.9 |
| 10 | - | - | NC | NT | NT |
| Chloroquine | <0.026 | 0.14 | NC | NT | NT |
| Artemisinin | <0.026 | <0.026 | NC | NT | NT |
| Pentamidine | NT | NT | NT | 1.4 | 6 |
| Amphotericin B | NT | NT | NT | 0.13 | 0.3 |
Chloroquine-sensetive clone;
Chloroquine-resistant clone;
=Not Active; NT = Not Tested; NC = Not cytotoxic (up to the maximum dose tested; 4.76 µg/mL for pure compounds and 47.6 mg/ml for crude extracts). IC50 is the concentration that affords 50% inhibition of growth.
Table 4.
Antibacterial activities of compounds 2-10.
| IC50/MIC, μg/mL |
|||||
|---|---|---|---|---|---|
| Compound | S. aureus | MRS | E. coli | P. aureginosa | M. intracellulare |
| 2 | −/− | −/− | −/− | −/− | −/− |
| 3 | 1.35/5 | 1.47/2.5 | −/− | −/− | 11.9/− |
| 6 | 1.33/2.5 | 0.96/2.5 | −/− | −/− | 14.5/− |
| 7 | 1.75/5 | 1.56/2.5 | −/− | −/− | −/− |
| 8 | 6.8/20 | 8.44/20 | −/− | −/− | −/− |
| 9 | −/− | −/− | −/− | −/− | −/− |
| 10 | −/− | −/− | −/− | −/− | −/− |
| Ciprofloxacin | 0.1/0.25 | 0.08/0.25 | 0.004/0.008 | 0.06/0.25 | 0.30/1.00 |
=Not Active; NT = Not Tested; IC50 is the concentration that affords 50% inhibition of growth; MIC is the lowest test concentration that allows no detectable growth.
On the other hand, the antiplasmodial activities of compounds 3, 7 and 8 were found to be very weak, with IC50 values of 1.2 – 4.8 µg/mL, when compared with the standard artemisinin (IC50 <0.026 µg/mL). Strong antifungal activities were also displayed by 3 and 7, showing IC50 values of 0.58 and 0.96 µg/mL, respectively, against C. neoformans, as compared with 0.44 µg/mL of the standard amphotericin B. Compounds 3, 6 and 7 showed strong antibacterial activity against Staphylococcus aureus and methicillin-resistant S. aureus with IC50/MIC values between 1.33–1.75/2.5–5 and 0.96–1.56/2.5 µg/mL, respectively. With regard to cytotoxicity, only 6-hydroxysalvinolone (7) showed moderate cytotoxic activity with an IC50 value of 4.5 µg/mL against monkey kidney fibroblasts (VERO). Finally, due to paucity of material, compound 1 could not be evaluated for its in vitro antiparasitic, antimicrobial and cytotoxic activities.
Experimental
General
Optical rotations were measured in CHCl3 or MeOH using an AUTOPOL IV® instrument at ambient temperature. Circular dichroism (CD) spectra were recorded in MeCN using an Olis DCM 20 CD spectrometer at ambient temperature. IR spectra were taken as films on a Bruker Tensor 27 FTIR instrument. UV spectra were obtained in MeCN using a Hewlett-Packard 8453 spectrophotometer; 1D and 2D NMR data were acquired on a Bruker BioSpin instrument at 600 MHz (1H), 150 (13C) in CDCl3 using the residual solvent as int. standard. HRMS were obtained by direct injection using a Bruker Bioapex-FTMS with electrospray ionization (ESI). For column chromatography (CC), Merck silica gel 60 (0.063–0.200 mm) and Fluka Sephadex LH-20 were used as stationary phases; For PTLC, Merck silica gel 60 PF254+366, coated on glass plates to make 1.0 mm layers was used; Analytical TLC was carried out using factory prepared aluminum plates (0.25 mm) coated with silica gel (60 F254, Merck); The isolated compounds were visualized by observing under UV light at 254 or 365 nm, followed by spraying with 1% vanillin-H2SO4 spray reagent.
Plant material
The roots of Clerodendrum eriophyllum were collected from Machakos, Eastern Kenya in November 2007 and identified at the Department of Botany, University of Nairobi, Kenya, where a voucher specimen No. JMFM/2007/11 has been deposited.
Extraction and isolation
The roots of C. eriophyllum were air dried and pulverized to give 1.8 kg of material. This was extracted by cold percolation at room temperature using 1:1 MeOH/ CH2Cl2 (3×4 L, 24 h each), followed by 100% methanol (1×4 L, 24 h) to give 65 g of brown gummy extract, of which 35 g was adsorbed onto 40 g of silica gel and subjected to CC on a silica gel column (300 g, 5×35 cm), eluted with n-hexane/CH2Cl2 (95:5, 3.5 L; 9:1, 1.25 L; 3:1, 2 L; 1:1, 3 L; 1:3, 1 L; 100% CH2Cl21.5 L) followed by CH2Cl2/MeOH (99:1, 1.25 L; 98:2, 1 L; 95:5, 1 L). Sixty-two fractions of eluents, collected in 250 mL aliquots, were concentrated using a rotary evaporator and similar fractions were combined on the basis of TLC analysis. Combination of fraction 5–9 crystallized in n-hexane/CH2Cl2 (95:5) gave 2 (260 mg). Fractions 11–19 (640 mg) were rechromatographed over silica gel (50 g, 2.0×30 cm) and eluted with n-hexane/CH2Cl2(95:5) to give 4 (8.7 mg) after 0.6 L of elution, and 6 (65 mg) after 1.4 L of elution. The latter was further purified by PTLC developed with n-hexane/CH2Cl2(8:2). Fraction 22–28 (160 mg) was purified on a Sephadex LH 20 column (100 g, 2.5×30 cm), eluted with MeOH/ CH2Cl2 1:1 (0.3 L), followed by PTLC developed with n-hexane/CH2Cl2 7:3 to give 3 (28 mg).
Fraction 34–45 (600 mg) was subjected to silica gel CC (50 g, 2.0×30 cm), eluted with CH2Cl2/ n-hexane (8:2) to give 1 (4.0 mg) after 0.28 L of elution, 9 (6.7 mg) after 0.35 L of elution, and 5 (10.3 g) after 0.8 L of elution. Fractions 52–57 (580 mg) were rechromatographed over silica gel (50 g, 2.0×30 cm) and eluted with CH2Cl2 to yield 7 (6.5 mg) and 8 (64.2 mg), both of which crystallized from CH2Cl2, after 0.52 L and 1.3 L of elution, respectively. Compound 10 (15 mg) was obtained after purifying fractions 58–60 on a Sephadex LH 20 column (100 g, 2.5×30 cm) eluted with MeOH/ CH2Cl2 1:1 (0.3 L).
Royleanone (2) [9], taxodione (3) [10], 11-hydroxy-7,9(11),13-abietatrien-12-one (4) [11], sugiol (5) [12], ferruginol (6) [13], 6-hydroxysalvinolone (7) [14], 6,11,12,16-tetrahydroxy-5,8,11,13-abietatetra-en-7-one (8) [15], uncinatone (9) [16] and 11-hydroxy-8,11,13-abietatriene-12-O-β-xylopyranoside (10) [15] were identified by comparison of their full physical (mp and optical rotation) and spectral data (UV, IR, 1H and 13C NMR, and MS) with those reported in the literatures.
12-Hydroxy-8,12-abietadiene-3,11,14-trione (1)
Yellow solid Rf 0.5 (n-hexane/CH2Cl2/MeOH 60:39:1) [αD25 +184 (c 0.16, CHCl3) UV (MeCN) λmax (lg ε), nm: 201 (3.94), 205 (3.86), 273 (3.98), 378 (2.78) CD (MeCN) λmax ([θ], deg·cm2/dmol), nm: 208 (+6.4·103), 275 (+18.9·103), 284 (+20.2·103), 330 (−2.3·103) IR (film) νmaxcm−1: 3430–3080 (OH), 2965 (C-H), 2934 (C-H), 2873 (C-H), 1704 (C=O), 1650 (C=O), 1633 (C=O), 1609 (C=C), 1462 (C-H), 1271 (C-O) 1H and 13C NMR: (see Table 1) HRESIMS: m/z 331.1910 [M+H+ (calcd. for C20H27O4, 331.1909); 329.1758 [M-H− (calcd. for C20H25O4, 329.1753).
Taxodione (3)
UV (MeCN) λmax (lg ε), nm: 211 (3.81), 315 (4.13), 325 (4.13), 334 (4.12), 337 (4.11), 394 (3.38); CD (MeCN) λmax ([θ], deg·cm2/dmol), nm: 205 (−20.2·103), 261 (−12.7·103), 321 (−18.7·103), 337 (19.2·103), 445 (+13.6·103).
Ferruginol (6)
UV (MeCN) λmax (lg ε), nm: 211 (3.90), 219 (3.87), 281 (3.58); CD (MeCN) λmax ([θ], deg·cm2/dmol), nm: 206 (−2.8·103), 211 (+12.9·103), 227 (+9.5·103), 265 (−0.9·103), 301 (−2.3·103).
6-Hydroxysalvinolone (7)
UV (MeCN) λmax (lg ε), nm: 219 (3.97), 250 (3.98), 284 (3.94), 335 (4.00); CD (MeCN) λmax ([θ], deg·cm2/dmol), nm: 213 (+36.3·103), 281 (+30.4·103), 338 (−19.7·103).
6,11,12,16-Tetrahydroxy-5,8,11,13-abietatetra-en-7-one (8)
UV (MeCN) λmax (lg ε), nm: 218 (3.98), 251 (4.01), 285 (3.89), 339 (3.97), 407 (2.90); CD (MeCN) λmax ([θ], deg·cm2/dmol), nm: 212 (+48.9·103), 284 (+29.7·103), 339 (−19.7·103).
Uncinatone (9)
UV (MeCN) λmax (lg ε), nm: 220 (4.06), 228 (4.08), 232 (4.09), 283 (4.17), 298 (4.17), 334 (4.01), 376 (3.95). CD (MeCN) λmax ([θ], deg·cm2/dmol), nm: 205 (−18.8·103), 213 (−18.9·103), 234 (+24.5·103), 241 (+23.9·103), 271 (sh) (−4.3·103), 297 (−17.1·103), 323 (+12.7·103), 350 (−2.7·103), 381 (−5.6·103).
Antimicrobial assay
All organisms were obtained from the American Type Culture Collection (Manassas, VA) and included the fungi Candida albicans ATCC 90028, C. glabrata ATCC 90030, C. krusei ATCC 6258, Cryptococcus neoformans ATCC 90113 and Aspergillus fumigatus ATCC 90906; the bacteria Staphylococcus aureus ATCC 29213, methicillin-resistant S. aureus ATCC 43300 (MRS), Escherichia coli ATCC 35218, Pseudomonas aeruginosa ATCC 27853 and Mycobacterium intracellulare ATCC 23068. Susceptibility testing was performed using a modified version of the CLSI methods [21, 22], as described by Samoylenko et al 23]. Drug controls, ciprofloxacin (ICN Biomedicals, Ohio) for bacteria and amphotericin B (ICN Biomedicals, Ohio) for fungi, were included in each assay.
Antimalarial/parasite LDH assay
The in vitro antimalarial activity was measured by a colorimetric assay that determines the parasitic lactate dehydrogenase (pLDH) activity [23, 24]. The assay was performed in a 96-well microplate and included two P. falciparum strains [Sierra Leone D6 (chloroquine-sensitive) and Indochina W2 (chloroquine-resistant)]. The IC50 values were computed from the dose response curves generated by plotting percent growth against test concentrations. DMSO, artemisinin and chloroquine were included in each assay as vehicle and drug controls, respectively.
Antileishmanial assay
Antileishmanial activity of the compounds was tested in vitro on a culture of Leishmania donovani promastigotes. In a 96 well microplate assay, compounds with appropriate dilution were added to the Leishmania promastigotes culture (2×106 cells/mL). The plates were incubated at 26°C for 72 h and growth of Leishmania promastigotes was determined by Alamar blue assay [25]. Pentamidine and amphotericin B were used as standard antileishmanial agents. IC50 values for each compound were computed from the growth inhibition curve.
Cytotoxicity assay
The in vitro cytotoxic activity was determined against monkey kidney fibroblasts (VERO) following the method described by Samoylenko et al 23]. Doxorubicin was used as the positive and DMSO as the negative (vehicle) control.
Figure 1.
Chemical structures of compounds 1-10 isolated from C. eriophyllum.
Table 3.
Antifungal activities of compounds 2-10.
| IC50/MIC, μg/mL |
|||||
|---|---|---|---|---|---|
| Compound | C. glabrata | C. krusei | C. neoformans | A. fumigatus | C. albicans |
| 2 | −/− | −/− | −/− | −/− | −/− |
| 3 | 5.2/10 | 12.0/− | 0.58/1.25 | 8.9/− | 12.5/− |
| 6 | −/− | −/− | −/− | −/− | −/− |
| 7 | −/− | −/− | 0.96/2.5 | 11.2/− | −/− |
| 8 | 14.9/20 | 14.5/20 | 5.9/20 | −/− | −/− |
| 9 | −/− | −/− | −/− | −/− | −/− |
| 10 | −/− | −/− | −/− | −/− | −/− |
| Amphotericin B | 0.31/0.65 | 0.95/1.25 | 0.44/1.25 | 1.29/2.50 | 0.43/1.25 |
=Not Active; NT = Not Tested; IC50 is the concentration that affords 50% inhibition of growth; MIC is the lowest test concentration that allows no detectable growth.
Acknowledgments
The authors sincerely thank Mr John P. Hester for database management and technical assistance, Mr John Trott and Ms Marsha Wright for assistance in biological work and Dr Bharathi Avula for recording mass spectra. One of our authors (FM) thanks DAAD-NAPRECA for a scholarship. This work was supported in part by the United States Department of Agriculture, Agricultural Research Service Specific Cooperative Agreement No. 58-6408-2-0009 and NIH, NIAID, Division of AIDS, Grant No. AI 27094.
References
- 1.Beenje HK. Kenyan trees, shrubs and lianas. Nairobi, Kenya: National Museums of Kenya; 1994. p. 613. [Google Scholar]
- 2.Muthaura CN, Rukunga GM, Chhabra SC, Omar SA, Guantai AN, Gathirwa JW, Tolo FM, Mwitari PG, Keter LK, Kirira PG, Kimani CW, Mungai GM, Njagi ENM. Antimalarial activity of some plants traditionally used in Meru district of Kenya. Phytotherapy Research. 2007;21:260–267. doi: 10.1002/ptr.2170. [DOI] [PubMed] [Google Scholar]
- 3.Tian J, Zhao QS, Zhang HJ, Lin ZW, Sun HD. New cleroindicins from Clerodendrum indicum. Journal of Natural Products. 1997;60:766–769. [Google Scholar]
- 4.Yang H, Hou AJ, Mei SX, Sun HD, Che CT. Constituents of Clerodendrum bungei. Journal of Asian Natural Products Research. 2002;4:165–169. doi: 10.1080/1028602021000000053. [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Zhu H, Zhang S, Zhang X, Yu Q, Xuan L. Abietane diterpenoids from Clerodendrum bungei. Journal of Natural Products. 2008;71:755–759. doi: 10.1021/np0703489. [DOI] [PubMed] [Google Scholar]
- 6.Fan TP, Min Z, Song G, Iinuma M, Tanaka T. Abietane diterpenoids from Clerodendrum mandarinorum. Phytochemistry. 1999;51:1005–1008. [Google Scholar]
- 7.Fan TP, Min Z, Iinuma M, Tanaka T. Rearranged abietane diterpenoids from Clerodendrum mandarinorum. Journal of Asian Natural Products Research. 2000;2:237–243. doi: 10.1080/10286020008039917. [DOI] [PubMed] [Google Scholar]
- 8.Pandey R, Verma RK, Singh SC, Gupta MM. 4-α-Methyl-24β-ethyl-5α-cholesta-14,25-dien-3β-ol and 24β-ethylcholesta-5,9(11),22E-trien-3β-ol, sterols from Clerodendrum inerme. Phytochemistry. 2003;63:415–420. doi: 10.1016/s0031-9422(03)00146-8. [DOI] [PubMed] [Google Scholar]
- 9.Edwards OE, Feniak G, Los M. Diterpenoid quinones of Inula royleana D.C. Canadian Journal of Chemistry. 1962;40:1540–1546. [Google Scholar]
- 10.Kupchan SM, Karim A, Marcks C. Taxodione and taxodone, two novel diterpenoid quinone methide tumor inhibitors from Taxodium distichum. Journal of the American Chemical Society. 1968;90:5923–5924. doi: 10.1021/ja01023a061. [DOI] [PubMed] [Google Scholar]
- 11.Dellar JE, Core MD, Waterman PG. Antimicrobial abietane diterpenoids from Plectranthus elegans. Phytochemistry. 1996;41:735–738. doi: 10.1016/0031-9422(95)00694-x. [DOI] [PubMed] [Google Scholar]
- 12.Ying BP, Kubo I. Complete proton and carbon-13 NMR assignments of totarol and its derivatives. Phytochemistry. 1991;30:1951–1955. [Google Scholar]
- 13.Samoylenko V, Dunbar DC, Gafur MA, Khan SI, Ross SA, Mossa JS, El-Ferali FS, Tekwani BL, Bosselaers J, Muhammad I. Antiparasitic, nematicidal and antifouling constituents from Juniperus berries. Phytotherapy Research. 2008;22:1570–1576. doi: 10.1002/ptr.2460. [DOI] [PubMed] [Google Scholar]
- 14.Hueso-Rodriguez JA, Jimeno ML, Rodriguez B, Savona G, Bruno M. Abietane diterpenoids from the root of Salvia phlomoides. Phytochemistry. 1983;22:2005–2009. [Google Scholar]
- 15.Han L, Huang X, Dahse H, Moellmann U, Grabley S, Lin W, Satler I. New abietane diterpenoids from the mangrove Avicennia marina. Planta Medica. 2008;74:432–437. doi: 10.1055/s-2008-1034318. [DOI] [PubMed] [Google Scholar]
- 16.Dorsaz A, Marston A, Stoeckli-Evans H, Msonthi JD, Hostettmann K. Uncinatone, a new antifungal hydroquinone diterpenoid from Clerodendrum uncinatum. Helvetica Chimica Acta. 1985;68:1605–1610. [Google Scholar]
- 17.Tian X, Min Z, Xie N, Lei Y, Tian Z, Zheng Q, Xu R, Tanaka T, Iinuma M, Mizuno M. Abietane diterpenes from Clerodendron cyrtophyllum. Chemical & Pharmaceutical Bulletin. 1993;41:1415–1417. [Google Scholar]
- 18.Katti SB, Ruedi P, Eugster CH. Diterpenoid quinomethans, vinylogous quinones and a phyllocladene derivative from Plectranthus purpuratus Harv. (Labiatae) Helvetica Chimica Acta. 1982;65:2189–2197. [Google Scholar]
- 19.Briggs LH, Cain BF, Davis BR, Wilmshurst JK. Absolute configuration of phyllocladene, mirene, rimuene, cupressene, and kaurene. Tetrahedron Letters. 1959;8:13–16. [Google Scholar]
- 20.Fun H, Quah CK, Chantrapromma S. Redetermination and absolute configuration of 6-hydroxysalvinolone. Acta Crystallographica, Section E. 2010;E66(1):o146–o147. doi: 10.1107/S1600536809053197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NCCLS. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi; Proposed Standard, M38-P. National Committee on Clinical Laboratory Standards. 1998;18(13) [Google Scholar]
- 22.NCCLS. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically M7-A5. National Committee on Clinical Laboratory Standards. 2000;20(2) [Google Scholar]
- 23.Samoylenko V, Jacob MR, Khan SI, Zhao J, Tekwani BL, Midiwo JO, Walker LA, Muhammad I. Antimicrobial, antiparasitic and cytotoxic spermine alkaloids from Albizia schimperiana. Natural Product Communications. 2009;4:791–796. [PMC free article] [PubMed] [Google Scholar]
- 24.Makler MT, Ries JM, Williams JA, Bancroft JE, Piper RC, Gibbins BL, Hinriches DJ. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. American Journal of Tropical Medicine Hygiene. 1993;48:739–741. doi: 10.4269/ajtmh.1993.48.739. [DOI] [PubMed] [Google Scholar]
- 25.Mikus J, Steverding D. A simple colorimetric method to screen drug cytotoxicity against Leishmania [by] using the dye Alamar Blue. Parasitology International. 2000;48:265–269. doi: 10.1016/s1383-5769(99)00020-3. [DOI] [PubMed] [Google Scholar]